Abstract
Introduction
Chronic sinusitis (CRS) is phenotypically divided into inflammation with polyps (CRSwNP) or without polyps (CRSsNP).
Aim
To try to explain the reasons for isolated sinusitis by analysing medical history as well as anatomical and bacteriological data.
Material and methods
In 2016, endoscopic surgery was performed in 103 patients with CRSsNP within 6 months. The authors evaluated 28 patients with lesions in one sinus.
Results
The patients did not report any previous facial trauma, dental procedures, there were no odontogenic causes of the disease. They had not been treated with antibiotics within 30 days prior to admission to hospital. Ninety-seven bacterial strains were grown, of which 32 in patients with isolated nasal sinusitis. Statistical analysis has shown that there is a trend toward a statistically significant (p = 0.0868) relationship between the presence of Staphylococcus aureus and the type of inflammation.
Conclusions
There is an indication that the presence of Staphylococcus aureus is associated with isolated sinusitis, especially in women.
Keywords: chronic sinusitis without polyps, isolated sinusitis, Staphylococcus aureus
Introduction
Rhinosinusitis lasting more than 12 weeks with at least two associated symptoms such as nasal discharge, swelling of the mucous membrane lining the nose, pain in the face or impaired sense of smell is classified as chronic, which is set out in detail in EPOS 2012 (European Guidelines on rhinosinusitis and nasal polyps 2012) [1]. Chronic sinusitis (CRS) is phenotypically divided into inflammation with polyps (CRSwNP) or without polyps (CRSsNP).
Isolated sinusitis is often found incidentally in radiological diagnostics, and rarely causes clinical problems that require surgical intervention [2, 3]. It is believed that isolated chronic sinusitis may be a result of trauma, surgery, dental procedures, anatomic abnormalities of the nasal cavity or allergy [4].
Aim
The authors evaluated patients operated for CRSsNP with lesions in one or many sinuses, and the aim was to try to explain the reasons for isolated sinusitis by analysing medical history, anatomical and bacteriological data.
Material and methods
The study examined 103 patients with CRSsNP subjected to functional endoscopic sinus surgery (FESS) in the period from March to November 2016 in the Department of Laryngology and Laryngological Oncology in Katowice and the Department of Otorhinolaryngology and Oncology in Zabrze. The patients were qualified for surgery after performing a computed tomography (CT) scan and endoscopic examination of sinuses as well as obtaining medical history data, which allowed, in accordance with the EPOS 2012 guidelines, to qualify them for surgery. The extent of FESS depended on the location of lesions. None of the patients reported any previous facial trauma, dental procedures; there were no odontogenic causes of disease. They had not been treated with antibiotics within 30 days prior to admission to hospital. Bacteriological and, in justified cases, histopathological material was collected during FESS. A swab specimen was taken from the middle nasal meatus, on the side of inflammatory lesions. It was decided to collect material from the middle nasal meatus, the place where discharge flows from the maxillary sinus, frontal sinus, and ethmoidal cells.
The material was collected using a swab stick with Amies transport medium. Aerobic colony grew on sheep blood agar (Columbia agar) – Gram-positive bacteria, MacConkey medium (Gram-negative bacteria), Sabouraud medium (fungal growth), chocolate-based medium in an environment of increased carbon dioxide content (Neisseria and Haemophilus species). Anaerobic bacteria grew on Schaedler agar. Each culture was stored at 35–37°C for 24–48 h, with the exception of fungi and bacteria on Schaedler agar which were stored for 7 days. During the cultivation of culture, the material that remained on the stick was stored for 7 days in a multiplying medium (cardio-cerebral broth). Identification and drug susceptibility were evaluated using the Vitek 2 Compact system.
Statistical analysis
The statistical analysis of the data was to assess whether the presence of some selected bacterial strains (in this case, Staphylococcus aureus) is associated with a specific type of inflammation (multi-sinus or isolated). The χ2 test of independence was used for this purpose. The data were organized in order to create a four-fold contingency table. Thus, the variable “strain type” was converted to the dichotomous form, where Staphylococcus aureus (S. aureus) was compared with other bacterial strains.
Results
The study included 103 patients, 54 women and 49 men, ranging in age from 17 to 78 years. In this group, there were 28 cases of isolated sinusitis, 17 women and 11 men aged 17 to 78 years. The group of patients with multi-sinusitis consisted of 32 women and 29 men aged 19 to 75 years. Nine men and 5 women from whom negative bacteriological cultures were obtained were excluded from the study. Allergic rhinitis to grass, trees, cats or mites was found in 9 patients. Two patients were affected by type II diabetes.
Isolated maxillary sinusitis was observed most frequently – in 24 cases. There were 2 cases of sphenoid sinusitis on the right side, one case of frontal sinusitis on the left side and one case of posterior ethmoidal sinusitis on the right side.
Two patients had nasal septum deviation toward the inflamed maxillary sinus. Pneumatic middle nasal concha (concha bullosa) was present in 1 case on the right side and in another case on the left side, always on the side of the inflamed maxillary sinus.
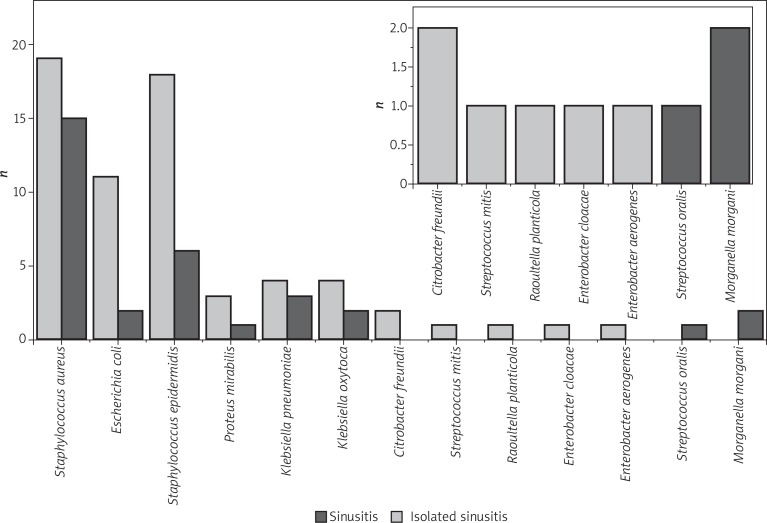
A total of 97 pathogens were cultured in all the examined patients, of which 32 in the case of isolated sinusitis. Two pathogens were cultured in 4 patients, and only one pathogen in the other 24. Figure 1 shows the distribution of the isolated bacterial flora.
Figure 1.
Distribution of bacterial flora in isolated sinusitis and multi-sinusitis
Among the 32 pathogens identified in the middle nasal meatus of the operated patients, S. aureus was present in 15 cases, and Staphylococcus epidermidis in 6. The other pathogens were Escherichia coli, Klebsiella oxytoca and pneumoniae, Streptococcus oralis, Proteus mirabilis and Morganella morganii. Staphylococcus aureus was isolated in 6 out of 9 patients with inhalant allergy.
No anaerobic bacteria or fungi were found.
In 3 cases, radiological images suggesting maxillary sinusitis masked the inverted papilloma confirmed histopathologically after surgery.
It has been found that there is a trend toward a statistically significant (p = 0.0868) relationship between the presence of S. aureus and the type of inflammation. There is an indication that the presence of S. aureus is associated with isolated sinusitis. In patients with isolated sinusitis, S. aureus occurred in 46.88%, while in patients with multi-sinusitis in 29.23%. It was decided to verify whether the described trend may be related to sex. It has been found that in women, the relationship between the number of S. aureus and the type of inflammation shows a trend towards significance (p = 0.0708), while in men the result is statistically insignificant (p = 0.4778). In women with isolated sinusitis, S. aureus accounted for 44.44% of all cases, and in those with multi-sinusitis, it represented 20.59%. In the case of men, S. aureus accounted for 50% and 38.71%, in the groups with isolated sinusitis and multi-sinusitis, respectively. Distributions of the number in each group and the interactions between the type of inflammation and the pathogen are shown in Figure 2.
Figure 2.
Distribution of the patients in each group and the interactions between the type of sinusitis and the pathogen
Discussion
Isolated sinusitis is more frequently studied in the case of CRSwNP due to a greater risk of carcinoma and inverted papilloma than in CRSsNP [5]. Besides maxillary sinusitis of odontogenic origin, aetiology of isolated sinusitis without polyps is unclear.
Frontal sinusitis is often a consequence of inflammatory processes in the anterior ethmoidal cells, whereas isolated sinusitis is rare. Significant pneumatisation of ethmoidal cells, agger nasi, superior attachment of the uncinate process, small sagittal diameter of recess are observed, and the frontal sinus is then secondarily altered to anatomical and inflammatory changes within the frontal recess [6, 7]. Landsberg and Friedman [6] observed isolated frontal sinusitis in 11 patients complaining of headaches, nasal discharge. Three of them had polyps, another 3 had pneumatic agger nasi, and 1 patient had type II frontal cells.
The causes of sphenoid sinusitis in a group of 50 patients studied by Friedman et al. [8] were fungi in 20%, carcinomas in 16%, mucocele in 12%, and chronic inflammation in 38% of all cases. Streptococcus and S. aureus are considered the most common causes of acute sphenoid sinusitis, whereas the role of Gram-negative and anaerobic bacteria is less important [9, 10]. A case of epidural abscess, being a complication of isolated chronic sphenoid sinusitis was described by Bielecki et al. [11]. The sample of fluid taken from the abscess showed no growth of bacteria or fungi. Likewise, there was no growth of bacteria in the case of sphenoid sinusitis complicated with sixth nerve palsy [12]. Massoubre et al. [13] analysed the bacterial flora of 79 patients with isolated chronic sphenoid sinusitis operated on via transnasal approach. Nonspecific inflammation without growth of pathological flora was found in 47 (59.5%) patients. There was fungus ball in 19 (24%) cases and bacterial flora was isolated in 13 (16%) patients. The main pathogens were Pseudomonas aeruginosa and S. aureus.
The study of the aetiology of isolated chronic maxillary sinusitis, was presented by Troeltzsch et al. [4] based on 174 cases. He found that 75% of all cases were of odontogenic origin, of which 64% were iatrogenic. Non-odontogenic cysts accounted for 6.3%, injuries – 10.3%, and carcinomas – 1.1%. Allergic rhinitis was reported by 3% of subjects. Highly diverse flora defined as non-specific flora of the oral cavity was isolated in 86.8%, Streptococcus spp., Enterococcus faecalis, Haemophilus influenzae and Escherichia coli were present in approx. 6%.
Staphylococcus colonization was prevalent in 64% of patients with CRSwNP and 87.5% in patients with asthma and hypersensitivity to aspirin compared to 33.3% in CRSsNP [14–17]. This preponderance of S. aureus reflects the specific inhibition of innate immunity against Staphylococcus in these Th2-mediated diseases similar to what is observed in atopic dermatitis [18].
Studies on the toll like receptors of the respiratory epithelium (TLR) demonstrated that TLR-2 binds to Gram-positive bacteria and certain fungi, TLR-3 to viruses, and TLR-4 to Gram-negative bacteria. An increased expression of TLR-2 and TLR-4 was found in CRSsNP [19]. The interaction between S. aureus and endothelial cells results from the connections between fibronectin-binding proteins (FnBPs) on bacteria and fibronectin and heat shock proteins 60 (Hsp60) [20]. Staphylococcus aureus may also bind to integrin α5β1 through fibronectin-binding proteins and thus hide from the immune system in endothelial cells [20–22].
Staphylococcal enterotoxins are superantigens and have the ability to stimulate a number of T cell clones that have a T-cell receptor (TCR). In addition, they atypically bind to MHC class II and class I molecules (enterotoxin B). Staphylococcal protein A is a B-cell superantigen and binds to immunoglobulin receptors outside the classical antigen-binding site [20].
Staphylococcal enterotoxin (SEA, SEB) is adjacent to the epithelial barrier. Once T cells are activated, they produce interleukins IL-4, IL-5, IL-13, eotaxins and many other inflammation mediators that lead to severe eosinophilic inflammation and local production of IgE. Moreover, direct action of superantigen on B cells, epithelial cells and eosinophils maintains this inflammation [21]. Staphylococcal toxin can change sensitivity of the steroid receptor beta, which limits the effectiveness of anti-inflammatory therapy [23].
Bachert et al. [24] found SEA and SEB-specific IgE in 50% of bilateral eosinophilic nasal polyps suggesting a common pathophysiologic mechanism in atopic dermatitis, CRSwNP and asthma.
The study by Kohanski and Lane [25] showed an increase in sensitivity of the mucosa cells of the nasal cavity and sinuses in patients with CRSsNP, which may be due to pathological changes in the innate immune response, which contributes to maintaining the inflammation secondarily to exposure to bacterial agents.
The frequent presence of S. aureus in isolated sinusitis without polyps is quite puzzling. In the examined material, in the patients with isolated sinusitis, S. aureus occurred in 46.88%, while in the patients with multi-sinusitis in 29.32%. Perhaps it is the effect of impaired microbiome of sinuses after past viral infections and antibiotic therapies, and hence, secondary proliferation of pathogenic flora, which is normally inhibited by commensals [26, 27].
There are conflicting reports in the literature concerning the significance of nasal cavity anatomy differences such as nasal septum deviation, concha bullosa, big agger nasi or the presence of Haller cells in the aetiology of chronic sinusitis [19]. We have found abnormalities in the anatomy of the nasal septum or middle nasal concha in four cases, which could have contributed to the formation of inflammatory lesions in the sinus, which seems to be less important in the aetiology of the disease compared with the bacteriological image. We believe that the problem of isolated sinusitis without polyps requires further observation.
Conclusions
It has been found that there is a trend toward a statistically significant (p = 0.0868) relationship between the presence of S. aureus and isolated sinusitis.
It seems that anatomical changes or allergy to inhalant allergens were not relevant to the development of the disease. Further research is needed to answer the question whether the frequent presence of coagulase-positive staphylococcus results from frequent antibiotic therapies, disruptions in the immune system, the effect of the external environment, or whether it is the primary cause of isolated sinusitis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Fokkens W, Lund V, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012;50:1–112. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Habesoglu T, Habesoglu M, Surmeli M, et al. Unilateral sinonasal symptoms. J Craniofac Surg. 2010;21:2019–22. doi: 10.1097/SCS.0b013e3181f5389a. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto Y, Ikeda T, Yokoi H, et al. Association between odontogenic infections and unilateral sinus opacification. Auris Nasus Larynx. 2015;42:288–93. doi: 10.1016/j.anl.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Troeltzsch M, Christoph P, Markus T, et al. Etiology and clinical characteristics of symptomatic unilateral maxillary sinusitis: a review of 174 cases. J Craniomaxillofacial Surg. 2015;43:1522–9. doi: 10.1016/j.jcms.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Paz Silva M, Jayant M, Corey J, et al. Diagnostic algorithm for unilateral sinus disease: a 15-year retrospective review. Intern Forum Allergy Rhinol. 2015;5:590–596. doi: 10.1002/alr.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landsberg R, Friedman M. Operative techniques in otolaryngology. Head Neck Surg. 2006;17:184–8. [Google Scholar]
- 7.Landsberg R, Friedman M. A computer-assisted anatomical study of frontal recess. Laryngoscope. 2001;111:2125–30. doi: 10.1097/00005537-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Friedman A, Elatra P. Isolated sphenoid sinus disease: etiology and management. Otolaryngol Head Neck Surg. 2005;133:544–50. doi: 10.1016/j.otohns.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Marseglia C, Pageeila F, Licari A, et al. Acute isolated sphenoid sinusitis in children. Int J Pediatr Otorhinolaryngol. 2006;70:2027–31. doi: 10.1016/j.ijporl.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Unlu H, Aslan A, Goktan C, et al. The intracranial complication of acute isolated sphenoid sinusitis. Auris Nasus Larynx. 2002;29:69–71. doi: 10.1016/s0385-8146(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 11.Bielecki I, Gierlotka A, Cofała M. An intracranial complication of acute isolated sphenoid sinusitis. Int J Pediatr Otorhinolaryngol. 2013;8:125–127. [Google Scholar]
- 12.Mehmet A, Kaytaz A, Tuskan K, et al. Isolated sphenoid sinusitis presenting with unilateral VI nerve palsy. Int J Pediatr Otorhinolaryngol. 2004;68:507–510. doi: 10.1016/j.ijporl.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Massoubre J, Saroul N, Vokwely JE, et al. Results of transnasal transostial sphenoidotomy in 79 cases of chronic sphenoidsinusitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:231–6. doi: 10.1016/j.anorl.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Hamilos D. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;133:640–53. doi: 10.1016/j.jaci.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brook L. Microbiology of chronic rhinosinusitis. Eur J Clin Microbiol Infect Dis. 2016;35:1059–68. doi: 10.1007/s10096-016-2640-x. [DOI] [PubMed] [Google Scholar]
- 16.Thanasumpun T, Batra P. Endoscopically-derived bacterial cultures in chronic rhinosinusitis: a systematic review. Am J Otolaryngol. 2015;36:686–91. doi: 10.1016/j.amjoto.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Boase S, Foreman A, Cleland E, et al. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis. 2013;13:210. doi: 10.1186/1471-2334-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy J, Borish L. Chronic rhinosinusitis and antibiotics: the good, the bad, and the ugly. Am J Rhinol Allergy. 2013;27:467–72. doi: 10.2500/ajra.2013.27.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anselmo-Lima W, Sakano E. Rhinosinusitis: evidence and experience. Braz J Otorhinolaryngol. 2015;81:1–49. doi: 10.1016/j.bjorl.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gołąb J, Jakóbisiak M, Lasek W, Stokłosa T. Immunologia. Warsaw: Wydawnictwo Naukowe PWN; 2014. [Google Scholar]
- 21.Zhang N, Gevaert P, Van Zele T, et al. An update on the impact of Staphylococcus aureus enterotoxins in chronic sinusitis with nasal polyposis. Rhinology. 2005;43:162–8. [PubMed] [Google Scholar]
- 22.Von Eiff C, Becker K, Metze D, et al. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier’s disease. Clin Infect Dis. 2001;32:321–5. doi: 10.1086/320519. [DOI] [PubMed] [Google Scholar]
- 23.Hauk P, Hamid Q, Chrousos G, et al. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000;105:782–7. doi: 10.1067/mai.2000.105807. [DOI] [PubMed] [Google Scholar]
- 24.Bachert C, Gevaert P, Holtappels G, et al. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–14. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 25.Kohanski M, Lane A. Sinonasal epithelial cell response to Staphylococcus aureus burden in chronic sinusitis. JAMA Otolaryngol Head Neck Surg. 2015;141:341–9. doi: 10.1001/jamaoto.2014.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam K, Schleimer R, Kem R. The etiology and pathogenesis of chronic rhinosinusitis: a review of current hypotheses. Curr Allergy Asthma Rep. 2015;15:41. doi: 10.1007/s11882-015-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abreu N, Nagalingam N, Song Y, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;115:1–9. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]