Abstract
Objective
To develop a short, 5-item measure of the core symptoms of depression based on the 16-item Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR16) and to evaluate psychometric properties of this new measure (Very Quick Inventory of Depressive Symptomatology – Self-Report: VQIDS-SR5).
Method
Using data from a convenience sample of the Combining Medications to Enhance Depression Outcomes (CO-MED) trial, we evaluated the psychometric properties of the VQIDS-SR5, its sensitivity to change, and its comparability to the QIDS-SR16 and clinician-rated scales (QIDS-C16 and VQIDS-C5).
Results
The VQIDS-SR5 has a single-factor structure with an acceptable internal consistency (Cronbach’s alpha: 0.67–0.81). The VQIDS-SR5 was as sensitive to change as its parent scale QIDS-SR16 and detected change at an earlier time frame. Additionally, the VQIDS-SR5 was comparable to the QIDS-SR16, QIDS-C16, and VQIDS-C5.
Conclusion
The VQIDS-SR5 can effectively evaluate the core symptoms of depression during the course of treatment.
Keywords: Depression, Self-Report, Psychometrics
Introduction
Measurement-based care (MBC) has been shown to improve the outcomes of patients treated for depression (1). Brief and convenient symptom and side effect self-report measures are essential for effective MBC. Currently, the 9-item Patient Health Questionnaire (PHQ-9) (2) and the 16-item (9 domain) Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR16) (3) are among the self-report measures commonly used to implement MBC in primary and psychiatric care settings. While both measures are relatively brief, there may be ways to further curtail assessment time, and thus facilitate the adoption of MBC in routine practice (4). Additionally, some criterion symptoms of a major depressive episode may simply be medication side effects (e.g., insomnia, lethargy, etc.). Thus, the rating of these symptoms may actually interfere with detecting a positive response to treatment in other core depressive symptoms.
Bech et al. found that a subset of 6 core depressive symptoms extracted from the 17-item Hamilton Rating Scale for Depression (HRSD17) accounted for 54.8% of the variance attributable to the HRSD17 (5, 6, 7). Further, Kyle et al. found that the briefer HRSD6 was better at detecting remission than the longer versions of the Hamilton Rating Scale (8). These findings suggest that a shorter version of the report may be just as effective while taking only one-third of the time to complete, thereby making it more practical to implement clinically. As such, it is logical to investigate an analogous question: can fewer items extracted from the QIDS-SR16 be sufficient and time-effective to gauge the symptomatic effects of treatment for depression?
To that end, we created the Very Quick Inventory of Depressive Symptomatology (VQIDS-SR5) by extracting 5 core depression domains from the QIDS-SR16: sad mood, self-outlook, involvement, fatigue, and psychomotor slowing. These items reflect those identified as the depressive symptoms in Bech’s HRSD6 (5), but excludes the anxiety item, which has the smallest loading when using Principle Component Analysis (7). It was not deemed core to major depressive disorder, but rather an associated symptom, and as such, it is not in the QIDS-SR16.
This report evaluates the psychometric features (internal consistency, concurrent validity, dimensionality, and sensitivity to change) of the VQIDS-SR5, as well as thresholds that define response and remission as compared to the QIDS-SR16. The data for this report came from individuals who participated in the Combining Medications to Enhance Depression Outcomes (CO-MED) study, a single blind, multi-center, randomized trial that compared three different medication treatments in outpatients with non-psychotic depression treated over 12 weeks (9).
Materials and Methods
The CO-MED study protocol and procedures were approved by the Institutional Review Board at the University of Texas Southwestern Medical Center (national coordinating center), the University of Pittsburgh (data coordinating center), and each participating center and clinic. Details of the study design, measurements, and primary outcomes are available elsewhere (9).
Study participants met criteria for chronic (current episode >2 years) or recurrent non-psychotic depression with a current episode lasting at least 2 months and a score ≥16 on the HRSD17. The study enrolled 665 participants from six primary and nine psychiatry care sites. Participants were assigned to one of three different treatments in a 1:1:1 ratio: 1) escitalopram plus placebo, 2) sustained-release bupropion plus escitalopram, and 3) extended-release venlafaxine plus mirtazapine. Study physicians implemented MBC (1, 4) at every visit to adjust participants’ medication dosage based on scores from the 16-item Quick Inventory of Depressive Symptomatology – Clinician-rated (QIDS-C16) scale (3) and the Frequency, Intensity, and Burden of Side Effects Rating scale (FIBSER) (10). The 30-item Inventory of Depressive Symptomatology – Clinician-rated (IDS-C30) scale (11) and QIDS-SR16 measurements were collected at baseline (week 0) and at follow-up visits (weeks 1, 2, 4, 6, 8, 10, and 12). The QIDS-C166-item Inventory of Depressive Symptomatology (IDS-C6) scale (12), and VQIDS-C5 were derived from the IDS-C30. Subsequently, the VQIDS-SR5 was extracted from the QIDS-SR16 core symptom items: sad mood, self-outlook, involvement, fatigue, and psychomotor slowing.
Statistical Analysis
The statistical analysis was divided into four parts. First, we evaluated the psychometric properties of the VQIDS-SR5 questionnaire using Principal Component Analysis and Mokken Analysis (13). To determine the number of factors present in the VQIDS-SR5, both the Parallel Analysis and Velicer's Minimum Average Partial (MAP) tests were performed. The tests were conducted and evaluated at 4 time points: baseline (week 0), critical decision point (week 4), middle of treatment (week 6), and end of acute treatment (week 12) (14). We then evaluated the performance of the VQIDS-SR5 and how it compares to the QIDS-SR16. Cronbach’s coefficient alpha was used to examine the reliability of both measures and individual items from each participant at each time point (15) Mokken Analysis allows for the use of the automated item selection algorithm (AISA) and the computation of Loevinger coefficient of homogeneity (H) (13).
The second analysis compared the relative sensitivities of the VQIDS-SR5 and QIDS-SR16 to their treatment effect over time. To compare the two scales’ sensitivities to change in this trial, two mixed model analyses were conducted using the VQIDS-SR5 and the QIDS-SR16 as outcomes. The model was limited to the main effects of treatment group (A, B, C), time (weeks 0, 1, 2, 4, 6, 8, 10, and 12), and the interaction between group by time. We also report three model-fit indices: the −2 log likelihood, the Akaike’s information criterion (AIC) and the Bayesian information criterion (BIC). These measures are based on the residual variance that is not explained by a model. Thus, a lower number indicates a better fit and greater sensitivity to change over time.
The third set of analyses were conducted to examine the comparability of the VQIDS-SR5 and the standard QIDS-SR16 in terms of their estimation of the two most common categorical clinical outcomes: response (defined as 50% symptom reduction from baseline) and remission (defined as QIDS-SR16 score of 5 or less; VQIDS-SR5 score of 3 or less).
Finally, to understand the relationship between the VQIDS-SR5 and clinician-rated scales, we report correlations between each of the two self-reports, VQIDS-SR5 and QIDS-SR16, and the IDS-C30, QIDS-C16, IDS-C6, and VQIDS-C5. These were all collected at week 6 to provide a more robust range of scores than at baseline, while being early enough in the study to minimize dropouts.
Results
Table 1 provides basic clinical and demographic information of the sample population.
Table 1.
Baseline demographic and clinical characteristics of the sample (N=665)
| Characteristic | Total | |
|---|---|---|
|
| ||
| N | % | |
| Sex | ||
| Male | 213 | 32.0 |
| Female | 452 | 69.0 |
| Race | ||
| White | 431 | 67.0 |
| Black | 174 | 27.1 |
| Other | 38 | 5.9 |
| Hispanic | 101 | 15.2 |
|
| ||
| Mean | SD | |
|
| ||
| Age (years) | 42.7 | 13.0 |
| Education (years) | 13.8 | 3.0 |
| Monthly Household Income (dollars) | 2,678 | 5,353 |
| IDS-C30 | 38.0 | 9.1 |
| QIDS-C16 | 15.8 | 3.4 |
| QIDS-SR16 | 15.5 | 4.3 |
| VQIDS-SR5 | 8.5 | 3.0 |
Abbreviations: IDS-C30 30-item Inventory of Depressive Symptomatology – Clinician-rated; QIDS-C16 16-item Quick Inventory of Depressive Symptomatology – Clinician-rated; QIDS-SR16 16-item Quick Inventory of Depressive Symptomatology – Self-Rated; VQIDS-SR5 Very Quick Inventory of Depressive Symptomatology – Self-Rated.
Psychometric Evaluation
The Principle Component Analysis revealed a single-factor structure for the VQIDS-SR5. All five items loaded on a single factor that ranged from .77 to .67 when analyzing data collected at week 6. Based on both Parallel Analysis and the MAP test, the VQIDS-SR5 is best described as a single-factor measure at baseline, week 4, week 6, and week 12. Based on the AISA, all 5 items load on a single factor for all weeks, except for self outlook at the baseline visit. The H coefficients ranged from .33 at baseline to .53 at week 12, indicating better discriminate ability of the scale over the course of treatment.
Internal consistency of the VQIDS-SR5 was measured using Cronbach's Alpha, which reported values ranging from 0.67 at baseline to 0.81 at week 12. Omitted Item Statistics showed that all VQIDS-SR5 items had a good fit based on improvement to the internal reliability throughout time. We also calculated Cronbach’s alpha for the QIDS-SR16 at different time intervals. Results ranged from 0.70 at week 0 to 0.81 at week 12. These results are similar to the ones observed for the VQIDS-SR5 (Table 2).
Table 2.
Cronbach’s alphas over the acute trial for the QIDS-SR16, the VQIDS-SR5, and its variations based on dropping each item
| Measure | Baseline | Week 4 | Week 6 | Week 12 |
|---|---|---|---|---|
| Alpha QIDS-SR16 | .70 | .78 | .81 | .81 |
| Alpha VQIDS-SR5 | .66 | .78 | .80 | .81 |
| VQIDS-SR5 alpha with dropped Item 1 | .57 | .73 | .75 | .75 |
| VQIDS-SR5 alpha with dropped Item 2 | .58 | .71 | .75 | .76 |
| VQIDS-SR5 alpha with dropped Item 3 | .63 | .74 | .76 | .77 |
| VQIDS-SR5 alpha with dropped Item 4 | .57 | .73 | .75 | .75 |
| VQIDS-SR5 alpha with dropped Item 5 | .63 | .76 | .78 | .77 |
Abbreviations: QIDS-SR16 16-item Quick Inventory of Depressive Symptomatology – Self-Rated, VQIDS-SR5 5-item Very Quick Inventory of Depressive Symptomatology – Self-Rated.
Sensitivity to Treatment Effects
The model using the VQIDS-SR5 showed no significant main effect for group, a main effect for time, and no group-by-time interaction. The same pattern of results was found when the QIDS-SR16 was evaluated, demonstrating that the shorter VQIDS-SR5 could detect the same changes as were found using the full scale (Table 3).
Table 3.
Comparison of sensitivity to change in the VQIDS-SR5 and QIDS-SR16 by group, time, and group by time
| VQIDS-SR5 | QIDS-SR16 | |
|---|---|---|
| Group | F(2, 630) =.44, p < 0.65 | F(2, 630)=1.76, p <0.18 |
| Time | F(7, 3471) =184.18, p < 0.0001 | F(7, 3471)= 209.01, p <0.0001 |
| Group by Time | F(14, 3471) = .44, p <0.65 | F(14, 3471) = .99, p <0.46 |
Abbreviations: QIDS-SR16 16-item Quick Inventory of Depressive Symptomatology – Self-Rated, VQIDS-SR5 5-item Very Quick Inventory of Depressive Symptomatology – Self-Rated.
An additional test was performed to compare the two measures based on the amount of systematic change that was not explained by the model. Specifically, the sensitivity of both mixed effect models was measured by calculating residual variance. For the VQIDS-SR5, the fits statistics were −2 log likelihoods = 19309.5, AIC = 19313.5, and BIC = 19322.4. In comparison, the analysis using the QIDS-SR16 reported −2 log likelihoods = 22252.9, AIC = 22256.9, and BIC = 22265.8. In all cases, smaller values are better. Therefore, the VQIDS-SR5 provided a better fit of the basic model.
Comparison of Response and Remission Defined by the VQIDS-SR5 and the QIDS-SR16
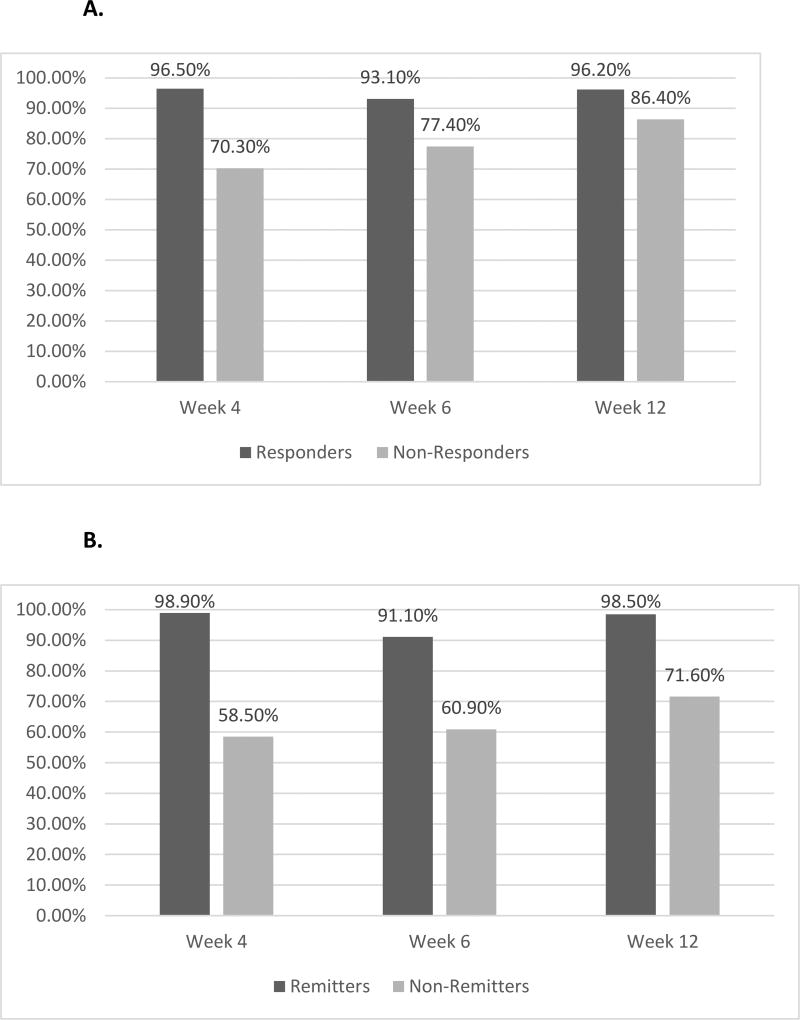
To compare both measures in terms of their equivalence when defining response and remission, data were evaluated at weeks 4, 6, and 12. Using the QIDS-SR16 as reference, week 4 agreement was 96.5% for responders, 70.3% for non-responders, 98.9% for remitters, and 58.5% for non-remitters. At week 6, agreement was 93.1% for responders, 77.4% for non-responders, 91.1% for remitters, and 60.9% for non-remitters. At week 12, agreement was 96.2% for responders, 86.4% for non-responders, 98.5% for remitters, and 71.6% for non-remitters. Thus, over the course of the acute treatment phase, there was high concordance between the two measures, although the VQIDS-SR5 consistently produced higher rates of both response and remission (Figure 1A and B).
Figure 1.
A. Agreement of the VQIDS-SR5 with the QIDS-SR16 on response status.
B. Agreement of the VQIDS-SR5 with the QIDS-SR16 on remission status.
Abbreviations: QIDS-SR16: 16-item Quick Inventory of Depressive Symptomatology – Self-Rated, VQIDS-SR5: 5-item Very Quick Inventory of Depressive Symptomatology – Self-Rated.
Relations among Self-report and Clinician-rated Scales
The correlations among the clinician-rated and self-report forms of the severity measures were examined at week 6. The correlation between the self-report scales, the VQIDS-SR5 and the QIDS-SR16, was r=0.90. The correlation between the VQIDS-SR5 and the IDS-C30, the clinician-rated scale with the most items, was r=0.57, which is comparable to the r=0.67 correlation between the longer QIDS-SR16 and the IDS-C30. While there is meaningful overlap between the self-report and the clinician-rated scales, there are also clear differences. The overlap and differences between self-report scales and clinician-rated scales appear to be proportionate to the length of the scale (Table 4).
Table 4.
Correlations among self-report and clinician-rated measures of depressive symptomatology
| VQIDS-SR5 | QIDS-SR16 | IDS-C30 | QIDS-C16 | IDS-C6 | VQIDS-C5 | |
|---|---|---|---|---|---|---|
| VQIDS-SR5 | 1 | 0.90 | 0.57 | 0.57 | 0.55 | 0.55 |
| QIDS-SR16 | 0.90 | 1 | 0.67 | 0.67 | 0.56 | 0.53 |
| IDS-C30 | 0.57 | 0.67 | 1 | 0.85 | 0.75 | 0.71 |
| QIDS-C16 | 0.57 | 0.67 | 0.85 | 1 | 0.83 | 0.85 |
| IDS-C6 | 0.55 | 0.55 | 0.75 | 0.83 | 1 | 0.95 |
| VQIDS-C5 | 0.55 | 0.53 | 0.71 | 0.85 | 0.95 | 1 |
Abbreviations: IDS-C: Inventory of Depressive Symptomatology – Clinician-rated (30- and 6-item version); QIDS-C16 16-item Quick Inventory of Depressive Symptomatology – Clinician-rated; QIDS-SR16 16-item Quick Inventory of Depressive Symptomatology – Self-Rated; VQIDS-C5 5-item Very Quick Inventory of Depressive Symptomatology – Clinician-rated; VQIDS-SR5 5-item Very Quick Inventory of Depressive Symptomatology – Self-Rated.
Discussion
To our knowledge, this is the first report to evaluate a much shorter self-report scale for measuring the core symptoms of major depression using a 5-item version of the Quick Inventory of Depressive Symptoms (VQIDS-SR5). The VQIDS-SR5 has a single-factor structure and an internal consistency comparable to the longer QIDS-SR16. The VQIDS-SR5 was as sensitive to change as the QIDS-SR16 and provided a better measure of change to treatment. Furthermore, in this initial study, the VQIDS-SR5 was better able to differentiate responders from non-responders at an earlier time point than the QIDS-SR16. Finally, the 5-item self-report was comparable to the 5-item clinician-rated version, as demonstrated by a strong correlation between both tools. Together, results suggest that the VQIDS-SR5 may be a useful alternative or supplement to the longer QIDS-SR16.
While there is no prior report specific to a shortened version of the QIDS16 with which to compare our study, the concept of shortening a longer scale to focus on core symptoms of depression has previously been reported using other clinical rating scales. For example, Bech et al. (6, 7) developed and tested a 6-item HRSD sub-scale to measure the core symptoms of depression, which has been reported as more robust and sensitive to change than its parent scale (16). Analogous efforts were undertaken by Maier et al. (17) and Gibbons et al. (18) with a focus on clinical trials. Recently, McIntyre et al. validated the use of a 7-item sub-scale of the HRSD in primary care settings (19). Shorter tools have the benefit of reducing the time burden of both patients and clinicians while simultaneously retaining or enhancing sensitivity due to their uni-factorial structure. In this report, we compare the self-reported 5-item Very Quick Inventory of Depressive Symptomatology to the longer self-reported 16-item Quick Inventory of Depressive Symptomatology. Our results are consistent with previous findings with shortened clinical ratings: The shorter single-factor scale is comparable to longer exams, sensitive to symptom change over time, and useful for tracking this change over time.
Of note, virtually all of the shorter versions of the clinically rated scales contain the core symptoms of depression. Bech et al. (6) includes the following items: depressed mood, feelings of guilt, work and activities, psychomotor retardation, psychic anxiety, and somatic symptoms. Maier et al. (17) also includes 6 items, although agitation replaces somatic symptoms as one of the scale’s items. The 7 items that McIntyre et al. (19) reported are depressed mood, feelings of guilt, suicide, work and activities, psychic anxiety, somatic anxiety, and somatic symptoms. These shorter scales were aimed at identifying specific items that accounted for the greatest amount of the variance in outcome as assessed by the longer parent scale (20). This coalescence of studies indicates that the core symptoms are pivotal indicators of therapeutic effect, giving credence to their use as a primary outcome tool.
This initial report suggests that the 5-item version of the QIDS-SR is adequately sensitive to change, such that it may suffice for the implementation of MBC in practice and between visits. That speculation, however, requires prospective testing. Clearly, a focus on the core symptoms limits the information that a clinician may take into consideration with MBC. For example, the presence of hypersomnia/insomnia or weight gain/loss may affect medication choice, yet these symptoms are not addressed on the VQIDS-SR5. It is possible that side effects may go unreported or dose adjustments may be poorly informed. On the other hand, a sharp focus on the core symptoms appears to detect positive change in depressive symptoms early in the course of treatment.
This secondary analysis has several limitations. The VQIDS-SR5 was compared to the parent QIDS-SR16 from which the 5-item version was extracted, which clearly inflates the similarity between these two measures. Secondly, this relatively small sample of convenience drawn from a single study makes generalizability uncertain. Thirdly, while we emulated the Bech et al. HRSD6 (6) item test, we did not carefully evaluate the pros and cons of including versus excluding the anxiety metric. Further evaluation of this question is necessary. Lastly, we have not yet compared the VQIDS-SR5 to other commonly-used scales.
Conclusions
The VQIDS-SR5, a 5-item self-report using selected items from the QIDS-SR16, is quick, easy, and provides a reliable and valid measure of the severity of the core symptoms of depression. It is also sensitive to early treatment change and may be a worthwhile report to employ clinically.
Significant Outcomes.
The psychometric properties of the VQIDS-SR5 demonstrate it as a single-factor tool with an internal consistency comparable to its parent scale, the QIDS-SR16.
The 5-item self-report was as sensitive to change as the 16-item QIDS-SR16.
The VQIDS-SR5 was able to differentiate non-responders from responders at an earlier time point than the QIDS-SR16.
Limitations.
The sample used may not fully represent the entire population of depressed individuals.
Replication with other populations is necessary for validation.
Comparison of the VQIDS-SR5 against other accepted depression severity rating scales was not performed.
Acknowledgments
This work was funded by NIMH under contract N01 MH-90003 to the University of Texas Southwestern Medical Center at Dallas (principal investigators, A.J. Rush and M.H. Trivedi). Forest Pharmaceuticals, GlaxoSmithKline, Organon, and Wyeth Pharmaceuticals provided medications for this trial at no cost. This work was also supported in part through the Hersh Foundation (principal investigator, M.H. Trivedi). The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. NIMH had no role in the drafting or review of the manuscript, or in the collection or analysis of the data. We thank Savitha Kalidas, Ph.D. and Jennifer Furman, Ph.D. for their editing and administrative support.
Footnotes
Declaration of Interest
Drs. De La Garza, Grannemann report no financial relationships with commercial interests. Dr. Rush has received consulting fees from the American Psychiatric Association, Brain Resource, Ltd, Curbstone Consultant LLC., Eli Lilly, Emmes Corp, Liva Nova Inc., Mindlinc Inc., Medavante I, Otsuka America Pharmaceuticals Inc., Santium Inc., Sunovion, Takeda USA, and the University of Texas Southwestern Medical Center; royalties from Guilford Publications and the University of Texas Southwestern Medical Center. Dr. Madhukar H. Trivedi is or has been an advisor/consultant and received fees from: Alkermes Inc., Allergan, AstraZeneca, Brintellix, BMS, Cerecor, Eli Lilly & Company, Forest, Health Research Associates, Johnson & Johnson, Lundbeck, Medscape, MSI Methylation Sciences Inc., Merck, Naurex Inc., Nestle Health Science – Pamlab Inc., One Carbon Therapeutics, Otsuka America Pharmaceuticals Inc., PamLab, Pfizer Inc., Roche, SHIRE Development, Takeda Pharmaceuticals Inc. In addition, he has received grants/research support from: Agency for Healthcare Research and Quality (AHRQ), National Institute of Mental Health (NIMH), Johnson and Johnson, National Institute of Diabetes and Digestive and Kidney diseases (NIDDK) and National Institute on Drug Abuse (NIDA).
Previous Presentation: The findings of this report have not been presented at any scientific meetings.
Author Contributions
Dr. Trivedi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rush, De La Garza, and Grannemann.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: De La Garza, Rush, Granneman, Trivedi.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Grannemann.
Obtained funding: Rush, Trivedi.
Administrative, technical, or material support: Clinical staff at each clinical site including site investigators and co-investigators, Data Safety Monitoring Board, Eric Nestler, M.D., Carol Tamminga, M.D.
Study supervision: Rush, Trivedi.
References
- 1.Rush AJ. Isn’t it about time to employ measurement-based care in practice? Am J Psychiatry. 2015;172(10):934–936. doi: 10.1176/appi.ajp.2015.15070928. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush A, Trivedi M, Ibrahim H, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi M, Rush A, Wisniewski S, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Bech P, Gram L, Dein E, Jacobsen O, Vitger J, Bolwig TG. Quantitative rating of depressive states. Acta Psychiatr Scand. 1975;51(3):161–170. doi: 10.1111/j.1600-0447.1975.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 6.Bech P, Allerup P, Gram L, et al. The Hamilton depression scale. Evaluation of objectivity using logistic models. Acta Psychiatrica Scand. 1981;63(3):290–299. doi: 10.1111/j.1600-0447.1981.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 7.Bech P, Fava M, Trivedi MH, Wisniewski SR, Rush AJ. Factor structure and dimensionality of the two depression scales in STAR*D using level 1 datasets. J Affect Disord. 2011;132:396–400. doi: 10.1016/j.jad.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Kyle PR, Lemming OM, Timmerby N, Søndergaard S, Andreasson K, Bech P. The validity of the different versions of the Hamilton depression scale in separating remission rates of placebo and antidepressants in clinical trials of major depression. J Clin Psychopharmacol. 2016;36(5):453–456. doi: 10.1097/JCP.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 9.Rush A, Trivedi M, Stewart J, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 10.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12(2):71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger JE, Burns CT. The Inventory of Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 12.Østergaard SD, Bech P, Trivedi MH, Wisniewski SR, Rush AJ, Fava M. Brief, unidimensional melancholia rating scales are highly sensitive to the effect of citalopram and may have biological validity: Implications for the Research Domain Criteria (RDoC) J Affect Disord. 2014;163:18–24. doi: 10.1016/j.jad.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Van de Ark LA. Mokken scale analysis. J Stat Soft. 2007;20(11):1–19. [Google Scholar]
- 14.O’Connor B. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer’s MAP test. Behav Res Methods Instrum Comput. 2000;32(3):396–402. doi: 10.3758/bf03200807. [DOI] [PubMed] [Google Scholar]
- 15.Nunnally J, Bernstein I. Psychometric Theory. New York: McGraw Hill; 1994. [Google Scholar]
- 16.Bech P, Boyer P, Germain JM, et al. HAM-D17 and HAM-D6 sensitivity to change in relation to Desvenlafaxine dose and baseline depression severity in major depressive disorder. Pharmacopsychiatry. 2010;43(7):271–276. doi: 10.1055/s-0030-1263173. [DOI] [PubMed] [Google Scholar]
- 17.Maier W, Philipp M. Comparative analysis of observer depression scales. Acta Psychiatr Scand. 1985;72(3):239–245. doi: 10.1111/j.1600-0447.1985.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons R, Clark D, Kupfer D. Exactly what does the Hamilton depression rating scale measure? J Psychiatr Res. 1993;27(3):259–273. doi: 10.1016/0022-3956(93)90037-3. [DOI] [PubMed] [Google Scholar]
- 19.McIntyre R, Konarski JZ, Mancini DA, et al. Measuring the severity of depression and remission in primary care: validation of the HAMD-7 scale. CMAJ. 2005;173(11):1327–1334. doi: 10.1503/cmaj.050786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boessen R, Groenwold R, Knol M, Grobbee DE, Roes KC. Comparing HAMD17 and HAMD subscales on their ability to differentiate active treatment from placebo in randomized controlled trials. J Affect Disord. 2013;145(3):363–369. doi: 10.1016/j.jad.2012.08.026. [DOI] [PubMed] [Google Scholar]