Abstract
To explore an optimal frequency of whole-body low-dose radiation (LDR) to protect the kidney from diabetes, type 1 diabetic mice were induced with multiple injections of low-dose streptozotocin in male C57BL/6J mice. Diabetic or age-matched normal mice received whole-body exposure to 12.5 or 25 mGy either every other day or weekly for 4 or 8 weeks. Diabetes decreased the urinary creatinine and increased the microalbumin in urine, renal accumulation of 3-nitrotyrosine and 4-hydroxynonenal, and renal expression of collagen IV and fibronectin. All these renal pathological and functional changes in diabetic mice were significantly attenuated by exposure to LDR at all regimens. However, whole-body exposure of diabetic mice to 25 mGy weekly and to 12.5 mGy every other day for 8 weeks provided a better prevention of diabetic nephropathy than other LDR regimens. Furthermore, whole-body exposure to 25 mGy weekly for 8 weeks showed no detectable effect on the kidney of normal mice, but whole-body exposure to normal mice at 12.5 mGy every other day for 8 weeks increased urinary microalbumin and renal expression of collagen IV and fibronectin. These results suggest that whole-body exposure to LDR at 25 mGy weekly is the optimal condition of LDR to protect the kidney from diabetes.
Keywords: low-dose radiation, diabetic nephropathy, renal remodeling, renal pathogenesis, radiation frequency
Introduction
Diabetes mellitus (DM) is a growing and costly public health problem worldwide. Most patients with DM will die from diabetic complications, among which, diabetic nephropathy (DN) is the most common and also the leading cause of end-stage renal disease in developed countries.1 Although the pathogenesis of DN remains unclear, previous studies have disclosed that many factors are implicated in the development of DN. These include hyperglycemia, advanced glycation end products, protein kinase C, oxidative stress, and inflammation.2-6 Based on these findings, several drugs targeting these factors have been developed potentially for the clinical application; however, the prevention of DN remains unsatisfactory.7 Particularly, the patients at the late stage of DM with renal dysfunction are restricted for giving multiple drugs to avoid the increase in renal drug metabolizing workload.8 Therefore, new therapeutic, particularly noninvasive, interventions are urgently needed for the prevention of DN at the late stage.
Low-dose radiation (LDR) has been proved to activate various bioeffects, especially antioxidative and anti-inflammatory effects.9-12 Therefore, LDR has been explored for its potential treatment of diseases caused by abnormal oxidative stress and inflammation, such as Parkinson disease and arthritic symptoms.13,14 Given the important roles of oxidative stress and inflammation in the progression of DN, we have explored the therapeutic effect of LDR on DN, showing that multiple whole-body exposures of diabetic mice to 25 mGy X-rays every other day significantly prevented renal dysfunction and fibrosis via reducing DM-induced systemic inflammation and oxidative damage.15 This finding was confirmed by Nomura study, in which, continuous exposure of type II diabetic mice to LDR also ameliorated DN through increasing the renal antioxidant activity.16 These studies implied that LDR may be a new strategy for the therapy of DN. More importantly, LDR is a noninvasive approach for patients with diabetes. However, obviously there are many questions needed to be addressed before LDR therapy for diabetic complications can be considered. For example, there is only a single fractional dose in our previous study.15 Thus, more dose–response relationships need to be established to find an optimal dose with the best therapeutic effect. In one of our recent studies, the preventive effect of more fractional doses, including 12.5, 25, and 50 mGy, was investigated using a diabetic mouse model, which was induced by streptozocin (STZ). The STZ is is an alkylating antineoplastic agent that is particularly toxic to the insulin-producing β cells of the pancreas; therefore, STZ was extensively used to induce type 1 diabetic animal models, and STZ-induced diabetic model has early renal injury 4 weeks after onset. We showed that exposure to LDR at 12.5 mGy every other day for 8 weeks ameliorated DN more effectively than the other 2 doses.17 Aside from dosage, the bioeffect of radiation also partly depends on frequency of radiation exposure (fractionation schedule).18 Therefore, a more detailed study, including different radiation frequencies, was done in the present study.
Our recent study showed a fractional dose of 12.5 mGy optimally preventing DN; in the current study, therefore, the diabetic or normal mice were irradiated to the optimal dose (12.5 mGy) or its double dose (25 mGy) every other day or once a week to compare their preventive effects on DN.
Materials and Methods
Animals
Ten-week-old male C57BL/6J mice from the Jilin University Animal Center were maintained in light (12:12-h light-dark cycle) and temperature-controlled quarters (22°C) with rodent chow and water for ≥2 weeks before being used for the experiments. All animal protocols were approved by the Institutional Animal Care and Use Committee at Jilin University (2011037), which is certified by the Chinese Association of Accreditation of Laboratory Animal Care.
Type 1 DM
Type 1 diabetic mice were induced by multiple injections of STZ (Sigma Chemical, St. Louis, Missouri) at 60 mg/kg daily for 5 days. One week after the last injection, blood glucose levels of mice were determined using a freestyle glucometer. When fasting blood glucose of the mice was ≥12 mmol/L for more than 2 weeks, mice were considered to be successful type 1 DM models for the subsequent experiments.
Low-Dose Radiation
Mice in both diabetic and age-matched control groups were randomly divided into 2 groups with and without LDR, namely, control, control/LDR (LDR), DM, and DM/LDR. Mice in both LDR and DM/LDR groups were further divided into 4 groups. Two groups were given multiple exposures of X-rays at either 12.5 or 25 mGy every other day. The remaining groups were given multiple exposures at dose of 12.5 or 25 mGy weekly. X-ray exposure was performed using an American PXi (Precision X-ray) machine (X-RAD 320 X-Ray Biological Irradiator with TouchRAD Control) with a dose rate of 12.5 mGy/min. Eight mice from each group were euthanized at the fourth and eighth week of exposure to LDR.
Measurements for Renal Function
To collect urine samples, mice were individually placed in metabolic cages for 24 hours prior to killing and only allowed access to tap water. Urinary microalbumin (Malb) and creatinine (Cre) contents as parameters of renal function were detected using the Mouse MAU/ALB enzyme-linked immunosorbent assay (ELISA) kit and Mouse UCR ELISA kit (Boster Biological Technology, Wuhan, China).
Histopathological Examination
Kidney tissues were fixed in 10% neutral-buffered formalin solution, followed by paraffin embedding, and cutting into 4-μm slices. After deparaffinization by xylene and ethanol dilutions and rehydration, sections were stained with hematoxylin and eosin (H&E) and Periodic acid–Schiff (PAS), respectively. A semiquantitative assessment of the degree of mesangial matrix expansion was performed on PAS-stained sections. Mesangial matrix expansion, one of the important pathological features of DN, was defined by the presence of increased amounts of PAS-positive material in the mesangial region. A minimum of 20 randomly selected intact glomeruli in the 2 slides from each mouse was evaluated independently by 2 investigators without prior knowledge of the origin of the slides. The degree of mesangial matrix expansion was evaluated according to the percentage of involved glomerular area, and the scores were as follows: 0, PAS-positive materials were not found in the mesangial region; 1, <25%; 2, 25% to 50%; 3, 51% to 75%; 4, >75%. The scores obtained by the 2 investigators were averaged.
Western Blotting
Renal tissues were homogenized in lysis buffer (1% Triton X-100, 150 mmol/L NaCl, 50 mmol/L Tris, pH 8.0, 1 mmol/L EDTA, 10 mg/L phenylmethylsulfonyl fluoride). Protein concentration was determined by the Bradford assay. Equal amounts (50 μg protein/lane) of proteins were loaded onto 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, electrophoresed, and transferred to polyvinyl difluoride membranes. Membranes were blocked with a 5% nonfat milk for 1 hour and then incubated with antimouse antibodies (Abcam, Cambridge, Massachusetts) against 3-nitrotyrosine (3-NT at 1:1400 dilution), 4-hydroxynonenal (4-HNE,1:50), collagen IV (Col IV,1:500), and fibronectin (FN,1:5000) overnight at 4°C. Membranes were washed to remove the unbound antibodies with Tris-buffered saline (pH 7.2), containing 0.05% Tween 20 3 times and then incubated with the appropriate secondary antibody. Antigen–antibody complexes were visualized by electrochemiluminisence, and the resulting image was analyzed with Bio-Rad Quantity One (Hercules, CA).
Statistical Analysis
Experimental data were collected from multiple animals under each experimental condition (n = 8) and are presented as the means (SD). Comparisons were performed by paired Student t test and 1-way analysis of variance followed by Student-Newman-Keuls q test for the different groups using statistical software SPSS 19.0. Differences were considered to be significant at P < .05.
Results
Exposure to LDR at 25 mGy Weekly and 12.5 mGy Every Other Day Improved DM-Induced Renal Dysfunction More Effectively Than Other Exposure Regimens
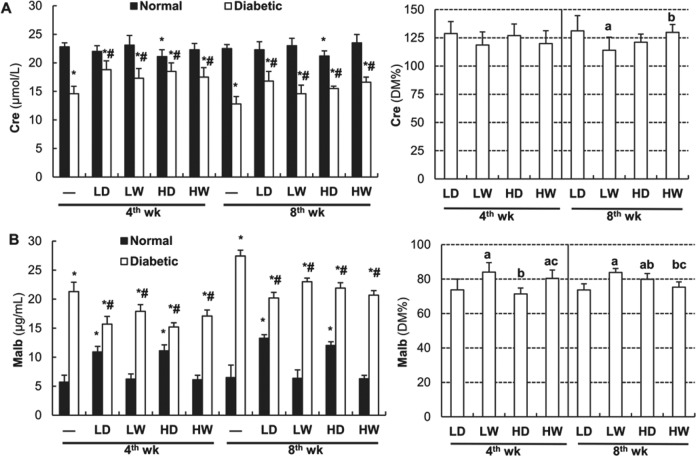
Table 1 shows that neither 4-week nor 8-week exposure to LDR affect blood glucose level in normal mice. However, alhthough 4-week exposure to LDR did not, 8-week exposure to LDR significantly reduced diabetes-increased blood glucose. As shown in left panel of Figure 1A and Table 2, DM resulted in a significant decrease in urinary Cre levels at both the fourth and eighth week of the study. Exposure of normal mice to LDR at 25 mGy every other day for either 4 weeks or 8 weeks also slightly reduced urinary Cre levels. However, exposure to LDR at all regimens significantly ameliorated DM-induced decease in urinary Cre levels. In order to easily compare the effect of different LDR regimens on DN, we calculated the percentage of each DM/LDR group urinary Cre level relative to DM urinary Cre (right panel of Figure 1A). It clearly indicates that there was no significant difference in urinary Cre levels among DM mice exposed to LDR for 4 weeks, while exposure to LDR at 12.5 mGy every other day or at 25 mGy weekly for 8 weeks more effectively attenuated DM-decreased urinary Cre than exposure to LDR at 12.5 mGy weekly for 8 weeks.
Table 1.
Effects of Different LDR Frequencies on Blood Glucose in Normal and Diabetic mice.a
| Time for LDR | — | N/L | DM | DM/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | LW | HD | HW | LD | LW | HD | HW | |||
| 4 weeks | 6.6 (1.1) | 6.7 (1.2) | 6.4 (1.6) | 6.2 (1.1) | 6.5 (1.3) | 24.6 (1.4)b | 24.4 (1.2)b | 23.5 (1.3)b | 22.9 (2.1)b | 25.0 (1.6)b |
| 8 weeks | 6.8 (1.0) | 7.0 (1.1) | 7.2 (1.5) | 6.9 (1.3) | 7.1 (1.2) | 27.9 (1.4)b | 23.0 (1.2)b,c | 22.6 (1.3)b,c | 21.7 (1.1)b,c | 21.2 (1.8)b,c |
Abbreviations: DM, diabetes mellitus; LDR, low-dose radiation.
aEffects of different LDR regimens on blood glucose in normal and diabetic mice. Blood samples of mice from each group were collected at the 4th and the 8th week of the study, respectively. Blood glucose levels were measured using a freestyle glucometer. “-” indicates normal mice without LDR (Sham group). “N/L” indicates normal mice with LDR. “DM/L” indicates diabetic mice with LDR. LD: 12.5 mGy every other day; LW: 12.5 mGy every week; HD: 25 mGy every other day; 25 mGy every week. “wk” indicates the time when the samples were collected and examined. Data are the means (SD; n = 8).
b P < .05 versus normal mice with or without LDR at the same time point.
c P < .05 versus unirradiated diabetic mice at the same time point.
Figure 1.
Effects of different doses and frequency of low-dose radiation (LDR) on renal function in normal and diabetic mice. Urine samples of mice from each group were collected at the fourth and eighth week of the study, respectively. Creatinine (Cre; left panel of A) and microalbumin (Malb; left panel of B) levels in the urine were measured by enzyme-linked immunosorbent assay. The Cre and Malb levels in the urine of different diabetes mellitus (DM)/LDR groups were further calculated relative to DM and presented by the percentage of DM group (right panels of A and B, respectively). “-” indicates mice without LDR (Sham group) LD: 12.5 mGy every other day; LW: 12.5 mGy every week; HD: 25 mGy every other day; 25 mGy every week. “wk” indicates the time when the samples were collected and examined. Data are the means (SD; n = 8). *P < .05 versus normal mice of “-” (sham) at the same time point; # P < .05 versus diabetic mice of “-” at the same time point; a P < .05 versus LD at the same time point; b P < .05 versus LW at the same time point; c P < .05 versus HD at the same time point.
Table 2.
Effects of Different LDR Frequencies on Urinary Creatinine in Normal and Diabetic Mice.a
| Time for LDR | — | N/L | DM | DM/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | LW | HD | HW | LD | LW | HD | HW | |||
| 4 weeks | 22.8 (0.7) | 22.0 (1.0) | 23.1 (1.4) | 21.1 (1.2)b | 22.3 (1.1) | 14.6 (1.3)b | 18.8 (1.6)b,c | 17.3 (1.7)b,c | 18.5 (1.5)b,c | 17.5 (1.6)b,c |
| 8 weeks | 22.5 (0.7) | 22.3 (1.4) | 23.0 (1.3) | 21.2 (0.9)b | 23.5 (1.5) | 12.8 (1.3)b | 16.8 (1.7)b,c | 14.6 (1.5)b,c | 15.5 (0.9)b,c | 16.6 (0.9)b,c |
Abbreviations: DM, diabetes mellitus; ELISA, enzyme-linked immunosorbent assay; LDR, low-dose radiation.
aEffects of different LDR regimens on renal function in normal and diabetic mice. Urine samples of mice from each group were collected at the 4th and 8th week of the study, respectively. Creatinine levels in the urine were measured by ELISA assay. “-” indicates normal mice without LDR (Sham group). “N/L” indicates normal mice with LDR. “DM/L” indicates diabetic mice with LDR. LD: 12.5 mGy every other day; LW: 12.5 mGy every week; HD: 25 mGy every other day; 25 mGy every week. “wk” indicates the time when the samples were collected and examined. Data are the means (SD; n = 8).
b P < .05 versus normal mice without LDR (Sham group) at the same time point.
c P < .05 versus unirradiated diabetic mice at the same time point.
The left panel of Figure 1B and Table 3 show that urinary Malb increased slightly in normal mice exposed to LDR at 12.5 and 25 mGy every other day, but not weekly, both for 4 weeks and 8 weeks. Diabetes mellitus exhibited a significant time-dependent increase in urinary Malb, which, however, were significantly reduced by all LDR regimens at both 4 and 8 weeks. When the effect of different LDR regiments on DM-increased Malb levels was directly compared by its percentage of DM group, we found that exposure to LDR at 12.5 or 25 mGy every other day for 4 weeks was more efficient in attenuating DM-increased urinary Malb than the weekly course, while exposure of diabetic mice to LDR at 12.5 mGy every other day or 25 mGy weekly for 8 weeks was more efficient than other exposure regimens (right panel of Figure 1B).
Table 3.
Effects of Different LDR Frequencies on Urinary Microalbumin in Normal and Diabetic Mice.a
| Time for LDR | — | N/L | DM | DM/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | LW | HD | HW | LD | LW | HD | HW | |||
| 4 weeks | 5.70 (1.20) | 10.90 (0.96)b | 6.23 (0.88) | 11.10 (1.03)b | 6.12 (0.77) | 21.30 (1.62)b | 15.70 (1.33)b,c | 17.90 (1.16)b,c | 15.20 (0.73)b,c | 17.10 (1.04)b,c |
| 8 weeks | 6.50 (2.15) | 13.26 (0.61)b | 6.37 (1.44) | 12.02 (0.64)b | 6.29 (0.58) | 27.40 (1.01)b | 20.20 (0.97)b,c | 23.00 (0.63)b,c | 21.91 (0.92)b,c | 20.70 (0.77)b,c |
Abbreviations: DM, diabetes mellitus; ELISA, enzyme-linked immunosorbent assay; LDR, low-dose radiation.
aEffects of different LDR regimens on renal function in normal and diabetic mice. Urine samples of mice from each group were collected at the 4th and 8th week of the study, respectively. Microalbumin levels in the urine were measured by ELISA assay. “-” indicates normal mice without LDR (Sham group). “N/L” indicates normal mice with LDR. “DM/L” indicates diabetic mice with LDR. LD: 12.5 mGy every other day; LW: 12.5 mGy every week; HD: 25 mGy every other day; 25 mGy every week. “wk” indicates the time when the samples were collected and examined. Data are the means (SD; n = 8).
b P < .05 versus normal mice without LDR (Sham group) at the same time point.
c P < .05 versus unirradiated diabetic mice at the same time point.
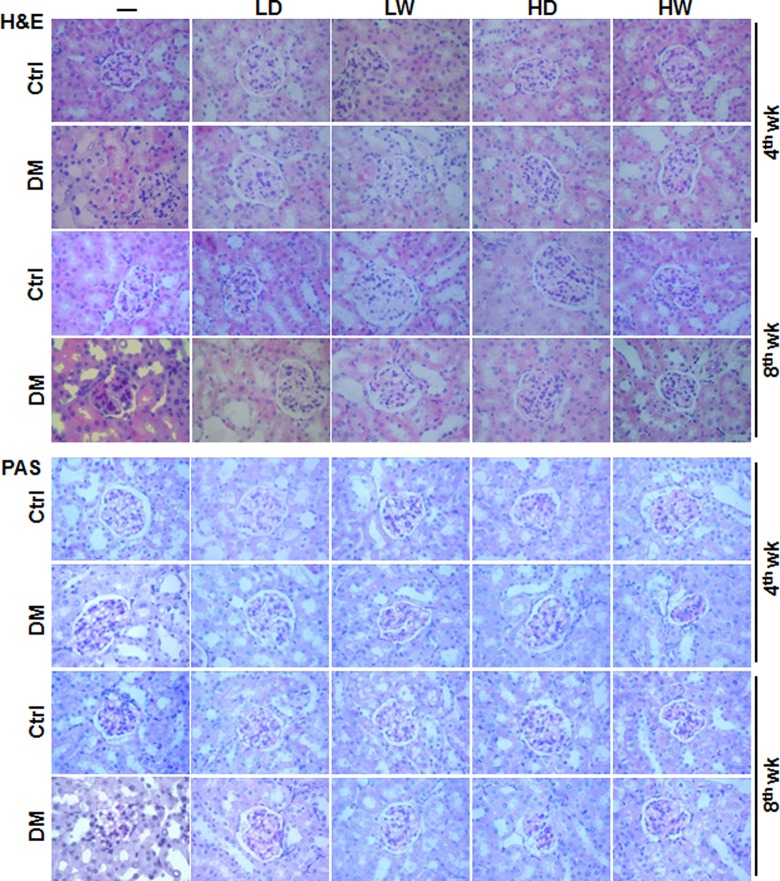
Evaluation of renal pathological changes was performed with H&E staining (top panel of Figure 2). Analysis showed that LDR for either 4 weeks or 8 weeks had almost no effect on the pathological features of kidneys from normal mice, except for causing rare inflammatory cell infiltration in renal interstitial areas. Diabetic mice without LDR displayed time-dependent pathological changes, including increased size of glomeruli, obvious mesangial cell proliferation and mesangial matrix expansion, pink exudation in renal tubules, collapse of partial capillary, fibroplasia in interstitial areas, and vacuolar degeneration of partial tubular epithelia. However, after these diabetic mice were exposed to any of 4 LDR regimens for 4 or 8 weeks, the renal glomerulus structure damage, glomerulus size enlargement, mesangial cell proliferation, and mesangial matrix expansion were markedly decreased compared to diabetic mice without LDR. Among all 8-week exposure DM/LDR groups, mice exposed to 12.5 mGy every other day or to 25 mGy weekly showed the least damage.
Figure 2.
Effects of different doses and frequency of low-dose radiation (LDR) on diabetes mellitus (DM)-induced renal histopathological changes. At the fourth and eighth week of the study, renal pathology of mice from different groups was examined with light microscope by hematoxylin and eosin (H&E; upper panel) and Periodic acid–Schiff (PAS; lower panel) staining. “Ctrl” and “DM” indicate normal and diabetic mice, respectively. LD: 12.5 mGy every other day; LW: 12.5 mGy every week; HD: 25 mGy every other day; 25 mGy every week. Other abbreviations were referred in Figure 1. Images are the representatives of each group with 3 fields (× 200) for each of the 8 mice at least examined.
Renal sections were subsequently subjected to PAS staining for further observation of glomerular basement membrane changes, mesangial cell proliferation, and mesangial matrix expansion (bottom panel of Figure 2 and their semiquantitative data in Table 4). There was no difference for these features between normal mice with and without LDR. Diabetic mice displayed enhanced deposition of PAS-positive materials in both the vascular plexus and the mesangial region of glomeruli and increased nuclei and dense staining in mesangial cells. After mice were exposed to different LDR regimens for 4 or 8 weeks, DM-increased deposition of PAS-positive materials in vascular plexus and mesangial region of glomeruli and thickness of glomerular capillary basement membrane were attenuated, the greatest degree of which was observed in the groups exposed to 12.5 mGy every other day or to 25 mGy weekly (Figure 2 and Table 4).
Table 4.
The Semiquantitative Data of PAS Staining Sections in Kidney From Different Groups.a
| Time for LDR | — | N/L | DM | DM/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | LW | HD | HW | LD | LW | HD | HW | |||
| 4 weeks | 0 | 0 | 1 | 0 | 0 | 4 | 2 | 3 | 2 | 2 |
| 8 weeks | 1 | 0 | 1 | 1 | 1 | 4 | 2 | 3 | 3 | 2 |
Abbreviations: DM, diabetes mellitus; LDR, low-dose radiation; PAS, Periodic acid–Schiff.
aThe semiquantitative data of PAS staining sections in kidney from different groups. Mesangial matrix expansion, one of the important pathological features of diabetic nephropathy, was defined by the presence of increased amounts of PAS positive material in the mesangial region. A minimum of 20 randomly selected intact glomeruli in the 2 slides from each rat was evaluated independently by 2 investigators without prior knowledge of the origin of the slides. The degree of mesangial matrix expansion was evaluated according to the percentage of involved glomerular area and the scores were as follows: 0, PAS positive materials were not found in the mesangial region; 1, <25%; 2, 25% to 50%; 3, 51% to 75%; 4, >75%. The scores obtained by the 2 investigators were averaged. “-” indicates normal mice without LDR (Sham group). “N/L” indicates normal mice with LDR. “DM/L” indicates diabetic mice with LDR. LD: 12.5 mGy every other day; LW: 12.5 mGy every week; HD: 25 mGy every other day; 25 mGy every week. “wk” indicates the time when the samples were collected and examined.
Exposure to LDR at 25 mGy Weekly and 12.5 mGy Every Other Day Improved DM-Induced Oxidative/Nitrosative Damage More Effectively Than Other Exposure Regimens
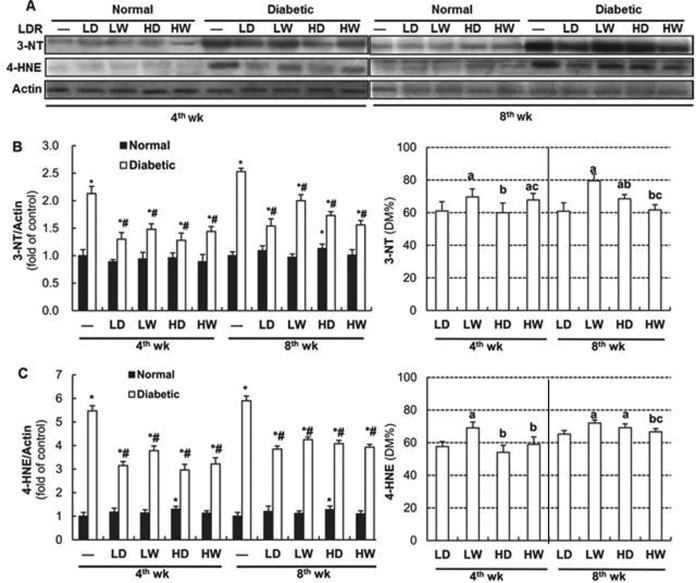
Our previous studies showed that LDR suppressed DN through increasing renal antioxidant activity,15 and the suppressive effect of different LDR regimens on DN was in line with their capacity to increase renal antioxidant activity.17 Therefore, the accumulation of oxidative and nitrosative damage products (lipid peroxidation-related 4-HNE and protein-nitration-related 3-NT) were assessed by Western blotting. As seen in Figure 3A and the left panels of 3B and 3C, normal mice exposed to LDR for either 4 weeks or 8 weeks showed little change in the amount of 3-NT and 4-HNE contents in the kidney. Notable exceptions are in 25 mGy exposure every other day for 8 weeks which saw slightly increased accumulation of 3-NT. Furthermore, both 4- and 8-week LDR exposure demonstrated slightly increased 4-HNE accumulation. However, exposure of diabetic mice to all LDR regimens for either 4 weeks or 8 weeks significantly attenuated DM-increased renal 3-NT and 4-HNE accumulation.
Figure 3.
Effects of different doses and frequency of low-dose radiation (LDR) on diabetes mellitus (DM)-induced renal oxidative and nitrosative damage. Renal tissues from normal or diabetic mice with and without exposure to LDR at different doses and different frequency were collected at the 4th and 8th week of the study. Renal accumulation of 4-hydroxynonenal (4-HNE) as oxidative lipid-peroxidation and 3-nitrotyrosine (3-NT) as protein nitration was detected by Western blotting (A) followed by quantitative analysis (left panels of B and C, respectively). Results of quantitative analysis were further shown as their percentages of that in DM group (right panels of B and C, respectively). Abbreviations are referred in Figure 1. Data are the means (SD; n = 8). *P < .05 versus normal mice of “-” at the same time point; # P < .05 versus diabetic mice of “-” at the same time point; a P < .05 versus LD at the same time point; b P < .05 versus LW at the same time point; c P < .05 versus HD at the same time point.
When the effects of LDR on DM-increased renal accumulation of 3-NT and 4-HNE were directly compared to their preventive percentages, we observed that exposure to LDR at 12.5 and 25 mGy every other day for 4 weeks attenuated 3-NT accumulation more effectively than other 2 LDR regimens, and exposure to LDR at 12.5 mGy weekly for 4 weeks provided a less suppressive effect on DM-caused accumulation of 4-HNE than the remaining 3 LDR regimens (right panels of 3B and 3C). However, exposure of diabetic mice to LDR at 25 mGy weekly or 12.5 mGy every other day for 8 weeks showed the greatest degree of prevention against 3-NT and 4-HNE accumulation among all DM/LDR groups.
Exposure to LDR at 25 mGy Weekly and 12.5 mGy Every Other Day Suppressed DM-Induced Renal Fibrosis More Effectively Than Other Exposure Regimens
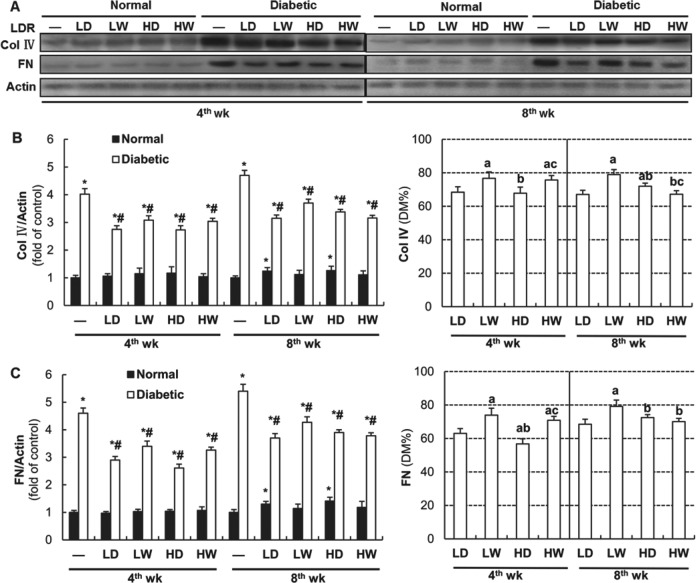
Renal fibrotic changes were subsequently investigated by Western blotting for Col IV and FN. As shown in Figure 4A and left panels of 4B and 4C, although exposure of normal mice to all LDR regimens for 4 weeks did not affect the renal Col IV and FN expression, exposure of normal mice to 12.5 and 25 mGy every other day for 8 weeks slightly increased renal expression of these 2 fibrotic factors. However, exposure of diabetic mice to all LDR regimens for either 4 weeks or 8 weeks significantly attenuated DM-increased renal accumulation of Col IV and FN. The right panel of Figure 4B indicates that exposure of diabetic mice to 12.5 and 25 mGy X-rays every other day for 4 weeks provided the best preventive effect on DM-increased renal expression of Col IV than other 2 LDR regimens; however, among the groups exposed to LDRs for 8 weeks, the most efficient attenuation was in the group of diabetic mice exposed to LDR at 12.5 mGy every other day and 25 mGy weekly. The right panel of Figure 4C shows that exposure of diabetic mice to 25 mGy every other day for 4 weeks prevented DM-increased renal accumulation of FN more effectively than other LDR groups. All LDR exposures over 8 weeks provided similar prevention except for the group exposed to 12.5 mGy weekly.
Figure 4.
Effects of different doses and frequency of low-dose radiation (LDR) on diabetes mellitus (DM)-increased expression of collagen IV (Col IV) and fibronectin (FN). Renal tissues from normal or diabetic mice with and without exposure to LDR at different doses and different frequency were collected at the 4th and 8th week of the study. The expression of Col IV and FN was detected by Western blotting (A) followed by quantitative analysis (left panels of B and C, respectively). The results of quantitative analysis were further shown as their percentages of that in DM group (right panels of B and C, respectively). LD: 12.5 mGy every other day; LW: 12.5 mGy every week; HD: 25 mGy every other day; 25 mGy every week. Abbreviations are referred in Figure 1. Data are the means (SD; n = 8). *P < .05 versus normal mice of “-” at the same time point; # P < .05 versus diabetic mice of “-” at the same time point; a P < .05 versus LD at the same time point; b P < .05 versus LW at the same time point; c P < .05 versus HD at the same time point.
Discussion
One of our previous studies demonstrated for the first time that exposure of diabetic mice to LDR significantly attenuated DN via suppression of renal oxidative stress and inflammation.15 Using a more systemic evaluation of exposure doses (12.5, 25, and 50 mGy) and times (4 and 8 weeks) every other day, our previous study showed that exposing diabetic mice to 12.5 mGy X-rays for 8 weeks more efficiently attenuated DM-induced renal damage.17 Based on our own previous study17 and other previous studies,19,20 we can assume that there should be a low-dose threshold, below which the protective effect/radioadaptive response is not inducible; however, when the dose, regardless of whether it is a single dose or an accumulated dose of multiple exposures, exceeds the threshold dose, the protective effect would be induced in a dose-dependent manner until reaching to the maximal level. Then, the protective effect will decrease with the further increased radiation dose. After the radiation dose reaches to the upper dose threshold, the protective effect will disappear. Considering that the biological effect of radiation depends on not only the dose of radiation but also the frequency of radiation, we further investigated the preventive effects of LDR at 12.5 or 25 mGy for different exposure frequencies (every other day vs weekly). Here, we demonstrate that whole-body exposure to 25 mGy X-rays weekly and 12.5 mGy every other day for 8 weeks similarly provided a more efficient prevention on DM-induced renal dysfunction, oxidative damage, and renal remodeling (fibrotic response) than whole-body exposure to 25 mGy every other day or to 12.5 mGy weekly for 8 weeks. Furthermore, whole-body exposure to 25 mGy X-rays weekly did not show any detectable side effect on normal mice with respect to detected markers of renal function, oxidative damage, and renal fibrosis.
We demonstrate here that exposure of diabetic mice to 12.5 and 25 mGy every other day (more than 37.5 mGy/wk and 75.0 mGy/wk, respectively) attenuated DM-increased nitrosative (3-NT) damage and fibrosis (Col IV and FN) and improved diabetic suppression of renal function more efficiently than exposure to 12.5 and 25 mGy weekly (12.5 mGy/wk and 25 mGy/wk, respectively, Figure 3 and Figure 4). However, when diabetic mice were exposed to LDR for 8 weeks, the best protective effect was found in the groups exposed to 25 mGy weekly or 12.5 mGy every other day in terms of renal function, remodeling, and oxidative damage (Figures 3 and 4). Furthermore, our recent study showed that exposure of diabetic mice to 12.5 mGy every other day for 8 weeks showed much better prevention of DN than exposure to 25 or 50 mGy every other day for 8 weeks.17 These results suggest that accumulating a certain level of total exposure (neither too low nor too high) is required for the induction of renal protection from DM by LDR. This is consistent with a previous report indicating the existence of specific dose windows for the adaptive response in mammalian cells and mammals.21 Similarly, Phan et al reported that in vivo repeated 20-mGy computed tomography (CT) scans to mice rather than a single CT scan induced an adaptive response.22
Aside from protective effects, potential side effect of LDR treatment is another key issue that needs to be considered. Although exposure of normal mice to 25 mGy weekly for 8 weeks provided a similar protective effect to those provided by exposure at 12.5 mGy every other day 8 weeks, the former showed no detectable effect on normal mice except that the latter showed increased urinary Malb and renal expression of Col IV and FN. This indicates that exposure to 25 mGy weekly would be safer than exposure to 12.5 mGy every other day possibly due to the relatively lower total dose given by the former.
In terms of potential mechanisms by which LDR prevents DN, it is largely unknown yet but should include multiple mechanisms such as the regulation by LDR of systemic hormones, immunologic factors, anti-inflammatory cytokines, antioxidants, Akt-mediated signaling, and even certain epigenetic modification.1,9,10,12,13,15,23 We have reported that exposure of mice to LDR that was able to protect DN9 also increased renal antioxidant levels such as SOD and Nrf2-mediated antioxidant pathways.23,24 Therefore, the antioxidant stimulation by LDR may play important role in the preventing DN from diabetic mice.
In summary, following our previous studies, the present study further clarified that whole-body exposure to 25 mGy weekly showed the best preventive effect among all the tested LDR regimens, in terms of biochemical, pathological, and functional changes with minimal side effect. Therefore, whole-body exposure to 25 mGy weekly might be the optimal exposure condition to efficiently reduce diabetic damage to the kidney from DN. Currently, the no linear threshold principle remains key for radiation protection, and there are no currently approved clinical applications of LDR to the prevention and therapy for diabetic patients. However, therapeutic LDR treatment may be advantageous in certain situations. For instance, in senior patients who have severe multiple organs dysfunction, exogenous drugs may increase the organ metabolizing burden, and these drugs would worsen the patient’s organ dysfunction. Under such conditions, therefore, administration of these drugs to these senior patients would not be a good idea, while the noninvasive approach of LDR to stimulate renal antioxidative and anti-inflammatory functions may be a good choice.
Acknowledgments
The authors would like to thank Mr Leroy R. Sachleben Jr for his help in editing this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by grants from the National Science Foundation of China (81071920 to W.G.; 81202151 and 31570854 to F.L.), and Ministry of Education Key Project of Science and Technology (311015 to J.C.).
ORCID iD: Lu Cai  http://orcid.org/0000-0003-3048-1135
http://orcid.org/0000-0003-3048-1135
References
- 1. Tuttle KR, Anderson PW. A novel potential therapy for diabetic nephropathy and vascular complications: protein kinase C beta inhibition. Am J Kidney Dis. 2003;42(3):456–465. [DOI] [PubMed] [Google Scholar]
- 2. Galler A, Muller G, Schinzel R, Kratzsch J, Kiess W, Munch G. Impact of metabolic control and serum lipids on the concentration of advanced glycation end products in the serum of children and adolescents with type 1 diabetes, as determined by fluorescence spectroscopy and nepsilon-(carboxymethyl) lysine ELISA. Diabetes Care. 2003;26(9):2609–2615. [DOI] [PubMed] [Google Scholar]
- 3. Sun YM, Su Y, Li J, Wang LF. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem Biophys Res Commun. 2013;433(4):359–361. [DOI] [PubMed] [Google Scholar]
- 4. Li J, Gobe G. Protein kinase C activation and its role in kidney disease. Nephrology. 2006; 11(5):428–434. [DOI] [PubMed] [Google Scholar]
- 5. Wu J, Mei C, Vlassara H, Striker GE, Zheng F. Oxidative stress-induced JNK activation contributes to proinflammatory phenotype of aging diabetic mesangial cells. Am J Physiol Renal Physiol. 2009;297(6):F1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology. 2006;11(3):226–231. [DOI] [PubMed] [Google Scholar]
- 7. Ke HL, Zhang YW, Zhou BF, Zhen RT. Effects of Danggui Buxue Tang, a traditional Chinese herbal decoction, on high glucose-induced proliferation and expression of extracellular matrix proteins in glomerular mesangial cells. Nat Prod Res. 2012;26(11):1022–1026. [DOI] [PubMed] [Google Scholar]
- 8. Oulahiane A, Anaddam S, Ouleghzal H, et al. Diabetes management issues for patients with chronic kidney disease. Nephrol Ther. 2012;8(3):135–140. [DOI] [PubMed] [Google Scholar]
- 9. Otsuka K, Koana T, Tauchi H, Sakai K. Activation of antioxidative enzymes induced by low-dose-rate whole-body gamma irradiation: adaptive response in terms of initial DNA damage. Radiat Res. 2006;166(3):474–478. [DOI] [PubMed] [Google Scholar]
- 10. Kataoka T. Study of antioxidative effects and anti-inflammatory effects in mice due to low-dose X-irradiation or radon inhalation. J Radiat Res. 2013;54(4):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schaue D, Jahns J, Hildebrandt G, Trott KR. Radiation treatment of acute inflammation in mice. Int J Radiat Biol. 2005;81(9):657–667. [DOI] [PubMed] [Google Scholar]
- 12. Eken A, Aydin A, Erdem O, Akay C, Sayal A, Somuncu I. Induced antioxidant activity in hospital staff occupationally exposed to ionizing radiation. Int J Radiat Biol. 2012;88(9):648–653. [DOI] [PubMed] [Google Scholar]
- 13. Calabrese EJ, Calabrese V. Reduction of arthritic symptoms by low dose radiation therapy (LD-RT) is associated with an anti-inflammatory phenotype. Int J Radiat Biol. 2013;89(4):278–286. [DOI] [PubMed] [Google Scholar]
- 14. El-Ghazaly MA, Sadik NA, Rashed ER, Abd-El-Fattah AA. Neuroprotective effect of EGb761(R) and low-dose whole-body gamma-irradiation in a rat model of Parkinson’s disease. Toxicol Ind Health. 2015;31(12):1128–1143. [DOI] [PubMed] [Google Scholar]
- 15. Zhang C, Tan Y, Guo W, et al. Attenuation of diabetes-induced renal dysfunction by multiple exposures to low-dose radiation is associated with the suppression of systemic and renal inflammation. Am J Physiol Endocrinol Metab. 2009;297(6):E1366–1377. [DOI] [PubMed] [Google Scholar]
- 16. Nomura T, Li XH, Ogata H, et al. Suppressive effects of continuous low-dose-rate gamma irradiation on diabetic nephropathy in type II diabetes mellitus model mice. Radiat Res. 2011;176(3):356–365. [DOI] [PubMed] [Google Scholar]
- 17. Cheng J, Li F, Cui J, et al. Optimal conditions of LDR to protect the kidney from diabetes: exposure to 12.5 mGy X-rays for 8 weeks efficiently protects the kidney from diabetes. Life Sci. 2014;103(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stewart FA, Akleyev AV, Hauer-Jensen M, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs—threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 2012;41(1-2):1–322. [DOI] [PubMed] [Google Scholar]
- 19. Ina Y, Sakai K. Prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice. Radiat Res. 2004;161(2):168–73. [DOI] [PubMed] [Google Scholar]
- 20. Ina Y, Sakai K. Further study of prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice: effects of whole-life irradiation. Radiat Res. 2005;163(4):418–23. [DOI] [PubMed] [Google Scholar]
- 21. Mitchel RE. The dose window for radiation-induced protective adaptive responses. Dose Response. 2009;8(2):192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phan N, De Lisio M, Parise G, Boreham DR. Biological effects and adaptive response from single and repeated computed tomography scans in reticulocytes and bone marrow of C57BL/6 mice. Radiat Res. 2012;177(2):164–175. [DOI] [PubMed] [Google Scholar]
- 23. Xing X, Zhang C, Shao M, et al. Low-dose radiation activates Akt and Nrf2 in the kidney of diabetic mice: a potential mechanism to prevent diabetic nephropathy. Oxid Med Cell Longev. 2012;2012:291087 doi:10.1155/2012/291087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Xing X, Zhang F, et al. Low-dose radiation induces renal SOD1 expression and activity in type 1 diabetic mice. Int J Radiat Biol. 2014;90(3):224–30. [DOI] [PubMed] [Google Scholar]