Abstract
Objectives:
In future healthcare systems, individuals are expected to be more involved in managing their health and preventing illness. A previous study of patient empowerment on a hip fracture pathway uncovered a gap between what the healthcare system provided and patients’ needs and wishes. The aim of this study was to investigate whether a user-driven approach and a participatory design could provide a solution that would bridge this gap.
Methods:
Four workshops and a laboratory test were conducted with healthcare professionals to co-create a final prototype. This was performed in iterative processes through continuous interviews and face-to-face evaluation with patients, together with field studies in patients’ homes, to maintain relevance to end-users, that is, patients and healthcare professionals. The data were analysed according to the plan, act, observe and reflect methodology of iterative processes in participatory design.
Results:
Our results contribute to a key research area within patient involvement. By using participatory design, patients and healthcare professionals gained a mutual understanding and collaborated to create a technological solution that would encompass needs and wishes. Patient empowerment also involved giving healthcare professionals a means of empowerment, by providing them with a platform to support patient education. We found that one solution to bridging the aforementioned gap could be an app, including a range of educational features that would accommodate different learning styles.
Conclusion:
In developing a technological solution, user involvement in a participatory design ensures usability and inclusion of the requested functionalities. This can help bridge the gap between what the healthcare system provided and patients’ needs and wishes and support patients’ individual empowerment needs and self-care capacity. Together with the tools and techniques, the setting in which PD unfolds should be thoughtfully planned.
Keywords: Participatory design, hip fracture, empowerment, self-care, user-driven, tele-health, app
Introduction
As a study on the worldwide impact of fragility fractures has pointed out, osteoporosis and its associated fragility fractures are common conditions that contribute significantly to morbidity, mortality, and healthcare spending.1 Although there is some evidence to suggest a current plateauing of fracture incidence, ageing populations mean that, globally, there is an increasing societal burden of osteoporosis.1 Hip fractures are the most common cause of hospitalisation and account for more than 70% of osteoporosis-related fracture cost.2 Thus, the incremental societal burden of hip fractures represents a significant challenge – for the patient, in the form of functional decline and pain, for their families, and for the society, from the health economics perspective.3 Patients with an osteoporotic hip fracture are at high risk of subsequent fractures; thus, further prevention of fractures is a major issue.4
Systematized programmes aimed at improving perioperative treatment and reducing length of stay (LOS) have become increasingly common as an essential tool for quality development and in improving efficiency in the hospital setting.5–7 Within our healthcare system, individuals are expected to have greater involvement in managing their health and health promotion strategies.8,9 In connection with hospitalisation, the significance of both written and oral information – to provide patients with knowledge and thereby support their self-care – has been highlighted in studies within general contexts, and specifically for older people who have sustained a hip fracture.10 The dissemination of such information and patient education can be challenging: while LOS is decreasing, thus imposing time constraints, requirements for and the magnitude of discharge education have increased.11–13
Patient empowerment is about strengthening and supporting patients’ own resources and capabilities to exercise self-care.14 Empowerment is a concept that focuses on the processes through which people can improve their ability to develop, control, and manage their resources. Patient empowerment is thus about strengthening and supporting patients’ own resources and capabilities. Empowerment also involves giving employees skills, resources, opportunities, and motivation.15 Thus, healthcare professionals (HPs) need to be provided with the skills and resources to empower patients. A study of patient empowerment on a hip fracture pathway with short LOS uncovered a gap between what the healthcare system provided and what patients wanted.11 The study showed that individuals want to oversee their own lives and they prefer to have autonomy. Experiencing a hip fracture and recovering from this serious injury have a physiological effect on individuals and on the way they live. Incurring a hip fracture is also a forceful reminder of the frailty of life and mortality, and this stressful situation complicates the ability to receive information and education.11 Thereby, the conventional way of communicating healthcare information to empower individuals is further challenged on pathways with short LOS. Low levels of patient health literacy are a further challenge, which can limit the accomplishment of important teaching elements.
A common finding in healthcare research is that patients report difficulties in navigating healthcare systems and that a major factor is lack of information at the right time and in the right place.16,17 Tele-health has been shown to be an effective method to support communication and cooperation between individuals and HPs.18–20 Tele-health encompasses a broad variety of technologies and tactics to deliver virtual medical, health, and education services. Tele-health is not a specific service, but a collection of means to enhance care and education delivery. As such, tele-health is defined in broad terms as digitally supported healthcare services over a distance.21 In orthopaedics, there have been some examples of tele-orthopaedic services for paediatric and adult patients.22–25
Successful development and implementation of a tele-health service in healthcare requires an understanding of the clinical challenges and requirements, and user participation.26,27 Participatory design (PD) is a research methodology that promotes and highlights the participation of users in the process of designing technological solutions.8 It has a potential to more closely align with users’ needs.27–29 For more than a decade, PD has been adopted in health research and has contributed to the development of technologies that have changed the way in which health services are being delivered.8,19,30–33 The rationale for adopting PD as the approach in this study was because we wanted to take advantage of new technologies that could help address the emerging needs of hip fracture patients.
The aim of this study was to investigate whether a user-driven approach in a PD can provide a solution to bridge the gap between what the healthcare system provides and what patients need after being treated for a hip fracture, during a short period of hospitalisation.
Methods
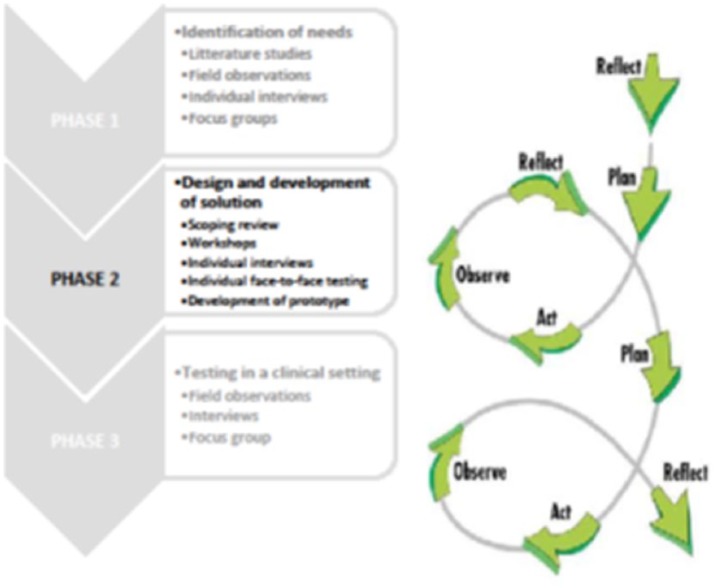
This study reports from the second phase of a research project in which PD is the overall research methodology. PD has its roots in action research (AR). AR is a research methodology that points to changes in the investigated field. The central idea in AR is the scientific approach to study social or organisational issues together with those who experience them. In this regard, AR is characterised by the collaborative relation between the researcher and practice in finding a solution to practical problems and results in changes to practice.34 Actions in AR are conducted in iterative cycles, involving parallel and ongoing actions throughout the research process.35 The iterative cycles consist of the elements plan, act, observe, and reflect. A new plan is made on the basis of reflections of previous plan. PD shares the characteristics of AR, but the focus is on development of future technological solutions and on technology as leverage for organisational change.36–38 PD is not defined by formulas, rules, and strict definitions, but by a commitment to core principles of participation in design. As such, PD is a research methodology that increases and highlights the participation of users in the design process of future technological solutions.28,39
PD in the health sciences is conducted in three phases.8 In phase 1, users’ needs are identified and discussed. In phase 2, the design and development of a prototype is made through several workshops, using creative tools and techniques. Mock-ups and preliminary suggestions towards the final prototype are designed, tested, and re-tested towards the development and final test of the prototype in a laboratory setting. In the third phase, this prototype is tested and evaluated in a clinical setting. The iterative nature of PD emphasises its focus on shared or mutual learning.40 Thus, the involvement of health IT designers in the processes is essential.40 To promote and achieve mutual learning, designers need to have knowledge of users’ needs and wishes. Users need knowledge of potential technological options as well as how these options can be provided. This knowledge and relevant design expertise can be provided by the designers.27
In PD, various methods and user activities are employed to reflect the aim of the study as well as the different phases in the project. Literature studies and field studies are conducted continuously, as part of the process of learning and understanding.9,41
Figure 1 illustrates the three phases in the PD project with a focus on phase 2 and with the iterative cycles illustrated on the right.
Figure 1.
The three phases in the participatory design project – with a focus on phase 2. The iterative processes comprising four steps – planning, acting, observing, and reflecting – are illustrated on the right.
Design
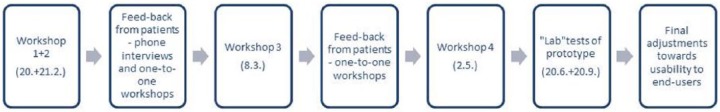
This study reflects phase 2 – the design and development of a solution to cover the needs identified in phase 1 (and reported in a prior study11). The phase was conducted from January to November 2017. Findings from phase 1 informed the phase 2 process. We conducted four workshops and one laboratory test at the Centre for Innovative Medical Technology (CIMT) at Odense University Hospital. A prototype was designed and tested in iterative processes, with patients, HPs, and healthcare software designers. Furthermore, the creation of the prototype was conducted through continuous evaluation and field studies to maintain relevance to end-users.37
In short, the needs identified in phase 1 revealed that patients felt unsecure and unprepared about their future. They accepted pathways with short time stay in hospital, but acquiring a hip fracture was a traumatic experience with chock-like implications on their state of mind. They had a strong desire to be in charge of their own lives, but they had no recollection of information given during hospital admittance. Accordingly, they were unsure of what to do and what to expect after discharge.
Figure 2 presents an overview of the phase 2 processes conducted in this study.
Figure 2.
An overview of the phase 2 processes conducted in this study.
Setting and sampling
The patient pathway of concern is patients with a hip fracture treated at a university hospital in Denmark. We focused on patients who were discharged to their own homes and who had been independent prior to the fracture. The patients were treated in the orthopaedic area, but geriatric doctors came on rounds every day.
As recommended when conducting a PD study, in January 2017 we set up a team;37 it comprised participants from healthcare professions, selected according to their involvement in the patient pathway. Likewise, all participants had in one way or another been involved in phase 1 of the overall project, by participating in either interviews or focus group discussions. Thus, participants were HPs from different healthcare professions all working at the same hospital and with a professional connection and collaboration along the given patient pathway. Convenience sampling was applied because the sampling process had to be practicable for the HPs during their busy working hours.42
The orthopaedic department has inpatient wards treating hip fracture patients in two hospitals situated approximately 50 km apart. Workshops 1 and 2 were conducted with clinical staff representing the separate wards, all of whom also had been interviewed in the first phase. An overview of participants’ characteristics and attendance at workshops and laboratory tests is outlined in Table 1. The two orthopaedic doctors also contributed with videos and other materials that were included in the final solution. Two of the authors (C.M.J., J.C.) were present at all workshops; C.M.J. acted as facilitator, assisted by J.C. as an experienced PD researcher.
Table 1.
Participants, their characteristics, and contributions to the workshop and laboratory test.
| Participants and characteristics | Workshop attendance |
Laboratory tests | Face-to-face workshops | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Patient, woman, age 76 | X | X | XXXX | |||
| Patient, woman, age 82 | X | XX | ||||
| Patient, male, age 80 | X | XXXXX | ||||
| From hospital A | ||||||
| Geriatrician, male, >20 years’ experience | X | X | ||||
| Nurse, female, >25 years’ experience | X | X | ||||
| Nurse, female, <5 years’ experience | X | X | ||||
| Physiotherapist, male, >10 years’ experience | X | X | ||||
| Orthopaedic doctor, male, >20 years’ experience | X | X | ||||
| Social and healthcare assistant, female, >20 years’ experience | X | X | X | X | ||
| From hospital B | ||||||
| Geriatrician, >10 years’ experience | X | X | ||||
| Nurse, female, 5 years’ experience | X | X | X | X | ||
| Nurse, female, <5 years’ experience | X | X | X | X | ||
| Social and healthcare assistant, female, >20 years’ experience | X | X | X | X | ||
| Researcher, female, experienced in PD | X | X | X | X | X | |
| Researcher, female, PhD student | X | X | X | X | X | XXXXX |
| Researcher, male, experienced in orthopaedics | X | |||||
| Researcher, male, experienced software engineer | X | |||||
| Software designer, male, experienced designer | X | |||||
| Software designer, female, illustrator | X | X | X | X | X | |
PD: participatory design.
At workshops 1 and 2, the aim of the study was introduced and the identified needs from phase 1 were presented on a large poster illustrating the patient pathway. Sound clips from the audio-recorded interviews with patients were played to support the findings from phase 1. To induce creativity in the process, the participants were invited to present ideas. Adhesive notes and writing materials were available and participants were asked to suggest and document ideas to improve the pathway.
In workshops 3 and 4, the design and development of a solution, a prototype, was processed on the basis of continuous feedback from face-to-face workshop with users. This prototype was then tested in laboratory tests involving users, researchers, and designers.
In phase 1 of the overall project, we had conducted 10 interviews with patients (age range: 67–92 years) and four relatives.11 All 10 patients and their relatives had, at that point, also given their consent to participate in the workshops. When the time came to invite patients to participate in the workshops, only one of the patients (a woman, aged 76) wished to participate. Therefore, we conducted a semi-structured interview by phone with each of the nine patients not wanting to participate in action in the workshop, where ideas from workshops 1 and 2 were presented. The focus of the interview was on the patient’s needs and what they imagined future patients would require.
We decided to recruit another two patients (a man aged 80 and a woman aged 82) with the aim of getting an evaluation of the ideas generated at the workshops with HPs, from patients with more recent experience. These two patients were recruited unsystematically by a nurse during their hospital stays. Eligible patients were asked whether they were interested in developing a new way of communicating healthcare knowledge. If interested, these patients were contacted by the first author (C.M.J.) and, approximately 2 weeks after hospital discharge, they were introduced in their homes to the ideas that had emerged from workshops 1 and 2. This was done in several iterative processes where C.M.J. drove back and forth from the patients’ homes to CIMT several times. Suggestions and mock-ups were also presented and evaluated by the one patient, the 76-year-old woman, from phase 1 interested in participating in the workshops. This continuous feedback from the patients was conducted throughout the phase.
All workshops were audio-recorded for transcription purposes. The duration of each workshop was 2 h. Further data consisted of notes taken by C.M.J. and drawings and notes made by the participants. Likewise, data were continuously collected throughout field studies during the joint workshops and the testing and throughout the semi-structured phone interviews and face-to-face workshops. At the end of each workshop and interview, we conducted an immediate first validation by summarising the contents of the workshop/interview so that participants could confirm the summary. Likewise, each new workshop was initiated with a summary of prior findings and the patients’ interpretation of suggestions and mock-ups. In addition, data to support the final content of the prototype were generated by way of a scoping search, in which we used the following search words: technology, ageing, learning, recall of information, acute settings, and hip fracture.
Ethical issues
In compliance with the Helsinki Declaration,43 the participants received oral and written information about the study and were included after providing written consent. Approval was obtained from the Regional Health Service and University Research Ethics Committee and the Danish Data Protection Agency (S-20110171; § 14, 1; 2008-58-0035) (case approval no. 15/11860).
Consent was confirmed prior to each workshop in line with good ethical practice in qualitative research with vulnerable populations.44 Accordingly, the design of the workshops was changed in accordance with the ethical concerns for the individuals participating in the study processes. Similarly, written consent was obtained from participating individuals to publish Figures 3 and 4.
Figure 3.
Pictures 1–4: The development process.
Figure 4.
Pictures 5–8: The contents of the app.
Analysis
Transcribed data from the workshops were analysed in ongoing iterations by C.M.J. according to the plan, act, observe, and reflect methodology of iterative processes.40,45 The transcribed text was analysed in these ongoing processes using qualitative content analysis,46 as the goal of content analysis is ‘to provide knowledge and understanding of the phenomenon under study’.47 We used qualitative content analysis to systematically process coding of text and identify themes or patterns in the interpretation of the content of text data. Themes that emerged from the analysis were additionally discussed in ongoing processes with co-authors (J.C., S.O., and U.K.W.) and all workshop participants, with a view to confirming findings.
Results
To empower patients and bridge the gap between what was provided by the system and patient needs, we furthermore discovered that HPs also needed to be empowered, that is, HPs needed to be provided with the skills and resources required to empower patients.
The iterative processes
As mentioned previously, all the activities and processes were based on the identified needs from phase 1. Based on idea-generating discussions on how to bridge the gap, the primary discussions in workshops 1 and 2 were centred around two overall themes: (1) How to communicate with individuals in a crisis-like state of mind, and (2) a new way of communicating healthcare knowledge in means of a ‘pick-and-choose’ solution.
HPs expressed a need for training in how to communicate with patients who are in a crisis-like state of mind. This was also seen as a focus on a change of mind-set of HPs, that is, changes from valuing all the objective and measurable tasks in connection with the patient pathway to an approach involving patients’ psychological concerns:
… we stress around and report all the measurable values … but we need to see the individual person as well … this requires a change of mind-set … (Geriatrician)
HPs also expressed a need for a technological training platform on which knowledge about how best to support the hip fracture patient postoperatively could be presented. This was seen as a way of disseminating experts’ tacit knowledge to the whole group of HPs connected with the patient pathway. The teams A and B generated ideas on different ways of presenting individualised information in systematised pathways with short LOS. Thus, the focus became the design of an application that would allow more individualised health knowledge to be imparted. Such an app would accommodate a ‘pick-and-choose’ solution and allow patients to access knowledge at a time convenient to them:
… we have a lot of focus on all the objective tasks that we as HPs have to do in connection with the pathway … and most of the information or education of the patients is done at a time convenient to us … we don’t consider if the time is convenient to the patient … (Nurse)
Another idea was to establish a representative group of patients (who have recovered from a hip fracture), as has been successfully established in other medical fields. This group could then disseminate their knowledge and experiences to the other patients and maybe thereby create a greater sense of security concerning conditions after discharge.
In the interviews with the 10 patients, it was found that they were not able to give specific suggestions when asked about the kind of information they would have liked and what would be meaningful to their situations and everyday lives. They found the questions difficult and responded with remarks such as ‘one size does not fit all’ and ‘we are all different, therefore we also have different needs’. However, several expressed the need for control and autonomy, as represented in this quotation:
When the incident of the broken hip took place I lost control … I would need something that gives me the possibility of regaining control. (Woman, aged 78)
We then presented the idea of an app and suggestions for its content, as generated from workshops 1 and 2, and patients’ reaction were ‘yes, that might be a good idea’. Therefore, the following workshops focused on creating a ‘pick-and-choose’ solution containing different features to accommodate patients’ individual needs along the pathway. The solution should also contain a means of accommodating eHealth literacy as several of the patients declared difficulty in reading and understanding the written information given during hospital admittance. None of the patients expressed an interest in being part of a corps of prior patients. Nevertheless, they supported the idea of disseminating knowledge to help in giving patients a sense of security:
If I had just known what I know now … I wouldn’t have been so terrified by the thought of going home … (Man, aged 80)
In workshop 3, reflections from the patients were introduced and incorporated into the app. One part in the app was initially meant as a training site for HPs, but was subsequently also made accessible to patients:
It would have been very nice for me to see these instructions (for HPs) … then I would know why they do what they do … and I am sure it would have been easier for me to collaborate … (Woman, aged 74)
A further focus in workshop 3 was on how HPs could minimise workload in the systematised pathway and coordinate the app with patients’ electronic patient journals. HPs found that they could individualise the information and create time to involve patients’ psychosocial concerns. Thus, the principal wish in workshop 3 was to create an app with an easy-to-use design. We ended up with a compromise that would ensure future usability. That is, we chose to build on an existing app called ‘My Patient Journey’ (English translation). This app had been introduced by the healthcare system in several hospitals in the Region of Southern Denmark and was planned as a future platform for digital communication and distribution of health knowledge in the whole region.48 Therefore, we wished to work with a solution that would be recognisable to the patients should they be introduced to ‘My Patient Journey’ in a separate encounter with the healthcare system in the Region. ‘My Patient Journey’ is a personal access point for patients involved in a number of specific treatment processes at the hospital. One of the core features of the app is that it is integrated into the electronic patient journal and, as such, is a secure way of communicating personal information.
Plans for the pick-and-choose solution were unfolded and combined with knowledge gleaned from the scoping search. This unfolded a desire to illustrate information using animated drawings. One of the app designers had an active role in coming up with drawing suggestions. In the end, it was decided to design an app providing general knowledge of a typical pathway, supplemented with patient stories. It was furthermore decided that the app should contain a personal information site.
These ideas and suggestions were presented to the patients in the face-to-face workshops, where the app was presented as a mock-up and contents were evaluated by the patients. Patients expressed a need for an easy-to-use application. All of the patients were familiar with using a computer or a smart phone, but none of them was familiar with downloading an app or all the processes of ‘going back and forth’ in an app. When asked about whether to change anything in the contents, they focused on usability and that it might take a little time for users to become familiar with the app:
Well … I have a tablet … and I enjoy using it … now … but I only use it for stuff like emails … and, and bank matters and such … but I think … now that you have introduced the ‘My hip fracture journey’ app to me, I can use it … but I wouldn’t have been able to do it on my own … (Man, aged 79)
At the fourth workshop, all of the contents (features) of the app were examined. Several disparities arose between the meanings of expressions as presented by the HPs and how they were perceived by the patients. For example, the HPs made an instructional video on how to mobilise the patient postoperatively. In the video, they said, ‘use the patient’s own resources’. On seeing the video, one patient perceived it as something she had to pay for. Similarly, several other expressions implied one thing for the HPs and another for the patients. This engendered mutual learning and changes were made accordingly. Some of the workshop processes are presented in illustrations 1–4 in Figure 3.
Finally, we held a joint and more technical workshop, the ‘laboratory test’, in August 2017, involving four HPs and one patient. Here, the functionalities of the prototype were tested one by one. The app was then tested using an iPad in the clinical area with four of the authors (C.M.J., S.O., U.K.W., J.C.) present and able to make final adjustments. The order of features on the app was changed so that they were presented more logically according to user feedback. When presenting the iPads in a clinical context, we found a need to equip the iPad with a protective shell that partly protected it for hygiene and infection control reasons. The iPad should be able to withstand being sent with the patients’ home and subsequently being properly cleaned and redeployed from the hospital.
To promote usability of the iPad, we only had one icon installed: ‘My Hip Fracture Journey’. A green dot was placed on the on/off button and stylus pens were bought to accommodate rheumatic fingers.
The app ‘My Hip Fracture Journey’
The HPs had multiple wishes regarding content and teaching methodologies to be contained in the app and a desire that they meet end-users’ individual needs and learning styles. With this in mind, the app contained five main features:
Pictographs;
Short videos;
Illustrated exercises;
Written information;
Audio recordings.
The first feature was an animated drawing of a typical treatment pathway for individuals with a hip fracture. Likewise, we made an animated drawing on ‘How to get back on my feet again’, containing elements of how to rehabilitate/change positions, how to manage pain, and so on. Some of the features in the app are shown in pictures 5–8 in Figure 4.
Messages about ‘being positive’ and ‘maintaining perspective’ in recognising milestones and potential setbacks were stressed as important elements in a Canadian study on patient perspectives on guiding a successful recovery process after hip fracture.49 This was contained in the second feature, where two individuals (a man of 80 and a woman of 76) presented their hip fracture stories and experiences in short movies. Likewise, educational movies were presented by different HPs on different topics. For example, one told about what to expect during hospital admission, another talked about the fracture and the operation, and another was about the importance of preventing new fractures – and how to do so.
Previous research on exploring older adults’ patterns and perceptions of exercise after hip fracture have shown that successful recovery from hip fracture is attainable.50 The group of older adults included in the study reported intrinsic factors, such as determination, seeing improvements, and making exercise part of their daily routine. This knowledge supported our third feature in which we illustrated eight filmed exercise programmes, introduced by a physiotherapist and with an elderly nurse acting as a patient.
The fourth feature contained short, factual, written information about, for instance, ‘FAQ’. These questions had been agreed upon by the HPs in questioning their colleagues over a period of 2 months. The font size of the writing was increased to accommodate potential vision impairment.
Recent studies have shown that the use of audio recordings to improve outcomes of patient consultation was successful.51–53 This supported the basis for a fifth feature in the app: an audio recording. The recording was of a real discharge-planning conference where the patient, their relatives, a physiotherapist, and a nurse or social and healthcare assistant from the hospital were present. In order to access this fifth and very personal feature in the app, the patient would have to be logged in, by using his or her social security number and a password.
Discussion
User-driven studies and studies on the importance of patients’ participation in decision-making in the medical field have been highlighted throughout the past 35 years.13,54,55 Patient participation is recognised as a key component in improving patient safety.56 Likewise, a focus on the individual patient’s needs is recognised as the most essential factor in an elderly patient’s successful participation in the discharge process.57
In our study, we found that in order to bridge the gap, the app should also contain a means of empowering HPs. Patient empowerment is not only about the healthcare system doing something for the patient’s sake.58 It is also about patients being supported in being active and equal partners in the healthcare process. For this to be effectively achieved, HPs must be aware of individual patients’ needs and wishes. Likewise, HPs must be aware of the impact of the disease – in this case a hip fracture – on individuals’ lives. Thus, our findings implied a need to adopt a much more holistic approach in healthcare.
The findings that arose from identifying patient needs indicated that patients desired autonomy.11 The application of design and technology can contribute to independent living and lifestyle support and thus contribute to autonomous ageing.59 The aim of the user-driven approach became to design a pick-and-choose solution. From this perspective, empowering individuals with a hip fracture would change their role from passive care receivers into active participants in their care decisions.
When conducting a PD process, one core ethical motivation is to support and enhance how people can engage with others in shaping their world.60 A second principle calls for use of processes and tools that enable designers, technology users, and other stakeholders to learn from each other through understanding each other’s perspectives and priorities. In the current study, one of the tools to enable this process was to establish face-to-face workshops in patients’ homes. Likewise, we presented ideas to patients through phone interviews. The user-driven approach in creating a solution to bridge the gap was mainly in means of how HPs could work together and find a future way of supporting patients – that is, working together in shaping a better future in order to accommodate future demands and also for the purposes of usability. Another core principle of PD is that ‘people have a basic right to make decisions about how they do their work and any other activities where they might use technology’.37 When stressing user participation as a principle in PD, two goals are followed. Initially, user participation increases the potential of visions produced by a design project to reflect the users’ true situation and needs. Later, user participation increases the potentials of the system to be used according to their intentions.36
When working alongside older adults, we found that they can engage in some PD activities; however, there can be some different requirements, compared to younger adults. While eyesight, hearing, memory, and physical coordination impairments are a common consequence of the ageing process, we do not believe that these challenges prevent the development of an app/tele-health service. We found that, when designing a technology intended to be used by older people, consideration should be given to avoiding terms they might not understand. This reflects the findings from another study on new horizons in the design for autonomous ageing.59 One of the findings in that study was that technologies must be familiar and usable to become an essential part of older people’s lives.
When engaging with a PD project, user activities in the second phase are normally very creative and experimental.40 We expanded the creativity to apply to the process itself. Thus, we did ‘one-to-one’ workshops in the patients’ homes and discussed findings from these workshops in iterative processes in the workshops with HPs. One of the basic perspectives in PD is that users ‘have a say’ in the design; this means not only expressing opinions but also having an impact on the outcome of an activity, by what one says.61 To achieve this, the first author would act as the ‘travelling messenger’ for all communication between the individual patients and the group of HPs. Through the iterative design process, the individuals were able to see that their ‘say’ affected the outcome. Likewise, when conducting a PD study, the testing and retesting of the mock-ups are important when producing a prototype ready for final testing in phase 3.62 By using this different way of conducting workshops and lab tests, we took the ethical stance that this would be the best way to engage individuals. Patients recovering from a hip fracture would prefer to concentrate on their own recovery and on their own future. They had a preconception that sessions focusing on ideas or solutions aimed at informing future patients with a hip fracture were tantamount to ‘thinking backwards’ and remaining unwell.
PD is progressively being used in the development of tele-health solutions to support patients or individuals with long-term illnesses and promote their participation in healthcare.63 Incurring a hip fracture is an acute and traumatic event, and individual’s recovery process was characterised by a desire to distance themselves from the event.11 Hence, previous patients were reluctant to attend workshops and get involved in the design process. Therefore, we tailored a concept of ‘one-to-one’ workshops as a tool to accommodate a genuine user participation approach. It is advocated that the setting in which PD unfolds should be thoughtfully planned and tools and techniques should not be applied strictly or by rote.37,64 Likewise, we also found that it is important to be aware of what can be accomplished by using these particular tools and techniques.
The clinical staff involved in this study were very engaged in the process and they were looking forward to testing the prototype in their clinical setting. Our study contributes to the growing body of research that shows that, by involving clinical staff in the developmental process of PD, the likelihood of a positive process in the organisational changes following testing of the app is optimised.
Strengths and limitations
In conducting a PD study, the researcher should be open-minded about the various suggestions that arise.40 In our study, it was also a question of ‘creating a vision of the possible’ in the time available. That is, time and resource limits characterised the end product and not all wishes expressed by the end-users could be accommodated.
It was considered at the planning stage that the physical participation of patients in the workshops would be beneficial to the development process. However, we found the iterative process, which involved the researcher acting as a messenger back and forth between patients’ homes and workshops with health professionals, to be an acceptable – albeit time-consuming – alternative. Nevertheless, the development process may have been different with a larger number of previous patients and their relatives present. It would have been beneficial to test the app’s usability with newly admitted patients. However, ethical considerations concerning patients’ state of mind in the acute phase after a hip fracture meant that this idea was not followed.
A strength in conducting the iterative processes was that the finding regarding the training need to empower HPs arose inadvertently out of the PD process.
The patients who participated in our study cannot be considered to be representative of the average individual incurring a hip fracture. First, the number of participants was very limited, and, second, they had expressed a wish to engage in something new, despite being in a stressful situation. Nevertheless, we consider that the content of the app and its accommodation of different learning styles and health literacy should help in its transferability to a wider group.
Further research
A thorough evaluation of the app in the clinical setting was conducted from December 2017 to June 2018. Patients were introduced to the app by means of an iPad which was loaned to them for 3–4 weeks. Their experiences will be analysed using semi-structured interviews. The usability of the app will be evaluated through focus group discussions with the HPs involved in the test phase.
Conclusion
Our study demonstrated that PD is an acceptable approach to co-design a solution to bridge the gap between what the system provides and patients’ needs and wishes in hip fracture treatment with short LOS. Nevertheless, the process of generating ideas and creating mutual learning in joint workshops in this study had to be re-thought. To this end, we tailored tools and techniques to create an environment that would support genuine involvement of participants who represented future end-users. The study showed that PD processes can support and inspire participation and engagement, and that patients, HPs, and app designers benefited from mutual learning and understanding.
Importantly, the technological solution of communicating health information and promoting patient empowerment must accommodate different learning styles, by way of some customised features. Likewise, the solution should support HPs in providing a reliable platform to address patients’ individual needs and to monitor patient progress during the recovery period after a hip fracture.
Acknowledgments
We wish to thank all the participants who participated in our study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Region of Southern Denmark, University of Southern Denmark, The Orthopaedic Department at Odense University Hospital, and Patient@home have funded the project and thereby the development of the prototype.
Ethical approval: Approval was obtained from the Regional Health Service and University Research Ethics Committee.
Informed consent: In compliance with the Helsinki Declaration, the participants received oral and written information about the study and were included after providing their written consent. Likewise, written consent was obtained from participating subjects to publish Figures 3 and 4.
Trial registration: The study was approved by the Danish Data Protection Agency (S-20110171; § 14, stk. 1; 2008-58-0035) (case approval no. 15/11860).
ORCID iDs: Charlotte Myhre Jensen  https://orcid.org/0000-0002-7058-4641
https://orcid.org/0000-0002-7058-4641
Anthony C Smith  https://orcid.org/0000-0002-7756-5136
https://orcid.org/0000-0002-7756-5136
References
- 1. Curtis EM, Moon RJ, Harvey NC, et al. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017; 104: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Silver Book, 1700 K Street, NW, Suite 740 Washington, DC, 2006, http://www.silverbook.org/ [Google Scholar]
- 3. Hansen L, Mathiesen AS, Vestergaard P, et al. A health economic analysis of osteoporotic fractures: who carries the burden? Arch Osteoporos 2013; 8: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sale JE, Beaton D, Bogoch E. Secondary prevention after an osteoporosis-related fracture: an overview. Clin Geriatr Med 2014; 30(2): 317–332. [DOI] [PubMed] [Google Scholar]
- 5. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl 2012; 83(346): 1–39. [DOI] [PubMed] [Google Scholar]
- 6. Walter CJ, Smith A, Guillou P. Perceptions of the application of fast-track surgical principles by general surgeons. Ann Roy Coll Surg 2006; 88(2): 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003; 362(9399): 1921–1928. [DOI] [PubMed] [Google Scholar]
- 8. Clemensen J, Rothmann MJ, Smith AC, et al. Participatory design methods in telemedicine research. J Telemed Telecare 2017; 23: 780–785. [DOI] [PubMed] [Google Scholar]
- 9. Ministry of Health. Healthcare in Denmark. 2017, http://www.sum.dk/English/Healthcare-in-Denmark-An-Overview.aspx
- 10. Zidén L, Scherman MH, Wenestam CG. The break remains – elderly people’s experiences of a hip fracture 1 year after discharge. Disabil Rehabil 2010; 32(2): 103–113. [DOI] [PubMed] [Google Scholar]
- 11. Jensen CM, Smith AC, Overgaard S, et al. ‘If only had I known’: a qualitative study investigating a treatment of patients with a hip fracture with short time stay in hospital. Int J Qual Stud Health Well-Being 2017; 12(1): 1307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norlyk A, Harder I. After colonic surgery: the lived experience of participating in a fast-track programme. Int J Qual Stud Heal Well-Being 2009; 4: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glasdam S, Oeye C, Thrysoee L. Patients’ participation in decision-making in the medical field – ‘projectification’ of patients in a neoliberal framed healthcare system. Nurs Philos 2015; 16(4): 226–238. [DOI] [PubMed] [Google Scholar]
- 14. Anderson RM, Funnell MM. Patient empowerment: myths and misconceptions. Patient Educ Couns 2010; 79(3): 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rappapon J. Studies in empowerment. Prevent Human Serv 1984; 3(2–3): 1–7. [Google Scholar]
- 16. Hesselink G, Flink M, Olsson M, et al. Are patients discharged with care? A qualitative study of perceptions and experiences of patients, family members and care providers. BMJ Qual Safe 2012; 21(Suppl. 1): i39–i49. [DOI] [PubMed] [Google Scholar]
- 17. Sims-Gould J, Stott-Eveneshen S, Fleig L, et al. Patient perspectives on engagement in recovery after hip fracture: a qualitative study. J Age Res 2017; 2017: 2171865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clemensen J, Larsen SB, Ejskjaer N. Telemedical treatment at home of diabetic foot ulcers. J Telemed Telecare 2005; 11(Suppl. 2): S14–S16. [DOI] [PubMed] [Google Scholar]
- 19. Danbjorg DB, Wagner L, Clemensen J. Do families after early postnatal discharge need new ways to communicate with the hospital? A feasibility study. Midwifery 2014; 30(6): 725–732. [DOI] [PubMed] [Google Scholar]
- 20. Garne K, Brødsgaard A, Zachariassen G, et al. Telemedicine in neonatal home care: identifying parental needs through participatory design. JMIR Res Protocol 2016; 5(3): e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Health and sustainable development. Telehealth, 2018, http://www.who.int/sustainable-development/health-sector/strategies/telehealth/en/
- 22. Bower P, Cartwright M, Hirani SP, et al. A comprehensive evaluation of the impact of telemonitoring in patients with long-term conditions and social care needs: protocol for the whole systems demonstrator cluster randomised trial. BMC Health Serv Res 2011; 11(1): 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLean S, Protti D, Sheikh A. Telehealthcare for long term conditions. BMJ 2011; 342: d120. [DOI] [PubMed] [Google Scholar]
- 24. Steventon A, Bardsley M, Billings J, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012; 344: e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The American Telemedicine Association. What is telemedicine? The American Telemedicine Association; 2016, https://thesource.americantelemed.org/resources/telemedicine-glossary
- 26. Armfield NR, Edirippulige SK, Bradford N, et al. Telemedicine – is the cart being put before the horse? Med J Australia 2014; 200(9): 530–533. [DOI] [PubMed] [Google Scholar]
- 27. Kushniruk A, Nohr C. Participatory design, user involvement and health IT evaluation. Stud Health Technol Inform 2016; 222: 139–151. [PubMed] [Google Scholar]
- 28. Kensing F. Methods and practices in participatory design, vol. viii Copenhagen: ITU Press, 2003, p. 493. [Google Scholar]
- 29. Simonsen J, Robertson T. Routledge international handbook of participatory design. Copenhagen: Nota, 2015, https://nota.dk/bibliotek/bogid/631290 [Google Scholar]
- 30. Clemensen J, Larsen SB, Kirkevold M, et al. Treatment of diabetic foot ulcers in the home: video consultations as an alternative to outpatient hospital care. Int J Telemed Appl 2008; 2008: 132890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dykes PC, Stade D, Chang F, et al. Participatory design and development of a patient-centered toolkit to engage hospitalized patients and care partners in their plan of care. AMIA Annual Symp Proc 2014; 2014: 486–495. [PMC free article] [PubMed] [Google Scholar]
- 32. Terp M, Bjørnes CD, Jørgensen R, et al. Collaborating with young adults diagnosed with schizophrenia: a participatory design study to shape the healthcare system. Open J Nurs 2017; 7(7): 743–758. [Google Scholar]
- 33. Garne Holm K, Brodsgaard A, Zachariassen G, et al. Participatory design methods for the development of a clinical telehealth service for neonatal homecare. SAGE Open Med. Epub ahead of print 21 September 2017. DOI: 10.1177/2050312117731252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bradbury H. The SAGE handbook of action research. Los Angeles, CA: SAGE, 2015, http://methods.sagepub.com/book/the-sage-handbook-of-action-research-3e [Google Scholar]
- 35. Kemmis S, McTaggart R. The action research planner. New York: Springer, 2014. [Google Scholar]
- 36. Bødker K, Kensing F, Simonsen J. Participatory IT design: designing for business and workplace realities, vol. xviii Cambridge, MA: MIT Press, 2004, p. 337. [Google Scholar]
- 37. Simonsen J, Robertson T. Routledge international handbook of participatory design. New York: Routledge, 2013, http://site.ebrary.com/lib/royallibrary/Doc?id=10617655 [Google Scholar]
- 38. Spinuzzi C. The methodology of participatory design. Tech Commun 2005; 52(2): 163–174. [Google Scholar]
- 39. Kensing F, Blomberg J. Participatory design: issues and concerns. Comput Support Cooperat Work 1998; 7(3): 167–185. [Google Scholar]
- 40. Clemensen J, Larsen SB, Kyng M, et al. Participatory design in health sciences: using cooperative experimental methods in developing health services and computer technology. Qual Health Res 2007; 17(1): 122–130. [DOI] [PubMed] [Google Scholar]
- 41. Clemensen J. Pervasive healthcare: home treatment of patients with diabetic foot ulcers. Aarhus: University of Aarhus, 2006. [Google Scholar]
- 42. Lavrakas P. Encyclopedia of survey research methods. Thousand Oaks, CA: SAGE, 2008. [Google Scholar]
- 43. World Medical Association. WMA declaration of Helsinki – ethical principles for medical research involving human subjects: The World Medical Association, Inc, 2018, https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [PubMed]
- 44. Seymour JE, Ingleton C. Ethical issues in qualitative research at the end of life …article reprinted from Int J Palliat Nurs 1999; 5(2): 65–73. Int J Palliat Nurs 2005; 11(3): 138–146. [Google Scholar]
- 45. Kemmis S, McTaggart R, Nixon R. The action research planner: doing critical participatory action research. Singapore: Springer, 2014, 10.1007/978-981-4560-67-2 [DOI] [Google Scholar]
- 46. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs 2008; 62(1): 107–115. [DOI] [PubMed] [Google Scholar]
- 47. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005; 15(9): 1277–1288. [DOI] [PubMed] [Google Scholar]
- 48. http://www.medware.dk/en/project/my-patient-journey/
- 49. Schiller C, Franke T, Belle J, et al. Words of wisdom – patient perspectives to guide recovery for older adults after hip fracture: a qualitative study. Patient Prefere Adher 2015; 9: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gorman E, Chudyk AM, Hoppmann CA, et al. Exploring older adults’ patterns and perceptions of exercise after hip fracture. Physiother Can 2013; 65(1): 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wolderslund M, Kofoed PE, Holst R, et al. Patients’ use of digital audio recordings in four different outpatient clinics. Int J Qual Health Care 2015; 27(6): 466–472. [DOI] [PubMed] [Google Scholar]
- 52. Wolderslund M, Kofoed PE, Holst R, et al. Digital audio recordings improve the outcomes of patient consultations: a randomised cluster trial. Patient Educ Couns 2017; 100(2): 242–249. [DOI] [PubMed] [Google Scholar]
- 53. van Bruinessen IR, Leegwater B, van Dulmen S. When patients take the initiative to audio-record a clinical consultation. Patient Educ Couns 2017; 100: 1552–1557. [DOI] [PubMed] [Google Scholar]
- 54. van de, Bovenkamp HM, Trappenburg MJ. Reconsidering patient participation in guideline development. Health Care Anal 2009; 17(3): 198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J General Intern Med 2012; 27(10): 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Longtin Y, Sax H, Leape LL, et al. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc 2010; 85(1): 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Foss C, Askautrud M. Measuring the participation of elderly patients in the discharge process from hospital: a critical review of existing instruments. Scand J Caring Sci 2010; 24: 46–55. [DOI] [PubMed] [Google Scholar]
- 58. Freire P. Pedagogy of the oppressed. 30th ed. London: Continuum International Publishing Group, 2007, p. 183. [Google Scholar]
- 59. Van Der Cammen TJM, Albayrak A, Voûte E, et al. New horizons in design for autonomous ageing. Age Ageing 2017; 46(1): 11–17. [DOI] [PubMed] [Google Scholar]
- 60. Robertson T, Wagner I. Ethics: engagement, representation and politics-in-action. In: Simonsen J, Robertson T. (eds) Routledge international handbook of participatory design. New York: Routledge, 2013, pp. 64–85. [Google Scholar]
- 61. Robertson T, Simonsen J. Participatory design: an introduction. In: Simonsen J, Robertson T. (eds) Routledge international handbook of participatory design. New York: Routledge, 2013, pp. 1–19. [Google Scholar]
- 62. Bratteig T, Bødker K, Dittrich Y, et al. Methods: organising principles and general guidelines for participatory design projects. In: Simonsen J, Robertson T. (eds) Routledge international handbook of participatory design. New York: Routledge, 2013, pp. 117–144. [Google Scholar]
- 63. Allin S, Shepherd J, Tomasone J, et al. Participatory design of an online self-management tool for users with spinal cord injury: qualitative study. JMIR Rehabil Assist Technol 2018; 5(1): e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brandt E, Binder T, Sanders EBN. Tools and techniques: ways to engage in telling, making and enacting. In: Simonsen J, Robertson T. (eds) Routledge international handbook of participatory design. New York: Routledge, 2013, pp. 145–181. [Google Scholar]