Abstract
Background
Adenomyosis is a cause of chronic pelvic pain in women of reproductive age. The aim of this study was to investigate the effects of subcutaneous etonogestrel implantation on adenomyosis.
Material/Methods
A clinical observational study included 17 women with adenomyosis who were treated with subcutaneous etonogestrel implants and followed-up for 12 months. Imaging and clinical observations were undertaken in the 17 patients at baseline (time 0), and at 3 months, 6 months, and 12 months following subcutaneous etonogestrel implantation. The following imaging and clinical findings were compared between baseline (time 0) and 12-month follow-up: menstrual bleeding pattern, dysmenorrhea, visual analog scale (VAS) pain score, uterine volume, serum cancer antigen 125 (CA125) levels, hemoglobin, follicle-stimulating hormone (FSH) levels, luteinizing hormone levels, serum estradiol levels, and any treatment side effects.
Results
All 17 patients treated with etonogestrel implants completed the 12-month follow-up, at which time, the mean hemoglobin level (127.08±2.56 g/L) was significantly higher compared with that at baseline (94.54±5.47 g/L; P<0.01); uterine volume, serum CA125, and VAS score for dysmenorrhea at 12 months (118.03±12.83 cm3, 34.58±9.66 U/mL, and 1.45±0.35, respectively) were significantly lower when compared with baseline (198.53±39.47 cm3, 100.41±49.89 U/mL, and 7.62±0.74, respectively) (P<0.01, for all). However, changes in bleeding pattern and amenorrhoea occurred after treatment in some women.
Conclusions
Subcutaneous etonogestrel was effective in reducing some symptoms and signs of adenomyosis, including dysmenorrhea, anemia, serum CA125, and uterine volume.
MeSH Keywords: Adenomyosis, Clinical Medicine, Dysmenorrhea
Background
Chronic pelvic pain is common in women of reproductive age, with the main causes including adenomyosis, endometriosis, and pelvic inflammatory disease. Chronic pelvic pain syndromes may present clinically with lower abdominal pain or back pain and can cause loss of appetite, fatigue, and insomnia. Adenomyosis is a common benign disease of women of childbearing age, characterized by endometrial glands and stroma within the myometrium. The clinical manifestations of adenomyosis include increased menstrual flow, prolonged menstruation, and progressive dysmenorrhea, and the uterus is often uniformly enlarged, or contains localized nodules, and is considered to be a form of intra-uterine endometriosis [1,2]. Medical, or non-surgical, treatments for adenomyosis include the use of gonadotropin-releasing hormone (GnRH) agonists and analogs, and more recently, the use of the long-term contraceptive device, the etonogestrel subdermal implant, Implanon®.
Both etonogestrel and levonorgestrel prevent pregnancy by blocking ovulation [3,4]. The implantable etonogestrel contraceptive known as Implanon® is a subcutaneous progestogen-releasing device that contains 68 mg of etonogestrel that is slowly released for up to three years after implantation [5]. Etonogestrel implants have been approved for use in the US since 2006 and are on the list of essential medicines of the World Health Organization (WHO) as safe and effective use as a contraceptive [6]. Etonogestrel exerts its contraceptive effect by inhibiting the secretion of luteinizing hormone (LH), and inhibiting ovulation. Etonogestrel also increases the viscosity of the cervical mucus and reduces the thickness of the endometrium.
The mechanisms of the contraceptive effect of etonogestrel also suggest a potential application for the use of etonogestrel in the treatment of adenomyosis. Therefore, the aim of this preliminary observational clinical study was to investigate the effects of subcutaneous etonogestrel implantation on the uterine involvement and symptoms of adenomyosis.
Material and Methods
Patients
The study population included 17 adult women, recruited between March 2015 to June 2015, mean age, 40.29±3.80 years (range, 33–47 years), with a clinical history of dysmenorrhea for a mean duration of 6.29±2.37 years (range, 3–12 years). The inclusion criteria for this observational clinical study included were women >18 years-of-age who preferred to have medical treatment, rather than surgical treatment, for confirmed adenomyosis. Women were excluded from this study who were undergoing fertility treatment, had uterine fibroids, or pelvic endometriosis, or who had any clinical contraindications for treatment with etonogestrel.
Three of the 17 patients had a previously used an intra-uterine implant placement, but the device was expelled due to excessive menstrual flow; two of the patients included in the study had previously been unsuccessfully treated with gonadotropin-releasing hormone (GnRH) agonists and analogs but were not currently on medical treatment. All 17 patients had a degree of adenomyosis that would have made them suitable for surgical management, and all patients had normal liver function and renal function, without blood disease, gynecological cancer or breast cancer, or recent reproductive tract infection. A detailed consultation and physical screening were performed before subcutaneous etonogestrel implantation. The Ethics Committee of the Medical Faculty of the First Affiliated Hospital of Fujian Medical University Medical Ethics and Audit unit (Ref: 2014: 064) approved the study protocol. All the patients provided written informed consent to participate in the study.
Preparation and imaging before subcutaneous etonogestrel implantation
Before subcutaneous implantation of the etonogestrel subcutaneous device containing 68 mg of etonogestrel, all patients underwent color flow ultrasound imaging using a GE Voluson E8 color flow ultrasound machine (GE-E8 device) with the trans-abdominal transducer set at IC-5-9D (GE Healthcare) (Figure 1). On the third day from the end of the menstruation cycle, a examination was performed of each patient, undertaken by specialized medical personnel, to measure the uterine volume and blood supply to the myometrium and to evaluate the adenomyosis lesions. Peripheral venous blood was collected to measure the baseline levels of hemoglobin, serum cancer antigen 125 (CA125) levels, and female hormones, including follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels, on the third day of menstruation. Subcutaneous implantation was performed by trained clinical staff at between days 1–5 of the menstrual cycle, and implantation was confirmed to be successful for each study participant.
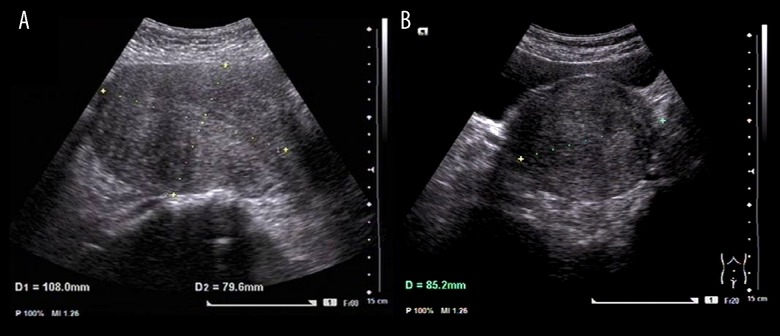
Figure 1.
Trans-abdominal color ultrasound imaging of the uterine corpus. Imaging was performed using a GE Voluson E8 color flow ultrasound machine (GE-E8 device), with the intracavity transducer set at IC-5-9D, including measurements of the uterine corpus length (L), width (W) and the anteroposterior diameter (AP). (A) The uterine length (L) and the anteroposterior diameter (AP) measurements are 108.0×79.6 mm. (B) The uterine width (W) measurement is 85.2 mm.
Patient follow-up
All patients were followed-up after subcutaneous etonogestrel implantation by trained specialized clinical personnel, using a specially designed chart for each woman. A specific phone number was used for follow-up consultations for all patients following implantation to facilitate timely feedback of clinical symptoms. The patients were followed-up at 3, 6, and 12 months after subcutaneous etonogestrel implantation.
The indicators noted on follow-up are summarized in Table 1, and included changes in bleeding pattern; severity of dysmenorrhea pain; hemoglobin measurements; serum CA125 levels; uterine volume, as measured by color flow ultrasound imaging (Figure 2); and female hormone levels, including FSH, LH, and estradiol, on days 3–5 of the menstrual cycle.
Table 1.
Definition of bleeding patterns.
| Bleeding patterns | Definition |
|---|---|
| Bleeding | Blood in the vaginal discharge, at least one sanitary pad or tampon is needed |
| Spotting | Blood in the vaginal discharge, no more than one sanitary pad or tampon is needed |
| No bleeding | No bleeding or spotting |
| Intermittent bleeding or spotting | Several days free of bleeding or spotting between days with bleeding or spotting |
| Amenorrhea | No bleeding or spotting within 90 days of reference period |
| Oligomenorrhea | Less than 3 times of bleeding or spotting within the 90 days of reference period, but not amenorrhea |
| Normal frequency | Three to 5 times of bleeding or spotting within the 90 days of reference period |
| Frequent bleeding | Six or more times of bleeding or spotting within the 90 days of reference period |
| Prolonged bleeding | Sustained bleeding or spotting for 14 days or more within the 90 days of reference period |
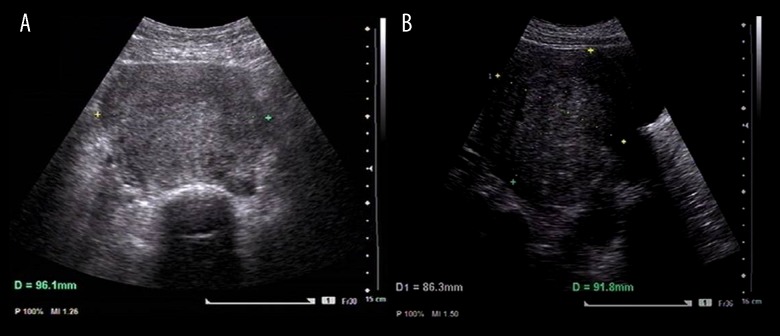
Figure 2.
Trans-abdominal color ultrasound imaging of the uterine corpus. Imaging was performed using a GE Voluson E8 color flow ultrasound machine (GE-E8 device), with the intracavity transducer set at IC-5-9D, including measurements of the uterine corpus length (L), width (W) and the anteroposterior diameter (AP). (A) The uterine width (W) diameter measurement is 96.1 mm. (B) The uterine length (L) and the anteroposterior diameter (AP) measurements are 86.3×91.8 mm.
Changes in the menstrual bleeding pattern were judged with reference to the descriptions and patterns recommended by the World Health Organization (WHO) [6]. Dysmenorrhea pain was scored using the visual analog scale (VAS) [7]. Each patient was provided with a 10 cm-long segment and asked to make a mark on the segment what level best described her pain, with the left end of the scale representing ‘no pain,’ and the right end of the scale representing severe or ‘unbearable’ pain. The measurement of the distance in cm between the left end of the scale and the level of the reported pain represented the VAS score for pain for each study participant at follow-up.
Statistical analysis
Data were analyzed using SPSS version 22.0 statistical software (SPSS, IBM, Chicago, IL, USA). P<0.05 was considered statistically significant. Measurement data with a normal distribution were shown as the mean ± standard deviation (SD). The significance of compared differences was tested using the paired t-test.
Results
Menstruation
Before subcutaneous etonogestrel implantation, all the patients had a normal menstrual cycle, without amenorrhea or irregular vaginal bleeding. Follow-up of the 17 patients at 12 months after implantation showed that five women (29.4%) had amenorrhea, four women (23.5%) had normal frequency of the menstrual cycle, one woman (5.9%) had increased frequency of bleeding, three women (17.6%) reported prolonged bleeding, and four women (23.5%) had oligomenorrhea (Table 2).
Table 2.
Changes in bleeding pattern after implantation [n, (%)].
| Menstruation changes | 3 mo | 6 mo | 12 mo |
|---|---|---|---|
| Amenorrhea | 3 (17.6) | 5 (29.4) | 5 (29.4) |
| Oligomenorrhe | 3 (17.6) | 4 (23.5) | 4 (23.5) |
| Frequent bleeding | 2 (11.7) | 2 (11.7) | 1 (5.9) |
| Prolonged bleeding | 5 (29.41) | 3 (17.6) | 3 (17.6) |
Dysmenorrhea
All the 17 study participants required pain before they started the study, including indomethacin and sustained-release ibuprofen capsules, to relieve dysmenorrhea, before implantation. Following subcutaneous etonogestrel implantation, 12 patients reported a milder degree of dysmenorrhea one month after implantation, and the remaining five patients reported milder dysmenorrhea at between 2–3 months following implantation. The VAS score significantly decreased, from 7.63±0.74 pre-implantation to 1.55±0.42 at three months after implantation (P<0.01) (Table 3).
Table 3.
Hemoglobin level, dysmenorrhea, uterine volume, and serum CA125 before and after implantation.
| Baseline | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Dysmenorrhea, VAS | 7.62±0.74 | 1.55±0.42* | 1.43±0.41* | 1.45±0.35* |
| Hemoglobin, g/mL | 94.54±5.47 | 120.84±4.94* | 127.00±3.98* | 127.08±2.56* |
| Serum CA125, U/mL | 100.41±49.89 | 64.11±22.41* | 45.35±11.15* | 34.58±9.66* |
| Uterine volume, cm3 | 198.53±39.47 | 148.17±24.37* | 124.78±13.55* | 118.03±12.83* |
P<0.05 compared to values before implantation.
Hemoglobin levels
Thirteen of the 17 patients had a hemoglobin level lower than 110 g/L before implantation, indicating anemia. The hemoglobin levels of these 13 patients significantly increased after implantation, from 94.54±5.47 g/L to 127.08±2.56 g/L at 12 months of follow-up (P<0.01) (Table 3).
Serum levels of cancer antigen 125 (CA125)
All 17 patients with had high levels of cancer antigen 125 (CA125) before implantation (100.41±49.89 U/mL), which decreased to 64.11±22.41 U/mL at three months after implantation, and 34.58±9.66 U/mL at 12 months of follow-up (Table 3).
Uterine volume measured by trans-abdominal color ultrasound
At baseline, at the beginning of the study, imaging with trans-abdominal color ultrasound showed that the uterine volumes of the 17 patients were equivalent to between 10–16 weeks of pregnancy, which would have been indications for surgical management, had the patients wanted this form of treatment, but all 17 patients refused surgery.
Using a GE Voluson E8 color flow ultrasound machine (GE-E8 device), with the intracavity transducer set at IC-5-9D, measurements included the uterine corpus length (L), width (W) and the anteroposterior diameter (AP). The measurements using color flow ultrasound were performed on the third day after clearance of menstruation. The uterine volume was calculated as V=0.523×L×W×AP (mm3), and the uterus was considered as an ellipsoid (Figure 3). The mean uterine volume was 198.53±39.47 cm3 before subcutaneous etonogestrel implantation, which decreased to 148.17±24.37 cm3 at three months after implantation, and 118.03±12.83 cm3 at 12 months of follow-up (Table 3; Figure 4).
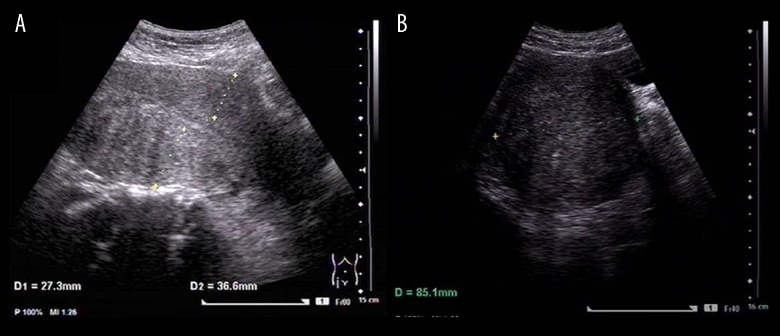
Figure 3.
Trans-abdominal color ultrasound imaging of the uterine corpus on the third day after clearance of menstruation. Imaging was performed using a GE Voluson E8 color flow ultrasound machine (GE-E8 device), with the intracavity transducer set at IC-5-9D, including measurements of the uterine corpus length (L), width (W) and the anteroposterior diameter (AP). (A) The uterine posterior wall thickness is 36.6 mm. (B) The uterine width (W) measurement is 85.1 mm.
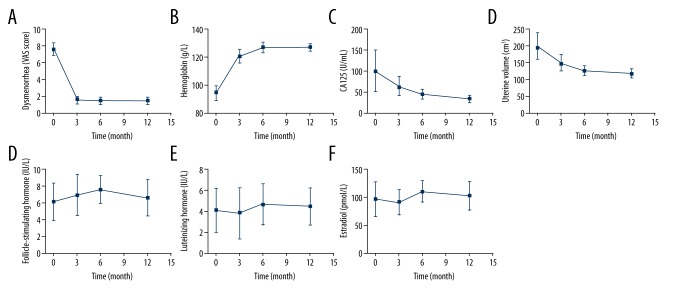
Figure 4.
Changes in dysmenorrhea, hemoglobin, serum cancer antigen 125 (CA125), uterine volume, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels before and after implantation of the subcutaneous device containing 68 mg of etonogestrel. (A) Dysmenorrhea, before and after subcutaneous etonogestrel implantation. (B) Hemoglobin (Hb) levels (g/dL), before and after subcutaneous etonogestrel implantation. (C) Serum cancer antigen 125 (CA125) levels (U/ml), before and after subcutaneous etonogestrel implantation. (D) Uterine volume measurements (mm), before and after subcutaneous etonogestrel implantation. (E) Follicle stimulating hormone (FSH) levels (mIU/mL), before and after subcutaneous etonogestrel implantation. (F) Luteinizing hormone (LH) levels (IU/L), before and after subcutaneous etonogestrel implantation. (G) Estradiol (E2) levels (pg/mL), before and after subcutaneous etonogestrel implantation.
Serum hormone levels
The levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) at the third day of menstruation after implantation were similar to baseline (Table 4).
Table 4.
Changes in serum FSH, LH, and E2 levels before and after implantation.
| Baseline | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Follicle-stimulating hormone, IU/L | 6.21±2.31 | 7.03±2.45 | 7.62±1.68 | 6.70±2.18 |
| Luteinizing hormone, IU/L | 4.06±2.12 | 3.81±2.41 | 4.66±1.93 | 4.42±1.75 |
| Estradiol, pmol/L | 96.73±31.91 | 90.56±22.79 | 109.68±19.36 | 102.31±26.53 |
P<0.05 compared to values before implantation (baseline).
Side effects and complications of subcutaneous etonogestrel implantation
Six of the 17 patients increased in body weight (mean, 2.82±1.24 kg) at 12 months after subcutaneous etonogestrel implantation; two patients had breast tenderness which gradually decreased. None of the patients experience acne, ovarian cysts, arm numbness, abnormalities in skin sensation, or skin pigmentation.
The rate of continued use of subcutaneous etonogestrel implants
All 17 patients were followed-up for 12 months, with careful consultation and clinical review. None of the women in this study experienced side effects or complications, and none of the devices were removed. No displacement or loss of the subcutaneous implant devices were detected. All 17 patients chose to continue to use the subcutaneous etonogestrel implant device beyond the end of the 12-month study period.
Discussion
Etonogestrel, also known as desogestrel, is the main component of the subcutaneous implantable contraceptive device, Implanon®. Etonogestrel inhibits ovulation by suppressing luteinizing hormone (LH). The pathogenesis of adenomyosis remains unclear, and the relationship between high levels of estrogen and the development of adenomyosis remains controversial. Etonogestrel inhibits ovulation, eliminating the ovulatory peak of estrogen and reducing the average estrogen levels during the menstrual cycle. The average estradiol concentration is maintained at a level above than that of the early follicular phase, a mechanism by which the symptoms of adenomyosis might be alleviated [8].
However, in the present study, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels on the third day of menstruation showed no significant differences before and after subcutaneous etonogestrel implantation. This finding suggests that etonogestrel had no significant effects on the serum level of basal female hormones of the women in this study who had adenomyosis. However, since only hormone levels on the third day of menstruation were measured, the effects of etonogestrel on ovulation and the dynamic monthly cycle of estrogen remain unclear. Therefore, further studies are needed to measure the hormone levels and changes in the endometrium at different phases of menstruation to clarify how Implanon® alleviates the clinical symptoms and signs of adenomyosis.
In the present study, 65% of the adenomyosis patients presented with clinical symptoms of adenomyosis, mainly with hypermenorrhea, secondary progressive dysmenorrhea, and uterine enlargement. The quality of life of the women with adenomyosis was severely affected by their symptoms. The usual treatment for adenomyosis includes medical and surgical treatment. In this study, all 17 women chose not to have surgery, and the efficacy of their medical treatment was evaluated according to the size of the uterine lesion, serum CA125 levels, resolution of dysmenorrhea, and improvement of hemoglobin levels and reduction of anemia.
Also, the findings of this study showed that the uterine volume reduced from a mean volume of 198.53±39.47 cm3 before treatment to 118.03±12.83 cm3 by 12 months following subcutaneous etonogestrel implantation, with the efficacy of treatment being most significant after three months of implantation. The study involved measurement serum cancer antigen 125 (CA125) levels and showed that the levels of CA125 were closely related to the development of the endometrium. Therefore, detection of CA125 expression levels in serum might have an important role in evaluating adenomyosis. In this study, the mean serum CA125 was 100.41±49.89 U/mL before treatment and decreased to 64.11±22.41 U/mL by three months after treatment, and to 34.58±9.66 U/mL at 12-month follow-up. These findings indicated that etonogestrel treatment, using the subcutaneous etonogestrel implant, was effective in reducing the endometriotic lesions of adenomyosis.
In this study, the effect of treatment with the subcutaneous etonogestrel implant on dysmenorrhea was evaluated using the visual analog scale (VAS) pain scoring method. The mean VAS scores decreased from 7.22±1.21 at baseline to 1.21±0.46 after treatment, and all the patients experienced complete remission of pain within three months after treatment. Previous studies have suggested that etonogestrel may alleviate dysmenorrhea either by reducing the size of the adenomyosis lesions, or by reducing menstrual volume, or even amenorrhea, leading to reduced production of endogenous prostaglandin I2 (PGI2) and thromboxane A2 (TXA2) [9].
Excessive menstrual flow is another common clinical manifestation of adenomyosis that can lead to anemia and is a reason for surgical resection of the uterus (hysterectomy) when medical treatments fail. This study also investigated the changes in hemoglobin levels after treatment with subcutaneous etonogestrel implantation as an indicator of the effectiveness of this treatment. The mean hemoglobin level was 94.54±5.47 g/L before etonogestrel implantation treatment and increased to a mean of 127.08±2.56 g/L after treatment. This degree of clinical improvement might indicate the use of subcutaneous etonogestrel implants as an alternative to surgical resection of the uterus for some patients with anemia due to excessive menstrual flow.
Subcutaneous etonogestrel implantation is an effective contraceptive device that releases up to 20 μg of etonogestrel every day. There have now been several published clinical studies that have shown that in addition to its contraceptive effects, the subcutaneous etonogestrel implant may be an effective treatment for endometriosis, adenomyosis, and chronic pelvic pain. He et al. studied 42 patients with adenomyosis who were treated with a levonorgestrel-releasing intrauterine device (IUD) and found that uterine volume significantly decreased in the three months after placement, menstrual flow decreased to 27% of the original amount, and dysmenorrhea was significantly reduced [10]. However, in this previously published study, most of the patients with adenomyosis had malposition of the uterus, and the uterine cavity was too large to place the levonorgestrel-releasing IUD [10]. In the present study, three of the 17 patients had a previous intra-uterine implant placement, but the device was expelled due to excessive menstrual flow, and nine patients had malposition of the uterus or a uterine depth >8 cm on ultrasound, which would have meant that they were not suitable for intra-uterine etonogestrel or levonorgestrel implantation placement. Because the sample size of this observational clinical study was relatively small, future studies should include more patients and use a randomized controlled study population to evaluate further the use of subcutaneous etonogestrel implants in adenomyosis.
In a previously published clinical trial of the efficacy of the subcutaneous etonogestrel implant Implanon® as a contraceptive device, irregular bleeding was the most common reason for discontinuing treatment [11]. The mechanism of bleeding remains unclear but might be explained by progestin-induced breakthrough bleeding [12]. Mansour et al. analyzed the bleeding pattern of 923 patients of adenomyosis treated with Implanon® and found that 6.7% and 17.7% of the patients experienced increased frequency or prolonged menstruation, and 22.2% and 33.6% of the patients experienced amenorrhea or oligomenorrhea, respectively [13]. In the present study, five of the 17 patients (29.4%) experienced amenorrhea, which was slightly higher than previously reported, and four of the 17 patients (23.5%) experienced frequent or prolonged bleeding.
Amenorrhea is an inevitable result of long-term simple progestin treatment, since it not only inhibits ovarian function but also antagonizes the pro-proliferative effects of estrogen on the endometrium, leading to endometrium thinning and decidual changes. Although these effects do not usually require special treatment, increase in body weight is another commonly observed side effect, as are changes in menstrual bleeding patterns.
In 2013, a controlled clinical study in the US to compare contraceptive efficacy showed that in a 12 month period, subcutaneous etonogestrel implant users gained an average of 2.1 kg, levonorgestrel-releasing IUD users gained 1.0 kg, depot medroxyprogesterone acetate users gained 2.2 kg, and copper IUD users gained 0.2 kg, but in an adjusted statistical analysis, no difference in weight gain between the devices was observed. [14]. In 2016, Wang et al. reported that 31 out of 150 women (28.7%) treated with etonogestrel implantation had increased body weight 12 months after implantation of between 2–10 kg [15]. In the present study, six of the 17 women (35.3%) had a mean weight gain during 12-month follow-up of 2.82±1.24 kg.
A multinational, multicenter study in the US showed that 11.8% of women experienced acne after using the contraceptive etonogestrel implant, Implanon® [6]. In 2005, Funk et al. showed that 61% of the women originally with acne had less acne and only 7% of them had more after the use of Implanon®, while 84% of those without acne experienced no significant changes in the skin [16]. No incidence of acne was observed in the present study, possibly due to the small sample size and possibly also due to racial difference. Some other common side effects, such as a headache, nausea, breast tenderness, and mood swings may also occur due to progestogen therapy but were not reported in the present study.
The findings of the present study have demonstrated the potential benefits of the use of subcutaneous etonogestrel implants for women with adenomyosis, and have shown that this form of treatment might be an alternative to surgery. Also, the results of the study have shown that the use of progestogen treatment with etonogestrel using a subcutaneous provides an alternative treatment approach for women who have a uterine cavity that is too large for placement an intra-uterine etonogestrel implant, and for women who do not have access to treatment with gonadotropin-releasing hormone (GnRH) agonists and analogs. However, it should also be noted that etonogestrel has some of the common side effects of long-acting progestogens, such as vaginal bleeding, amenorrhea, weight gain, and breast tenderness. Therefore the patients should be properly informed about these side effects, to reduce the rate of non-compliance with treatment.
Conclusions
The findings of this observational clinical study have shown that subcutaneous etonogestrel implant is an alternative treatment device that can be used to treat adenomyosis by the inhibitory effects of progesterone on hypothalamic and ovarian function when other forms of treatment are ineffective or are not preferred by the patient.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Fujian Provincial Science and Technology Foundation (Number: 2014Y6001)
References
- 1.Benagiano G, Brosens I. History of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:449–63. doi: 10.1016/j.bpobgyn.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Levy G, Dehaene A, Laurent N, et al. An update on adenomyosis. Diagn Interv Imaging. 2013;94:3–25. doi: 10.1016/j.diii.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Hynes EF, Handasyde KA, Shaw G, Renfree MB. Levonorgestrel, not etonogestrel, provides contraception in free-ranging koalas. Reprod Fertil Dev. 2010;22:913–19. doi: 10.1071/RD09253. [DOI] [PubMed] [Google Scholar]
- 4.Chanana C, Gupta N, Bansal I, et al. Different sonographic faces of ectopic pregnancy. J Clin Imaging Sci. 2017;7:6. doi: 10.4103/jcis.JCIS_105_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensouda-Grimaldi L, Jonville-Bera AP, Beau-Salinas F, et al. le reseau des centres regionaux de pharmacovigilance. [Insertion problems, removal problems, and contraception failures with implanon]. Gynecol Obstet Fertil. 2005;33:986–90. doi: 10.1016/j.gyobfe.2005.10.016. [in French] [DOI] [PubMed] [Google Scholar]
- 6.Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon®): Results from 11 international clinical trials. Fertil Steril. 2009;91:1646–53. doi: 10.1016/j.fertnstert.2008.02.140. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty SA. Pain measurement tools for clinical practice and research. AANA J. 1996;64:133–40. [PubMed] [Google Scholar]
- 8.Bhatia P, Nangia S, Aggarwal S, Tewari C. Implanon®: Subdermal single rod contraceptive implant. J Obstet Gynaecol India. 2011;61:422–25. doi: 10.1007/s13224-011-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He T, Qi F, Jia L, et al. MicroRNA-542-3p inhibits tumour angiogenesis by targeting angiopoietin-2. J Pathol. 2014;232:499–508. doi: 10.1002/path.4324. [DOI] [PubMed] [Google Scholar]
- 10.He SM, Weu MX, Han YH. The clinical observation of Levonorgestrel-releasing intrauterine device in treatment of uterine adenomyosis. Chin J Obstet Gynecol. 2005;40:536–38. [Google Scholar]
- 11.Maddox DD, Rahman Z. Etonogestrel (implanon), another treatment option for contraception. Drug Forecast. 2008;33:337–47. [Google Scholar]
- 12.Varma R, Mascarenhas L. Endometrial effects of etonogestrel (Implanon®) contraceptive implant. Curr Opin Obstet Gynecol. 2001;13:335–41. doi: 10.1097/00001703-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Mansour D, Korver TP, Marintcheva-Petrova M, Fraser IS. The effects of Implanon® on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(S1):13–28. doi: 10.1080/13625180801959931. [DOI] [PubMed] [Google Scholar]
- 14.Vickery Z, Madden T, Zhao Q, et al. Weight change at 12 months in users of three progestin-only contraceptive methods. Contraception. 2013;88:503–8. doi: 10.1016/j.contraception.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CF, Zhu LH, Li YH. Observation of clinical effect of etonogestrel implant in 12 months. Chin J Family Plann. 2016;1:28–32. [Google Scholar]
- 16.Funk S, Miller MM, Mishell DR, Jr, et al. Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319–26. doi: 10.1016/j.contraception.2004.11.007. [DOI] [PubMed] [Google Scholar]