Abstract
Recent phase I/II adeno-associated viral vector-mediated gene therapy clinical trials have reported remarkable success in ameliorating disease phenotype in hemophilia A and B. These trials, which highlight the challenges overcome through decades of preclinical and first in human clinical studies, have generated considerable excitement for patients and caregivers alike. Optimization of vector and transgene expression has significantly improved the ability to achieve therapeutic factor levels in these subjects. Long-term follow-up studies will guide standardization of the approach with respect to the combination of serotype, promoter, dose, and manufacturing processes and inform safety for inclusion of young patients. Certain limitations preclude universal applicability of gene therapy, including transient liver transaminase elevations due to the immune responses to vector capsids or as yet undefined mechanisms, underlying liver disease from iatrogenic viral hepatitis, and neutralizing antibodies to clotting factors. Integrating vectors show promising preclinical results, but manufacturing and safety concerns still remain. The prospect of gene editing for correction of the underlying mutation is on the horizon with considerable potential. Herein, we review the advances and limitations that have resulted in these recent successful clinical trials and outline avenues that will allow for broader applicability of gene therapy.
Keywords: adeno-associated virus, gene therapy, hemophilia, lentivirus
Introduction
Hemophilia is an X-linked bleeding disorder caused by deficiency of coagulation factor VIII (FVIII) or factor IX (FIX) function due to mutations in the F8 or F9 gene, respectively.1 FVIII deficiency, or hemophilia A (HA), accounts for 80% of cases and affects 1:5000 male births while FIX deficiency, or hemophilia B (HB), affects 1:30,000 male births worldwide.1 In both disorders, bleeding severity correlates with residual factor activity wherein patients who have less than 1% activity (severe disease) present with frequent, spontaneous hemorrhages in joints (hemarthrosis), soft tissue, and muscles. Bleeding may also occur into closed spaces (e.g. intracranial or retroperitoneal), which can be life threatening. Patients with moderate disease (1–5% activity) only rarely have spontaneous bleeds, and those with mild disease (5–30% activity) generally only present with trauma-induced or postsurgical bleeding.1 The current standard of care for patients with severe disease is initiation, in childhood, of lifelong prophylaxis with exogenous factor replacement to limit spontaneous bleeds in an effort to curtail morbidity and mortality.2 However, in the United States, only around 60% of young adults and adults report adherence to prophylaxis due to its inherent complexity,3 resulting in unacceptable rates of bleeding and long-term joint complications. Further, challenges of intravenous factor administration in young patients and lack of access to factor concentrates in developing countries pose additional barriers to optimal prophylaxis administration.
Cloning of the F84 and F95 genes not only allowed for the generation of recombinant clotting factors6,7 for therapy but also instigated gene therapy efforts to, at first, ameliorate phenotype or give simplified prophylaxis and, more recently, potentially cure disease. Hemophilia is an optimal target for gene therapy due to the monogenic nature of inheritance and the observation that even minimal increases in clotting factor activity can significantly improve quality of life.1 Lessons from early gene therapy trials8,9 have been integrated into recent successful early phase clinical trials for both HA and HB.10–14 Although gene therapy targeting the liver has come a long way, certain limitations preclude its ability to become a definitive cure for all patients; overcoming these will be of utmost importance in the coming years.15 Here, we review the advances that have resulted in recent successful phase I/II clinical trials and outline avenues that will allow for broader applicability of gene therapy. In particular, we focus on the needs of patients with pre-existing neutralizing alloantibodies to the vector or the clotting factors (clinically termed inhibitors), those with liver disease, and pediatric patients.
Recent successes in gene therapy for hemophilia
Decades of preclinical and clinical work have resulted in the current successful phase I/II gene therapy trials for hemophilia. All published and currently open studies use adeno-associated viral (AAV) vectors.8–14 AAV is a replication defective member of the parvovirus family and is nonpathogenic in humans.15 The vector genome is manipulated to replace the AAV coding sequences with the transgene of interest under the control of a tissue-specific promoter, such as in liver gene therapy, to yield a recombinant AAV (rAAV) vector (Figure 1). rAAV is a nonintegrating vector and the vector genome is maintained episomally in transduced cells. The size of the AAV genome (~4.7 kb) limits the size of the packaged transgene,15 such that the F9 cDNA at 1.6 kb5 was easier to incorporate than the 7 kb F8 cDNA.16 Even after removal of the F8 B domain (~2.6 kb), which is not required for coagulation function,16 incorporation of F8 cDNA has been accomplished slowly by a combination of distinct strategies.17,18 Thus, early studies focused on HB given the smaller size of the F9 gene, despite its lower prevalence.
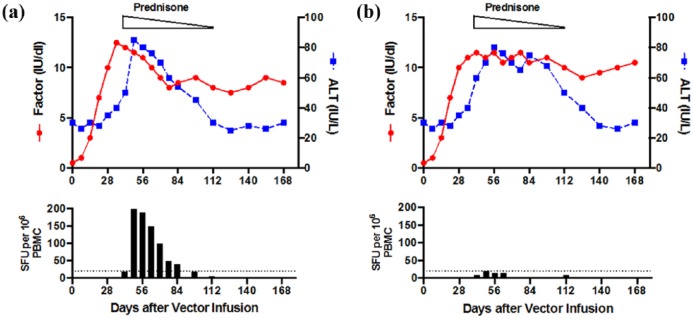
Figure 1.
Overview of adeno-associated virus (AAV) mediated liver-directed gene therapy for hemophilia. The wildtype AAV genome consists of two inverted tandem repeat (ITR) regions flanking the rep (replication) and cap (capsid) genes. These genes are replaced by a tissue-specific promoter with enhancer, intron, and transgene of interest in the recombinant (r)AAV vector genome, which is packaged into capsids and injected into subjects via a peripheral venous infusion. Once infused, rAAV vector can be neutralized by pre-existing antibodies in a serotype-specific manner or transduce hepatocytes where the capsid is degraded and the genetic material maintained as an episome in the nucleus to produce the transgene product. Capsid peptides can be presented on the surface of hepatocytes to CD8+ T cells, thought to lead to a cellular immune response coinciding with loss of transgene and rise in liver transaminases in some clinical trials. Modifications in the transgene, serotype, infusion of empty capsids, and production process may all affect efficacy. Options to bypass the pre-existing humoral response or liver disease are listed. Additional hurdles to general application of liver-directed AAV gene therapy include inhibitors to factors VIII and IX as well as infusion in young patients. FVIII, factor VIII; FIX, factor IX; CpG, cytosine-guanine residues.
In an early AAV clinical study with percutaneous injection of AAV2-FIX into skeletal muscle, the levels of neutralizing antibodies (NAbs) to AAV2 did not preclude local gene transfer or FIX transgene expression.9 Thus, in the subsequent first liver-directed gene therapy trial for HB, the presence of NAbs to AAV was not listed in the exclusion criteria.8 This trial demonstrated the ability to achieve therapeutic FIX levels in the highest dose cohort [2 × 1012 vector genomes/kg (vg/kg)] using AAV2 and a wild-type FIX transgene (FIX-WT).8 The low and intermediate dose cohorts (8 × 1010–4 × 1011 vg/kg) were designed as subtherapeutic doses to assess safety. The first patient in the high-dose cohort achieved a FIX level of around 12% but then developed a cellular immune response against the AAV capsid, as noted by a transient increase in liver enzymes and decrease in transgene expression.8 In contrast to the intramuscular trial, the second patient’s transduction efficiency was hindered by pre-existing NAbs to the AAV capsid.8 Pre-existing NAbs can be present in 30–70% of the general population for a given AAV serotype.19 Subsequent liver-directed trials, therefore, excluded patients with pre-existing NAbs to the trial AAV serotype and planned for immunosuppression with steroids if liver enzymes increased or factor expression decreased in order to limit the capsid-triggered cellular immune response.10–14,20 All studies to date have used an AAV serotype with liver tropism utilizing a codon optimized transgene under the control of a liver-specific promoter.8,10–14,20 Differences in AAV serotype, CpG content of the transgene, vector inverted tandem repeats (ITRs), presence of empty capsids as byproducts, and manufacturing cell line comprise the major differences between products21 (Figure 1, Tables 1 and 2). Some trials are utilizing hyperactive transgenes in an effort to decrease vector dose, which has been successful.10
Table 1.
AAV gene therapy trials for hemophilia B.
| Sponsor | Serotype | Transgene | Highest dose (vg/kg) | Mean FIX:C (%) | T-cell ELISPOT | ↑ ALT | ↓ FIX:C with ↑ ALT | Steroid treatment | Status$ | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| CHOP/UPENN | rAAV2 | FIX-WT | 2 × 1012 | 12* | N/A | 1/2 | 1/2 | 0/2 | Closed | 8 |
| UCL/SJCRH | rAAV8 | FIX-WT | 2 × 1012 | 5.1 | 4/6 | 4/6 | 4/6 | 4/6 | Recruiting | 13,14 |
| Shire | rAAV8 | FIX-Padua | 3 × 1012 | 0 | 2/3 | Closed | 20,22 | |||
| Spark Therapeutics | Spark100 | FIX-Padua | 5 × 1011 | 33 | 2/10 | 2/10 | 2/10 | 2/10 | Closed | 10 |
| uniQure | rAAV5 | FIX-WT | 2 × 1013 | 6.9 | 0/5 | 2/5 | 0/5 | 2/5 | Closed | 11 |
| Dimension | rAAV-rh10 | FIX-WT | 5 × 1012 | 6.7 | 2/3 | 3/3 | 3/3 | 3/3 | Closed | 23 |
| Sangamo Therapeutics | rAAV6 | Zinc finger-FIX | N/A | N/A | N/A | N/A | N/A | N/A | Recruiting | N/A |
| Freeline Therapeutics | rAAV-engineered | FIX-Padua | N/A | N/A | N/A | N/A | N/A | N/A | Not yet recruiting | N/A |
One subject had peak activity of 12% before immune response and subsequent decline to less than 1%.
Status determined by data available to ClinicalTrials.gov as of March 2018.
AAV, adeno-associated virus; ALT, alanine aminotransferase; CHOP, Children’s Hospital of Philadelphia; ELISPOT, enzyme-linked immunospot; FIX, factor IX; N/A, not available; rAAV, recombinant adeno-associated virus; Ref., reference; SJCRH, St Jude Children’s Research Hospital; UCL, University College of London; UPENN, University of Pennsylvania; WT, wild type.
Table 2.
AAV gene therapy trials for hemophilia A.
| Sponsor | Serotype | Transgene | Highest dose (vg/kg) | Mean FVIII:C (%) | T-cell ELISPOT | ↑ ALT | ↓ FVII:C with ↑ ALT | Steroid treatment | Status* | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarin Therapeutics | rAAV5 | BDD-FVIII | 6 × 1013 | 4–270 | 0/7 | 7/7 | 1/7 | 7/7 | Recruiting | 12 |
| Spark Therapeutics | Spark200/LK03 | BDD-FVIII | 2 × 1012 | N/A | N/A | N/A | N/A | N/A | Recruiting | 24 |
| Shire | rAAV8 | BDD-FVIII | N/A | N/A | N/A | N/A | N/A | N/A | Recruiting | N/A |
| Sangamo Therapeutics | rAAV2/6 | BDD-FVIII | N/A | N/A | N/A | N/A | N/A | N/A | Recruiting | N/A |
| UCL/SJCRH | rAAV8 | BDD-FVIII | N/A | N/A | N/A | N/A | N/A | N/A | Recruiting | N/A |
Status determined by data available to ClinicalTrials.gov as of March 2018.
AAV, adeno-associated virus; ALT, alanine aminotransferase; BDD-FVIII, B-domain-deleted factor VIII; ELISPOT, enzyme-linked immunospot; FVII, factor VII; FVIII, factor VIII; N/A, not available; rAAV, recombinant adeno-associated virus; Ref., reference; SJCRH, St Jude Children’s Research Hospital; UCL, University College of London.
AAV liver-directed HB clinical trials
Table 1 provides a summary of recently completed and open AAV-based trials for HB. The initial AAV2-FIX-WT trial by Manno and colleagues infused patients via the hepatic artery to enhance hepatocyte transduction.8 AAV8, compared with AAV2, has higher liver-specific tropism allowing for peripheral infusion of the vector.13,14 Building on the lessons from the AAV2 trial, the next HB study sponsored by St Jude Children’s Research Hospital (SJCRH) and University College of London (UCL) infused AAV8-FIX-WT in 10 subjects at doses between 2 × 1011 and 2 × 1012 vg/kg and excluded patients with anti-AAV8 NAbs.13,14 This study demonstrated sustained FIX activity of 3–5%, in a dose-dependent manner, with no long-term safety issues identified at 3 years, with ongoing follow up.13,14 However, four of six subjects in the high-dose cohort had elevated liver transaminases concomitant with a positive T-cell enzyme-linked immunospot (ELISPOT) assay against the AAV capsid, signifying a capsid-triggered cellular immune response [Figure 2(a)]. Gaining from the experience of the earlier AAV2 study,8 this study was the first to identify that prompt initiation (<48 h) of steroids in the presence of increased liver enzymes or drop in transgene expression levels prevented further loss of FIX activity.13,14 In the follow-up study with an additional four patients infused in the high-dose cohort, the annualized bleed rate (ABR) dropped from a median of 16.5 to 1 (94%) and overall ABR decreased from 15.5 to 1.5 (90%) in a vector dose-dependent manner.13,14
Figure 2.
Modeling of capsid-triggered cellular immune response, liver
transaminase, and factor level correlation in published adeno-associated
virus (AAV) liver-directed hemophilia gene therapy clinical trials.
Correlation between alanine aminotransferase (ALT,  ), factor activity
(
), factor activity
( )
and T-cell enzyme-linked immunospot (ELISPOT). (a) The observation of a
rise in liver alanine aminotransferase (ALT) with coincident decline in
transgene expression is thought to be associated with a cellular immune
response against the AAV capsid as confirmed by positive ELISPOT (bottom
panel). This effect can be rescued with a short course of
corticosteroids (prednisone). (b) In two recent trials, a rise in ALT
does not always correlate with loss of factor activity or positive
T-cell ELISPOT. Although steroids are used in these trials, ALT response
is not uniform in all cases. PBMC, peripheral blood mononuclear cells;
SFU, spot-forming units
)
and T-cell enzyme-linked immunospot (ELISPOT). (a) The observation of a
rise in liver alanine aminotransferase (ALT) with coincident decline in
transgene expression is thought to be associated with a cellular immune
response against the AAV capsid as confirmed by positive ELISPOT (bottom
panel). This effect can be rescued with a short course of
corticosteroids (prednisone). (b) In two recent trials, a rise in ALT
does not always correlate with loss of factor activity or positive
T-cell ELISPOT. Although steroids are used in these trials, ALT response
is not uniform in all cases. PBMC, peripheral blood mononuclear cells;
SFU, spot-forming units
The observation that there is vector dose dependency on the rate and presence of immune response to the vector capsid prompted the development of strategies to allow lower therapeutic vector doses. The identification of a naturally occurring hyperactive FIX variant (FIX-Padua), which yields approximately eightfold increased specific activity,25 afforded the opportunity to achieve therapeutic FIX levels with a lower vector dose. Our laboratory reported success with this strategy in inhibitor-prone HB dogs treated with AAV8 carrying canine (c) FIX-Padua at 1–3 × 1012 vg/kg with therapeutic FIX levels of 25–200%.26 There was no clinical or laboratory evidence of thrombosis and no antibody to the transgene after recombinant cFIX-WT infusion. In these dogs, cFIX-Padua specific activity was similar to that of FIX-Padua in the original family.26
Subsequently, a clinical study by Shire (Dublin, Ireland) utilizing AAV8-FIX-Padua at doses of 2 × 1011 to 3 × 1012 vg/kg demonstrated long-term expression of FIX at 20% in a single subject infused with 1 × 1012 vg/kg without development of inhibitors to the transgene.22 However, in the mid- and high-dose cohorts, after near normal FIX activity, immunosuppression with prednisone did not prevent loss of transgene expression caused by the cellular immune response to the capsid.22,27 In the study sponsored by Spark Therapeutics, (Philadelphia, PA, USA) a novel AAV serotype (Spark100) with FIX-Padua was infused in 10 subjects at a fixed dose of 5 × 1011 vg/kg and resulted in sustained FIX activity of around 30% with lower rates of immune response to the vector capsid.10 Only two of 10 subjects required steroids compared with four of six in the SJCRH/UCL trial. Notably, these patients retained FIX levels within the mild deficiency to normal range even after prednisone was discontinued (30–80%). All patients have stopped prophylaxis with a concurrent decrease in ABR from 11.1 to 0.4 (96%) and there has been no evidence of inhibitor formation to FIX-Padua.10 The planned phase III trial utilizing this vector will be sponsored by Pfizer (New York, NY, USA).28
The trials mentioned above used AAV vectors generated in mammalian cells using triple transfection of plasmid DNA for vector production.8,10,13,14,20 The uniQure-sponsored HB trial utilized AAV5-FIX-WT generated in an insect cell line using a baculovirus production system at doses of 5 × 1012 and 2 × 1013 vg/kg, with five subjects in each cohort.11 Results show mean steady-state FIX activity of 3–12% with a decrease in spontaneous ABR from 9.8 to 4.6 (53%) in the low-dose cohort and from 3 to 0.9 (70%) in the high-dose cohort.11 However, there was not a clear linear dose response to the vector, as despite a fourfold higher vector dose there was only 1.6-fold higher FIX activity.11 Furthermore, although three of ten subjects had an increase in alanine aminotransferase (ALT) and received steroids, this was neither associated with anti-capsid cellular immune response nor did it have an effect on FIX activity11 [Table 1, Figure 2(b)], in contrast to the other studies.8,13,14,22 The underlying mechanism of this complication is unknown. Of note, the phase III study announced by uniQure (Amsterdam, Netherlands) will use AAV5-FIX-Padua instead of wild type (WT) transgene.11 The trials to date have clearly demonstrated that FIX-Padua is likely the optimal transgene to ameliorate the disease phenotype as well as decrease the likelihood of a capsid-triggered immune response by allowing lower vector doses. FIX-Padua has no increased immunogenicity in preclinical models, and in these models, gene therapy with FIX-Padua can actually eradicate pre-existing inhibitors to FIX-WT.26
AAV liver-directed HA clinical trials
Ongoing and published AAV-based clinical trials for HA are summarized in Table 2. HA gene therapy was initially limited by the large size of the F8 gene; however evidence that removal of the B domain, which comprises 40% of the gene, does not affect coagulation function16 as well as further vector optimization have resulted in successful generation of B-domain-deleted (BDD) FVIII AAV vectors for clinical trials.17,18 Biomarin (Novato, CA, USA) has reported success with its HA gene therapy trial utilizing rAAV5 with BDD-FVIII at doses of 2 × 1012 to 6 × 1013 vg/kg12. Surprisingly, in the low (2 × 1012 vg/kg) and intermediate (2 × 1013 vg/kg) dose cohorts, FVIII expression was either unchanged or only 1–3% (n = 1/dose).12 In the high-dose cohort (n = 7), the first treated subject had an increase in ALT (1.5 fold baseline but below the upper limit of normal) between weeks 4 and 7, prompting the use of steroids for a presumed capsid-triggered immune response. All subsequent patients in the high-dose cohort were treated prophylactically with steroids. As in the AAV5-FIX uniQure trial,11 in the Biomarin trial there was no clear evidence of a connection between increased ALT, anti-capsid T-cell response, steroid use, and FVIII activity [Table 2, Figure 2(b)]. In four of the seven patients on high-dose treatment, steroid therapy did not halt ALT elevation. Normalization of ALT with respect to steroid discontinuation varied widely, with some patients having increases even after steroids were stopped. The steroid dose was titrated down as per the protocol in all patients. Prior studies have shown correlation between ALT and capsid-triggered immune response8,10,13,14 but no capsid-directed T-cell response was seen in these HA subjects, raising the question of whether the ALT elevation seen here is not an immune response but rather potential hepatocyte toxicity or other unknown effects.12 The exact mechanism behind this outcome is not completely understood and remains under investigation but raises the question of whether the insect-line, baculovirus system manufacturing process, or the AAV5 immune response may differ from other rAAV vectors produced in mammalian lines using plasmid DNA. Over a 12-month observation period, ABR decreased 94% from 16 to 1 and the reported FVIII activity ranged between 19% and 164% without a linear dose response (threefold higher dose between cohorts with 10- to 80-fold increased FVIII activity).12 The safety of chronic supraphysiologic FVIII activity is unknown and may carry a risk of thrombosis;29,30 furthermore, these levels are unnecessary for phenotypic improvement. Long-term follow-up data from these subjects may guide the efficacy and further understanding of the mechanism of this strategy. A phase III trial is planned using vector doses from 4 × 1013 to 6 × 1013 vg/kg.12
Spark Therapeutics has also reported early results from its phase I/II clinical trial using a novel AAV serotype (Spark200/LK0331) with BDD-FVIII at doses of 5 × 1011 (low dose), 1 × 1012 (intermediate dose), and 2 × 1012 vg/kg (high dose). The two patients each treated at the low and intermediate dose had steady-state FVIII levels of 10–13% and 9–13%, respectively.24 The first intermediate dose subject (1 × 1012 vg/kg) had increased ALT and was started on immunosuppression with stable FVIII activity thereafter, and the second subject was treated with steroids empirically. Data from the high-dose (2 × 1012 vg/kg) subjects have not yet been released. As in the HB trials, there is wide variation in vector dose between these HA trials (~60-fold higher vector dose for Biomarin compared with Spark HA trial), and whether one strategy may be more successful remains to be determined. There are also concerns about manufacturing feasibility, and the short duration of follow up of these recent trials precludes definite safety assessment at this point.
Future and challenges of gene therapy
The variety of AAV serotypes, the wide range of therapeutic vector doses, and differing manufacturing processes make a comparison of these products very difficult. However, the common threads amongst these trials are the following exclusion criteria: patients under 18 years of age; patients with less than 30–50 exposure days to FVIII/FIX concentrates; underlying liver disease or active viral hepatitis B or C infections (HBV/HCV); presence of NAbs to AAV serotype higher than a cutoff; and current or prior history of inhibitors to FVIII or FIX. Despite promising results, due to the limits imposed by these exclusion criteria, gene therapy is not yet at the point of being universally applicable to all persons with hemophilia. Notably, in the United States, like many other countries, the high prevalence of HCV in patients with hemophilia over the age of 35 (in 2018) means that over 35% of adult patients who could benefit from gene therapy will be ineligible for these liver-directed approaches.3 What impact, if any, the ability to cure HCV with new antivirals will have remains to be seen.
The ability to mitigate pre-existing NAbs to AAV will also expand the eligible population as around 30–70% of patients have NAbs to specific serotypes.19,32,33 However, attempts to eliminate pre- existing NAbs utilizing either immunosuppression,34,35 plasmapheresis,36 inclusion of empty capsids37 to serve as decoys, novel bioengineered capsids, or localized vector infusion38 have only modestly decreased titers and not eliminated the NAbs. As expected, the development of a robust serotype-specific humoral response after vector infusion would preclude reinfusion with the same vector should response wane either due to capsid-triggered T-cell response or dilution of transduced hepatocytes with liver growth and increased blood volume, as when the vector is delivered to young patients.15
Finally, up to 30% of patients with severe HA and 5% of patients with severe HB develop inhibitors to the infused protein,39 which decrease or nullify achievement of hemostasis with replacement protein. Currently, the only known curative strategy for inhibitors is infusion of frequent, high doses of factor concentrates over a period of months to years termed immune tolerance induction (ITI), which is moderately (~60%) successful in patients with favorable risk characteristics.40 The goal of ITI is to allow the use of FVIII/FIX protein for replacement therapy as they more effectively achieve hemostasis than alternative bypassing agents (BPAs), such as activated prothrombin complex concentrates (aPCCs) or recombinant factor VIIa (rFVIIa),41 which circumvent the need for FVIII and thereby provide alternate pathways for generation of thrombin. While ITI is more cost effective than life-long BPA therapy for hemostasis, it comes at great economic burden (~$1 million for a 20 kg patient) and is demanding, as evidenced by around 20% of patients withdrawing from participation in the International ITI (I-ITI) study.40,42,43 There is mounting preclinical evidence in HA/HB dogs and mice26,44–47 that AAV liver-directed gene therapy in animal inhibitor models is tolerance inducing and successful application to this population would result in dramatic improvement in quality of life for these patients. Efforts to overcome these obstacles should broaden the availability of gene therapy for the vast majority of patients with hemophilia.
Gene therapy for eradication of inhibitors and immune tolerance induction
Inhibitors to FVIII and FIX are the most significant and challenging clinical complication to replacement therapy at the moment,48,49 Inhibitors are measured in Bethesda units (BU), where 1 BU is defined as the amount that neutralizes 50% of the normal clotting factor activity. Patients with high-titer inhibitors (BU >5) are generally treated with BPA instead of FVIII/FIX concentrates to induce hemostasis. Despite the initiation of ITI in the 1970s, a prospective study (I-ITI), was only recently completed in 2012.40 This study identified the characteristics of patients who were more likely to achieve tolerance as follows: BU less than 10 at start of ITI; historical peak BU less than 200; young age; and short time between diagnosis and ITI start.40 While around 60% of patients with these favorable risk factors will achieve tolerance with ITI, there are not good options for those with refractory or relapsed inhibitors.50 Furthermore, the time to reach BU less than 10 ranged from 3 to 8 months in I-ITI, and a subset of patients were unable to be randomized in the trial due to persistent BU greater than 10.40 Prior attempts at immunosuppressive regimens alone or coupled with ITI have had mixed and modest success at inhibitor eradication.51,52 Alternative strategies that simplify ITI are clinically necessary and could provide tremendous benefits to inhibitor patients. Indeed, the preclinical data below support the hypothesis that liver-directed gene therapy can provide the dual benefits of both eradicating inhibitors to the clotting factor and providing simplified prophylaxis thereafter.53
While several animal models of hemophilia have been characterized,54 the canine model55 is unique for several reasons: dogs naturally have hemophilia resulting from a spectrum of genetic mutations similar to humans; the disease phenotype is similar to humans; compared with mice, the colonies are outbred and of various genetic (and thereby immune) backgrounds; and characterization of cFVIII56 and cFIX57 cDNA affords the ability to produce recombinant cFVIII/cFIX protein for species-specific immunologic studies.53 The canine HA and HB models differ in their backgrounds, genetic mutations, and predisposition to antifactor immune responses (Table 3). While the HA dogs from Queens University (QU)58 and University of North Carolina (UNC-CH)59 both have mutations similar to the human F8 intron 22 inversion, the QU HA dogs were initially more inhibitor prone after exposure to cFVIII. Subsequent introduction of an outside male breeder to the UNC-CH HA colony has created a subset of inhibitor-prone HA dogs there as well.46 Murine hemophilia models, in contrast, are generally limited to two strains with very similar mutations, and immune studies are complicated by infusion of human xenoprotein.60 Nonetheless, in both canine and murine models, mounting preclinical data demonstrate that consistent endogenous expression of FVIII/FIX after liver-directed gene therapy can result in transgene-specific immune tolerance and eradicate pre-existing inhibitors.26,46,61–66 In contrast, collective data from nonhuman primates (NHPs) in AAV liver gene therapy for HB or HA resulted in inhibitor formation due to the use of a human transgene and precluded the consideration of transgene-specific ITI by gene therapy.17 In the canine and murine liver-directed gene therapy studies, it was demonstrated that tolerance occurs through induction of a regulatory T-cell (Treg) response and other mechanisms.46,61,62 Further evidence supporting the role of Tregs comes from a NHP study where the use of an anti-CD25 antibody, which depletes both T effector and Tregs, during the early phase of AAV2 liver-directed FIX expression resulted in inhibitor formation.67
Table 3.
Canine models of hemophilia.
| Mutation | Inhibitor risk* | Nomenclature | |
|---|---|---|---|
| HA | |||
| Queens University | Intron-22 inversion | High | QU HA |
| University of North Carolina | Intron-22 inversion | Low/high$ | UNC-CH HA |
| HB | |||
| University of North Carolina | Missense | Low | UNC-CH HB |
| University of Alabama | Premature stop | High | UAB HB |
Risk of inhibitor development to canine FVIII/FIX.
Initial colony did not develop inhibitors until introduction of an outside male breeder.
HA, hemophilia A; HB, hemophilia B; FVIII, factor VIII; FIX, factor IX.
The exact mechanism behind ITI by frequent exposure of FVIII/FIX protein concentrates is not completely understood, but the rationale is that prolonged and consistent antigen exposure would tilt the balance towards tolerance. The advantage of gene therapy for ITI is the opportunity to provide this continuous antigen endogenously with a single infusion.68 Our laboratory investigated whether gene therapy could eradicate pre-existing inhibitors and thereafter allow for simplified prophylaxis with endogenous FVIII/FIX production. Within the UNC-CH HA inhibitor prone model, three dogs with historically high titers were able to eradicate their inhibitors 4–5 weeks after liver-directed AAV gene therapy with progressively increasing FVIII levels and improvement in the bleeding phenotype. In addition, they demonstrated normal pharmacokinetics to infused cFVIII recombinant protein thereafter,46 in line with criteria for successful tolerance from I-ITI. A QU-HA dog who had developed inhibitors after prior exposure to human FVIII, which can cross react with cFVIII, given the same therapy had an anamnestic response that peaked at 216 BU but achieved tolerance by 18 months post infusion.46 In fact, in ITI an anamnestic response greater than 200 BU was negatively correlated with tolerance induction.69 Although this dog took longer to achieve FVIII tolerance, consistent endogenous antigen exposure via gene therapy was ultimately successful at eradicating the pre-existing inhibitor to both human and canine FVIII (unpublished observation, V.R.A. and Finn and colleagues46). In the canine HA inhibitor model, we have also noted an increase in Tregs with liver-directed gene therapy that precedes antibody eradication,46,68 whereas in dogs without inhibitors the Treg population does not change after gene therapy (unpublished observation, V.R.A.). Subsequent studies showed that administration of liver-directed AAV-cFVIII transgene in eight naïve UNC-CH HA dogs with intron 22 inversion resulted in only transient low-titer inhibitor formation in one dog, who was subsequently discovered to be a member of the inhibitor-prone subcolony.70 This dog achieved FVIII tolerance without further intervention and all eight remained without inhibitors even upon subsequent protein challenges.70
Inhibitors to FIX are less common, occurring in only 3–5% of patients with severe HB, but have a higher prevalence in patients with large gene deletions or early stop codon compared with other mutations.39 FIX inhibitors are more problematic clinically as they can be associated with allergic reactions to protein and nephrotic syndrome, posing additional challenges for ITI.71,72 Moreover, ITI is effective in only around 30% of the cases in patients with HB. Our group has investigated whether liver-directed gene therapy may promote transgene tolerance in HB as well. With liver-directed gene therapy, HB dogs showed long-term expression of cFIX-WT.63,64 We further demonstrated that AAV liver gene therapy with cFIX-Padua resulted in FIX activity of 25–40% and did not result in anti-FIX antibody formation in two inhibitor-prone University of Alabama (UAB) HB dogs.26 Furthermore, a third dog with pre-existing inhibitor to cFIX-WT had a transient anamnestic response followed by subsequent spontaneous eradication of the inhibitor with FIX activity around 200% after ITI with cFIX-Padua gene therapy.26 An additional dog with inhibitor to cFIX-WT was also tolerized with cFIX-Padua gene therapy (unpublished observation, V.R.A.). Thus, liver-directed gene therapy with FIX-Padua may be able to induce tolerance for patients with pre-existing anti-FIX-WT inhibitors as well.
Combined, these studies suggest that liver-directed gene therapy with AAV vectors can tilt the immune system towards transgene-specific tolerance. Inhibitors are most likely to develop in young patients with severe hemophilia, typically within their first 50 exposure days.73 ITI via protein infusion or BPA prophylaxis in these patients may require the placement of a central venous catheter, which increases the risk of infectious and thrombotic complications.74 Young patients may also need central venous catheters for routine FVIII/FIX prophylaxis. Furthermore, ITI takes several months to years to induce tolerance,15,40 and patients can have several breakthrough bleeds leading to significant joint damage. Although recent developments such as emicizumab,75 a bispecific antibody that mimics FVIIIa function by binding FIXa and FX, and inhibitors of natural anticoagulants76–78 such as fitusiran, a small interfering RNA to antithrombin, may improve hemostasis in patients with inhibitors,21 this still ties them to life-long therapy and does not eradicate the inhibitor in order to allow the use of factor for breakthrough bleeds,75,76 which is the main goal of ITI. Given the inability to predict exact prohemostatic effects from these drugs, they may pose thrombotic risks21 when combined with other clotting factors for bleeding, as evidenced by thrombosis (including thrombotic microangiopathy) in five patients in the emicizumab trial75 when treated concurrently with aPCC for more than 24 h due to bleeding and one patient in the fitusiran trial79 whose cavernous sinus thrombosis was initially thought to be a subarachnoid hemorrhage and received several doses of FVIII. Thus, likely the best therapeutic choice for a clotting factor deficiency is replacement of the clotting factor41 as the ideal replacement or BPA therapy, and dosage in the setting of these newer agents remain unclear. The tremendous physical, financial, and psychosocial morbidity80–83 for inhibitor patients can be significantly ameliorated if gene therapy is able to eradicate their inhibitors with a one-time infusion. Moreover, the presence of inhibitor in adolescent boys is also associated with delayed growth and maturation,84 thus it is imperative to eradicate inhibitors rather than just using BPAs. As clinical gene therapy trials move into phase III studies, a powerful and meaningful next step would be to include patients with inhibitors as this can provide the dual benefits of eradicating the inhibitor and improving the disease phenotype with one infusion.
Patients with underlying liver disease
Prior to the advent of recombinant factors, patients with hemophilia were infused with pooled cryoprecipitate or plasma-derived factors.1 The first evidence of liver injury in people with hemophilia treated with these products emerged in the 1970s with asymptomatic elevated transaminases documented in 45% of patients,85 which persisted for several years and was found in the 1980s to cause progressive cirrhosis. Although HBV was known to play a role, the identification of HCV as a contributor to this phenomenon did not occur until 1987.86 Recent epidemiological studies from hemophilia treatment centers in the United States demonstrate that although no cases of HCV have been documented in patients born after 1992, approximately 35% of patients born in the US between 1983 and 1992 have HCV and the prevalence is greater than 80% for patients born between 1958 and 1982.3 For safety concerns, clinical gene therapy trials have excluded patients with concomitant HBV or HCV infection, or liver disease from participation in early phase trials. Whether and how to include patients who have successfully been treated with anti-HCV antivirals remains an unsolved issue. New direct-acting antivirals demonstrate over 90% sustained virologic response, even in patients with advanced HCV liver disease, but whether the risk of hepatocellular carcinoma in these patients decreases will require further study.87,88 Safety of liver-directed gene therapy in these patients is unknown.
Another safety issue is the concern for both acute and long-term hepatic genotoxicity from AAV vectors themselves. Studies have suggested a protective role of WT AAV in cancer.89–91 Indeed, athymic nude mice injected with a colon cancer cell line treated with WT AAV2 had decreased tumor growth due to selective apoptosis induced by AAV in cells that lack activity of the tumor suppressor p53.89 Preclinical studies have assessed the risk of genotoxicity from hepatocyte transduction with AAV vectors.92 Despite the fact that AAV vector is nonreplicative and nonintegrating, studies in mice have shown that some integration can occur93 and has been demonstrated to cause hepatocellular carcinoma (HCC) in neonatal mice given rAAV gene therapy94,95 due to integration at a transcriptionally active Rian locus in neonatal mice, which is not present in humans.96 Studies in older rodents, dogs, and NHPs63,97–101 did not demonstrate increased HCC with AAV gene therapy. A comprehensive evaluation found that the HCC risk can be mitigated by modulating both vector characteristics (dose, promoter, enhancer) and subject characteristics (quiescent state of target cells).92 Of note, the promoters used in most current clinical trials have the lowest risk.92 To date, over 60 patients with hemophilia have been treated with AAV liver-directed gene therapy with no reported significant safety or toxicity concerns,8,10–14,22,24 however long-term follow up data are still being accumulated.
An alternative consideration for patients with HB and concomitant liver disease is the delivery of AAV to skeletal muscle.9,102 Skeletal muscle gene therapy offers sufficient vascularity to allow secretion of synthesized FIX into the circulation and provides a way to overcome pre-existing NAbs. Canine studies demonstrated that intramuscular injection of cFIX in the UNC-CH HB dogs had an excellent safety profile,103 but a similar injection protocol in the UAB HB inhibitor-prone dogs resulted in inhibitor formation.104 These studies laid the foundation for a clinical trial of intramuscularly injected AAV2-FIX-WT,9,102 which comprised the first in vivo parenteral gene therapy trial in humans. This study demonstrated safety and tolerability, albeit without significant increase in circulating plasma FIX activity.9 However, all subjects demonstrated detectable FIX in muscle at biopsies collected at 2 and 10 months after gene therapy. In addition, two subjects demonstrated detectable FIX or vector in muscle biopsy samples obtained 3.7 and 10 years post intramuscular injection of the vector.105,106 This study was limited to subjects with missense mutations based upon the HB canine experience. In preclinical studies, we demonstrated that the vector dose per site was the strongest predictor of immune response.107 As the highest dose cohort in the human HB intramuscular trial required 80–90 injections9 and did not result in circulating FIX activity, a different method of delivering FIX to the muscle was needed to facilitate higher vector doses. To this end, our group has demonstrated the ability to use isolated limb perfusion with transvenular delivery under hydrostatic pressure for skeletal muscle for gene therapy in HB dogs108 with transient cyclophosphamide around vector infusion to prevent inhibitor development. Combining this delivery system with cFIX-Padua transgene, we recently demonstrated in both the UNC-CH and the UAB inhibitor-prone HB dogs not only therapeutic circulating FIX levels but also complete normalization of FIX for the first time,109 without evidence of inhibitor formation despite multiple subsequent FIX-WT protein challenges.109,110
Overcoming pre-existing NAbs to AAV
As noted before, NAbs preclude effective viral transduction when delivered intravascularly.8,111 Depending upon patient age, AAV serotype tested, and geographic region, between 30% and 70% of patients may have pre-existing NAbs to specific AAV serotypes.19,33 The anti-AAV2 antibody prevalence seems highest but there is cross reactivity with other serotypes.33 NAbs represent one of the most significant challenges to overcome in order to allow broad applicability of gene therapy to the hemophilia population at large. To date, studies have excluded patients with pre-existing NAbs to the study AAV serotype. Furthermore, differences in seroprevalence of immunoglobulin G (IgG) antibodies and neutralizing titer112 suggest that non-NAbs may impact vector dose needed for efficacy.
Simple strategies to overcome the NAb titer include switching AAV serotype or trying to outcompete the antibody by increasing vector dose. Unfortunately, the cross reactivity of some NAbs limits the success of the first strategy. Other strategies to overcome NAbs have been studied, including adding empty capsids,37 administration of immunosuppressive34,35 drugs or plasmapheresis36 to decrease titer, alteration of the AAV capsid,113 and regional isolated AAV delivery to limit systemic exposure.38 Unfortunately, all of these strategies are fraught with complications that make them suboptimal. Infusion of empty capsids allows for the adsorption of some of the circulating antibodies to allow transduction with transgene-encoded vector.37 However, this strategy may be limited in those with high NAb titers and may increase the cellular immune response against the capsid as the total dose of vector would also need to be increased. Immunosuppression has been modestly effective at dampening but not eliminating the humoral response to AAV.35 Further, B-cell depletion comes with associated lymphopenia and risk of infections. Plasmapheresis is a relatively safe and effective strategy for low-titer inhibitors (⩽1:20); however, despite five sessions plasmapheresis was not able to decrease NAb titers below 1:5 for patients with initially high titers (>1:20).36 Patients with high-titer antibodies are more acutely in need of successful strategies and the limited efficacy of plasmapheresis is unlikely to be beneficial for this population. Localized liver perfusion38 or isolated limb perfusion108,114 with skeletal muscle delivery of vector, therefore, may be a viable option for patients with high NAb titers to liver tropic AAV serotypes. Likely, a combinatorial approach of these strategies will be needed to overcome pre-existing NAbs.
Lentiviral gene therapy
While AAV has emerged as the most successful vector for in vivo hemophilia gene therapy to date, there has been considerable effort in developing integrating vectors, such as retroviruses.115 A potential advantage of this system is the ability to propagate the integrated transgene into progeny of the transduced cells, thereby avoiding potential dilution from organ or blood volume growth and allowing long-term therapy in young patients. Retroviruses are single-stranded RNA viruses containing a reverse transcriptase to allow for viral DNA production, which can then integrate into the host genome via an integrase. Initial studies demonstrated the ability to achieve therapeutic FIX (12–36%) and FVIII (116–139%) levels in neonatal hemophilic dogs treated with γ-retrovirus vector at 0.8–1.3 × 1010 transduction units (TU)/kg and 0.7–0.9 × 1010 TU/kg, respectively.65,116 A phase I clinical trial sponsored by Chiron (San Diego, CA, USA) utilizing Moloney murine leukemia virus (MoMLV) carrying BDD-FVIII at doses of 2.8 × 107 to 8.8 × 108 TU/kg in patients with severe HA was shown to be safe and well tolerated.117 However, there was a question of germline transmission of retrovirus via semen and FVIII activity was only transiently measured after infusion with no clear dose response. Furthermore, a clinical trial also using a retroviral vector in severe combined immunodeficiency (SCID) was complicated by leukemogenesis in four patients due to insertional mutagenesis,118,119 which raised significant safety concerns.
More recently, studies have focused on lentiviruses, which are members of the retrovirus family but are different from simple MoMLV in that they are able to infect nondividing cells.120 Of note, the oncogenesis seen in retroviral-mediated gene therapy for SCID defined above has not been seen in lentivirus-mediated gene therapy trials to date.121 Lentiviral vectors are easily capable of carrying the F8 cDNA and have decreased likelihood of preexisting NAbs in the population. Lentiviral transduction can occur either ex vivo with infusion of transduced autologous hematopoietic stem cells (HSCs) from the recipient or in vivo via intravenous infusion of vector targeting the liver. The ex vivo approach in HSCs can either be lineage restricted122 or not.123 In HA mice, transplantation of lentivirus-transduced HSCs with a human-porcine hybrid BDD-FVIII demonstrated therapeutic levels of 1–10%,123,124 even in mice with pre-existing inhibitors. Delivery of platelet FVIII via transplantation of HSCs in a similar manner did result in disease amelioration in HA dogs,122 but further studies are needed to understand if this is tolerogenic. Ex vivo HSC lentivirus gene therapy is complicated by the need for conditioning regimens, bone marrow engraftment, or invasive procedures, which are likely to be significant hindrances for the subjects. Further large animal model studies are needed to understand the efficacy and safety profile of this approach. One approach that avoids the need for conditioning is the use of transduced autologous blood outgrowth endothelial cells (BOECs).125 Ex vivo transduced autologous BOECs in three HA dogs via omental implantation with or without transient immunosuppression did initially result in therapeutic FVIII levels; however, this was not sustained due to the development of anti-FVIII IgG antibodies.125
Recently, in vivo intravascular delivery of lentiviral vectors carrying FVIII and FIX targeting the liver has been carried out in animal models.126–128 The advantage of this vector-integrating system compared with AAV is the ability to allow long-term sustained transgene expression by avoiding potential transgene dilution when delivered to pediatric patients. In these studies, the lentivirus is modified through the addition of microRNA target sites to silence expression in nontarget cells. Recent studies have shown that the endogenous production of FVIII from the liver is mainly from liver sinusoidal endothelial cells rather than hepatocytes.129,130 Building upon this observation, Merlin and colleagues treated HA mice with 1 × 109 TU of lentivirus containing BDD-FVIII under the control of an endothelial promoter and demonstrated therapeutic FVIII levels of around 3–8% with no inhibitor formation.126 Even higher levels of FVIII activity were observed with further FVIII transgene optimization.131 Furthermore, HA mice with inhibitors to FVIII treated with the same approach were able to eradicate pre-existing inhibitors and subsequently achieve expression of therapeutic FVIII levels. In HB, lentivirus expressing FIX-Padua modified to target the liver was successful in ameliorating disease phenotype in murine and canine HB models.127,128 However, at doses of 5.7 × 108 to 2.3 × 109 TU/kg, FIX activity was only around 1% in the two dogs treated at the highest dose, despite use of cFIX-Padua in one dog.128 Additionally, the first two dogs developed anaphylactoid infusion reactions necessitating steroid and antihistamine use in the third dog. Encouragingly, this vector was not associated with genotoxicity in a murine model128 followed for around 12 months. However, the risks of insertional mutagenesis and genotoxicity in hemophilia subjects with a life expectancy over 60 years remains to be answered. Finally, in vivo lentivirus delivery is also complicated by vector stability,132 immune response by antigen presenting cells to pseudotyped vector,120 pre-existing antibodies to vesicular stomatitis virus used to pseudotype vector,133 and complement binding to vector which can result in infusion reactions.128 While lentivirus may offer significant advantages in hematopoietic and other stem-cell based disorders, there are significant challenges yet to overcome.
Gene-editing approaches
Gene-editing technologies are based on harnessing the natural repair machinery of the cell to modify DNA. Following a double-stranded break induced by a nuclease, repair progresses either through nonhomologous end joining (NHEJ) or homology-directed repair (HDR). NHEJ repair results in insertions or deletions (indels) of various lengths while HDR can be used to create precise site- and sequence-specific changes from a template.134–136 Nucleases used to induce these breaks include zinc-finger nucleases (ZFN), transcription-activator-like effector nucleases and clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR associated protein 9 (CRISPR/Cas9).137 Gene editing offers several advantages over AAV and integrating vectors, including precise editing, decreased insertional oncogenesis, and control under the endogenous promoter. While early animal studies utilizing ZFN and CRISPR/Cas9 did ameliorate disease phenotype in animal models,138–140 more promising results were obtained by introducing the F8/F9 genes into the albumin gene using 2A peptides by ZFN for targeting, resulting in complete and functional FVIII/FIX mRNA141 under the control of the endogenous albumin promoter. A similar approach is now in clinical trials for HA and HB sponsored by Sangamo Therapeutics (Richmond, CA, USA) (Table 1) utilizing distinct AAV vectors to deliver the nuclease and two separate templates for the transgene (i.e. three different AAV vectors), but no data have been reported to date. Although gene editing holds significant promise for hemophilia, in its current state, the approach still requires AAV for delivery of the nuclease or transgene. Therefore, the same limitations for AAV gene therapy are present for gene-editing approaches and additional complications may arise as a result of the multitude of vectors needed. Moreover, limited data on off-target effects of these technologies prevents broader clinical application at this point.
Conclusion
Recent phase I/II AAV liver-directed gene therapy trials for hemophilia have shown remarkable decreases in ABR and factor usage, and engender optimism about the ability to significantly improve quality of life for persons with hemophilia. Lessons from early trials incorporating strategies for vector and transgene optimization and exclusion of subjects with pre-existing NAb to vector serotype have created the ability of a one-time infusion to provide simplified, lifelong prophylaxis which is a tremendous paradigm shift for patients and healthcare providers. Notably, there is convincing evidence that FIX-Padua may be the optimal transgene in HB gene therapy and there is considerable preclinical evidence that liver-directed gene therapy for ITI in HA has translational potential for a population with limited, suboptimal, and demanding therapeutic options otherwise. A one-time infusion that allows both tolerance induction and lifelong prophylaxis after inhibitor eradication with a single infusion would have immense benefits. The preclinical and clinical trial data have yielded a wealth of information regarding factors that permit success, but additional studies are needed to answer questions of optimal transgene, serotype, dose, target clotting factor activity, and manufacturing process as well as to understand the mechanistic processes underlying the immune response and rise in liver transaminases.
Currently, AAV gene therapy is limited in its general applicability due to pre-existing NAbs and pre-existent HCV or HBV infection. Long-term follow-up studies will be critical to assessing and addressing the safety concerns and therefore allow enrollment of younger patients, as further evidence of safety around risk of genotoxicity is amassed and ways to overcome NAbs for potential reinfusion are established. In the coming years, as some trials move beyond phase I/II, we anticipate the recognition of promising strategies that would yield benefits for many more patients. Further, these studies will likely help standardize aspects such as NAb titers, manufacturing process, and serotype. Finally, there are considerations of the cost of gene therapy, both to patients and payers. Arguably, the most striking benefit would be in the developing world, where routine access to clotting factors is limited, but how gene therapy is priced will affect its availability to these patients. There is a moral imperative to develop economically priced strategies to improve the burden of disease for patients in the developing world.
Footnotes
Funding: Primary results from the V.R.A. lab were supported by grants from the National Institutes of Health, the National Heart, Lung and Blood Institute P01 HL64190 Project 1, HL084220, NIH/NLHBI grant U54, HL142012 and from the Hemophilia Association of New York.
Conflict of interest statement: The authors declare that they have no conflicts of interest.
ORCID iD: Valder R. Arruda  https://orcid.org/0000-0002-8100-5481
https://orcid.org/0000-0002-8100-5481
Contributor Information
Bhavya S. Doshi, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Valder R. Arruda, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, 5056 Colket Translational Research Center, Philadelphia, PA 19104, USA.
References
- 1. Mannucci PM, Tuddenham EG. The hemophilias – from royal genes to gene therapy. N Engl J Med 2001; 344: 1773–1779. DOI: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 2. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357: 535–544. DOI: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 3. Mazepa MA, Monahan PE, Baker JR, et al. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood 2016; 127: 3073–3081. DOI: 10.1182/blood-2015-10-675140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gitschier J, Wood WI, Goralka TM, et al. Characterization of the human factor VIII gene. Nature 1984; 312: 326–330. [DOI] [PubMed] [Google Scholar]
- 5. Choo KH, Gould KG, Rees DJ, et al. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature 1982; 299: 178–180. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz RS, Abildgaard CF, Aledort LM, et al. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A. recombinant Factor VIII Study Group. N Engl J Med 1990; 323: 1800–1805. Clinical Trial Comparative Study Research Support, Non-U.S. Gov’t 1990/12/27. DOI: 10.1056/NEJM199012273232604. [DOI] [PubMed] [Google Scholar]
- 7. Kaufman RJ, Wasley LC, Furie BC, et al. Expression, purification, and characterization of recombinant g -carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem 1986; 261: 9622–9628. [PubMed] [Google Scholar]
- 8. Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347. DOI: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 9. Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003; 101: 2963–2972. DOI: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 10. George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017; 377: 2215–2227. DOI: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miesbach W, Meijer K, Coppens M, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2017. DOI: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rangarajan S, Walsh L, Lester W, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med 2017; 377: 2519–2530. DOI: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 13. Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365. DOI: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014; 371: 1994–2004. DOI: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arruda VR, Samelson-Jones BJ. Obstacles and future of gene therapy for hemophilia. Expert Opin Orphan Drugs 2015; 3: 997–1010. DOI: 10.1517/21678707.2015.1069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lind P, Larsson K, Spira J, et al. Novel forms of B-domain deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur J Biochem 1995; 232: 19–27. [DOI] [PubMed] [Google Scholar]
- 17. McIntosh J, Lenting PJ, Rosales C, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 2013; 121: 3335–3344. DOI: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siner JI, Samelson-Jones BJ, Crudele JM, et al. Circumventing furin enhances factor VIII biological activity and ameliorates bleeding phenotypes in hemophilia models. JCI Insight 2016; 1: e89371 DOI: 10.1172/jci.insight.89371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calcedo R, Morizono H, Wang L, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol 2011; 18: 1586–1588. DOI: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monahan PE, Sun J, Gui T, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther 2015; 26: 69–81. DOI: 10.1089/hum.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arruda VR, Doshi BS, Samelson-Jones BJ. Novel approaches to hemophilia therapy: successes and challenges. Blood 2017; 130: 2251–2256. DOI: 10.1182/blood-2017-08-742312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monahan PE, Walsh CE, Powell JS, et al. Update on phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy program for hemophilia B. J Thromb Haemost 2015; 13: 87. [Google Scholar]
- 23. Pipe S, Stine K, Rajasekhar A, et al. 101HEMB01, a phase 1/2 open-label single ascending dose-finding trial of DTX101 (AAVrh10FIX) in patients with moderate/severe to severe hemophilia B demonstrated meaningful but transient expression of human factor IX. Blood 2017; 130: 3333. [Google Scholar]
- 24. George LA, Ragni MV, Samelson-Jones BJ, et al. Spk-8011: preliminary results from a phase 1/2 dose escalation trial of an investigational AAV-mediated gene therapy for hemophilia A. Blood 2017; 130: 604. [Google Scholar]
- 25. Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med 2009; 361: 1671–1675. DOI: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 26. Crudele JM, Finn JD, Siner JI, et al. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood 2015; 125: 1553–1561. DOI: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chapin J, Rottensteiner H, Scheiflinger F, et al. Factor IX Consumption in the Phase I/II BAX 335 Gene Therapy Trial in Subjects with Hemophilia B. Res Prac Thromb Haemost 2017; 1: 144. [Google Scholar]
- 28. Pfizer. Spark therapeutics and Pfizer amend license agreement for investigational SPK-9001 in hemophilia B. Spark Therapeutics, 2017. [Google Scholar]
- 29. Kyrle PA, Minar E, Hirschl M, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med 2000; 343: 457–462. DOI: 10.1056/NEJM200008173430702. [DOI] [PubMed] [Google Scholar]
- 30. Koster T, Blann AD, Briet E, et al. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345: 152–155. [DOI] [PubMed] [Google Scholar]
- 31. Lisowski L, Dane AP, Chu K, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 2014; 506: 382–386. DOI: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013; 4: 341 DOI: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Narkbunnam N, Samulski RJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012; 19: 288–294. DOI: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- 34. Corti M, Elder M, Falk D, et al. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol Ther Methods Clin Dev 2014; 1 DOI: 10.1038/mtm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Unzu C, Hervas-Stubbs S, Sampedro A, et al. Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates. J Transl Med 2012; 10: 122. DOI: 10.1186/1479-5876-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monteilhet V, Saheb S, Boutin S, et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther 2011; 19: 2084–2091. DOI: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mingozzi F, Anguela XM, Pavani G, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med 2013; 5: 194ra192. DOI: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mimuro J, Mizukami H, Hishikawa S, et al. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther 2013; 21: 318–323. DOI: 10.1038/mt.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller CH, Benson J, Ellingsen D, et al. F8 and F9 mutations in US haemophilia patients: correlation with history of inhibitor and race/ethnicity. Haemophilia 2012; 18: 375–382. DOI: 10.1111/j.1365-2516.2011.02700.x. [DOI] [PubMed] [Google Scholar]
- 40. Hay CR, DiMichele DM; International Immune Tolerance Study. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012; 119: 1335–1344. DOI: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 41. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood 2014; 124: 3365–3372. DOI: 10.1182/blood-2014-05-577643. [DOI] [PubMed] [Google Scholar]
- 42. Colowick AB, Bohn RL, Avorn J, et al. Immune tolerance induction in hemophilia patients with inhibitors: costly can be cheaper. Blood 2000; 96: 1698–1702. [PubMed] [Google Scholar]
- 43. Rocino A, Cortesi PA, Scalone L, et al. Immune tolerance induction in patients with haemophilia a and inhibitors: effectiveness and cost analysis in an European Cohort (The ITER Study). Haemophilia 2016; 22: 96–102. DOI: 10.1111/hae.12780. [DOI] [PubMed] [Google Scholar]
- 44. Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003; 111: 1347–1356. DOI: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Markusic DM, Hoffman BE, Perrin GQ, et al. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med 2013; 5: 1698–1709. DOI: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Finn JD, Ozelo MC, Sabatino DE, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood 2010; 116: 5842–5848. DOI: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sack BK, Merchant S, Markusic DM, et al. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One 2012; 7: e37671 DOI: 10.1371/journal.pone.0037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol 2007; 138: 305–315. [DOI] [PubMed] [Google Scholar]
- 49. DiMichele DM. Immune tolerance in haemophilia: the long journey to the fork in the road. Br J Haematol 2012; 159: 123–134. DOI: 10.1111/bjh.12028. [DOI] [PubMed] [Google Scholar]
- 50. Valentino LA, Kempton CL, Kruse-Jarres R, et al. US Guidelines for immune tolerance induction in patients with haemophilia a and inhibitors. Haemophilia 2015; 21: 559–567. DOI: 10.1111/hae.12730. [DOI] [PubMed] [Google Scholar]
- 51. Laros-van Gorkom BA, Falaise C, Astermark J. Immunosuppressive agents in the treatment of inhibitors in congenital haemophilia A and B – a systematic literature review. Eur J Haematol Suppl 2014; 76: 26–38. DOI: 10.1111/ejh.12372. [DOI] [PubMed] [Google Scholar]
- 52. Leissinger C, Josephson CD, Granger S, et al. Rituximab for treatment of inhibitors in haemophilia A. A phase II study. Thromb Haemost 2014; 112: 445–458. DOI: 10.1160/TH14-01-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arruda VR, Samelson-Jones BJ. Gene therapy for immune tolerance induction in hemophilia with inhibitors. J Thromb Haemost 2016; 14: 1121–1134. DOI: 10.1111/jth.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reipert B, Arruda V, Lillicrap D. Animal models of inhibitors. Haemophilia 2010; 16(Suppl. 5): 47–53. DOI: 10.1111/j.1365-2516.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 55. Nichols TC, Hough C, Agersø H, et al. Canine models of inherited bleeding disorders in the development of coagulation assays, novel protein replacement and gene therapies. J Thromb Haemost 2016; 14: 894–905. [DOI] [PubMed] [Google Scholar]
- 56. Cameron C, Notley C, Hoyle S, et al. The canine factor VIII cDNA and 5’flanking sequence. Thromb Haemost 1998; 79: 317–322. [PubMed] [Google Scholar]
- 57. Evans JP, Brinkhous KM, Brayer GD, et al. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A 1989; 86: 10095–10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hough C, Kamisue S, Cameron C, et al. Aberrant splicing and premature termination of transcription of the FVIII gene as a cause of severe canine hemophilia A: similarities with the intron 22 inversion mutation in human hemophilia. Thromb Haemost 2002; 87: 659–665. [PubMed] [Google Scholar]
- 59. Lozier JN, Dutra A, Pak E, et al. The Chapel Hill hemophilia A dog colony exhibits a factor VIII gene inversion. Proc Natl Acad Sci U S A 2002; 99: 12991–12996. DOI: 10.1073/pnas.192219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sabatino DE, Nichols TC, Merricks E, et al. Animal models of hemophilia. Prog Mol Biol Transl Sci 2012; 105: 151–209. DOI: 10.1016/B978-0-12-394596-9.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sack BK, Herzog RW, Terhorst C, et al. Development of Gene Transfer for Induction of Antigen-specific Tolerance. Mol Ther Methods Clin Dev 2014; 1: 14013 DOI: 10.1038/mtm.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Cur Gene Ther 2009; 9: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niemeyer GP, Herzog RW, Mount J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood 2009; 113: 797–806. DOI: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood 2002; 99: 2670–2676. [DOI] [PubMed] [Google Scholar]
- 65. Xu L, Nichols TC, Sarkar R, et al. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci U S A 2005; 102: 6080–6085. DOI: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu L, Mei M, Haskins ME, et al. Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice. Thromb Res 2007; 120: 269–280. DOI: 10.1016/j.thromres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 67. Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 2007; 110: 2334–2341. DOI: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arruda VR. Gene therapy for immune tolerance induction in hemophilia with inhibitors. JCI Insight 2016; 14: 1121–1134. 2016/10/14. DOI: 10.1172/jci.insight.8937110.1111/jth.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. DiMichele DM, Kroner BL; North American Immune Tolerance Study Group. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost 2002; 87: 52–57. [PubMed] [Google Scholar]
- 70. Sabatino DE, Lange AM, Altynova ES, et al. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther 2011; 19: 442–449. DOI: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ewenstein BM, Takemoto C, Warrier I, et al. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood 1997; 89: 1115–1116. [PubMed] [Google Scholar]
- 72. Warrier I, Ewenstein BM, Koerper MA, et al. Factor IX inhibitors and anaphylaxis in hemophilia B. J Pediat Hematol Onc 1997; 19: 23–27. [DOI] [PubMed] [Google Scholar]
- 73. DiMichele DM. Inhibitors in childhood hemophilia A: genetic and treatment-related risk factors for development and eradication. Pediatr Blood Cancer 2013; 60(Suppl. 1): S30–S33. DOI: 10.1002/pbc.24338. [DOI] [PubMed] [Google Scholar]
- 74. Rodriguez V, Mancuso ME, Warad D, et al. Central venous access device (CVAD) complications in haemophilia with inhibitors undergoing immune tolerance induction: lessons from the international immune tolerance study. Haemophilia 2015; 21: e369–e374. DOI: 10.1111/hae.12740. [DOI] [PubMed] [Google Scholar]
- 75. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med 2017; 377: 809–818. DOI: 10.1056/NEJMoa1703068. [DOI] [PubMed] [Google Scholar]
- 76. Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med 2017; 377: 819–828. DOI: 10.1056/NEJMoa1616569. [DOI] [PubMed] [Google Scholar]
- 77. Chowdary P, Lethagen S, Friedrich U, et al. Safety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trial. J Thromb Haemost 2015. DOI: 10.1111/jth.12864. [DOI] [PubMed] [Google Scholar]
- 78. Polderdijk SG, Adams TE, Ivanciu L, et al. Design and characterization of an APC-specific serpin for the treatment of hemophilia. Blood 2017; 129: 105–113. DOI: 10.1182/blood-2016-05-718635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mathias T. Alnylam stock plummets after patient death in hemophilia study, https://www.reuters.com/article/us-alnylam-pharms-trial/alnylam-stock-plummets-after-patient-death-in-hemophilia-study-idUSKCN1BI1I9 (2017, accessed 15 February 2018).
- 80. Walsh CE, Jimenez-Yuste V, Auerswald G, et al. The burden of inhibitors in haemophilia patients. Thromb Haemost 2016; 116 Suppl 1: S10–S17. DOI: 10.1160/TH16-01-0049. [DOI] [PubMed] [Google Scholar]
- 81. Kulkarni R, Presley RJ, Lusher JM, et al. Complications of haemophilia in babies (first two years of life): a report from the Centers for Disease Control and Prevention Universal Data Collection System. Haemophilia 2017; 23: 207–214. DOI: 10.1111/hae.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. DeKoven M, Karkare S, Lee WC, et al. Impact of haemophilia with inhibitors on caregiver burden in the United States. Haemophilia 2014; 20: 822–830. DOI: 10.1111/hae.12501. [DOI] [PubMed] [Google Scholar]
- 83. Eckhardt CL, Loomans JI, van Velzen AS, et al. Inhibitor development and mortality in non-severe hemophilia A. J Thromb Haemost 2015; 13: 1217–1225. DOI: 10.1111/jth.12990. [DOI] [PubMed] [Google Scholar]
- 84. Donfield SM, Lynn HS, Lail AE, et al. Delays in maturation among adolescents with hemophilia and a history of inhibitors. Blood 2007; 110: 3656–3661. DOI: 10.1182/blood-2007-05-088062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mannucci PM, Capitanio A, Del Ninno E, et al. Asymptomatic liver disease in haemophiliacs. J Clin Pathol 1975; 28: 620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Choo QL, Kuo G, Weiner AJ, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989; 244: 359–362. [DOI] [PubMed] [Google Scholar]
- 87. Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016; 65: 727–733. DOI: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 88. Llovet JM, Villanueva A. Liver cancer: effect of HCV clearance with direct-acting antiviral agents on HCC. Nat Rev Gastroenterol Hepatol 2016; 13: 561–562. DOI: 10.1038/nrgastro.2016.140. [DOI] [PubMed] [Google Scholar]
- 89. Raj K, Ogston P, Beard P. Virus-mediated killing of cells that lack p53 activity. Nature 2001; 412: 914–917. DOI: 10.1038/35091082. [DOI] [PubMed] [Google Scholar]
- 90. Logan GJ, Dane AP, Hallwirth CV, et al. Identification of liver-specific enhancer-promoter activity in the 3’ untranslated region of the wild-type AAV2 genome. Nat Genet 2017; 49: 1267–1273. DOI: 10.1038/ng.3893. [DOI] [PubMed] [Google Scholar]
- 91. Khleif SN, Myers T, Carter BJ, et al. Inhibition of cellular transformation by the adeno-associated virus rep gene. Virology 1991; 181: 738–741. [DOI] [PubMed] [Google Scholar]
- 92. Chandler RJ, LaFave MC, Varshney GK, et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest 2015; 125: 870–880. DOI: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nakai H, Iwaki Y, Kay MA, et al. Isolation of recombinant adeno-associated virus vector-cellular DNA junctions from mouse liver. J Virol 1999; 73: 5438–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007; 317: 477 DOI: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 95. Donsante A, Vogler C, Muzyczka N, et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther 2001; 8: 1343–1346. DOI: 10.1038/sj.gt.3301541. [DOI] [PubMed] [Google Scholar]
- 96. Srivastava A, Carter BJ. AAV infection: protection from cancer. Hum Gene Ther 2017; 28: 323–327. DOI: 10.1089/hum.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gauttier V, Pichard V, Aubert D, et al. No tumour-initiating risk associated with scAAV transduction in newborn rat liver. Gene Ther 2013; 20: 779–784. DOI: 10.1038/gt.2013.7. [DOI] [PubMed] [Google Scholar]
- 98. Bell P, Wang L, Lebherz C, et al. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol Ther 2005; 12: 299–306. DOI: 10.1016/j.ymthe.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 99. Li H, Malani N, Hamilton SR, et al. Assessing the potential for AAV vector genotoxicity in a murine model. Blood 2011; 117: 3311–3319. DOI: 10.1182/blood-2010-08-302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kaeppel C, Beattie SG, Fronza R, et al. A largely random AAV integration profile after LPLD gene therapy. Nat Med 2013; 19: 889–891. DOI: 10.1038/nm.3230. [DOI] [PubMed] [Google Scholar]
- 101. Gil-Farina I, Fronza R, Kaeppel C, et al. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol Ther 2016; 24: 1100–1105. DOI: 10.1038/mt.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000; 24: 257–261. DOI: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 103. Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med 1999; 5: 56–63. DOI: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 104. Herzog RW, Mount JD, Arruda VR, et al. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther 2001; 4: 192–200. DOI: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 105. Buchlis G, Podsakoff GM, Radu A, et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood 2012; 119: 3038–3041. DOI: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jiang H, Pierce GF, Ozelo MC, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther 2006; 14: 452–455. DOI: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 107. Herzog RW, Fields PA, Arruda VR, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther 2002; 13: 1281–1291. DOI: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 108. Arruda VR, Stedman HH, Haurigot V, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood 2010; 115: 4678–4688. DOI: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. French RA, Samelson-Jones BJ, Niemeyer GP, et al. Complete correction of hemophilia B phenotype by FIX-Padua skeletal muscle gene therapy in an inhibitor-prone dog model. Blood Advances 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Finn JD, Nichols TC, Svoronos N, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood 2012; 120: 4521–4523. DOI: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006; 108: 3321–3328. DOI: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010; 21: 704–712. DOI: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 113. Asuri P, Bartel MA, Vazin T, et al. Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Mol Ther 2012; 20: 329–338. DOI: 10.1038/mt.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Arruda VR, Stedman HH, Nichols TC, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood 2005; 105: 3458–3464. DOI: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rothe M, Schambach A, Biasco L. Safety of gene therapy: new insights to a puzzling case. Cur Gene Ther 2014; 14: 429–436. [DOI] [PubMed] [Google Scholar]
- 116. Xu L, Gao C, Sands MS, et al. Neonatal or hepatocyte growth factor-potentiated adult gene therapy with a retroviral vector results in therapeutic levels of canine factor IX for hemophilia B. Blood 2003; 101: 3924–3932. DOI: 10.1182/blood-2002-10-3050. [DOI] [PubMed] [Google Scholar]
- 117. Powell JS, Josephson NC, Quon D, et al. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood 2012; 119: 3031–3037. DOI: 10.1182/blood-2011-09-382846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 2008; 118: 3132–3142. DOI: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cavazzana M, Six E, Lagresle-Peyrou C, et al. Gene therapy for X-linked severe combined immunodeficiency: where do we stand? Hum Gene Ther 2016; 27: 108–116. DOI: 10.1089/hum.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Matrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther 2010; 18: 477–490. DOI: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. McGarrity GJ, Hoyah G, Winemiller A, et al. Patient monitoring and follow-up in lentiviral clinical trials. J Gene Med 2013; 15: 78–82. DOI: 10.1002/jgm.2691. [DOI] [PubMed] [Google Scholar]
- 122. Du LM, Nurden P, Nurden AT, et al. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat Commun 2013; 4: 2773. DOI: 10.1038/ncomms3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ide LM, Iwakoshi NN, Gangadharan B, et al. Functional aspects of factor VIII expression after transplantation of genetically-modified hematopoietic stem cells for hemophilia A. J Gene Med 2010; 12: 333–344. DOI: 10.1002/jgm.1442. [DOI] [PubMed] [Google Scholar]
- 124. Ide LM, Gangadharan B, Chiang KY, et al. Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood 2007; 110: 2855–2863. DOI: 10.1182/blood-2007-04-082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ozelo MC, Vidal B, Brown C, et al. Omental implantation of BOECs in hemophilia dogs results in circulating FVIII antigen and a complex immune response. Blood 2014; 123: 4045–4053. DOI: 10.1182/blood-2013-12-545780. [DOI] [PubMed] [Google Scholar]
- 126. Merlin S, Cannizzo ES, Borroni E, et al. A novel platform for immune tolerance induction in hemophilia A mice. Mol Ther 2017; 25: 1815–1830. DOI: 10.1016/j.ymthe.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cantore A, Nair N, Della Valle P, et al. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood 2012; 120: 4517–4520. DOI: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 128. Cantore A, Ranzani M, Bartholomae CC, et al. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci Transl Med 2015; 7: 277ra228 DOI: 10.1126/scitranslmed.aaa1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Follenzi A, Benten D, Novikoff P, et al. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest 2008; 118: 935–945. DOI: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shahani T, Covens K, Lavend’homme R, et al. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J Thromb Haemost 2014; 12: 36–42. DOI: 10.1111/jth.12412. [DOI] [PubMed] [Google Scholar]
- 131. Siner JI, Iacobelli NP, Sabatino DE, et al. Minimal modification in the factor VIII B-domain sequence ameliorates the murine hemophilia A phenotype. Blood 2013; 121: 4396–4403. DOI: 10.1182/blood-2012-10-464164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. DePolo NJ, Reed JD, Sheridan PL, et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther 2000; 2: 218–222. DOI: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- 133. Milani M, Annoni A, Bartolaccini S, et al. Genome editing for scalable production of alloantigen-free lentiviral vectors for in vivo gene therapy. EMBO Mol Med 2017; 9: 1558–1573. DOI: 10.15252/emmm.201708148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 2013; 14: 49–55. DOI: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rahman SH, Maeder ML, Joung JK, et al. Zinc-finger nucleases for somatic gene therapy: the next frontier. Hum Gene Ther 2011; 22: 925–933. DOI: 10.1089/hum.2011.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tsai SQ, Joung JK. Defining and improving the genome-wide specificities of CRISPR-Cas9 nucleases. Nat Rev Genet 2016; 17: 300–312. DOI: 10.1038/nrg.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Swystun LL, Lillicrap D. Gene therapy for coagulation disorders. Circ Res 2016; 118: 1443–1452. DOI: 10.1161/CIRCRESAHA.115.307015. [DOI] [PubMed] [Google Scholar]
- 138. Li H, Haurigot V, Doyon Y, et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 2011; 475: 217–221. DOI: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Anguela XM, Sharma R, Doyon Y, et al. Robust ZFN-mediated genome editing in adult hemophilic mice. Blood 2013; 122: 3283–3287. DOI: 10.1182/blood-2013-04-497354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Huai C, Jia C, Sun R, et al. CRISPR/Cas9-mediated somatic and germline gene correction to restore hemostasis in hemophilia B mice. Hum Genet 2017; 136: 875–883. DOI: 10.1007/s00439-017-1801-z. [DOI] [PubMed] [Google Scholar]
- 141. Sharma R, Anguela XM, Doyon Y, et al. In vivo genome editing of the albumin locus as a platform for protein replacement therapy. Blood 2015; 126: 1777–1784. DOI: 10.1182/blood-2014-12-615492. [DOI] [PMC free article] [PubMed] [Google Scholar]