Abstract
Study Design:
Review of literature and case series.
Objectives:
Update and review of current treatment concepts for spine fractures in patients with ankylosing spinal disorders.
Methods:
Case presentation and description of a diagnostic and therapeutic algorithm for unstable spinal injuries with an underlying ankylosing spinal disorder (ASD) of the cervical and thoracolumbar spine.
Results:
Nondisplaced fractures can be missed easily using conventional X-rays. Thus, computed tomography (CT) scans are recommended for all trauma patients with ASD. In doubt or presence of any neurologic involvement additional magnetic resonance imaging (MRI) scans should be obtained. Spine precautions should be maintained all times and until definitive treatment (<24 h). Nonoperative fracture treatment is not recommended given the mechanical instability of the most commonly seen fracture patterns (AOSpine B- and C-type, M2) in patients with ASD and inherent high risk of secondary neurologic deterioration. For patients with ankylosing spondylitis (AS) or diffuse idiopathic hyperostosis (DISH) sustaining cervical spine fractures, a combined anterior-posterior instrumentation for fracture fixation is recommended. Closed reduction and patient positioning can be challenging in presence of preexisting kyphotic deformities. In the thoracolumbar (TL) spine, a posterior instrumentation extending 2 to 3 levels above and below the fracture level is recommended to maintain adequate reduction and stability until fracture healing. Minimally invasive percutaneous pedicle screws and cement augmentation can help to minimize the surgical trauma and strengthen the construct stability in patients with diminished minor bone quality (osteopenia, osteoporosis).
Conclusions:
Current concepts, treatment options, and recommendations of the German Orthopedic Trauma Society–Spine Section for spinal fractures in the ankylosed spine have been outlined.
Keywords: ankylosing spondylitis, Morbus Bechterew, trauma, cervical spine, thoracolumbar spine, diffuse idiopathic skeletal hyperostosis, DISH, senile ankylosing hyperostosis, Morbus Forestier, spinal fractures, medical treatment, nonoperative fracture treatment, operative treatment, iatrogenic spine fracture, complication
Introduction
Almost everyone knows someone or has recognized patients with ankylosing spinal disorders (ASDs), because of its distinct pathognomonic features and their clinical presentation. Typically, patients suffer from one or more of the following commonly associated symptoms: pain, stiffness, and kyphotic deformity with a stooped forward or rigid posture. Neck and/or back pain, loss of flexibly, and inability to walk upright without a horizontal line of vision makes these patients more susceptible to falls and spinal fractures. Simple ground-level falls (40%) and minor trauma are the most common mechanisms of injury.1 Most elderly patients (mean age 69 years) with ASD will have diminished bone quality, for example, osteopenia or osteoporosis, and are prone to sustain mechanically unstable fractures (AOSpine classification B- and C-type injuries with case-specific modifier M2 [TL system], M3 [subaxial system]).1 Mechanically highly unstable injuries render a higher risk of secondary neurologic injuries. Despite the need to expedite treatment, a delay in diagnosis of fractures and even facture dislocations in ASDs is commonplace. Interpretation of radiographic images and consequently treatment decisions can be challenging. Treatment strategies of spinal fractures in ASD are different from those for the young and otherwise healthy trauma patients with a nonrigid spine. A sound understanding of patient-specific needs, individual comorbidities, and knowledge of medical treatment options of the underlying diseases are helpful. More so, a multidisciplinary approach with the intent to prevent ASDs and fractures by offering the right treatment is essential.
The most common etiologies of ASD are seronegative (associated with HLA-27) spondyloarthritis (ankylosing spondylitis [AS]/Morbus Bechterew, Reiters syndrome, psoriasis arthritis, and other rheumatologic conditions), and senile ankylosing hyperostosis (Morbus Forestier), also known as diffuse idiopathic skeletal hyperostosis (DISH), which will be discussed as well.
These recommendations are the result of a literature review, clinical experience, and informal consent by a German Society for Orthopaedics and Trauma (DGOU) working group formed for the purpose to write treatment recommendations for spine fractures in ankylosing diseases from a German perspective. The working group members have been assigned by the Board of the Spine Section of the German Society for Orthopaedics and Trauma (DGOU) based on their expertise and clinical experience in the field of spine trauma care.
This review article summarizes current treatment concepts along with recommendations of the German Orthopedic and Trauma Society for cervical and thoracolumbar spinal injuries in the ankylosed spine.
Diffuse Idiopathic Skeletal Hyperostosis
Diffuse idiopathic skeletal hyperostosis, also known as Forestier’s disease,2 is a spondyloarthropathy characterized by an ankylosing hyperostosis, usually found in the anterior and lateral spine. The disease selectively affects the spine and takes place at the bony attachments (entheses) of tendons, ligaments, and joint capsules. Ossification occurs in the absence of inflammation. To match the diagnostic criteria 2/3 (according to Forestier/Resnick), adjacent vertebras have to show bony bridges with nearly normal intervertebral discs and without sacroiliac or facet joint fusion.3,4 Besides flowing ossification along the anterior and anterolateral aspects of the vertebrae, further radiographic criteria for the diagnosis include the presence of preserved intervertebral disc height, absence of facet joint ankylosis, and finally no erosion, sclerosis, or bony ankylosis of the sacroiliac joints.5
Most of the DISH patients show no symptoms and are diagnosed by chance. That might be the reason that the rate of DISH patients is underestimated and further displayed by a lack of publications.6 An article by Holton and coworkers, in men from the general population over 65 years of age, showed that 42% fulfilled the diagnostic criteria by Resnick. The prevalence further rises with age.7 Its progression is comparable to AS and directly associated to overweight and diabetes. Compared to AS, patients suffering from DISH presenting with vertebral fractures are significantly older and have higher comorbidity rates.1
DISH is associated with a higher prevalence of thoracolumbar vertebral fractures in the elderly,8 and it is more commonly seen in men than in women.9
Quantitative computed tomography (CT) densitometry has shown significantly lower bone mass density in elderly men with both DISH and fractures when compared to men with DISH but no fractures, despite higher bone mass density measurements caused by the presence of paravertebral calcifications.8 Spinal column rigidity and osteoporosis contribute to an increased fracture risk in individuals with ASDs.1
Ankylosing Spondylitis (Morbus Bechterew)
Ankylosing spondylitis, also known as Morbus Bechterew, is one of the most common seronegative (associated with HLA-27) spondyloarthritis subtypes. It is characterized by 2 key pathological findings: sacroiliac joint and spinal inflammation and new bone formation with the possible consequence of bone fusion, usually in the axial skeleton with ankylosis of the sacroiliac and facet joints.10 Mostly men (15-50 years of age) are effected with an overall prevalence of 0.5% in Europe.11 Typical findings include bone marrow edema, lymphocytic infiltrates, and increased microvessel density. Enthesitis is characterized by lymphocytic infiltrates around fibrous cartilage followed by subsequent ankylosis. Acute inflammation can be observed on magnetic resonance imaging (MRI; SI joints) earlier on during the disease. The molecular mechanisms that promote the transition from inflammation to new bone formation in patients with AS are not well understood.12
Patterns of radiographic involvement can be assessed using the Bath Ankylosing Spondylitis Radiology Index (BASRI). Usually, symmetric sacroiliitis can be seen in 86% of patients, complete spinal fusion in 28% of patients for more than 30 years, and in 43% of patients with AS for more than 40 years.13
Within the literature several synonyms for different clinical patterns and diseases causing spinal ankylosis can be found. Table 1 is a summary of names and common terminology used in this article.
Table 1.
Ankylosing Spinal Disorders (ASDs).
| Group | (1) Seronegative axial spondyloarthritis (SpA) | (2) Degenerative diseases | ||
| Definition: | Definition: | |||
| • Genetic disposition (HLA-B27 positive) | • Primary cause unknown | |||
| • Missing rheumatoid factors (IgG, IgM, or IgA autoantibodies); “seronegative” | • Noninflammatory | |||
| Name/Terminology | Disease | Synonym | Disease | Synonym |
| Ankylosing spondylitis (AS) | Morbus Bechterew | Diffuse idiopathic skeletal hyperostosis (DISH) | Morbus Forestier, spondylosis hyperostotica, senile ankylosing hyperostosis | |
| Definition: | Definition: | |||
| • Evident radiographic SI-joint changes (New York criteria14) | • Spondyloarthropathy and ossification with lateral and anterior osteophytes | |||
| Nonradiographic axial spondyloarthritis (nr-axSpA) | ||||
| Definition: | ||||
| • Sacroiliitis visible on magnetic resonance imaging only | ||||
| • Clinical features and/or HLA-B27 detection15 |
Nonoperative Management of Patients With Axial Spondyloarthritis (Ankylosing Spondylitis) and Nonradiographic Axial Spondyloarthritis (nr-axSpA)
As result of modern disease-modifying drugs (biologics) and constantly optimized rheumatologic treatment regimens, patient numbers with completely ankylosed spines have significantly dropped over time. Consequently, the numbers of injuries and complications associated with have also reduced.
Surgeons should have a basic understanding of the underlying medical condition, its pathomechanisms, and current clinical rheumatologic classifications systems. This knowledge will ease interdisciplinary communication and help better encompass specific substances and side effects (hemostasis, immunosuppression) that can have a significant impact on surgical planning and clinical outcome.
Nonoperative medical management options for the 2 most common rheumatologic disease categories (AS and nr-axSpA) associated with spinal ankylosis will be discussed in the following.
Inflammatory back pain is the crucial clinical parameter for differential diagnosis between degenerative and inflammatory spine disease. While evident radiographic changes in the sacroiliacal joints according to the modified New York criteria14 classify patients for diagnosis of AS, sacroiliitis visible only by magnetic resonance imaging and/or other clinical features as well as the detection of HLA-B27 may lead to the diagnosis of nr-axSpA.15 The new ASAS classification criteria for axial SpA are the following: the presence of sacroiliitis by radiography or by MRI plus at least 1 SpA feature (“imaging arm”) or the presence of HLA-B27 plus at least 2 SpA features (“clinical arm”).15
Standard of care is physiotherapy and nonsteroidal anti-inflammatory drugs. Disease activity regularly is measured by BASDAI, a composite index based on different questions referring to activity of the disease. In the case of persisting disease, activity escalation of drug therapy is indicated. Biologics, especially TNF-blockers (adalimumab, certolizumab, etanercept, golimumab, infliximab), have demonstrated a high grade of efficacy in clinical trials with AS patients.16 As of late secukinumab, a blocker of IL-17, has also been approved for treatment of axial spondyloarthritis. Selection of the most suitable drug for the respective patient depends on clinical parameters. Clinical parameters are involvement of the eyes, presence of psoriasis, chronic inflammatory intestinal disease, and individual preferences of the patient like interval of application or administration or experience with former therapies. Sheared decision making should be envisaged.17
Clinical efficacy of drug therapy must be measured regularly. In the case of good and validated response (50% improvement in BASDAI, positive expert opinion), therapy should proceed.18 The primary goal of treating the patient with SpA is to maximize long-term health-related quality of life and social participation through control of signs and symptoms, prevention of structural damage, normalization or preservation of function, avoidance of toxicities, and minimization of comorbidities.17 As side effects are rare, one should be aware of infections. Therefore, latent tuberculosis or active/chronic hepatitis B or C should be excluded before start of therapy. To avoid infections vaccinations should be performed as recommend by regional authorities. One should be aware of allergic reactions. However, severe clinical cases are rare. There are some more contraindications and side effects, which should be considered before start and during therapy. Therapy with biologics should be stopped in the case of severe infections and in good time before an operation is scheduled (at least 2 half-life periods).
Biomechanical Considerations
The main reason for the significantly elevated fracture risk in patients with ASD compared to the normal population is the characteristic of continuous pathological spinal remodeling. Both ectopic bone formation19 and osteopenia20 occur simultaneously as an essential part of the pathologic pathways. Unusual osteoproliferative processes lead to a ligamentous ossification progressively bridging the whole spine, while osteopenia in part results from a stress shielding of the cancellous vertebral parts. The change from a highly flexible, load transferring, and resisting spine to a multilevel-fused spinal column usually takes several years through the course of the disease. During this time the biomechanical behavior of the spine is stepwise transferred from articulation to a long-bone-like rigid lever.21 It is commonly referred to as “bamboo-spine” in AS. This structure is not able to appropriately neutralize loads of a traumatic event. While trabecular bone is rarefied the external parts with the cortical shell of the anterior and posterior column are progressively loaded. The typical nonphysiologic kyphotic deformation22 further triggers the loss of stress absorbing abilities. Furthermore, patients with fixed kyphosis have an impaired horizontal gaze and have a higher general risk of falls.23 Furthermore, it has been shown that the degenerative processes are not limited to the bony structures: ligaments and muscles also undergo disease-specific modifications.24 Patients have an impaired muscle strength with less proprioceptive abilities, which will have a negative impact during accidents and falls.23 The result is a more than 10 times increased fracture risk and higher incidence of associated neurological deficits than in non-ASD patients.25
Diagnostic Approach
The history, mechanism of injury, and physical examination of patients with ASD is not always conclusive. A delay in the diagnosis, defined as lack of documentation of an existing fracture within 24 hours of a patient’s initial assessment, has been reported for 10% (DISH) to 37% (AS) of the cases.1 In the preclinical setting following any trauma in patients with known stiffening spinal conditions, it is advised to maintain spine precautions with external fixation (eg, hard cervical collar, vacuum mattresses) or any other available means of fixation until spine clearance has been obtained, for example, instability has been ruled out.
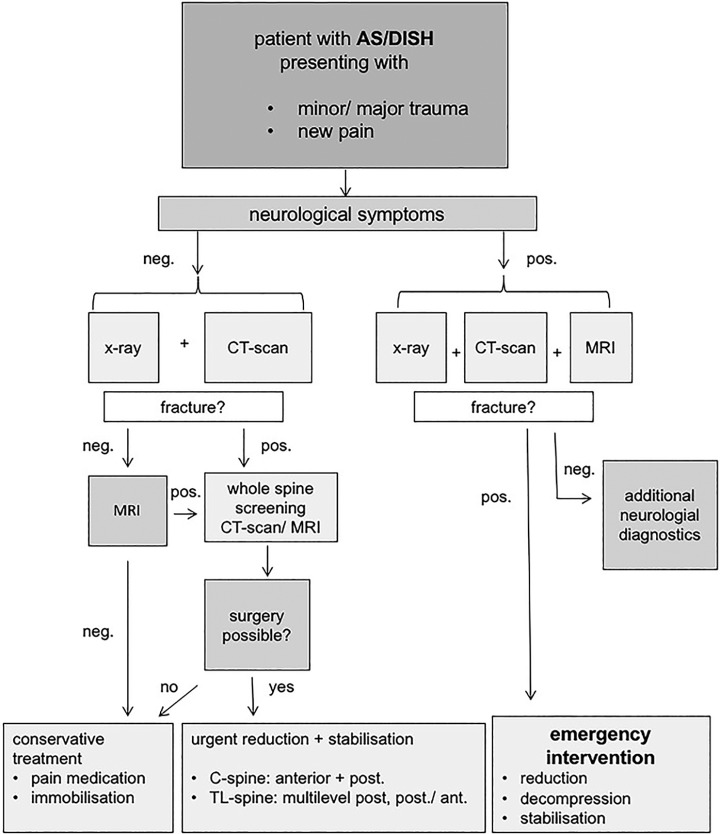
The diagnostic algorithm shown in Figure 1 is proposed. On conventional X-rays in both planes the common hyperextension fractures can be easily missed, especially if they are taken in an upright position. For exact classification and preoperative planning, CT scans with biplanar reconstruction or 3D imaging at least 3 vertebrae above and below the level of injury are strongly recommended.
Figure 1.
Diagnostic and therapeutic algorithm.
Multisegment or multilevel injuries have been reported in 6% to 8% of the cases.1,26 Thus, multisegment and multilevel injuries should be taken into consideration and excluded as well. The authors recommend whole spine CT scan or MRI for fracture screening in any case of relevant injury to patients with an ankylosed spine. Especially in the presence of neurologic deficits, MRI scans are helpful to assess all aspects of injury such as disco-ligamentous, spinal cord, and nerve root injuries, or reasonable option for the exclusion of occult fractures.27 In cases with neurologic involvement MRI scans are always mandatory.
Radiographic fracture assessment can be based on the level of injury and different patterns of ankylosis. Caron et al classified ASD spine fractures into 4 groups based on anterior fracture excursion through the intervertebral disc, vertebral body, and/or posterior disco-ligamentous or osseous elements.1
For fracture classification we recommend the recently published and validated AOSpine subaxial and thoracolumbar classification systems.28,29 The M2 case-specific modifier (TL system) is designated for patients with ASD (SA, DISH), rheumatologic conditions, or osteopenia/osteoporosis, respectively, the M3 modifier for the cervical spine in the subaxial system (ossification of the posterior longitudinal ligament, ligamentum flavum, and others).
Physicians and every medical personnel (X-ray technician, nurses, etc) involved should be informed and alerted about the presence of an ASD beforehand.
At all times during the entire diagnostic process and until definite treatment, it is strongly advised to maintain strict external fixation and other necessary precaution measures to avoid fracture dislocation or secondary neurologic injuries.
Nonoperative Management of Spine Fractures in Patients With ASD
Patients with surgical contraindications or simple A-type fractures may be treated nonoperatively. Nevertheless, the likelihood to sustain an isolated simple compression injury (AOSpine A-type injury) in patients with ASD is low and their differentiation from unstable B- and C-type injuries sometimes difficult.
As mentioned before, ASD renders the spine much more susceptible for mechanically unstable AOSpine B- and C-type injuries, defined as failure of the posterior or anterior tension band (B-type injury), or failure of all elements leading to dislocation or displacement (AOSpine C-type injury). Nonoperative management for the latter is not recommended.
Any patient with nonoperative spine fracture treatment and concomitant ASD that has been immobilized in a halo, collar, TLSO, or plaster jacket must be monitored closely given the high risk of fracture dislocation, potential for progressive deformity, secondary neurologic deterioration, and generally poor clinical outcome associated with nonoperative treatment. Traction therapy in an unstable spinal injury is not an option. Options for nonoperative management are further limited given the fact that bracing of a fixed kyphosis is difficult, if not impossible. External halo-fixation is associated with a high risk of pin pull-out, pin infections, loss or reduction, and respiratory insufficiency in this patient population. Prolonged bed rest as a means of nonoperative fracture treatment is associated with a high rate of pulmonary complications and fatal outcome.30 Overall, patients suffering from fractures of ASD present with an increased complication and mortality rate. In general, nonoperative treatment (immobilization) is only the second line of choice with inferior clinical outcomes when compared to surgical treatment and operative fracture fixation.21
Operative Management of Cervical Spine Fractures in Patients With ASD
At a level 1 trauma center, Caron et al in a retrospective case series identified 570 patients diagnosed with ASD (either DISH or AS) in their radiology database. A total of 112 (20%) of the 570 patients had 122 fractures. Injury distribution showed a majority of cases at the cervical spine level (55%; thoracic: 32%, lumbar 13%).1
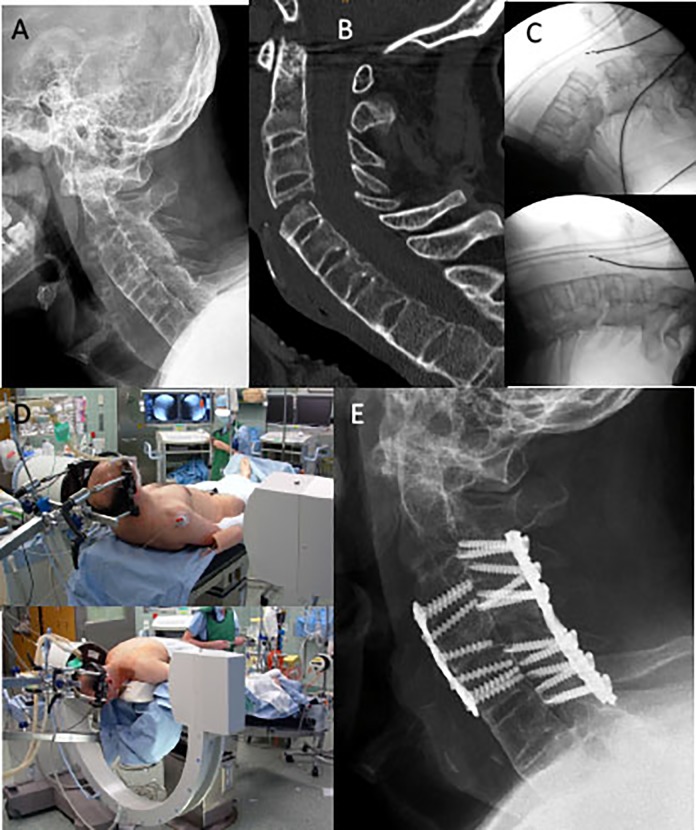
Two representative clinical cases have been chosen to highlight reasons and recommendations for cervical spine fractures treatment in ASD: the first case is a 49-year-old male with AS sustaining a ground-level fall, presenting to the emergency room with severe neck pain and bilateral C5/C6 radiculopathy (Figure 2). The fracture was harmless-appearing on lateral and AP cervical spine films extending through anterior (C4 vertebral body) and posterior elements (C4/5 level) in a completely ankylosed subaxial spine. CT scans were obtained to confirm an unstable AOSpine subaxial classification type C4-5 C, F4 BL, N2, M3 injury. This case highlight the well-documented difficulties to visualize and fully appreciate the extent of the injury in ASD with plain films only; thus, screening of the entire spinal column with advanced neuroimaging (CT or MRI imaging) has been recommended.31
Figure 2.
Case 1: Ankylosing spondylitis (AS) patient (AOSpine C4-5 C, F4 BL, N2, M3) with anterior-posterior fixation. (A) Initial lateral c-spine films; (B) Preoperative CT scan; (C) Instability and fracture dislocation following patient intubation and transfer in the supine position before and (D) after closed reduction on the operation table with a halo-reduction device fixed to the operation table; and (E) postoperative results.
After endotracheal intubation and transfer to the operating table in the supine position, the noncontiguous, highly unstable fracture with marked dislocation could be seen once more (Figure 2C). For these cases, video-laryngoscopic assisted or fiber-optic endonasal intubation is recommended and should be discussed with the anesthesiologist. Preoperatively, a manual closed-reduction maneuver under fluoroscopic control should be attempted. Temporary external fixation after fracture reduction in the prone or supine position can be best obtained with a halo-reduction device or Mayfield clamp. Preexisting spinal deformities must be considered for an appropriate adaption of the intraoperative patient positioning. A rigid cervicothoracic kyphosis can be challenging during fracture reduction and patient positioning. At times, a marked deformity can prohibit routine anterior or posterior surgical exposure.
For unstable B- and C-type cervical spine injuries in ASD, a combined anterior-posterior instrumentation is recommended. Whenever there is no option for a safe anterior approach and placement of an anterior plate with angular stable screws with bicortical purchase, we recommend a longer posterior lateral mass and/or pedicle screw construct for internal fixation.
For the first case and at the time of injury, a combined anterior-posterior plate construct with lateral mass screw was chosen and the radiculopathy resolved shortly after surgery.
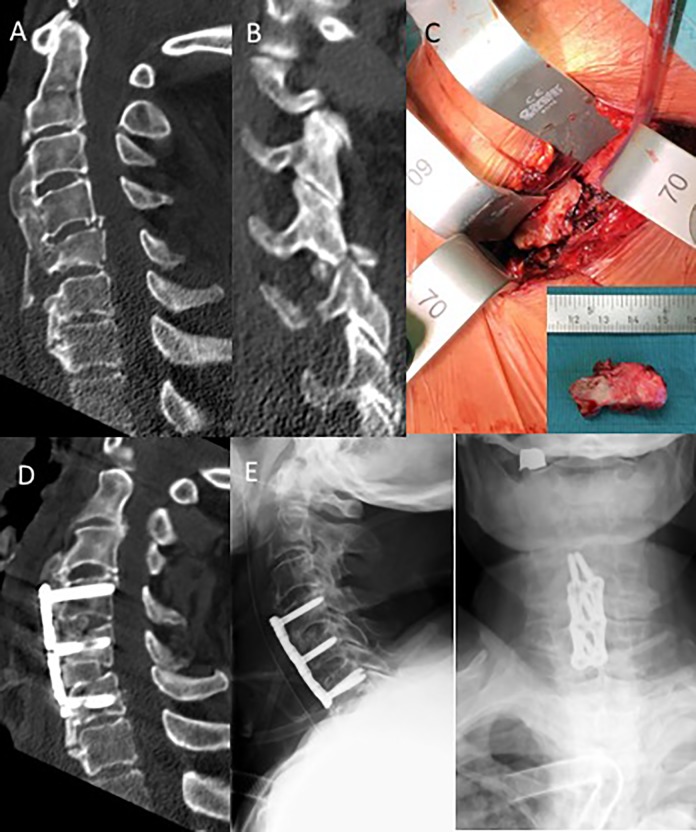
The second clinical case is a patient 86 years of age, who felt out of his bed and onto his head (Figure 3). His Glasgow Coma Scale score was 15 on admission to the hospital with complaint of severe neck pain. X-rays and CT scans of his head and c-spine were obtained and revealed an unstable C4-5 right facet fracture dislocation (AOSpine: C4-5 B2, F4, N0, M3) further extending through the C5 vertebral body and flowing anterior osteophyte. The large anterior bridging osteophyte (DISH) extending from C3-C7 was fractures. He was placed in a hard cervical collar and taken to the operating room after video-assisted intubation. The cervical collar was removed and a manual closed-reduction maneuver with Mayfield clamp under biplanar fluoroscopic control was carried out to reduce the unstable facet fracture dislocation. A left-sided standard anterolateral approach was chosen. After the exposure, a large prevertebral hematoma and loosened, broken off bony fragment of the flowing anterior osteophyte was removed. A 2-level ACDF (anterior cervical discectomy and fusion) C4-6 with autologous tricortical anterior iliac crest bone grafts with 6 angular stable bicortical screws was chosen for fracture fixation.
Figure 3.
Case 2: Diffuse idiopathic skeletal hyperostosis (DISH) patient (AOSpine C4-5 B2, F4, N0, M3) with 2-level ACDF and anterior angular stable plate fixation. (A) Initial CT scan with (B) C4-5 facet fracture dislocation, (C) fractures and broken off flowing anterior osteophyte; (D) Postoperative CT scans; (E) lateral and AP X-ray films.
The patient was extubated and remained neurologically intact. Postoperative imaging confirmed adequate implant positioning and realignment of the facet fracture dislocation. On the third postoperative day, the patient developed a pneumonia that required further medical attention and further delayed his discharged to a rehabilitation facility because of multiple other medical comorbidities. Otherwise the surgical wounds healed uneventful.
Operative Management of Thoracolumbar (TL) Fractures in Patients With ASD
The overall incidence of TL fractures in patients suffering from ankylosing spine diseases is yet not clear.27 The so-called “clinical” fractures present with associated pain while all others are called “morphometric” fractures. The incidence of the later type widely varies throughout the literature.32,33
In the literature, the proportion of ASD patients suffering from TL fractures varies between 30% and 50%.1,34 Caron et al reported an incidence of 21% thoracic, 19% thoracolumbar, and 8% lumbar fractures in ASD patients.1 Thoracic fractures are more likely in patients suffering from DISH while lumbar locations are more common in AS patients. At the segment level most fractures in DISH patients are observed through the vertebral body. In contrast, fractures in AS are evenly located at the level of the vertebral body and the intervertebral disc.21 In early stages of AS chondroid metaplasia and calcification of the anulus fibrosus and the nucleus pulposus occur, turning the intervertebral disc into the weakest point. In more advanced stages osteopenia coupled with ossification transfer this area to the vertebral body as the primary fracture site.27,35 Injuries at more than one level are common, making a diagnostic evaluation of the complete spine necessary36 (Figure 4).
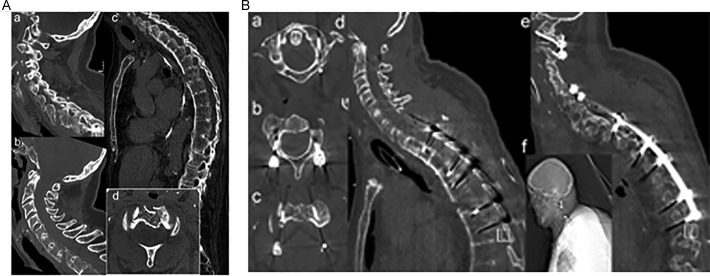
Figure 4.
(A) Case 3: 74-year-old female with AS sustaining a multilevel cervical and thoracic spine fracture and intracranial bleeding (SAB) after a trip and fall onto stairways (C1/2 fracture dislocation with AOSpine subaxial classification: C7-Th1 C M3-type injury (a, b, d), and AOSpine TL classification T6-7 B3, M2-type injury) (c). (B) One stage posterior segmental fixation with C1/2 fixation using Harms/Goel technique (a-c), posterior lateral mass screw and pedicle screw/rod construct C5/6-T3-T5/6 and T7/8 (d-f).
Most of the patients present with a history of low-energy hyperextension impacts, such as falls from a standing/sitting position. However, flexion, compression, and rotational fracture types are also reported.21 Involvement of all vertebral columns, ossification of ligaments, and degradation of surrounding tissues result in highly unstable fracture patterns. According to the new AOSpine TL classification system,29 the classic ASD TL spine fracture subtype is a B3, M2-type, but other A-, B-, and C-type injuries can be found as well. Based on high numbers with highly unstable injury patterns, the number of neurological deterioration is equally high. With an underlying ASD the risk to sustain a spinal cord injury, nerve root lesions, intramedullary edema, or spinal epidural hematoma is 11.4 times higher when compared to the general spine trauma population.25 Caron et al reported 33%, 23%, and 33% of neurological complications in thoracic, thoraco-lumbar, and lumbar fracture localizations, respectively. A common complication is the appearance of secondary neurological deficits in up to 15% of the cases due to insufficient immobilization and patient transfers. Most likely these numbers are underreported and even higher. The neurological status in most patients is not influenced by treatment and does not change in the early postoperative and follow-up period.34 Surgical intervention seems to positively increase the recovery rate.
Most cases, for example, any fracture with neurological deficits or mechanically unstable TL fractures in patients with ASD are indications for surgical intervention. Nonoperative treatment should be limited to cases with contraindications, such as poor general state of health or other comorbidities making general anesthesia impossible. If indicated, surgery should be carried out without further delay.
Sufficient preoperative closed reduction is one of the surgical key steps. However, closed and subsequently intraoperative open reduction can be hindered by long lever arms and high mechanical loads taking effect in the ankylosed spine. To avoid complications and dangers such as implant failure and screw pull-out by overstraining the construct, careful preoperative planning and patient positioning is mandatory. In the presence of a deformity, pads and rolls on the operation table can as a hypomochlion and help obtain the necessary fracture reduction. In extreme situations, an inclined upright sitting position is necessary, in order to close larger gaps of the widened anterior spinal elements in hyperextension injuries (B3-type).
Once the fracture is reduced, a posterior instrumentation is the treatment of choice in the TL spine. If mandatory, surgical decompression can be carried out in the usual fashion form posterior as well. Modern percutaneous pedicle screw and rod constructs are available as an option for less-invasive surgery and lower surgical comorbidities.37 From a mechanical design standpoint, usually monoaxial pedicle screw systems are recommended to achieve better fracture reduction and obtain higher intrinsic construct stability than polyaxial pedicle screw systems would inherently allow for. Biomechanically, pedicle screw rod systems are favorable because they can better counteract high mechanical stresses with known screw loosening rates of up to 15%. For the same reason, we strongly recommended to extent the instrumentation up to a minimum of 2 levels above and below the level of injury.1,36 In recent prospective studies the prevalence of osteoporosis was about 25% and thereby lower than originally assumed.38 In cases with diminished bone quality (osteoporosis, osteopenia), the load should be distributed to a higher number of screws and/or combined with a cement augmentation as well. In the literature, a rising number of percutaneous procedures with good clinical results for minimally invasive interventions and sufficient stability were reported.37,39 Given the large numbers of other medical comorbidities in patients with ankylosing spine fractures, percutaneous surgical technique seems highly attractive. Moussallem and coworkers showed lower blood loss, shorter operative times, decreased need for blood transfusion, shorter hospitalization time, and a lower perioperative complication rate.40
Under the circumstance of an insufficient fracture reduction, for example, presence of a remaining larger fracture gap after the posterior instrumentation, a combined posterior-anterior instrumentation can help avoid the elevated risks of an early implant failure or pseudarthrosis. Expandable cage systems in combination with anterior angular stable plating systems are recommended to achieve the highest possible stability. Technically demanding open or closed wedge osteotomy with the intent to correct preexisting kyphotic deformities are not routinely recommended in the acute spine fracture care setting, because these procedures will further destabilize the spine and are associated with higher complication rates (pseudarthrosis and implant failure). In presence of gross preexisting deformities, fracture fixation and simultaneous surgical deformity correction can be considered. Risks and benefits of any additional surgical measures to reduce and stabilize the spine, for example, osteotomies for kyphosis correction, must be discussed with the patient.
Fractures of the ankylosed spine can present with a wide range of complications. Aortic dissection, aortic pseudoaneurysm, and tracheal or esophageal ruptures were reported and are associated to a high mortality rate.34 General complications are elevated numbers of wound healing problems and infection, venous thrombosis and lung embolism, pneumonia, and respiratory insufficiency. Nevertheless, the overall complication and mortality rates in the nonoperative patient group are even higher compared to the operative treatment.34
Iatrogenic Spine Fractures in Patients With ASD After Patient Positioning Following Routine, Elective (Nonspinal) Surgeries
When searching PubMed with the MESH terms “patient,” “positioning,” “spine fracture,” and “iatrogenic complication” no item was found as on the search date.
On the contrary, it has come to the authors’ attention that there are several patients sustaining iatrogenic spinal fractures having undergone routine, elective, nonspinal surgical procedures without any trauma or fall after the surgery.
For the first time, we report “patient positioning” as a cause for iatrogenic spinal fractures in ASD patients.
In the following we will present a representative iatrogenic spine fracture case that once again should alert everyone involved in the treatment of patients with underlying ASD, especially in the elderly with diminished bone quality.
The case is an iatrogenic unstable thoracolumbar vertebral hyperextension fracture without neurologic deficits (AOSpine TL classification: T12B3, N0, M2) in a geriatric patient with DISH with surgical management of a symptomatic cholelithiasis following a laparoscopic cholecystectomy. This 84-year-old woman was schedule for a routine endoscopic cholecystectomy by general surgery at an outside institution. Besides the symptomatic cholecystolithiasis her past medical history included a decompensated cardiac insufficiency with pleural, pericardial effusions, mitral insufficiency, coronary artery disease, chronic atrial fibrillations, and osteoporosis. The patient was placed in a supine position in the usual fashion and underwent an otherwise uneventful routine laparoscopic cholecystectomy.
Postoperatively the patient immediately complained of severe back pain but required attention and medical treatment in the intensive care unit for other reasons. On the second postoperative day, conventional radiographs and subsequently CT imaging of the thoracolumbar spine were obtained, which revealed an iatrogenic T12 hyperextension fracture-dislocation with an ankylosed spine (Figure 5). Spine surgery was consulted. A repeat CT scan demonstrated progressive anterior widening with a noncontiguous spine fracture distraction through the T12 vertebral body, confirming gross instability of the injury in an ankylosed DISH spine (Figure 5C).
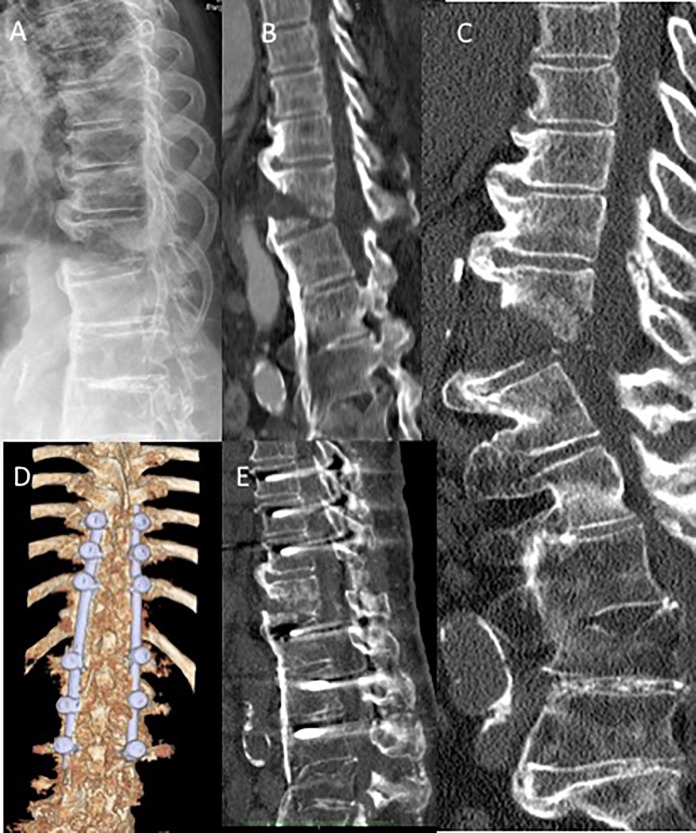
Figure 5.
Case 4: DISH patient with iatrogenic T12 hyperextension injury (AOSpine T12 B3, N0, M2) following laparoscopic cholecystectomy. (A) Lateral X-ray of TL spine and (B) sagittal CT image 2 days after laparoscopic cholecystectomy; (C) Repeat sagittal CT image 15 days after laparoscopic cholecystectomy, demonstrating further displacement of the T12 vertebral body fracture dislocation; (D, E) Open reduction internal fixation with monoaxial screws and rods 3 levels above and below.
Because of her advanced age, medical comorbidities, and slow recovery from the index surgery, transfer to the authors’ institution for spine fracture treatment had to be postponed not before the 22nd postoperative day. Open posterior decompression (T11-12 laminectomy) and fusion was obtained with a monoaxial pedicle screw-rod instrumentation extending 3 levels above and below (T9 to L3) the level of injury. Postoperative CT images revealed an improved realignment at the TL junction without bony spinal canal encroachment (Figure 5D and E). The remaining postoperative course was uneventful except for the presence of an arrhythmia, treated with the implantation of a cardiac pacemaker.
The patient was transferred back to the referring institution for rehabilitation on the 13th postoperative day. She remained neurologically intact throughout her treatment and was ambulating with assistance at the day of her discharge.
Discussion
From a demographic standpoint, an increasing numbers of elderly patients with ASDs will be seen requiring special attention in spine fracture care.
The susceptibility to sustain a spinal fracture even after trivial trauma in ASD patients, such as DISH and AS, has been described in the literature.41 These particular spinal fractures usually follow low-energy trauma, seemingly trivial injuries, and in a majority of cases are accompanied by spinal cord injuries.41 Delayed diagnosis and treatment can cause secondary neurological deterioration because of increasing fracture dislocation and instability with long lever-arms in an otherwise stiffened spine. Therefore, whole spine CT scan or MRI is recommended for clearance of the spine, even after identification of an injured segment. The frequency of multilevel injuries is increased in patients with ankylosing disorders. The behavior of spine fracture in ASD can be somehow compared to those of diaphyseal long bone fractures and should be treated as such. Special care and consideration for closed reduction, patient positioning, and obtaining surgical access is mandatory.
At the cervical spine combined anterior-posterior instrumentations demonstrated better stability and outcomes in different studies.34 At the TL level, fracture fixation usually requires long posterior pedicle screw instrumentations with or without decompression and cement augmentation. Additional anterior instrumentation can be considered if there is a remaining anterior fracture gap besides previous posterior interventions. Technically advanced percutaneous instrumentation techniques and perforated pedicle screws allowing for easy intraoperative cement augmentation are promising in cases with diminished bone quality. They might further help lower the overall increased risks for spine fracture patients with ASD when compared to the general spine trauma population as well as the associated risks with open surgery (intraoperative blood loss, wound healing problems, infection rates).27
Until now, complication rates and elevated mortality rates still remain significantly higher than in the usual spine trauma patient population without preexisting bony abnormalities.21 The complexity of fracture treatment in patients with an ASD renders the necessity for standardized diagnostic pathways, fracture classification, and surgical intervention for mostly mechanically unstable fractures without any delay. Internal fixation offers superior means of reduction, decompression of neural elements, maintained stability during bone healing, and faster sufficient patient mobilization than nonoperative treatment.
Few case reports of iatrogenic spinal fractures in patients with AS combined with severe spinal deformity following orthopedic42 and laparoscopic procedures43 have been published. Lessons learned from the presented geriatric DISH patient that luckily remained neurologically intact following a routine endoscopic cholecystectomy was that a significant delay of treatment must be avoided by immediately ruling out spinal fractures if there is any complaint or new onset of backache after the surgical intervention.
This case confirms Chowbey and coworkers’ finding that a marked kyphoscoliosis with a fixed rigidity deformity should be considered a contraindication for laparoscopic surgery as the altered body habitus can prohibit routine positioning and surgical access in these patients.43 Obtaining preoperative conventional radiographs could easily confirm the diagnosis of DISH and should trigger appropriate precautions.5 During the transfer of the intubated patients into the supine positioning any excessive hyperextension of the spine should be strictly avoided. Instead the patient should be placed and supported in the preexisting kyphotic position or habitus.
Key Points
Summary of the main recommendations for the treatment options for spinal fractures in the ankylosed spine:
ASD significantly increases the risk of unstable spinal fractures from potentially harmless, low-energy trauma. A delay in diagnosis and misinterpretation is common. Thus, a good understanding of the clinical presentation, fracture pathomorphology, altered biomechanical status, and understanding of the underlying pathology is essential.
A standardized diagnostic and therapeutic approach is recommended with imaging studies of the whole spine.
Unstable hyperextension, distraction, or translation injuries (AOSpine B3, M2/M3 type, or C, M2/M3 type fractures) require urgent surgical intervention and spine fracture fixation.
Closed reduction maneuvers and routine surgical approaches can be significantly compromised by preexisting kyphotic deformities and must be considered. For fracture fixation combined anterior-posterior or long posterior instrumentations are recommended. Minimally invasive surgical techniques (percutaneous screws, cement augmentation) are helpful to minimize the extent of instrumentation and approach-related surgical comorbidity.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Caron T, Bransford R, Nguyen Q, Agel J, Chapman J, Bellabarba C. Spine fractures in patients with ankylosing spinal disorders. Spine (Phila Pa 1976). 2010;35:E458–E464. doi:10.1097/BRS.0b013e3181cc764f. [DOI] [PubMed] [Google Scholar]
- 2. Forestier J, Lagier R. Ankylosing hyperostosis of the spine. Clin Orthop Relat Res. 1971;74:65–83. [PubMed] [Google Scholar]
- 3. Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology. 1976;119:559–568. doi:10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- 4. Forestier J, Rotes-Querol J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950;9:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cammisa M, De Serio A, Guglielmi G. Diffuse idiopathic skeletal hyperostosis. Eur J Radiol. 1998;27(suppl 1): S7–S11. [DOI] [PubMed] [Google Scholar]
- 6. Mazières B. Diffuse idiopathic skeletal hyperostosis (Forestier-Rotes-Querol disease): what’s new? Joint Bone Spine. 2013;80:466–470. doi:10.1016/j.jbspin.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 7. Holton KF, Denard PJ, Yoo JU, et al. Diffuse idiopathic skeletal hyperostosis and its relation to back pain among older men: the MrOS study. Semin Arthritis Rheum. 2011;41:131–138. doi:10.1016/j.semarthrit.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diederichs G, Engelken F, Marshall LM, et al. Diffuse idiopathic skeletal hyperostosis (DISH): relation to vertebral fractures and bone density. Osteoporos Int. 2011;22:1789–1797. doi:10.1007/s00198-010-1409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Resnick D, Dwosh IL, Goergen TG, et al. Clinical and radiographic abnormalities in ankylosing spondylitis: a comparison of men and women. Radiology. 1976;119:293–297. doi:10.1148/119.2.293. [DOI] [PubMed] [Google Scholar]
- 10. Appel H, Loddenkemper C, Miossec P. Rheumatoid arthritis and ankylosing spondylitis—pathology of acute inflammation. Clin Exp Rheumatol. 2009;27(4 suppl 55):S15–S19. [PubMed] [Google Scholar]
- 11. Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67. doi:10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12. Appel H, Loddenkemper C, Sieper J. Immunopathology of ankylosing spondylitis and other spondyloarthritides [in German]. Z Rheumatol. 2008;67:25–31. doi:10.1007/s00393-007-0242-9. [DOI] [PubMed] [Google Scholar]
- 13. Jang JH, Ward MM, Rucker AN, et al. Ankylosing spondylitis: patterns of radiographic involvement—a re-examination of accepted principles in a cohort of 769 patients. Radiology. 2011;258:192–198. doi:10.1148/radiol.10100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. [DOI] [PubMed] [Google Scholar]
- 15. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi:10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 16. Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016;68:282–298. doi:10.1002/acr.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smolen JS, Braun J, Dougados M, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis. 2014;73:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiltz U, Sieper J, Kellner H, et al. German Society for Rheumatology S3 guidelines on axial spondyloarthritis including Bechterew’s disease and early forms: 8.4 Pharmaceutical therapy, 8.5 Evaluation of therapy success of pharmaceutical measures [in German]. Z Rheumatol. 2014;73(suppl 2):78–96. doi:10.1007/s00393-014-1443-7. [DOI] [PubMed] [Google Scholar]
- 19. Jacobs WB, Fehlings MG. Ankylosing spondylitis and spinal cord injury: origin, incidence, management, and avoidance. Neurosurg Focus. 2008;24:E12 doi:10.3171/FOC/2008/24/1/E12. [DOI] [PubMed] [Google Scholar]
- 20. Briot K, Roux C. Inflammation, bone loss and fracture risk in spondyloarthritis. RMD Open. 2015;1:e000052 doi:10.1136/rmdopen-2015-000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18:145–156. doi:10.1007/s00586-008-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Debarge R, Demey G, Roussouly P. Radiological analysis of ankylosing spondylitis patients with severe kyphosis before and after pedicle subtraction osteotomy. Eur Spine J. 2010;19:65–70. doi:10.1007/s00586-009-1158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray HC, Elliott C, Barton SE, Murray A. Do patients with ankylosing spondylitis have poorer balance than normal subjects? Rheumatology (Oxford). 2000;39:497–500. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Xu H, Hu X, et al. Histopathological changes in supraspinous ligaments, ligamentum flava and paraspinal muscle tissues of patients with ankylosing spondylitis. Int J Rheum Dis. 2016;19:420–429. doi:10.1111/1756-185X.12305. [DOI] [PubMed] [Google Scholar]
- 25. Alaranta H, Luoto S, Konttinen YT. Traumatic spinal cord injury as a complication to ankylosing spondylitis. An extended report. Clin Exp Rheumatol. 2002;20:66–68. [PubMed] [Google Scholar]
- 26. Zdichavsky M, Blauth M, Knop C, Lange U, Krettek C, Bastian L. Ankylosing spondylitis. Therapy and complications of 34 spine fractures [in German]. Chirurg. 2005;76:967–976. doi:10.1007/s00104-005-1023-0. [DOI] [PubMed] [Google Scholar]
- 27. Leone A, Marino M, Dell’Atti C, Zecchi V, Magarelli N, Colosimo C. Spinal fractures in patients with ankylosing spondylitis. Rheumatol Int. 2016;36:1335–1346. doi:10.1007/s00296-016-3524-1. [DOI] [PubMed] [Google Scholar]
- 28. Vaccaro AR, Koerner JD, Radcliff KE, et al. AOSpine subaxial cervical spine injury classification system. Eur Spine J. 2016;25:2173–2184. doi:10.1007/s00586-015-3831-3. [DOI] [PubMed] [Google Scholar]
- 29. Vaccaro AR, Oner C, Kepler CK, et al. AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976). 2013;38:2028–2037. doi:10.1097/BRS.0b013e3182a8a381. [DOI] [PubMed] [Google Scholar]
- 30. Charles YP, Buy X, Gangi A, Steib JP. Fracture in ankylosing spondylitis after minor trauma: radiological pitfalls and treatment by percutaneous instrumentation. A case report. Orthop Traumatol Surg Res. 2013;99:115–119. doi:10.1016/j.otsr.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 31. Finkelstein JA, Chapman JR, Mirza S. Occult vertebral fractures in ankylosing spondylitis. Spinal Cord. 1999;37:444–447. [DOI] [PubMed] [Google Scholar]
- 32. Vosse D, van der Heijde D, Landewe R, et al. Determinants of hyperkyphosis in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:770–774. doi:10.1136/ard.2005.044081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montala N, Juanola X, Collantes E, et al. Prevalence of vertebral fractures by semiautomated morphometry in patients with ankylosing spondylitis. J Rheumatol. 2011;38:893–897. doi:10.3899/jrheum.100851. [DOI] [PubMed] [Google Scholar]
- 34. Westerveld LA, van Bemmel JC, Dhert WJA, Oner FC, Verlaan JJ. Clinical outcome after traumatic spinal fractures in patients with ankylosing spinal disorders compared with control patients. Spine J. 2014;14:729–740. doi:10.1016/j.spinee.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 35. Paley D, Schwartz M, Cooper P, Harris WR, Levine AM. Fractures of the spine in diffuse idiopathic skeletal hyperostosis. Clin Orthop Relat Res. 1991;267:22–32. [PubMed] [Google Scholar]
- 36. Balling H, Weckbach A. Hyperextension injuries of the thoracolumbar spine in diffuse idiopathic skeletal hyperostosis. Spine (Phila Pa 1976). 2015;40:E61–E67. doi:10.1097/BRS.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 37. Krüger A, Frink M, Oberkircher L, El-Zayat BF, Ruchholtz S, Lechler P. Percutaneous dorsal instrumentation for thoracolumbar extension-distraction fractures in patients with ankylosing spinal disorders: a case series. Spine J. 2014;14:2897–2904. doi:10.1016/j.spinee.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 38. Klingberg E, Nurkkala M, Carlsten H, Forsblad-d’Elia H. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol. 2014;41:1349–1356. doi:10.3899/jrheum.131199. [DOI] [PubMed] [Google Scholar]
- 39. Nayak NR, Pisapia JM, Abdullah KG, Schuster JM. Minimally invasive surgery for traumatic fractures in ankylosing spinal diseases. Global Spine J. 2015;5:266–273. doi:10.1055/s-0034-1397341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moussallem CD, McCutcheon BA, Clarke MJ, et al. Perioperative complications in open versus percutaneous treatment of spinal fractures in patients with an ankylosed spine. J Clin Neurosci. 2016;30:88–92. doi:10.1016/j.jocn.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 41. Hendrix R, Melany M, Miller F, Rogers LF. Fracture of the spine in patients with ankylosis due to diffuse skeletal hyperostosis: clinical and imaging findings. AJR Am J Roentgenol. 1994;162:899–904. [DOI] [PubMed] [Google Scholar]
- 42. Danish SF, Wilden JA, Schuster J. Iatrogenic paraplegia in 2 morbidly obese patients with ankylosing spondylitis undergoing total hip arthroplasty. J Neurosurg Spine. 2008;8:80–83. doi:10.3171/SPI-08/01/080. [DOI] [PubMed] [Google Scholar]
- 43. Chowbey P, Panse R, Khullar R, et al. Laparoscopic cholecystectomy in a patient with ankylosing spondylitis with severe spinal deformity. Surg Laparosc Endosc Percutan Tech. 2005;15:234–237. [DOI] [PubMed] [Google Scholar]