Abstract
BACKGROUND:
In prior studies, the use of standard breast cancer treatments has varied by race, but previous analyses were not nationally representative. Therefore, in a comprehensive, national cohort of Medicare patients, racial disparities in the use of radiotherapy (RT) after breast-conserving surgery (BCS) for invasive breast cancer were quantified.
METHODS:
A national Medicare database was used to identify all beneficiaries (age >65 years) treated with BCS for incident invasive breast cancer in 2003. Claims codes identified RT use, and Medicare demographic data indicated race. Logistic regression modeled RT use in white, black, and other-race patients, adjusted for demographic, clinical, and socioeconomic covariates.
RESULTS:
Of 34,080 women, 91% were white, 6% were black, and 3% were another race. The mean age of the patients was 76 ± 7 years. Approximately 74% of whites, 65% of blacks, and 66% of other-race patients received RT (P < .001). After covariate adjustment, whites were found to be significantly more likely to receive RT than blacks (odds ratio, 1.48; 95% confidence interval, 1.34–1.63 [P < .001]). Disparities between white and black patients varied by geographic region, with blacks in areas of the northeastern and southern United States demonstrating the lowest rates of RT use (57% in these regions). In patients age <70 years, racial disparities persisted. Specifically, 83% of whites, 73% of blacks, and 78% of other races in this younger group received RT (P < .001).
CONCLUSIONS:
In this comprehensive national sample of older breast cancer patients, substantial racial disparities were identified in RT use after BCS across much of the United States. Efforts to improve breast cancer care require overcoming these disparities, which exist on a national scale.
Keywords: breast cancer, Medicare, radiotherapy, race, disparities
Postoperative radiotherapy (RT) is generally considered a standard component of therapy for patients diagnosed with early invasive breast cancer who undergo breast-conserving surgery (BCS). Cumulative results from randomized trials have demonstrated local control and survival benefits with the addition of RT after BCS.1–4 However, despite the evidence supporting the use of RT after BCS, a recent analysis of treatment patterns in breast cancer patients enrolled in the North American Fareston versus Tamoxifen Adjuvant (NAFTA) trial suggested that racial disparities may exist in RT use after BCS. Specifically, this study found that postoperative RT was administered in 82% of white patients but only 70% of black patients, although the difference was not found to be statistically significant.5
Other studies of breast cancer patients have also suggested racial disparities in the receipt of standard treatments for locally advanced and regional disease, including differences among white and nonwhite patients in the use of RT, surgery, and chemotherapy.5–15 Results from many of these studies suggest that nonwhite patients may more frequently encounter barriers to receiving standard care. However, prior analyses were conducted on limited samples, including singlestate, single-center, or clinical trial patient cohorts, and studies of population-based cohorts have not to our knowledge been nationally comprehensive. Consequently, the true magnitude of and regional variation in racial disparities in breast cancer treatment across the United States remain uncertain.
Quantifying the magnitude of racial disparities in the use of standard treatments across the nation is an important step toward identifying and ultimately reducing barriers to high-quality breast cancer care. Therefore, using data from a comprehensive, national sample of Medicare patients, we sought to quantify racial differences in the use of RT after BCS for women with invasive breast cancer. In addition, we sought to determine whether there was geographic variation across the United States in the association between race and RT use.
MATERIALS AND METHODS
National Medicare Data Set and Study Sample Derivation
The national Medicare data set includes comprehensive claims data for all Medicare beneficiaries in the United States. Files contain data collected by Medicare for reimbursement of healthcare services for each beneficiary and include institutional (inpatient and outpatient) and noninstitutional (physicians or other providers) final action claims.16 The University of Texas M. D. Anderson Cancer Center’s institutional review board approved use of the Medicare database for this study.
We used the following algorithm to identify patients with incident breast cancer, which we based on a prior validated algorithm using claims data.17,18 Our initial study population was comprised of 853,273 women who had any diagnosis of invasive breast cancer in 2003, defined as an International Classification of Diseases-Ninth Revision (ICD-9) diagnosis code of 174. Because this denominator would have included incident and prevalent cases in 2003, as well as potentially disease-free patients undergoing breast cancer screening, we applied the following algorithm to refine our study sample to identify incident cases only. From our initial study population, we included 83,611 patients aged ≥65 years who underwent BCS between January 1, 2003, and December 31, 2003. From this sample, we excluded 17,872 patients who did not have at least 2 claims (on different dates) specifying a diagnosis of invasive breast cancer between January 1, 2003, and December 31, 2004 (at least 1 claim must have occurred during 2003), with no more than 6 months between the date of BCS and the earliest breast cancer diagnosis claim date. To exclude the prevalent cases, we excluded 10,362 patients who had a breast cancer-related diagnosis or procedure claim between January 1, 2002, and December 31, 2002. To ensure that BCS was intended to be the primary cancer-directed surgery, we excluded 13,470 patients who underwent mastectomy within 3 months of BCS. To limit our sample to patients with early stage invasive breast cancer, we then excluded 1082 patients who had ≥2 claims specifying metastatic breast cancer from 3 months before to 3 months after the diagnosis date. To improve sample homogeneity, we also excluded 2964 patients who were receiving Medicare coverage because of end-stage renal disease or disability. Finally, to ensure we had complete claims information to determine patients’ cancer treatment course and comorbidities, we excluded 3781 patients who lacked Medicare Part A or B coverage or who had intermittent health maintenance organization coverage in the 9 months after or in the 1 year before their breast cancer diagnosis date (of these patients, 2143 had incomplete information in the year before diagnosis because they were aged <66 years). For our study, the breast cancer diagnosis date was considered the date of the earliest claim for a diagnosis of breast cancer. This left a final sample size of 34,080 patients. The complete list of claims codes applied in our algorithm can be found in Table 1.
Table 1.
Claims Codes
| Variable | ICD-9 Code | CPT Code |
|---|---|---|
| Breast-conserving surgery | 85.20, 85.21, 85.22, 85.23 | 19120, 19125, 19126, 19160, 19162 |
| Breast cancer-related diagnosis and procedure claims | ||
| Biopsy | 85.1–85.19 | 19000, 19001, 19100, 19101, 19110, 19112 |
| Breast-conserving surgery | As above | As above |
| Mastectomy | 85.33–85.48 | 19180–19255 |
| Lymph node dissection | 40.3 | 38525, 38740, 38745 |
| Radiotherapy | 92.2–92.29, 0330*, 0333* | 77400–77499, 77520–77525, 77750–77799 |
| History of breast cancer | V10.3 | — |
ICD-9 indicates International Classification of Diseases, Ninth Revision; CPT, Current Procedural Terminology.
Revenue Center code.
RT and Race
Patients were considered to have received breast RT if a claim for RT (Appendix A) occurred within 9 months of the breast cancer diagnosis date. This definition has been validated in prior studies of breast cancer patients.19–24 In addition, for patients classified as receiving RT, completion of the therapeutic course was defined as at least 3 complete weeks of therapy (5 treatments per week). Patient race was determined using theMedicare denominator file, which contains demographic information on each beneficiary. In this file, race and ethnicity data are based on the patients’ self-report. For our analysis, we categorized patients as white, black, and other (nonwhite, nonblack) race based on a prior study of the sensitivity and specificity of Medicare race and ethnicity groupings.25
Other Covariates
Demographic and clinical covariates were derived from Medicare files, including the denominator file and claims files. Demographic data included age at diagnosis and state of residence, with geographic regions based on the Census Divisions.26 Disease-related and treatment-related variables included axillary lymph node involvement; axillary lymph node dissection; sentinel lymph node biopsy; receipt of any chemotherapy; receipt of doxorubicin or paclitaxel; any staging imaging; number of hospitalizations in the year after diagnosis; and number of medical oncology, radiation oncology, and surgery visits in the year after diagnosis. Variables indicating preventive healthcare and interactions with the healthcare system included mammography in the year before diagnosis and number of physician visits in the year before diagnosis. In addition, we calculated the severity of comorbid disease for each patient based on a modified Charlson comorbidity score validated in a prior claims-based study: 0 (no comorbidity), 1 (mild to moderate), or ≥2 (severe).27 This score combined comorbidities recorded in Medicare claims during the 12 months before the patient’s cancer diagnosis. To enhance the specificity of comorbid disease diagnoses, patients must have had at least 1 inpatient (Part A) claim or at least 2 outpatient (Part B) claims more than 30 days apart.27 Socioeconomic covariates were derived from the 2003 Area Resource File28 linked to the Medicare files by county and state, and included rural/urban status, percentage of population (by county) living in poverty, median income, education level, density of surgeons, and density of radiation oncologists.
Statistical Analysis
All analyses were conducted using SAS software (version 9.1.3; SAS Institute Inc, Cary, NC), and all statistical tests assumed a 2-tailed a of 0.05. We calculated percentage RT use for the entire sample, by state and by region, and tested the unadjusted association between receipt of RT and race using the Pearson chi-square test. Bivariate associations between receipt of RT and other covariates were tested using the Pearson chi-square test for categoric variables and the Wilcoxon rank sum test for continuous variables. A multivariate logistic model tested the adjusted association between receipt of RT and race. Covariates were selected a priori based on significance in bivariate analyses (P < .25) and significance in prior studies of cancer patients.19-24 Goodness of fit was assessed using the Hosmer and Lemeshow test. The final parsimonious multivariate logistic model was then also used to calculate adjusted rates of RT use.29 A subsidiary model included socioeconomic covariates and tested the association in the subset of 33,172 patients who had socioeconomic covariate data available.
We further examined the association between receipt of RT and race in the subset of patients age <70 years (n = 7270), because prior evidence has suggested that the use of RT after BCS is more common in younger patients.5 In sensitivity analyses, we tested the association between race and receipt of RT in the subset of patients with a more restrictive (specific) definition of invasive breast cancer, which required the 2 diagnosis codes for invasive breast cancer to be ≥30 days apart (n = 33,114), and in a more restrictive definition of breast cancer, which excluded patients with any diagnosis code of ductal carcinoma in situ (DCIS) (ICD-9 diagnosis code 233.0) within 6 months of the invasive breast cancer diagnosis date (n = 22,301). We also tested a less restrictive treatment window of RT within 12 months of breast cancer diagnosis to take into account possible racial differences in treatment delays. Finally, in patients (of any age) who received RT, we also examined the association between completion of RT course and race.
RESULTS
Patient Characteristics
Of the 34,080 women with invasive breast cancer who were treated with BCS, 91% (N = 31,127) were white, 6% (N = 2077) were black, and 3% (N = 876) were another race. The mean age of the sample was 76 (standard deviation, 7) years. Approximately 73% of patients received RT after BCS, and 13% received chemotherapy as part of their initial treatment course. Approxmately 39% of patients had a claim indicating axillary lymph node dissection or axillary lymph node involvement, and 55% had a claim indicating sentinel lymph node biopsy (Table 2).
Table 2.
Sample Characteristics
| Characteristic | Percentage |
|---|---|
| Demographic | |
| Mean age (SD), y | 76 (7) |
| Race | |
| White | 91 |
| Black | 6 |
| Other | 3 |
| Region | |
| West, Pacific West | 11 |
| West, Mountain West | 5 |
| Midwest, West North Central | 7 |
| Midwest, East North Central | 19 |
| Northeast, New England | 7 |
| Northeast, Mid-Atlantic | 16 |
| South, South Atlantic | 21 |
| South, West South Central | 9 |
| South, East South Central | 5 |
| Not known | 1 |
| Clinical | |
| No comorbid diseases | 32 |
| Axillary lymph node involvement | 39 |
| Sentinel lymph node biopsy | 55 |
| Radiotherapy | 73 |
| Chemotherapy | 13 |
| Socioeconomic | |
| Mean income (SD), $US | 43,429 (10,649) |
| Education | |
| Mean percent completed less than ninth grade (SD) | 7 (4) |
| Mean percent completed college or higher (SD) | 24 (9) |
| Mean surgeon density (per 100,000) (SD) | 13 (10) |
| Mean radiation oncologist density (per 100,000) (SD) | 1.5 (1.4) |
SD indicates standard deviation.
Race and RT Use
Significant racial differences were found to exist in the receipt of RT; 74% of white women, 65% of black women, and 66% of other-race women received RT after BCS (P < .001). After adjustment for demographic, clinical, and socioeconomic covariates, white women were still significantly more likely than black women to have received RT after BCS (odds ratio [OR], 1.48; 95% confidence interval [95% CI], 1.34–1.63 [P < .001]). In addition, there was a trend toward white women also being more likely than other-race women to have received RT after BCS (OR, 1.22; 95% CI, 1.04–1.42 [P = .01]) (Table 3). Other variables found to be independently associated with higher rates of RT use included geographic region, younger age, lower comorbidity score, treatment with doxorubicin, axillary lymph node dissection, use of staging imaging, use of mammography before cancer diagnosis, and greater number of physician visits (interactions with the healthcare system) in the year before cancer diagnosis. In the subset of patients with complete socioeconomic covariate data available, the association between receipt of RT and race was unchanged. In this model, lower poverty level, higher median income, higher education level, higher surgeon density, and higher radiation oncologist density were also found to be associated with receipt of RT.
Table 3.
Racial Disparities in the Use of Radiotherapy After Breast-Conserving Surgery for Breast Cancer
| Model: RT use | OR | 95% CI | P |
|---|---|---|---|
| Unadjusted | |||
| White vs black patients | 1.52 | 1.38–167 | <.001 |
| White vs other* patients | 1.46 | 1.27–1.68 | <.001 |
| Adjusted† | |||
| White vs black patients | 1.48 | 1.34–1.63 | <.001 |
| White vs other* patients | 1.22 | 1.04–1.42 | .01 |
RT indicates radiotherapy; OR, odds ratio; 95% CI, 95% confidence interval.
† Adjusted for age, comorbidity, chemotherapy (doxorubicin or paclitaxel), axillary lymph node involvement, staging, imaging, surgeon visits, mammography, physician visits, and region.
Sensitivity analyses using more restrictive definitions of invasive breast cancer did not appear to alter the magnitude or significance of the associations between race and RT use significantly. Sensitivity analyses using a less restrictive treatment window also did not appear to alter the magnitude or significance of these associations.
In the subset of patients age <70 years, 82% received RT after BCS. Specifically, 83% of white women, 73% of black women, and 78% of other-race women received RT after BCS (P < .001). On multivariate analysis of this younger group, white women were still found to be significantly more likely than black women to have received RT after BCS (OR, 1.73; 95% CI, 1.40– 2.14 [P = .003]), although there was no significant difference noted between white women and other-race women (OR, 1.28; 95% CI, 0.86–1.90 [P = .22]). The interaction term between race (black vs white race) and age (age <70 years vs ≥70 years) was not found to be statistically significant (P = .19).
In the subset of patients who received RT and had the number of treatments documented, 85% were found to have completed a course of therapy, whereas 15% had an incomplete course. Approximately 85% of white women, 85% of black women, and 82% of other-race women completed their RT course (P = .02). After adjusting for covariates, there remained no significant difference in rates of completion by race (black vs white women: OR, 1.00; 95% CI, 0.83–1.20 [P = .99] and other-race vs white women: OR, 1.02; 95% CI, 0.73–1.43 [P = .89]).
Geographic Variation in Racial Disparities
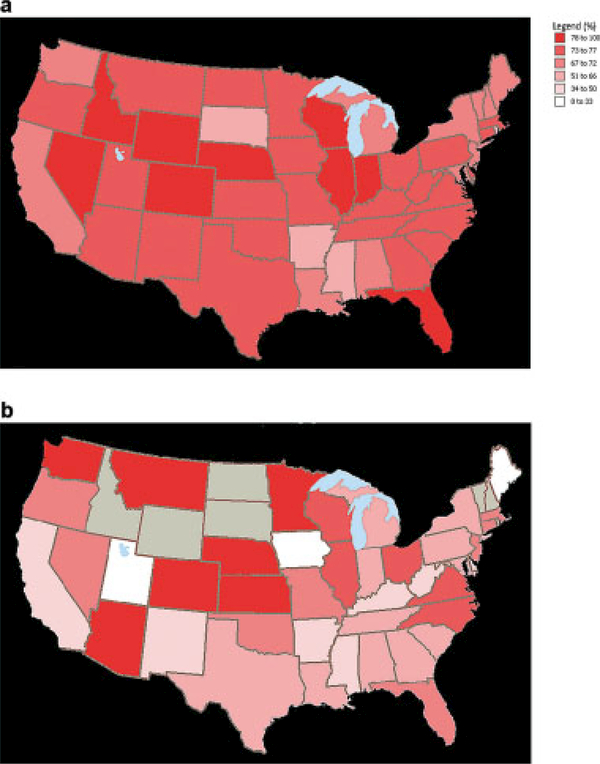
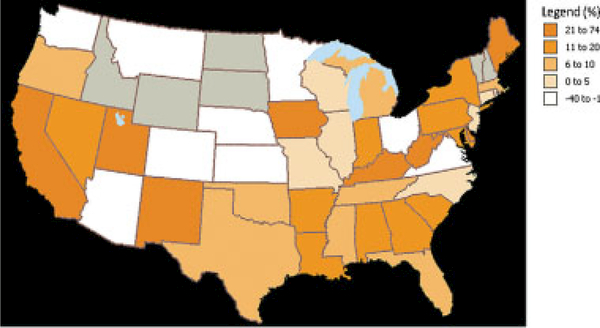
We observed significant geographic variations in racial disparities. The absolute difference in the percentage of RT use between white and black women by state ranged from −28% to 74%. However, for most states, the percentage of white women who received RT was greater than the percentage of black women who received RT, and in no state was the rate of RT use found to be significantly greater in black women compared with white women (Fig. 1). In areas of the northeastern and southern United States, the racial disparity between white and black women with regard to RT use was particularly pronounced (Fig. 2) (Table 4). Our data were insufficient to assess variations by state or region for other-race women.
Figure 1.
Percentage radiotherapy use in (a) white patients versus (b) black patients is shown. Gray shading indicates that the sample size was too small to provide meaningful data.
Figure 2.
Absolute difference in the rate of radiotherapy (RT) use between white and black patients is shown. An absolute difference <0% indicates that the percentage of black women receiving RT was greater than the percentage of white women. Gray shading indicates that the sample size was too small to provide meaningful data.
Table 4.
Unadjusted and Adjusted Regional Variations in the Rate of Radiotherapy Usage Between White and Black Patients With Breast Cancer
| Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
| Region | States | % Whites | % Blacks | P | % Whites | % Blacks | P |
| West, Pacific West | AK, CA, HI, OR, WA | 72 | 54 | <.001 | 74 | 61 | .07 |
| West, Mountain West | AZ, CO, ID, MT, NV, NM, UT, WY |
77 | 78 | .88 | 72 | 67 | .59 |
| Midwest, West North Central | IA, KS, MN, MO, NE, ND, SD |
74 | 73 | .94 | 74 | 70 | .67 |
| Midwest, East North Central | IL, IN, MI, OH, WI | 76 | 72 | .04 | 79 | 73 | .15 |
| Northeast, New England | CT, MA, NH, ME, RI, VT | 72 | 69 | .53 | 78 | 71 | <.001 |
| Northeast, Mid-Atlantic | NJ, NY, PA | 71 | 57 | <.001 | 70 | 62 | .01 |
| South, South Atlantic | DE, DC, FL, GA, MD, NC, SC, VA, WV |
76 | 68 | <.001 | 73 | 64 | .009 |
| South, West South Central | AR, LA, OK, TX | 74 | 63 | .001 | 69 | 59 | <.001 |
| South, East South Central | AL, KY, MS, TN | 72 | 57 | <.001 | 78 | 60 | .001 |
Adjusted for all covariates except state/region.
DISCUSSION
In our analysis of a national sample of US women aged ≥65 years who were diagnosed with invasive breast cancer, white women (74%) were significantly more likely than black women (65%) to receive RT after BCS. The results of the current study also suggested that white patients were also more likely than patients of other races (66%) to receive RT after BCS. Furthermore, even after we took into consideration other covariates such as patient comorbidity, white women were still nearly 50% more likely than black women to receive RT. The racial disparity between whites and blacks persisted even among the younger patients in the cohort, those age <70 years, in whom RT after BCS was more common. Our analysis also identified geographic variations in the magnitude of treatment disparities between white and black patients across the United States, although some degree of treatment disparity existed in most states. Furthermore, the disparities across the nation persisted even after adjusting for variations in socioeconomic factors such as income, education, and access to healthcare resources. The large magnitude of racial disparities in RT use identified in the current study is concerning given that the administration of RT after BCS in patients with early invasive breast cancer reduces the risk of disease recurrence and breast cancer-related death and is considered the standard of care.1–4,30
Other studies have also described disparities in breast cancer care between black patients and white patients.15 In a recent secondary analysis of the NAFTA trial, in which all patients in the study cohort were treated with BCS and hormonal therapy, 80% of all enrollees received RT. Similar to the 9% to 10% absolute difference noted in the current study, there was a 12% absolute difference found between white and black patients in the rate of RT use in the NAFTA study.5 A recent analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare cohort reported an 8% absolute difference between white and black patients with regard to the receipt of RT after BCS, with 86% of white women and 78% of black women between the ages of 66 and 85 years with invasive breast cancer receiving the treatment.31 A limitation of this study was that SEER-Medicare sampled ≤ 26% of the US Medicare population, compared with the current study, which sampled the Medicare population in its entirety.
A previous study of breast cancer patients who were treated in the state of Florida found that Hispanic women were significantly less likely to receive local therapy for breast cancer than non-Hispanic white women,14 whereas another study of patients in Hawaii found that Asian women may be less likely to receive therapy than white women.32 In our cross-sectional study, we were unable to explore the use of RT in specific nonwhite, nonblack ethnic groups because of the limited sample size of each ethnic group in our sample. Future analyses may seek to focus on exploring national patterns in potential treatment disparities affecting women of other racial/ethnic groups.
The underlying reasons for the racial disparities observed in the current study cohort remain to be determined.33 Prior studies have identified access to care and socioeconomic factors (such as the patients’ income and education, the cost of RT, or the availability of supplemental insurance) as factors that may influence racial disparities in cancer care,34,35 but even after adjustment for markers of healthcare access in the current analysis, such as number of physician visits and use of mammography before cancer diagnosis, racial disparities persisted. Other unmeasured potential explanatory factors include the impact of the physician-patient interaction.36 For example, it is not known whether physicians offer treatment less frequently to nonwhite patients, whether substandard care occurs more frequently in predominantly nonwhite communities, or whether nonwhite patients are more likely to decline treatment. Additional social factors, such as culturally specific health beliefs, the presence of social support, and marital status,37 also could affect racial disparities in care and may be important variables to explore in future studies seeking to identify specific barriers in breast cancer care.
The underlying causes of the geographic variations in racial disparities also require further study. Several published analyses have indicated that geographic variations in breast cancer care exist,7,8,38 but to the best of our knowledge no prior study has described how geographic patterns in care may be modified by patient race. Our analysis found that disparities between white and black patients in RT after BCS appeared most pronounced in the northeastern and southern regions of the United States. Given that racial disparities do not appear to be concentrated in a single area of the country, the underlying reasons for disparities in the different regions of the United States are likely to be varied, complex, and multifactorial. For example, rural/urban differences may play a role in the geographic variations in treatment disparities. Prior studies have identified patients’ distance from RT facilities as a potential barrier to treatment,12,39 and suggested that patients living in rural areas may have less access to RT.39,40 However, a recent study reported similar rates of RT use after mastectomy and after lumpectomy in urban versus rural locales.41 The study’s results suggest that disadvantaged patients in either setting may experience distinct but persistent treatment barriers. Prospective efforts to overcome disparities in cancer treatment, such as the Patient Navigation program,42 are currently being evaluated and may serve as an important source for identifying the key barriers to cancer care in different geographic regions of the United States.
The current study has limitations to consider. Our sample focused on older breast cancer patients with continuous fee-for-service Medicare Parts A and B insurance coverage. Therefore, future studies will be required to validate the magnitude and significance of racial disparities in the treatment of younger patients. In addition, studies are also needed to determine the magnitude of racial disparities among patients with other insurance status, such as those with Medicaid, given that prior evidence suggests that patients with Medicare insurance coverage are actually more likely to receive appropriate breast cancer treatment.43 Furthermore, our definitions of invasive breast cancer and RT were claims-based and, therefore, may be subject to misclassification bias. However, the results of the current study demonstrated reproducibility, even after applying the more restrictive criteria to exclude potential metastatic disease and DCIS. Prior studies indicate a high degree of validity for claims-based treatment variables and suggest that, although our treatment definition was unlikely to be 100% sensitive, our percentage use may underestimate true use rates by only approximately 2%.37 Finally, the racial distribution of patients in the current study sample differs from the general population distribution. Notably, 6% of our sample was black, whereas 9% of US women (age >65 years) are black.26 This difference is likely due to the tendency of nonwhite patients to receive mastectomy over BCS even for the treatment of early stage disease.44–46
Conclusions
To the best of our knowledge, the current study provides 1 of the first available data sets describing a quality indicator for breast cancer care across a comprehensive national sample of older breast cancer patients and was able to identify substantial racial disparities in care across the United States. Our analysis helps define the scope of the treatment disparities in RT after BCS and underscores the concern that this treatment disparity occurs not merely in isolation but is instead a problem that exists on a national scale. Future efforts to improve breast cancer care will require identifying and overcoming the underlying causes of these racial disparities. As additional data become available, future studies may also explore changes in the magnitude of disparities over time and the effect of these disparities in care on breast cancer outcomes.
Acknowledgments
We thank Ms. Alyson Todd (funding by Department of Scientific Publications, The University of Texas M. D. Anderson Cancer Center) for her assistance in editing of this article.
Supported by the Department of Defense Multidisciplinary Postdoctoral Award; and the Odyssey Program and Theodore N. Law Endowment for Scientific Achievement at The University of Texas M. D. Anderson Cancer Center (G.L.S.).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
REFERENCES
- 1.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year followup of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347: 1233–1241. [DOI] [PubMed] [Google Scholar]
- 3.Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol. 2005; 28:289–294. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 5.Chagpar AB, McMasters KM, Scoggins CR, Martin RC Jr,Thoene C, Edwards MJ. The use of radiation therapy after breast-conserving surgery in hormonally treated breast cancer patients is dependent on patient age, geographic region, and surgeon specialty. Am J Surg. 2008;195:793–798. [DOI] [PubMed] [Google Scholar]
- 6.Ballard-Barbash R, Potosky AL, Harlan LC, Nayfield SG,Kessler LG. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996;88:716–726. [DOI] [PubMed] [Google Scholar]
- 7.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz TA, Theriault RL, Niland JC, et al. The use of radiation as a component of breast conservation therapy in National Comprehensive Cancer Network Centers. J Clin Oncol. 2006;24:361–369. [DOI] [PubMed] [Google Scholar]
- 9.Gold HT, Dick AW. Variations in treatment for ductal carcinoma in situ in elderly women. Med Care. 2004;42: 267–275. [DOI] [PubMed] [Google Scholar]
- 10.Griggs JJ, Culakova E, Sorbero ME, et al. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25:277–284. [DOI] [PubMed] [Google Scholar]
- 11.Katz SJ, Lantz PM, Janz NK, et al. Patterns and correlates of local therapy for women with ductal carcinoma-in-situ. J Clin Oncol. 2005;23:3001–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punglia RS, Weeks JC, Neville BA, Earle CC. Radiation therapy after mastectomy between 1991 and 1999 in elderly women: response to clinical trial information. J Clin Oncol. 2006;24:3474–3482. [DOI] [PubMed] [Google Scholar]
- 13.Richardson LC, Tian L, Voti L, et al. The roles of teaching hospitals, insurance status, and race/ethnicity in receipt of adjuvant therapy for regional-stage breast cancer in Florida. Am J Public Health. 2006;96:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voti L, Richardson LC, Reis I, Fleming LE, Mackinnon J, Coebergh JW. The effect of race/ethnicity and insurance in the administration of standard therapy for local breast cancer in Florida. Breast Cancer Res Treat. 2006;95:89–95. [DOI] [PubMed] [Google Scholar]
- 15.Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. [DOI] [PubMed] [Google Scholar]
- 16.Research Data Assistance Center Medicare Data File Descriptions. Available at: http://www.resdac.umn.edu/Medicare Accessed December 2008.
- 17.Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold HT, Do HT. Evaluation of three algorithms to identify incident breast cancer in Medicare claims data. Health Serv Res. 2007;42:2056–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–690. [DOI] [PubMed] [Google Scholar]
- 20.Smith BD, Haffty BG, Buchholz TA, et al. Effectiveness of radiation therapy in older women with ductal carcinoma in situ. J Natl Cancer Inst. 2006;98:1302–1310. [DOI] [PubMed] [Google Scholar]
- 21.Smith BD, Haffty BG, Smith GL, Hurria A, Buchholz TA, Gross CP. Use of postmastectomy radiotherapy in older women. Int J Radiat Oncol Biol Phys. 2008;71:98–106. [DOI] [PubMed] [Google Scholar]
- 22.Smith BD, Smith GL, Buchholz TA. Controversies over the role of radiation therapy for ductal carcinoma in situ. Expert Rev Anticancer Ther. 2008;8:433–441. [DOI] [PubMed] [Google Scholar]
- 23.Smith BD, Smith GL, Haffty BG. Postmastectomy radiation and mortality in women with T1–2 node-positive breast cancer. J Clin Oncol. 2005;23:1409–1419. [DOI] [PubMed] [Google Scholar]
- 24.Smith GL, Smith BD, Giordano SH, et al. Risk of hypo-thyroidism in older breast cancer patients treated with radiation. Cancer. 2008;112:1371–1379. [DOI] [PubMed] [Google Scholar]
- 25.Arday SL, Arday DR, Monroe S, Zhang J. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 26.US Census Bureau. Available at: http://www.census.gov. Accessed December 2008
- 27.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 28.Health Resources and Services Administration. Area Resource File. Available at: http://www.arfsys.com/overview. htm Accessed February 2009.
- 29.Hannan EL, Wu C, DeLong ER, Raudenbush SW. Predicting risk-adjusted mortality for CABG surgery: logistic versus hierarchical logistic models. Med Care. 2005;43:726–735. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. Accessed January 2009
- 31.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelber RP, McCarthy EP, Davis JW, Seto TB. Ethnic disparities in breast cancer management among Asian Americans and Pacific Islanders. Ann Surg Oncol. 2006;13:977–984. [DOI] [PubMed] [Google Scholar]
- 33.Freedman RA, Winer EP. Reducing disparities in breast cancer care: a daunting but essential responsibility. J Natl Cancer Inst. 2008;100:1661–1663. [DOI] [PubMed] [Google Scholar]
- 34.Fontana V, Castro T, Polynice A. Preferences of healthy inner city women and the surgical treatment of early stage breast cancer. Am Surg. 2007;73:215–221. [PubMed] [Google Scholar]
- 35.Shih YC, Zhao L, Elting LS. Does Medicare coverage of colonoscopy reduce racial/ethnic disparities in cancer screening among the elderly? Health Aff (Millwood). 2006; 25:1153–1162. [DOI] [PubMed] [Google Scholar]
- 36.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportu-nities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25:2516–2521. [DOI] [PubMed] [Google Scholar]
- 37.Smith BD, Smith GL, Roberts KB, Buchholz TA. Baseline utilization of breast radiotherapy prior to institution of the Medicare Practice Quality Reporting Initiative. Int J Radiat Oncol Biol Phys. In press. [DOI] [PubMed] [Google Scholar]
- 38.Smith GL, Smith BD, Haffty BG. Rationalization and regionalization of treatment for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys. 2006;65:1397–1403. [DOI] [PubMed] [Google Scholar]
- 39.Schroen AT, Brenin DR, Kelly MD, Knaus WA, Slingluff CL Jr. Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. J Clin Oncol. 2005;23:7074–7080. [DOI] [PubMed] [Google Scholar]
- 40.Hershman DL, Buono D, McBride RB, et al. Surgeon characteristics and receipt of adjuvant radiotherapy in women with breast cancer. J Natl Cancer Inst. 2008;100: 199–206. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs LK, Kelley K, Chang D. Radiation therapy in urban and rural breast cancer patients [abstract]. Paper presented at 2008. ASCO Annual Meeting, May 30-June 3, 2008; Chicago, Illinois Abstract 204A. [Google Scholar]
- 42.National Cancer Institute. NCI’s Patient Navigator Research Program. Available at: http://www.cancer.gov/cancertopics/fact-sheet/PatientNavigator Accessed December 2008.
- 43.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J,Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–231. [DOI] [PubMed] [Google Scholar]
- 44.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–1107. [DOI] [PubMed] [Google Scholar]
- 45.Gilligan MA, Kneusel RT, Hoffmann RG, Greer AL, Nattinger AB. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care. 2002;40:181–189. [DOI] [PubMed] [Google Scholar]
- 46.Michalski TA, Nattinger AB. The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer. 1997;79(suppl): 314–319. [PubMed] [Google Scholar]