Abstract
Objective:
Animal studies demonstrated that glucagon-like peptide-1 receptor agonists reduce myocardial necrosis following regional ischaemia induction. This effect may improve cardiovascular outcomes after myocardial infarction. Risk of cardiovascular death or hospitalisation for heart failure after myocardial infarction was evaluated in patients with type 2 diabetes at high cardiovascular risk in the LEADER trial.
Methods:
Data from patients randomised to liraglutide or placebo, in addition to standard of care, in Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) (NCT01179048) were analysed post hoc. Cox regression, with myocardial infarction as a time-dependent covariate, was used to analyse time from randomisation to a composite of cardiovascular death or hospitalisation for heart failure.
Results:
Patients who experienced myocardial infarction had a sevenfold higher risk of the composite endpoint (with myocardial infarction: n = 148, 25.0%; without myocardial infarction: n = 716, 8.2%; hazard ratio: 7.0; 95% confidence interval: 5.8, 8.4). The risk of the composite endpoint after myocardial infarction was not significantly lower in the liraglutide group (n = 63, 23.0%) compared with placebo (n = 85, 26.7%; hazard ratio: 0.91; 95% confidence interval: 0.66, 1.26).
Conclusion:
The data demonstrated that having myocardial infarction significantly increased the risk of subsequent cardiovascular death or hospitalisation for heart failure. However, we did not find evidence for a reduced risk in these cardiovascular outcomes following myocardial infarction in patients treated with liraglutide versus placebo.
Keywords: Glucagon-like peptide-1 receptor agonists, cardiovascular outcomes, myocardial infarction, cardiovascular death, heart failure
Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) are used in patients with type 2 diabetes (T2D) to reduce blood glucose with low risk of hypoglycaemia1,2 and they also lower systolic blood pressure and body weight.1,2 Furthermore, the long-acting GLP-1RAs liraglutide (once daily)3 and semaglutide (once weekly)4 reduce the risk of cardiovascular (CV) events [measured using a composite of myocardial infarction (MI), stroke and CV death] in patients with T2D at high risk of CV events (post hoc analysis for semaglutide).4 Many biological actions are elicited by GLP-1RAs, and it is unknown if CV event prevention is due to a favourable influence on diabetes-related parameters [glycated haemoglobin (HbA1c), body weight, blood pressure, lipids], direct action on the heart and/or blood vessels or a combination of these.5
Multiple CV effects of GLP-1 receptor stimulation have been demonstrated,5 including a reduction in myocardial necrosis size after experimentally induced acute MI in rats,6 mice7 and pigs.8 These effects were evident with native GLP-16 and GLP-1RAs (exenatide8 and liraglutide).7 Furthermore, exenatide preserved left ventricular function in pigs,8 liraglutide improved survival post-acute MI in mice7 and cardiac function improved in humans with both GLP-1RAs when administered acutely.9,10
Whether these mechanisms apply to patients with T2D treated chronically with GLP-1RAs is unknown. We hypothesise that a potential reduction in necrosis after spontaneous MI leads to reduced post-MI (CV) mortality and/or prevents hospitalisation for heart failure (HHF). To explore this potential association, we evaluated follow-up data from patients in the LEADER trial who experienced an MI during the trial.
Methods
This post hoc analysis used data from patients randomised to liraglutide (up to 1.8 mg/day) or placebo (both in addition to standard of care) in LEADER (NCT01179048), a placebo-controlled, double-blind, CV outcomes trial, in 9340 patients with T2D and high risk of CV events.3 The trial duration was 3.5–5 years,3 allowing follow-up beyond primary CV events and monitoring of other safety events. Ethical approval was obtained at each study site and patients provided written informed consent.3
All potential MIs, HHF and CV deaths were adjudicated by an external event adjudication committee (EAC). There were four pre-defined ways to identify events for adjudication: by site investigators, by central electrocardiogram readers, by the EAC or external contract research organisation during review of documents submitted for another event, and through pre-defined Medical Dictionary for Regulatory Activities searches among all adverse events, performed by the sponsor. All first MIs confirmed by adjudication (fatal and non-fatal) were included in this analysis. MI was classified as fatal if assessed by the EAC as precipitating a subsequent CV death.
The main endpoint was time from randomisation to first occurrence of a composite of EAC-confirmed CV death or HHF, analysed using Cox regression for all randomised patients (full analysis set), with treatment as a fixed covariate and MI as a time-dependent covariate. At the time of a first MI, a patient’s status changed from ‘non-MI exposed’ to ‘MI-exposed’ for the rest of the trial. An interaction term between treatment and MI was included. The two individual composite components were also analysed independently of each other. Patients without events were censored at time of death or last follow-up.
As per the protocol, patients were allowed to discontinue and resume trial treatment if advised by the investigator, and sensitivity analyses accounted for this. These included patients who had at least 30 days of consecutive treatment prior to the first MI and using exposure to trial drug on/off as an additional time-dependent covariate.
Results
In total, 292 patients treated with liraglutide and 339 with placebo experienced an MI during LEADER. Baseline characteristics were balanced between the two groups, except for: prior coronary artery bypass graft (CABG; liraglutide: 30.8%, placebo: 21.5%, p = 0.008); peripheral arterial disease in lower extremities (liraglutide: 9.9%, placebo: 17.7%, p = 0.005); and >50% stenosis of coronary, carotid or other arteries (liraglutide: 33.2%, placebo: 41.0%, p = 0.044) (Tables S1 and S2). There was a numerical difference in the proportion of patients who underwent a percutaneous coronary intervention (liraglutide: 47.6%, placebo: 40.4%, p = 0.07; Table S2).
Irrespective of treatment group, patients who experienced an MI during the trial had a sevenfold higher risk of the composite endpoint (CV death or HHF; n = 148, 25.0%) versus those without MI during the trial [n = 716, 8.2%; hazard ratio (HR): 7.0; 95% confidence interval (CI): 5.8, 8.4; Figure S1]. The corresponding HRs were 6.3 (95% CI: 5.0, 7.9) for CV death and 8.2 (95% CI: 6.5, 10.5) for HHF (Figure S1).
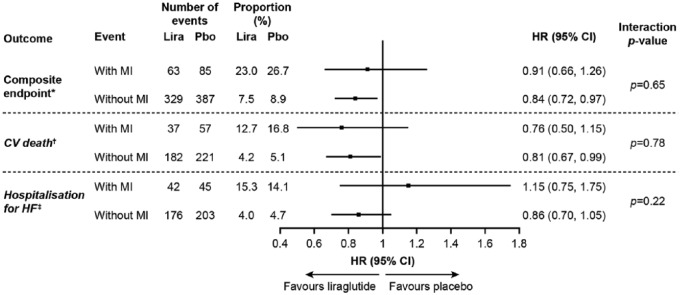
For the total trial population, the risk for the composite endpoint was reduced for liraglutide versus placebo; HR: 0.82; 95% CI: 0.72, 0.94 (see footnote in Figure 1). There was no significant difference for the risk of the composite endpoint after MI (liraglutide: 23.0%; placebo: 26.7%; HR: 0.91; 95% CI: 0.66, 1.26) and of the two individual components (Figure 1). There was no interaction between treatments and MI for these three endpoints (interaction p-values: not significant; Figure 1).
Figure 1.
Risk of CV events (composite endpoint of CV death and hospitalisation for heart failure) among patients treated with liraglutide or placebo analysed by MI as a time-dependent dichotomous variable.
*Although 292 patients in the liraglutide group and 339 patients in the placebo group experienced an MI during the trial, 274 and 319 patients, respectively, were included in this time-dependent analysis. This was because 18 and 20 patients were hospitalised for HF before the occurrence of MI in the liraglutide and placebo group, respectively. HR for first occurrence of hospitalisation for HF/CV death (FAS): 0.82 (95% CI: 0.72, 0.94; p = 0.005).
†HR for CV death in the total population (FAS): 0.78 (95% CI: 0.66, 0.93; p = 0.007).3
‡HR for hospitalisation for HF in the total population (FAS): 0.87 (95% CI: 0.73, 1.05; p = 0.14).3
CI: confidence interval; CV: cardiovascular; FAS: full analysis set; HF: heart failure; HR: hazard ratio of liraglutide/placebo; Lira: liraglutide; MI: myocardial infarction; Pbo: placebo.
Similar results were obtained if patients had at least 30 days’ consecutive treatment prior to MI and when analysed according to exposure to trial drug (on/off; data not shown). Overall, mean time on randomised treatment was 84% (liraglutide) and 83% (placebo).3
Discussion
Animal studies found that native GLP-1 and GLP-1RAs reduce myocardial necrosis following standardised induction of regional ischaemia.6–8 Proof-of-concept studies employing liraglutide or exenatide seem to support similar effects in patients with acute MI, when administered acutely at hospital admission.10–12 Some degree of protection from ischaemic damage post-MI was therefore expected in the LEADER trial. Since LEADER did not examine structural ischaemic damage post-MI, our approach was to examine clinical consequences of potential differences in necrotic area size, namely, CV death and HHF.
In this analysis, as expected, data demonstrated that an MI significantly increased the risk of subsequent CV death or HHF. Although the risk of this composite following an MI appeared lower with liraglutide versus placebo, the difference was not significant. Importantly, even though a specific cardioprotective effect following MI was not evident here, the data did not support a differential CV benefit of liraglutide in those with versus without MI (i.e. non-significant interaction p-value). In addition, a cardioprotective effect was evident in the overall trial population, where liraglutide significantly reduced the risk of the composite endpoint and of CV death alone.3
Several factors could explain why our hypothesis was not confirmed. Notably, this post hoc analysis included patients who experienced an MI during the trial. Accordingly, the influence of confounding factors removed by randomisation at baseline per se may have been enhanced, as evident from the significant differences in several of the CV history baseline characteristics between the two cohorts with MI. Notably, significantly more patients treated with liraglutide who experienced an MI had a CABG performed prior to baseline, but significantly fewer had peripheral arterial disease in lower extremities at this time point, both compared with those treated with placebo. It is difficult to assess the impact of these differences on subsequent MI/CV events. Also, fewer MI events occurred in liraglutide-treated patients versus placebo, and patient characteristics at the time of first MI were not collected. Furthermore, the trial drug exposure pattern for patients after the first non-fatal MI may have differed from the exposure pattern in patients without MI.
LEADER was not designed to evaluate CV mortality or HHF following MI; therefore, these analyses may have been underpowered to evaluate our hypothesis. Moreover, the endpoints used to estimate clinical consequences of putatively different necrotic area sizes post-MI may not adequately represent or be sensitive enough to capture this. The endpoints chosen were considered most likely to be sensitive to changes in necrotic area size. If a prospective study was designed, other parameters could be considered, but the present analysis was limited by the data available.
Although neither treatment HR for the individual composite endpoint components was statistically significant, they appeared different from each other, with a ratio of CV death < 1 but a ratio of HHF > 1. We did not take into account competing risk in the HHF analyses and hence this difference (notwithstanding randomness alone) could be due to liraglutide reducing CV death risk versus placebo.3 Hence, the lower CV death risk with liraglutide may have impacted the risk of experiencing a more severe MI leading to subsequent HHF.
Other more fundamental/physiological reasons may explain why our hypothesis was not confirmed. For example, the impact of stimulating GLP-1 receptors during spontaneous MI in humans may differ from experimentally induced MI in other species. Although there was high trial drug exposure in LEADER,3 the liraglutide dose/regimen used was probably not equivalent to animal studies, where administration was acute at the time of MI. MIs observed in a clinical trial setting are likely more heterogeneous than those in experimental animal models. Considering these reasons, this post hoc analysis was limited in its ability to test our hypothesis.
Given the homogeneity of results from animal studies testing cardioprotection from ischaemic damage with GLP-1/GLP-1RAs,6–8 it may help to look for beneficial effects in dedicated, appropriately powered studies, using direct measures of ischaemic necrosis in humans. Only such studies could prove if this potentially important GLP-1RA mechanism of action functions in humans; whether it is of clinical significance needs to be investigated in large outcome studies using hard endpoints.
Supplemental Material
Supplemental material, DVR_Supplementary_Material for Cardiovascular outcomes in patients who experienced a myocardial infarction while treated with liraglutide versus placebo in the LEADER trial by Michael A Nauck, Karen Tornøe, Søren Rasmussen, Marianne Bach Treppendahl and Steven P Marso in Diabetes & Vascular Disease Research
Footnotes
Declaration of conflicting interests: M.A.N. is on advisory boards or consulted for AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Fractyl, GlaxoSmithKline, Menarini/Berlin-Chemie, Merck, Sharp & Dohme and Novo Nordisk; received grant support from Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Merck, Sharp & Dohme, Novartis Pharma and Novo Nordisk; served on the speakers’ bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Menarini/Berlin-Chemie, Merck, Sharp & Dohme and Novo Nordisk. K.T., S.R. and M.B.T. are employees of and stockholders in Novo Nordisk. S.P.M. received consulting fees from Novo Nordisk and St Jude Medical and research support from Novo Nordisk, Terumo, The Medicines Company, AstraZeneca and Bristol Myers-Squibb.
Funding: Editorial support was provided by Gillian Groeger, PhD, and Izabel James, MBBS, of Watermeadow Medical, an Ashfield company, part of UDG Healthcare PLC, funded by Novo Nordisk.
Supplementary Material: Supplementary material (Table S1, S2 and Figure S1) is available online.
References
- 1. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 2. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016; 18: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 5. Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab 2016; 24: 15–30. [DOI] [PubMed] [Google Scholar]
- 6. Bose AK, Mocanu MM, Carr RD, et al. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 2005; 54: 146–151. [DOI] [PubMed] [Google Scholar]
- 7. Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009; 58: 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timmers L, Henriques JP, de Kleijn DP, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol 2009; 53: 501–510. [DOI] [PubMed] [Google Scholar]
- 9. Chen WR, Chen YD, Tian F, et al. Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging 2016; 9: pii:e005146. [DOI] [PubMed] [Google Scholar]
- 10. Lønborg J, Vejlstrup N, Kelbaek H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012; 33: 1491–1499. [DOI] [PubMed] [Google Scholar]
- 11. Woo JS, Kim W, Ha SJ, et al. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol 2013; 33: 2252–2260. [DOI] [PubMed] [Google Scholar]
- 12. Bernink FJ, Timmers L, Diamant M, et al. Effect of additional treatment with EXenatide in patients with an acute myocardial infarction: the EXAMI study. Int J Cardiol 2013; 167: 289–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DVR_Supplementary_Material for Cardiovascular outcomes in patients who experienced a myocardial infarction while treated with liraglutide versus placebo in the LEADER trial by Michael A Nauck, Karen Tornøe, Søren Rasmussen, Marianne Bach Treppendahl and Steven P Marso in Diabetes & Vascular Disease Research