Abstract
Exposure to low-dose ionizing radiation can have positive impacts on biological performance—a concept known as hormesis. Although radiation hormesis is well-documented, the predominant focus has been medical. In comparison, little research has examined potential effects of early life radiation stress on organismal investment in life history traits that closely influence evolutionary fitness (eg, patterns of growth, survival, and reproduction). Evaluating the fitness consequences of radiation stress is important, given that low-level radiation pollution from anthropogenic sources is considered a major threat to natural ecosystems. Using the cricket (Acheta domesticus), we tested a wide range of doses to assess whether a single juvenile exposure to radiation could induce hormetic benefits on lifetime fitness measures. Consistent with hormesis, we found that low-dose juvenile radiation positively impacted female fecundity, offspring size, and offspring performance. Remarkably, even a single low dose of radiation in early juvenile development can elicit a range of positive fitness effects emerging over the life span and even into the next generation.
Keywords: hormesis, ionizing radiation, early life stress, development, reproduction, Acheta domesticus
Introduction
Biological responses to ionizing radiation are often assumed to conform to a “linear-no-threshold” (LNT) model, which predicts that the degree of damage scales linearly with the dosage of radiation received.1,2 Indeed, radiation exposure is well known to have a range of negative outcomes on cellular, developmental, and behavioral traits,2 and indicators of poor fitness have been reported in feral animals from radio-contaminated areas.3 Considerable evidence, however, supports an alternative model known as hormesis,4 in which low-level stress exposures can have beneficial or stimulatory effects on biological performance even if higher levels are inhibitory or damaging.5,6 Hormetic dose–response relationships have been documented across broad phylogenies (ie, bacteria to plants to mammals) and in response to ∼1000 different stressors,7 suggesting that it could be an evolutionarily important phenomenon.
It is now well established that low-dose radiation can positively affect biological processes such as immunity, DNA repair, and cellular stress resistance.2,7-10 However, radiation hormesis has rarely been studied in a life history framework, that is, with reference to whole-organism traits that closely influence evolutionary and demographic processes (eg, growth, survival, and reproduction). Understanding fitness-related impacts of radiation is important because organisms in nature are subjected to low-level anthropogenic radiation pollution including nuclear power production, weapons testing, and nuclear accidents.11-13 Demonstrating a link between radiation hormesis and fitness would be significant not only for ecological and conservation purposes but also for understanding the nature of responses to previous radiation stress in the evolutionary past (eg, UV exposure).
Here, we examine consequences of a single, early juvenile exposure to ionizing radiation on a broad range of fitness-related traits emerging across the organismal life history. Specifically, we exposed cohorts of juvenile crickets (Acheta domesticus) to ionizing radiation at 14 days of age and established dose–response relationships for subsequent investments in lifetime fecundity, survival, age-specific reproductive effort, egg size, and offspring performance. The cricket is ideal for testing impacts of developmental stress on lifetime performance because its rapid development, small size, and short life span allow rearing of large populations for controlled dose–response analyses.14-16 Ionizing radiation is an ideal experimental stressor, given that dosages can be easily and accurately manipulated in the laboratory. Unlike other experimental stressors (eg, many heavy metals), radiation does not have confounding nutritional benefits at low doses.
Consistent with the hormetic model, we predicted that early-life exposures to low-level radiation stress would have stimulatory effects on trait development, whereas higher levels of stress would be inhibitory or damaging. Hormetic effects may be particularly robust when mild stress is encountered early in life,17-20 as the early period of juvenile growth is thought to represent a window of high sensitivity to environmental stress.21-23
Methods
Study Animals and Experimental Groups
Acheta domesticus were generated from a long-term breeding colony as described previously.16 For all experiments, juvenile A domesticus were collected on day 14 after hatching and assigned to one of 6 experimental treatments (n = 180 crickets per treatment), consisting of one of the following acute doses of γ-radiation (caesium-137): 0, 0.5, 1, 2, 4, or 5.5 Gy. Crickets were irradiated at the Taylor Radiobiology Source at McMaster University (dose rate = 0.25 Gy/min). During the irradiation period, crickets were housed in plastic cylindrical vials (9.5 cm height × 2.5 cm width) with 6 to 7 crickets per vial. Crickets were irradiated in groups of 45 (ie, 6 vials were irradiated at a time). Vials were evenly spaced around a 2.5-cm diameter to increase accuracy and consistency of dosing between sessions.
Following irradiation, crickets were housed in plastic containers (30 × 19 × 12 cm; n = 45 per container) and maintained in incubators at 30°C on a 12-hour light/12-hour dark photoperiod with egg carton shelters, water-soaked cellulose sponges, and ad libitum access to a diet of ground guinea pig food (Little Friends: 16.5% crude protein, 4% crude fat, and 19% crude fiber) mixed into a paste with distilled water (3 g of food per 8 mL of water).
Survival to Reproductive Maturity, Mating, and Body Condition
We quantified survival to reproductive maturity across doses as the number of crickets in each radiation treatment surviving to the mature molt (ie, total number of crickets per treatment out of n = 180). Immediately upon maturity, sexes were separated to avoid uncontrolled mating. On postmaturation day 13, females from each dose group were individually mated for 1 night with an age-matched, nonirradiated male. Body mass of each female was recorded immediately prior to mating.
At the end of the study, female crickets were euthanized with CO2, and both rear legs were removed and measured for femur length (a reliable estimate of adult body size). An electronic digital caliper (Titan) was employed. Average femur length and premating body mass were used to calculate the “scaled mass index” to estimate body condition as described by Peig and Green.24
Fecundity and Reproductive Effort
Reproductive effort was recorded for 2 separate periods over the adult female life span (n = 15-32 individuals per radiation treatment group): in early adulthood (postmaturation day 14-18) and in late adulthood (postmaturation day 20-24). Reproductive effort was calculated for each female as the product of oviposition rate (number of eggs laid over a 4-day period) and average egg mass (average dry mass of 50 eggs laid by each female). Lifetime fecundity was estimated for each female by adding the total number of eggs laid over both reproductive periods. Oviposition substrate (moist medical gauze in a petri dish) was replaced daily, and food and water were provided ad libitum. As an additional measure of total reproductive investment, we measured dry ovary mass dissected from separate populations of nonmated females from each radiation dose treatment on postmaturation day 13 (n = 11-25 individuals per radiation dose treatment group). Body mass of each female was recorded prior to dissection.
Offspring Investment and Performance
From radiation-stressed females, we measured egg size as an indicator of investment per offspring and egg hatching success as an indicator of offspring performance (n = 11-20 individuals per radiation treatment group). For egg size measurements, 5 to 11 randomly selected eggs (average = 6.7) laid by each female on postmaturation day 14 were individually visualized under a microscope (Motic, Xiamen, China). Images of each egg were captured using Motic Images Plus 2.0 software, and egg size (length × width) was measured using ImageJ software (NIH, Bethesda, Maryland). All egg size measurements were taken within 1 day from the time eggs were laid. For hatching success, we incubated 20 eggs per female in moist medical gauze at 30°C and measured the percentage of eggs that hatched. Lifetime reproductive success for each female was estimated as the product of lifetime fecundity and hatching success.
Statistics
Dose–response relationships were established for the effect of radiation on each trait measured. For each dose–response relationship, we used the extra sum-of-squares F test to compare the goodness of fit between a first-order polynomial (ie, the LNT model) and a second-order polynomial (ie, a quadratic function characteristic of the J-shaped hormetic curve). This test compares the improvement of fit of the more complicated hormetic model with the number of degrees of freedom lost from the simpler LNT model. Statistical significance (P < .05) indicates that the improvement in goodness of fit of the more complicated model over the simpler model is greater than what would be expected by chance. Dose–response analyses were performed in Prism 7.
Prior to analyses, normality was confirmed using the D’Agostino-Pearson omnibus normality test. All measures of reproductive effort and fecundity were strongly and significantly correlated with body size (average femur length), so dose–response values for these traits are presented with and without correcting for body size. Neither egg size or hatching success were significantly affected by body size, so these values were not transformed. To test for the effects of radiation dose on survival to reproductive maturity, we used a nominal logistic model. We repeated the model with individual comparisons of each radiation dose treatment group with the 0 Gy (control) group to determine which comparisons were significant. Survival analyses were performed in JMP 13.0 (SAS Institute, Cary, North Carolina).
Results
Dose–Response Analyses of Life History Traits
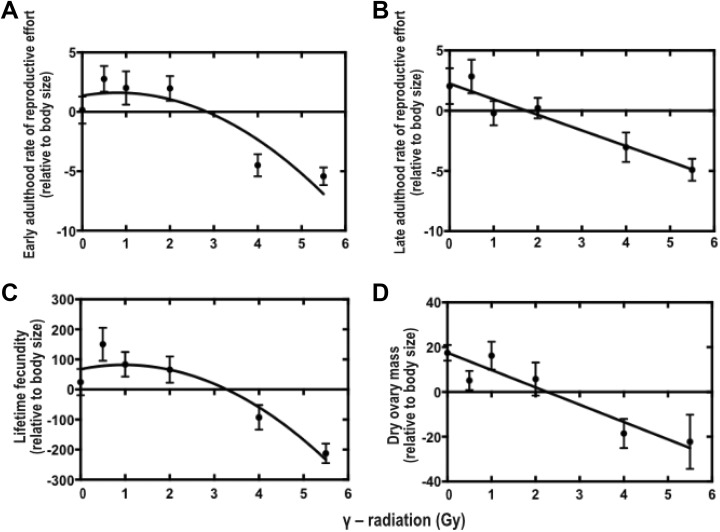
Reproductive effort in early adulthood (corrected for body size) was significantly described by a quadratic function, with hormetic stimulation occurring in the 0.5 to 2 Gy dose range (Table 1, Figure 1A). Without correcting for body size, early-life reproductive effort was best described by the LNT (Table 1). For reproductive effort in late adulthood (with and without correcting for body size), the quadratic fit was not resolved (Table 1, Figure 1B), and the trend was better described by the LNT. A quadratic function significantly explained the dose–response relationship for lifetime fecundity (corrected for body size), with hormetic stimulation occurring in the 0.5 to 2 Gy range (Table 1, Figure 1C), but when values were not corrected for body size, this relationship was best described by the LNT (Table 1). Ovary dry mass (an estimate of total reproductive investment) was not resolved by the quadratic fit, and the trend was better described by the LNT (Table 1, Figure 1D).
Table 1.
Extra Sum-of-Squares F Test Results for Linear Versus Quadratic Model Fits for Trait Dose–Responses Measured in Adult Female Acheta domesticus Exposed to Radiation Stress in Early Life.
| Trait | F (DFn, DFd) | P | Preferred Model | Equation for Preferred Model | R 2 for Preferred Model |
|---|---|---|---|---|---|
| Lifetime fecundity | 2.484 (1, 156) | .12 | Linear | y = −74.7x + 630.1 | 0.941 |
| Lifetime fecundity (relative to body size) | 5.203 (1, 156) | .024 | Quadratic | y = −13.369x 2 + 35.789x + 37.271 | 0.905 |
| Early adulthood reproductive effort rate | 2.484 (1, 148) | .12 | Linear | y = −2.026x + 17.15 | 0.923 |
| Early adulthood reproductive effort rate (relative to body size) | 4.37 (1, 148) | .038 | Quadratic | y = −3.002x 2+ 0.1913x + 1.6586 | 0.838 |
| Late adulthood reproductive effort rate | 0.352 (1, 108) | .55 | Linear | y = −1.735x + 15.44 | 0.973 |
| Late adulthood reproductive effort rate (relative to body size) | 0.003 (1, 108) | .95 | Linear | y = −1.3238x + 2.3642 | 0.927 |
| Ovary dry mass (relative to body size) | 0.073 (1, 111) | .79 | Linear | y = −7.472x + 16.83 | 0.891 |
| Adult body size (average femur length) | 2.99 (1, 156) | .091 | Linear | y = −1.042x + 10.73 | 0.816 |
| Adult body condition | 1.7 (1, 298) | .19 | Linear | y = −0.02507x + 0.06447 | 0.936 |
| Average egg size (mm2) | 18.7 (1, 89) | <.0001 | Quadratic | y = −0.0125x 2 + 0.0581x + 1.0835 | 0.512 |
| Hatching success (%) | 12.7 (1, 97) | .0005 | Quadratic | y = −0.0169x 2 + 0.069x + 0.6797 | 0.984 |
| Lifetime reproductive success (% hatched of eggs laid) | 8.401 (1, 96) | .0046 | Quadratic | y = −16.482x 2 + 27.589x + 438.71 | 0.953 |
Figure 1.
Dose–response relationships showing effects of early-life γ-radiation exposure on measures of reproductive investment in adult Acheta domesticus: rate of reproductive effort (eggs laid × mg × day−1) in early (A) and late (B) adulthood, lifetime fecundity (C), dry ovary mass (mg), and an estimate of total reproductive investment (D). All crickets were irradiated at 14 days of age at 0, 0.5, 1, 2, 4, or 5.5 Gy (dose rate = 0.25 Gy/min). Values are presented as body size-corrected residuals. All values are means ± standard error of the mean (SEM).
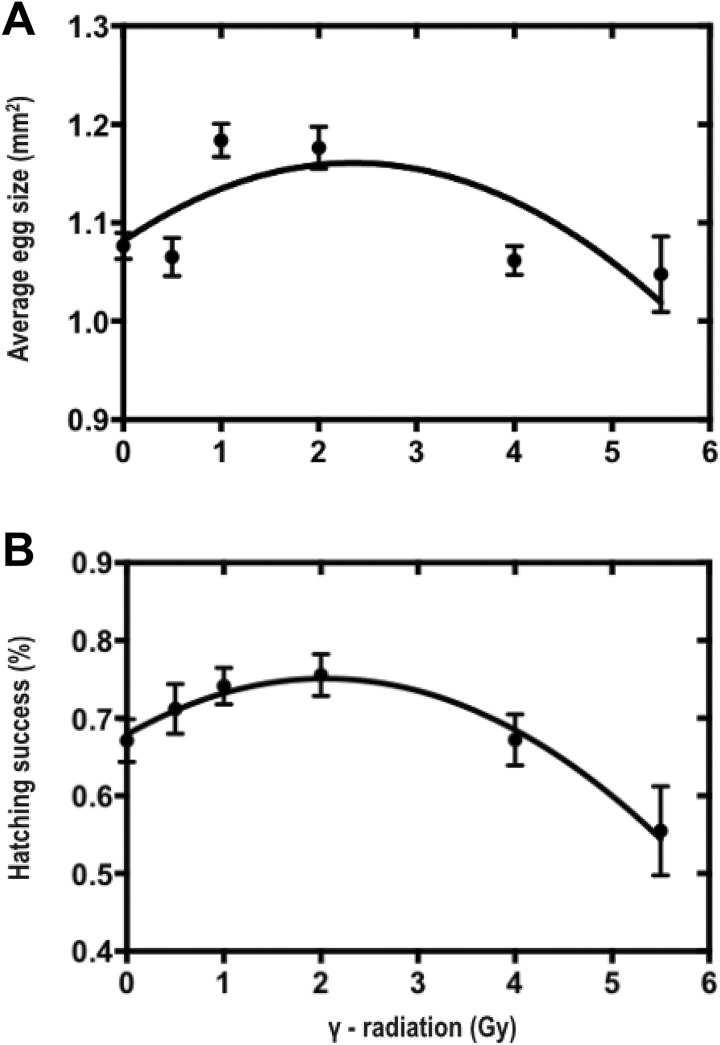
Average egg size of radiation-stressed females was significantly explained by a quadratic function with hormetic stimulation in the 1 to 2 Gy dose range (Table 1, Figure 2A). Hatching success was also significantly explained by a quadratic with hormetic stimulation across the 0.5 to 2 Gy range (Table 1, Figure 2B). Across all females, average egg size was not significantly correlated with hatching success (Pearson r = 0.184, P = .155).
Figure 2.
Egg size (A) and hatching success (B) of eggs laid by adult Acheta domesticus exposed to γ-radiation in early life. All mothers were irradiated at 14 days of age at 0, 0.5, 1, 2, 4, or 5.5 Gy (dose rate = 0.25 Gy/min). All values are means ± standard error of the mean (SEM).
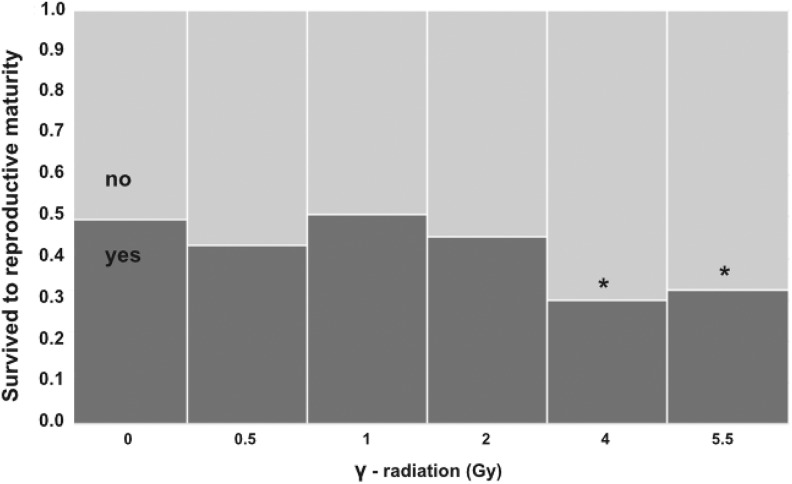
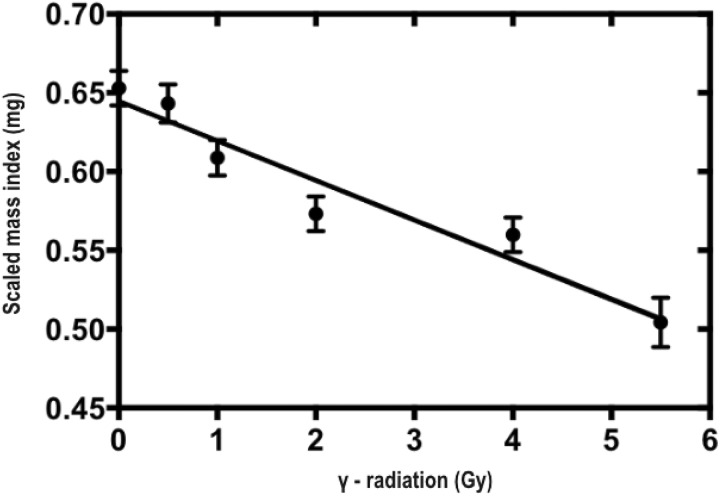
The effects of early-life radiation stress on survival to reproductive maturity was affected by radiation dose treatment (n = 1080, χ2 = 15.5, P < .0001) and was significantly lower in only the 4 and 5.5 Gy treatments (Figure 3). Analysis of variance revealed no significant differences in age at reproductive maturity among radiation dose treatments (F 5, 454 = 0.864, P = .0994). Effects of radiation stress on adult female premating body condition were best described by the LNT (Table 1, Figure 4).
Figure 3.
Effects of early-life ionizing radiation stress on Acheta domesticus survival to reproductive maturity (dark bars). There was an overall significant effect of radiation dose on survival. Follow-up nominal logistic models comparing each dose treatment to the 0 Gy (control) treatment found that survival was significantly lower in the 4 Gy (χ2 = 14.3, P = .0002) and 5.5 Gy (χ2 = 11.1, P = .001) treatments. All crickets were irradiated at 14 days of age at 0, 0.5, 1, 2, 4, or 5.5 Gy (dose rate = 0.25 Gy/min).
Figure 4.
Premating body condition (scaled mass index) of adult female Acheta domesticus exposed to ionizing radiation in early juvenile development. Data represent body mass on postmaturation day 13 standardized to a femur length of 10.59 mm (average adult femur length of the whole population). All females were irradiated at 14 days of age at 0, 0.5, 1, 2, 4, or 5.5 Gy (dose rate = 0.25 Gy/min). Values are means ± standard error of the mean SEM).
Discussion
As hypothesized, we found that early-life radiation stress had hormetic effects on multiple key fitness-related traits, including lifetime fecundity and early adulthood reproductive effort (Figure 1A, C) as well as egg size and hatching success (Figure 2). In contrast, late adulthood reproductive effort (Figure 1B) and adult body condition (Figure 4) were better described by the LNT model. Survival to reproductive maturity was negatively affected at only high doses of radiation but was unaffected at lower doses that were associated with hormetic responses in other traits (Figure 3). Together, our results show that a single, acute exposure to radiation stress in early life can induce hormetic benefits on multiple traits closely related to lifetime organismal fitness, and notably, such benefits can even extend into the next generation. These results are consistent with the idea that hormetic responses to early-life stress may represent an important form of “developmental plasticity,” perhaps mediated by epigenetic mechanisms.25-27 In this regard, hormesis could be facilitator of adaptive phenotypic variation available to natural selection.
A hormetic response was observed for reproductive effort in early adulthood, but not in late adulthood (Figure 1A, B). Thus, the hormetic effect on reproductive effort was not sustained over the adult life span, perhaps because it was offset by aging. One possible explanation for this could be that the energetic demands required to mount the hormetic response into late adulthood could not be met. An alternative possibility is that females enhance early reproductive investment at the expense of later investment, since iteroparous animals gain greater reproductive fitness from offspring contributing their own offspring earlier.28 Such a strategy could be particularly adaptive if the total reproductive life span is shortened by radiation stress.
Overlapping hormetic responses were observed for lifetime fecundity (Figure 1C), egg size, and hatching success (Figure 2) across the 0 to 2 Gy radiation dose range. The production of larger and potentially higher quality offspring following mild stress in early life is a hormetic response that could be adaptive in stressful environments. For instance, low-level stress could serve as a cue for parents to increase early investments in offspring numbers and quality in anticipation of adverse conditions.29,30 The adaptive value of such priming, however, may crucially depend on the degree to which early and late environments are positively correlated, as it has been shown that such responses can be costly under environmental mismatch.22 Thus, we might expect hormetic responses to evolve in animals with short generation times, such as insects, where there may be a relatively higher degree of correlation between the developmental environment of the mother and her offspring.31
The hormetic effect of early-life radiation stress on lifetime fecundity was resolved when corrected with covariation in body size (Table 1, Figure 1C). A potential explanation for this hormetic effect could be that crickets in the hormetic range invested more in total egg production relative to their body size. However, this seems unlikely since total ovary mass (relative to body size) showed a negative linear response across the entire radiation dose range (Figure 1D). It is more likely that the hormetic effect on lifetime fecundity was due to an increase in oviposition behavior. The capacity to lay more eggs than expected for a given body size (ie, increase reproductive effort) may be especially likely in highly fecund insects such as A domesticus. This species continuously develops and stores eggs32 and may retain more eggs than will be laid at any given time.33 This could allow for a high degree of plasticity in reproductive output, allowing females to buffer developmental constraints on body size or egg production imposed by environmental stressors or resource limitation.34,35 Whether such plasticity in oviposition behavior has costs or is a general feature of hormetic responses in high-fecundity species (eg, many insects and other invertebrates) remains to be seen.36
Even though hormetic responses to early-life irradiation were observed for many aspects of reproductive performance, premating body condition (ie, the scaled mass index) expressed a negative linear response (Figure 4). This is surprising given the often assumed positive relationship between body condition and reproductive fitness.24 One possibility is that females prioritized investment in reproduction over other demands (eg, tissue maintenance or lipid storage). Such changes in body condition could result from growth deficits caused by reduced feeding37 or even direct damage to midgut cells,38 both of which are noted effects of radiation exposure in insects. To the extent that reduced body condition reflects potential fitness deficits (eg, reduced predator avoidance or immunity), there may be “hidden” costs associated with hormesis, not directly observed in our study.
Hormetic effects of early-life ionizing radiation stress on adult phenotypes may be due to several proximate mechanisms that could generalize across invertebrate and vertebrate taxa. For instance, one of the primary effects of ionizing radiation on biological systems is the generation of free radicals via the ionization of water.39,40 While oxidative challenges in early life are well known to negatively affect subsequent fecundity and longevity,41,42 an emerging body of evidence indicates that low levels of oxidative stress in early life may have an adaptive role in shaping the adult phenotype.43 For instance, low levels of free radicals coordinate many important regulatory mechanisms that could underlie hormetic responses,44 such as generalized stress response mechanisms (eg, antioxidant production and repair processes) and mitochondrial biogenesis.45,46 To the extent that oxidative stress is a cost of reproductive investment,47-49 it seems plausible to speculate that the reproductive hormesis observed in our study could be a consequence of enhanced oxidative stress resistance due to early-life priming (ie, the cost of increased reproductive investment becomes lower if the organism is better able to endure the associated oxidative stress). Future work should test the role of oxidative stress resistance in facilitating reproductive hormesis.
This work adds to a growing body of literature on hormesis in insects, particularly in relation to insecticide, heavy metals, and radiation exposure.10,50-53 Due to their relatively small body sizes and short generation times, insects have been intriguing systems for examining lifetime and transgenerational impacts of hormesis, as well as its genetic regulation.36,50 Understanding hormetic responses in insects is also relevant to important practical issues such as the evolution and development of insecticide resistance and the ability for economically important pollinator species to adapt to stressful environments.50,51
Understanding the developmental and fitness consequences of hormesis is particularly relevant today, given that organisms are faced with a range of evolutionarily novel stressors from anthropogenic sources.51,54 Our results show that early-life exposures to low-dose ionizing radiation have multiple positive effects on organismal fitness across the life span. Hormesis might seem puzzling from an evolutionary perspective because it raises the question of why organisms do not produce these beneficial responses in the absence of a sensitizing stress exposure. One possibility is that hormetic responses are associated with fitness-related costs,22,55 and this requires greater attention. Other important directions for future research will be to understand the types of environmental conditions under which hormetic responses are adaptive, elucidate the proximate mechanisms underlying these responses, and determine how hormetic responses and their potential fitness costs may vary across populations and species with varying life history strategies.
Acknowledgments
This manuscript was improved from advice provided by Emilie Snell-Rood, Kristin Sikkink, Aamod Zambre, and Megan Kobiela. Special thanks go to Paul Andrews, Jonathon Stone, and Colin Seymour, who provided helpful advice over the course of the project. Help with data collection was provided by Zulal Ozkan, Ross Edwards, and Chloe Darling. The authors acknowledge funding from MITACS and Bruce Power awarded to DRB and CDR.
Authors’ Note: Alexander M. Shephard, C. David Rollo, Vadim Aksenov, Jonathan Tran, and Douglas R. Boreham conceived the ideas and designed the methodology. Alexander M. Shephard and Connor J. Nelson collected the data. Alexander M. Shephard analyzed the data and led the writing of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by MITACS Accelerate Grant #IT05851.
ORCID iD: Alexander M. Shephard  http://orcid.org/0000-0002-6926-1773
http://orcid.org/0000-0002-6926-1773
References
- 1. Calabrese EJ. Getting the dose-response wrong: why hormesis became marginalized and the threshold model accepted. Arch Toxicol. 2009;83(3):227–247. [DOI] [PubMed] [Google Scholar]
- 2. Vaiserman AM. Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose Response. 2010;8(2):172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Møller AP, Mousseau TA. Biological consequences of Chernobyl: 20 years on. Trends Ecol Evol. 2006;21(4):200–207. [DOI] [PubMed] [Google Scholar]
- 4. Southam CM, Ehrlich J. Decay resistance and physical characteristics of wood. J For. 1943;41(9):666–673. [Google Scholar]
- 5. Calabrese E, Baldwin L. Defining hormesis. Hum Exp Toxicol. 2002;21(2):91–97. [DOI] [PubMed] [Google Scholar]
- 6. Calabrese EJ, Blain RB. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Regul Toxicol Pharmacol. 2011;61(1):73–81. [DOI] [PubMed] [Google Scholar]
- 7. Mattson MP, Calabrese EJ. Hormesis: A Revolution in Biology, Toxicology and Medicine. New York, NY: Springer; 2010. [Google Scholar]
- 8. Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78(925):3–7. [DOI] [PubMed] [Google Scholar]
- 9. Luckey TD. Atomic bomb health benefits. Dose-Response. 2008;6(4):369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calabrese EJ. Low doses of radiation can enhance insect lifespans. Biogerontology. 2013;14(4):365–381. [DOI] [PubMed] [Google Scholar]
- 11. Little M. Risks associated with ionizing radiation. Br Med Bull. 2003;68(1):259–275. [DOI] [PubMed] [Google Scholar]
- 12. Tykva R, Podracká E. Bioaccumulation of 226Ra in the plants growing near uranium facilities. Nukleonika. 2005;50:25–27. [Google Scholar]
- 13. Mothersill C, Smith R, Lariviere D, Seymour C. Chronic exposure by ingestion of environmentally relevant doses of 226Ra leads to transient growth perturbations in fathead minnow (Pimephales promelas, Rafinesque, 1820). Int J Radiat Biol. 2013;89(11):950–964. [DOI] [PubMed] [Google Scholar]
- 14. Lyn J, Aksenov V, LeBlanc Z, Rollo CD. Life history features and aging rates: insights from intra-specific patterns in the cricket Acheta domesticus. Evol Biol. 2012;39(3):371–387. [Google Scholar]
- 15. Lyn JC, Naikkhwah W, Aksenov V, Rollo CD. Influence of two methods of dietary restriction on life history features and aging of the cricket Acheta domesticus. Age (Omaha). 2011;33(4):509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hans H, Lone A, Aksenov V, Rollo CD. Impacts of metformin and aspirin on life history features and longevity of crickets: trade-offs versus cost-free life extension? Age (Omaha). 2015;37(2):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costantini D. Oxidative stress and hormetic responses in the early life of birds In: Laviola G, Macri S. eds. Adaptive and Maladaptive Aspects of Developmental Stress. New York, NY: Springer; 2013:257–273. [Google Scholar]
- 18. Costantini D, Metcalfe NB, Monaghan P. Ecological processes in a hormetic framework. Ecol Lett. 2010;13(11):1435–1447. [DOI] [PubMed] [Google Scholar]
- 19. Costantini D, Monaghan P, Metcalfe NB. Early life experience primes resistance to oxidative stress. J Exp Biol. 2012;215(16):2820–2826. [DOI] [PubMed] [Google Scholar]
- 20. Burger JMS, Hwangbo DS, Corby-Harris V, Promislow DEL. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell. 2007;6(1):63–71. [DOI] [PubMed] [Google Scholar]
- 21. Taborsky B. Mothers determine offspring size in response to own juvenile growth conditions. Biol Lett. 2006;2(2):225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costantini D, Monaghan P, Metcalfe NB. Prior hormetic priming is costly under environmental mismatch. Biol Lett. 2014;10(2):20131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burton T, Metcalfe NB. Can environmental conditions experienced in early life influence future generations? Proc R Soc B Biol Sci. 2014;281(1785):20140311-20140311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118(12):1883–1891. [Google Scholar]
- 25. West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 26. Gilbert SF. Ecological developmental biology: developmental biology meets the real world. Dev Biol. 2001;233(1):1–12. [DOI] [PubMed] [Google Scholar]
- 27. Monaghan P. Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond B Biol Sci. 2008;363(1497):1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat. 1966;100(916):687–690. [Google Scholar]
- 29. Mousseau T, Fox C. The adaptive significance of maternal effects. Trends Ecol Evol. 1998;13(10):403–407. [DOI] [PubMed] [Google Scholar]
- 30. Qvarnström A, Price TD. Maternal effects, paternal effects and sexual selection. Trends Ecol Evol. 2001;16(2):95–100. [DOI] [PubMed] [Google Scholar]
- 31. Taborsky B. Developmental plasticity: preparing for life in a complex world. Adv Study Behav. 2017;49:49–99. [Google Scholar]
- 32. Woodring JP, Clifford CW, Beckman BR. Food utilization and metabolic efficiency in larval and adult house crickets. J Insect Physiol. 1979;25:903–912. [Google Scholar]
- 33. Adamo S. Evidence for adaptive changes in egg laying in crickets exposed to bacteria and parasites. Anim Behav. 1999;57:117–124. [DOI] [PubMed] [Google Scholar]
- 34. Whitman DW. The significance of body size in the orthoptera: a review. J Orthoptera Res. 2008;17(2):117–134. [Google Scholar]
- 35. Davidowitz G. Population and environmental effects on the size-fecundity relationship in a common grasshopper across an aridity gradient. J Orthoptera Res. 2008;17(2):265–271. [Google Scholar]
- 36. Ayyanath MM, Cutler GC, Scott-Dupree CD, Sibley PK. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. Plos One. 2013;8(9):e74532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. York JM, Blevins NA, Meling DD, et al. The biobehavioral and neuroimmune impact of low-dose ionizing radiation. Brain Behav Immun. 2012;26(2):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasan M, Khan AR. Control of stored product pests by ionizing radiation. J Stored Prod Res. 1998;3(1):15–29. [Google Scholar]
- 39. Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27–33. [DOI] [PubMed] [Google Scholar]
- 40. Koch RE, Hill GE. An assessment of techniques to manipulate oxidative stress in animals. Funct Ecol. 2017;31(1):9–21. [Google Scholar]
- 41. Monaghan P, Haussmann MF. The positive and negative consequences of stressors during early life. Early Hum Dev. 2015;91(11):643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alonso-Alvarez C, Bertrand S, Devevey G, et al. An experimental manipulation of life history trajectories and resistance to oxidative stress. Evolution (N Y). 2006;60(9):1913–1924. [PubMed] [Google Scholar]
- 43. Costantini D. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage between Mechanistic and Evolutionary Approaches. Berlin, Germany: Springer; 2014. [Google Scholar]
- 44. Zhang Y, Hood WR. Current versus future reproduction and longevity: a re-evaluation of predictions and mechanisms. J Exp Biol. 2016;219(20):3177–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. [DOI] [PubMed] [Google Scholar]
- 46. Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20(7):709–711. [DOI] [PubMed] [Google Scholar]
- 47. Metcalfe NB, Monaghan P. Does reproduction cause oxidative stress? An open question. Trends Ecol Evol. 2013;28(6):347–350. [DOI] [PubMed] [Google Scholar]
- 48. Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett. 2009;12(1):75–92. [DOI] [PubMed] [Google Scholar]
- 49. Speakman JR, Garratt M. Oxidative stress as a cost of reproduction: beyond the simplistic trade-off model. BioEssays. 2014;36(1):93–106. [DOI] [PubMed] [Google Scholar]
- 50. Guedes RNC, Cutler GC. Insecticide-induced hormesis and arthropod pest management. Pest Manag Sci. 2014;70(5):690–697. [DOI] [PubMed] [Google Scholar]
- 51. Cutler GC. Insects, insecticides and hormesis: evidence and considerations for study. Dose Response. 2013;11(2): 154-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ayyanath MM, Cutler GC, Scott-Dupree CD, Prithivirai B, Kandasamy S, Prithivirai K. Gene expression during imidacloprid-induced hormesis in green peach aphid. Dose Response. 2014;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nascarella MA, Stoffolano Jr JG, Stanek III EJ, Kostecki PT, Calabrese EJ. Hormesis and stage specific toxicity induced by cadmium in an insect model, the queen blowfly. Phormia regina Meig. Environ Pollut. 2003;124(2):256–262. [DOI] [PubMed] [Google Scholar]
- 54. Costantini D. Does hormesis foster organism resistance to extreme events? Front Ecol Environ. 2014;12(4):209–210. [Google Scholar]
- 55. Mitz C, Thome C, Cybulski ME, et al. Is there a trade-off between radiation-stimulated growth and metabolic efficiency? Radiat Res. 2017;494:486–494. [DOI] [PubMed] [Google Scholar]