Abstract
Aim
To describe factors associated with transfer from paediatric to adult care and poor glycaemic control among young adults with Type 2 diabetes, using the SEARCH for Diabetes in Youth study.
Methods
Young adults with Type 2 diabetes were included if they had a baseline SEARCH visit while in paediatric care at < 18 years and ≥ 1 follow-up SEARCH visit thereafter at 18–25 years. At each visit, HbA1c, BMI, self-reported demographic and healthcare provider data were collected. Associations of demographic factors with transfer of care and poor glycaemic control (HbA1c ≥ 75 mmol/mol; 9.0%) were explored with multivariable logistic regression.
Results
182 young adults with Type 2 diabetes (36% male, 75% minority, 87% with obesity) were included. Most (n = 102, 56%) reported transfer to adult care at follow-up; a substantial proportion (n = 28, 15%) reported no care and 29% did not transfer. Duration of diabetes [odds ratio (OR) 1.4, 95% confidence interval (95% CI) 1.1, 1.8] and age at diagnosis (OR 1.8, 95% CI 1.4, 2.4) predicted leaving paediatric care. Transfer to adult or no care was associated with a higher likelihood of poor glycaemic control at follow-up (adult: OR 4.5, 95% CI 1.8, 11.2; none: OR 4.6, 95% CI 1.4, 14.6), independent of sex, age, race/ethnicity or baseline HbA1c level.
Conclusions
Young adults with Type 2 diabetes exhibit worsening glycaemic control and loss to follow-up during the transfer from paediatric to adult care. Our study highlights the need for development of tailored clinical programmes and healthcare system policies to support the growing population of young adults with youth-onset Type 2 diabetes.
Introduction
Type 2 diabetes in youth has increased steadily on a global scale in the past decade, demanding attention to address this evolving public health emergency [1–4]. In the USA, from 2001 to 2009, the SEARCH for Diabetes in Youth Study (SEARCH) estimated a 30.5% overall increase in the prevalence of Type 2 diabetes in US youth [3]. Compared with adult-onset Type 2 diabetes, youth tend to have more severe disease requiring earlier initiation of insulin, and experience more micro- and macrovascular complications as well as higher mortality [5–8]. Thus, this growing population has the potential to place significant burden on health systems. The American Diabetes Association (ADA) recently published a consensus report on youth with Type 2 diabetes, citing an ‘urgent need for targeted treatments and patient-centered care in what appears to be a more aggressive disease in youth’ [9]. However, thus far, efforts to understand patient-centered issues remain limited.
Diabetes management requires continued lifestyle modification and effective treatments, which need to be developmentally tailored to youth. The period of young adulthood, defined as ages 18–30 years, is a stage of life that involves progressively higher levels of independence from the family unit and the assumption of more responsibility for self-care [10]. For young adults with chronic disease, self-management and health-related care tasks present a particular challenge during this transitional period, which is rife with competing demands of geographical and educational/vocational changes, as well as social and financial priorities [10]. Studies in young adults with chronic disease have demonstrated that healthcare transition from paediatric to adult care places additional burden and may result in loss to follow-up, deterioration of disease control, increased hospitalizations and early mortality [11–15]. The vast majority of research has focused on Type 1 diabetes and healthcare transition, because of its historical predominance in paediatric diabetes. Given the growing population of paediatric cases of Type 2 diabetes, it is imperative to understand how healthcare transition affects this population, which has differing treatment paradigms and needs than Type 1 diabetes.
The objective of this study was to determine how the healthcare transition affects outcomes in young adults with Type 2 diabetes. Specifically, we examined factors associated with transfer and poor glycaemic control after leaving paediatric care, using data from SEARCH. We hypothesized that the healthcare transition would increase the risk for loss to medical follow-up and deterioration of glycaemic control.
Patients and methods
Study overview and procedures
SEARCH is a multicentre study aimed at understanding the burden and clinical course of diabetes among youth in the USA. The SEARCH study has identified over 20 000 individuals from 2000 to 2015 who were diagnosed with diabetes at < 20 years of age. Youth were recruited from five centres based on geographical sites in the USA: Ohio, Colorado, Washington, South Carolina and California [16]. Institutional review boards for each of the study sites approved this study protocol.
As part of the SEARCH protocol, participants completed a brief initial survey, after which those with diabetes not secondary to other conditions (steroid-induced or genetic cause) were invited to a research visit where consent/assent were obtained, questionnaires administered, and physical exam and fasting blood samples taken. Individuals in selected incident years were invited for follow-up visits, in which a physical exam was conducted, and additional questionnaires and blood samples were obtained. In addition to demographic and clinical characteristics, questionnaires included information on type of provider, which was used in the current study to define transfer of care.
Study population and eligibility
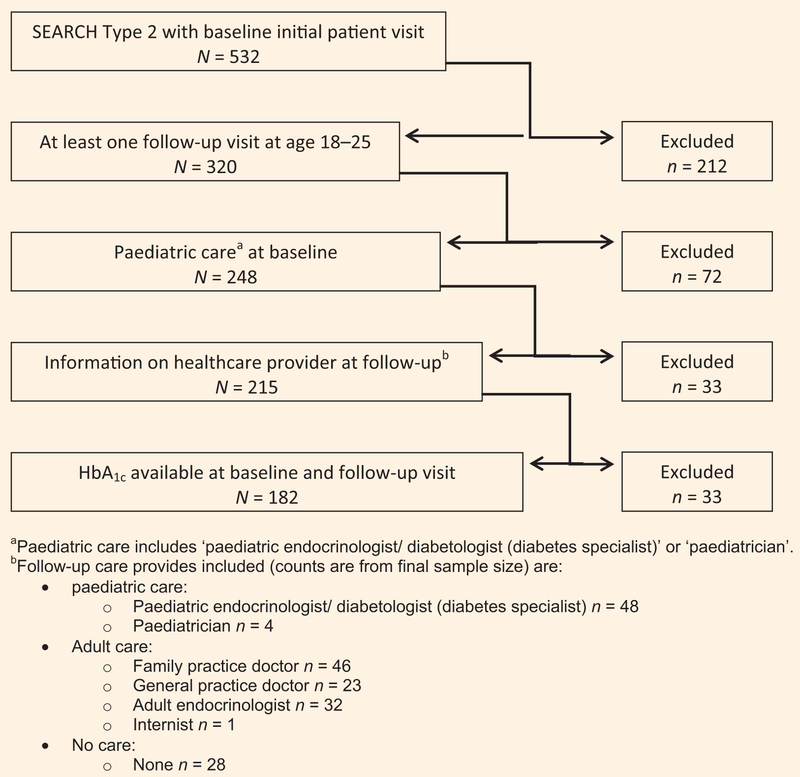
SEARCH participants were included in these analyses if they were diagnosed with Type 2 diabetes by their healthcare provider between 2002 and 2005, had an initial SEARCH visit before age 18 years while in paediatric care, and had at least one follow-up SEARCH visit thereafter at ages 18–25 years. Follow-up visits spanned from 2005 to 2015. If more than one follow-up visit was available, the first visit where a non-paediatric provider was reported was used. If all visits indicated paediatric care providers, the last visit was used for the analysis. Only one follow-up visit was used for all analysis. Participants were included if they had available information on HbA1c levels and type of healthcare provider at both the baseline and follow-up visits (Fig. 1).
FIGURE 1.
Inclusion criteria for analysis.
Some 532 SEARCH participants had Type 2 diabetes diagnosed between 2002 and 2005, and completed an initial study visit prior to 18 years of age; 212 were excluded because they did not have a follow-up SEARCH visit after age 18 years. Of the remaining 320 participants, 72 were excluded because they did not report paediatric care at the initial visit, and 33 were excluded because there was no information on healthcare provider at follow-up. Of the 215 remaining participants, 33 did not have HbA1c values available at the baseline and follow-up visits. A total of 182 participants were included for analysis (Fig. 1).
Variables
Demographic characteristics
Demographic characteristics collected as part of the initial SEARCH visit included age at diagnosis, age at the study visit and sex. Race/ethnicity was self-reported using the 2000 census question and categorized as non-Hispanic white, non-Hispanic black, Hispanic and other race/ethnicity. Highest parental education was self or parent-reported during the initial visit. Health insurance was reported at each visit as ‘private’; ‘Medicaid/Medicare/other’ (other state-funded plans, Indian Health Service, student health clinics, military or other/unknown sources) and ‘none’.
Clinical characteristics
BMI was calculated as weight in kilograms divided by height in metres squared and converted to a z-score [17]. Diabetes duration was calculated as the difference between the diagnosis date and each visit date. Concurrent medical comorbidity was self-reported based on pre-specified categories and included asthma, renal disease, coeliac disease, hypertension, hyperthyroidism, hypothyroidism and polycystic ovary syndrome. HbA1c was measured in whole blood with an automated nonporous ion-exchange high-performance liquid chromatography system (model G-7; Tosoh Bioscience, Montgomeryville, PA, USA) on blood samples obtained at baseline and follow-up visits.
Healthcare provider characterization
The current healthcare provider was self-reported in prespecified categories at each visit. Paediatric care was assigned if participants selected ‘paediatrician’ or ‘paediatric endocrinologist’ as their healthcare provider at the time of the SEARCH visit. Adult care was assigned if participants selected ‘family practice doctor’, ‘general practice doctor’, ‘adult endocrinologist’ or ‘internist’. Participants who selected ‘nurse practitioner/PA’, ‘nurse diabetes educator’ or ‘other/don’t know’ were excluded (n = 33) because it could not be determined whether nurse practitioners or physician assistants were affiliated with paediatric or adult care. Participants could also select ‘none’, which was assigned to the ‘no care’ group.
Outcomes
‘Leaving paediatric care’ was defined as reporting a non-paediatric provider or no provider at any SEARCH follow-up visit when participants were ≥ 18 years. If the participant reported that they had a paediatric provider at the last SEARCH follow-up visit on record at age ≥ 18 years, they were considered as not having left pediatric care. Glycaemic control was measured as HbA1c. Poor glycaemic control was defined as HbA1c ≥ 75 mmol/mol (9.0%) based on data from the Diabetes Control and Complications Trial, which found elevated risk of complications for young adults (age > 18 years) with HbA1c levels above this range [18].
Statistical analyses
All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA). Descriptive statistics were used to explore baseline characteristics, stratified by type of healthcare provider at follow-up. Multivariable logistic regression models were used to identify predictors of the two outcomes of interest: (1) leaving paediatric care, and (2) poor glycaemic control at follow-up. Variables included in the multivariable models were selected a priori based on their established contribution to glycaemic control. They included age at follow-up, sex, race/ethnicity (Hispanic/non-Hispanic black/ other vs. non-Hispanic white), HbA1c at baseline, duration of diabetes at follow-up and paediatric vs. adult vs. no care at follow-up. More specific racial/ethnic subgroups and insurance status could not be added to models due to sample size. Models were adjusted for SEARCH study site. The distribution of the residuals for the final models were reviewed for outliers and leverage points. Sensitivity analysis conducted after removing questionable observations arrived at similar results as the primary analysis.
Results
Participant characteristics
For the 182 individuals included in analyses, mean age at diagnosis was 14.2 years and diabetes duration at the baseline SEARCH visit was 6.9 months. Thirty-six per cent of participants were male (n = 65). The participants were racially and ethnically diverse; 45% (n = 82) were non-Hispanic black, 25% (n = 46) were non-Hispanic white, 25% (n = 45) were Hispanic, and 5% (n = 9) were ‘other’ race/ethnicities. At baseline, 58% (n = 104) were privately insured, 41% (n = 75) were publicly insured, and 1% (n = 3) were uninsured. Fifty-one per cent (n = 92) had received high school education or less, 33% (n = 59) had some college education, and 17% (n = 30) had at least a bachelor’s degree. Mean baseline HbA1c was 53 mmol/mol (7.0%) and 15% (n = 28) had poor glycaemic control at baseline. The majority (87%) were obese (Table 1).
Table 1.
Descriptive characteristics of individuals with Type 2 diabetes, overall and by healthcare provider at baseline and follow-up study visits (N = 182)
| Characteristics | Baseline | Healthcare provider at follow-up visit |
|||
|---|---|---|---|---|---|
| Paediatric | Adult | No care | P-value | ||
| Total | 182 | 52 (28.6) | 102 (56.6) | 28 (15.3) | |
| Age at diagnosis a (years) * | 14.2 ± 2.1 | 13.2 ± 2.3 | 14.7 ± 1.9 | 14.3 ± 1.9 | < 0.0001 |
| Duration of follow-up (years)* | 7.2 ± 0.7 | 6.8 ± 0.9 | 7.1 ± 0.6 | 7.6 ± 0.8 | < 0.0001 |
| Male sex a | 65 (35.7) | 19 (36.5) | 34 (33.3) | 12 (42.9) | 0.6410 |
| Race/ethnicity a | 0.3826 | ||||
| Non-Hispanic white | 46 (25.3) | 17 (32.7) | 20 (19.6) | 9 (32.1) | |
| Non-Hispanic black | 82 (45.1) | 23 (44.2) | 47 (46.1) | 12 (42.9) | |
| Hispanic | 45 (24.7) | 9 (17.3) | 29 (28.4) | 7 (25.0) | |
| Other | 9 (4.9) | 3 (5.8) | 6 (5.9) | 0 (0.0) | |
| Insurance status at visit | < 0.0001 | ||||
| Has private health insurance | 104 (57.5) | 25 (47.1) | 48 (46.5) | 1 (4.3) | |
| Medicaid/Medicare/other | 75 (40.9) | 23 (45.1) | 39 (38.6) | 6 (21.7) | |
| Uninsured | 3 (1.6) | 4 (7.8) | 15 (14.9) | 21 (73.9) | |
| Highest parental educationa | 0.2003 | ||||
| High school or less | 92 (50.8) | 28 (53.8) | 45 (44.6) | 19 (67.9) | |
| Some college | 59 (32.6) | 14 (26.9) | 39 (38.6) | 6 (21.4) | |
| Bachelor’s degree or more | 30 (16.6) | 10 (19.2) | 17 (16.8) | 3 (10.7) | |
| Diabetes duration at visit, months* | 9.8 ± 6.8 | 85.0 ± 21.1 | 82.1 ± 24.7 | 91.5 ± 24.9 | 0.1801 |
| HbA1c at baseline (mmol/mol [%])* | 53 ± 9 [7.0 ± 1.9] | 49 ± 6 [6.6 ± 1.6] | 54 ± 10 [7.1 ± 2.0] | 55 ± 11 [7.2 ± 2.1] | 0.2002 |
| HBA1c at follow-up (mmol/mol [%])* | – | 64 ± 13 [8.0 ± 2.8] | 75 ± 15 [9.0 ± 2.8] | 78 ± 20 [9.3 ± 3.8] | 0.0823 |
| Poor glycaemic control at visit c | 28 (15.4) | 14 (26.9) | 54 (52.9) | 15 (53.6) | 0.0060 |
| Have a comorbidity at visit b | 54 (40.6) | 35 (67.3) | 61 (59.8) | 16 (57.1) | 0.5800 |
| BMI categories at visit d | 0.4913 | ||||
| Normal or underweight | 12 (6.8) | 8 (15.7) | 10 (9.8) | 2 (7.1) | |
| Overweight | 11 (6.2) | 6 (11.8) | 18 (18.0) | 7 (25.0) | |
| Obese | 154 (87.0) | 37 (72.5) | 72 (72.0) | 19 (67.9) | |
| Number self-reported clinical | 2.4 ± 4.6 | 2.6 ± 2.4 | – | 0.7090 | |
| visits in 6 months prior to | |||||
| follow-up SEARCH visit*e | |||||
| Type of diabetes medication(s) | 0.0295 | ||||
| Metformin only | 81 (44.5) | 11 (21.2) | 22 (21.6) | 4 (14.3) | |
| Insulin only | 23 (12.6) | 12 (23.1) | 27 (26.5) | 6 (21.4) | |
| Insulin + other medication | 54 (29.7) | 14 (26.9) | 27 (26.5) | 1 (3.6) | |
| Other oral (sulfonylurea, incretin) | 12 (6.6) | 3 (5.8) | 10 (9.8) | 4 (14.3) | |
| None | 12 (6.6) | 12 (23.1) | 16 (15.7) | 13 (46.4) | |
Values are given as number (%), except
mean sd.
Measured only at baseline; one person missing data.
Have a medical comorbidity: presence of at least one of the following physician-diagnosed conditions (as reported by study participants): asthma, polycystic ovarian disease, kidney disease, celiac disease, hypertension, hyperthyroidism, or hypothyroidism.
Poor glycaemic control: HbA1c ≥ 75 mmol/mol (9.0%).
For participants aged under 18 years, BMI groups are defined by using z-score based on age and sex norms, and for those 18 and older groups are based on BMI score (normal or underweight < 85th percentile OR BMI < 25; overweight: 85th to 95th percentiles OR BMI between ≥ 25 and < 30; obese: 95th percentile or higher OR BMI ≥ 30.
Some missing data (n = 151; paediatric n = 49, adult n = 94, no care n = 8).
P-values are from Chi-square test for categorical measures and Type 3 P-value from an ANOVA for continuous measures.
Characteristics by care at follow-up
Most participants (n = 102, 57%) reported transfer to adult care at follow-up; a substantial proportion (n = 28, 15%) reported no care and 28% (n = 52) had not transferred out of paediatric care. Male sex, racial/ethnic minority proportions, and parental education were similar across the three care groups (paediatric care, adult care, none) (P > 0.05) (Table 1). The major difference between groups was in health insurance status; 74% of participants in the no care group were uninsured compared with 15% and 8% in the adult and paediatric care groups, respectively (P < 0.0001; Table 1).
Average duration of follow-up time after the SEARCH baseline visit ranged from 6.8 to 7.6 years, and was ~ 6 months longer for the no care group (Table 1). At follow-up, HbA1c levels were higher for those in adult care (75 mmol/mol; 9.0%) and no care (78 mmol/mol; 9.3%) groups compared with those in the paediatric care group (64 mmol/mol; 8.0%) (P = 0.0402 and 0.0772, respectively) (Table 1). This resulted in higher proportions of participants with poor glycaemic control, similar to baseline levels, in the adult (52.9%) and no care (53.6%) groups compared with the paediatric care group (26.9%) (P = 0.0022 and 0.0277, respectively) (Table 1). Mean number of medical visits in the previous 6 months did not differ between the adult and paediatric care groups (2.4 and 2.6 visits per year, respectively) (Table 1). There was also no significant difference in the likelihood of poor glycaemic control in specialty (59%) vs. primary (50%) adult care (P = 0.40) (data not shown). Forty-six per cent of the no care group was not taking diabetes medication, compared with 23% and 16% in the paediatric and adult care groups, respectively (Table 1).
Factors associated with transfer from paediatric care
Table 2 presents factors associated with transferring out of paediatric care to either adult care or no care. Higher age at diagnosis (per year) and diabetes duration at follow-up (per year) were each associated with higher likelihood (1.8 and 1.4, respectively) of leaving compared with remaining in paediatric care (P < 0.0001, P = 0.006), after adjusting for race/ethnicity, sex, and baseline HbA1c (Table 2). There was no significant interaction between age at diagnosis and diabetes duration (data not shown). Race/ethnicity and sex, were not significantly associated with transfer from paediatric care (Table 2). There was a trend towards worse baseline glycaemic control and higher likelihood of transfer to adult care. Likely, this might have been significant, but this study may have been inadequately powered to detect these differences.
Table 2.
Factors predicting transfer from paediatric care to either adult care or no care at follow-up study visit
| Adjusted results (N = 182) | |||
|---|---|---|---|
| Variable | Odds ratio | 95% CI | P-value |
| Duration of diabetes at follow-up (years) |
1.4 | 1.1, 1.8 | 0.0064 |
| Age at diagnosis (years) | 1.8 | 1.4, 2.4 | < 0.0001 |
| Sex (female vs. male) | 1.7 | 0.8, 3.7 | 0.1986 |
| Race/ethnicity (Hispanic/non-Hispanic black/other vs. non-Hispanic white) |
1.5 | 0.6, 3.4 | 0.3510 |
| HbA1c at baseline | 1.2 | 1.0, 1.5 | 0.0824 |
Model controls for all variables presented in the table plus SEARCH study site. P-values are type 3 chi-square tests of the model’s maximum likelihood estimates.
Poor glycaemic control at follow-up
Transferring from paediatric care was associated with a 4.5 and 4.6 higher odds of poor glycaemic control at follow-up in adult and no care, respectively, after adjusting for baseline HbA1c, age at diagnosis, duration of diabetes, sex, and race/ethnicity (Table 3). Additionally, poor glycaemic control at baseline [per unit increase above target value of 53 mmol/ mol (7%)] was associated with a greater likelihood of having poor glycaemic control at follow-up, regardless of transfer status [odds ratio (OR) = 1.4, P < 0.001) (Table 3). Proportions of participants taking insulin (insulin alone or insulin + other medication) did not differ by care group at follow-up, but those taking insulin were more likely to have poor glycaemic control (P < 0.0001) (data not shown). Increasing age at diagnosis was protective against poor glycaemic control (OR = 0.8, P = 0.01), independent of sex, race/ ethnicity, and insulin treatment (Table 3). Duration of diabetes at follow-up, race/ethnicity and sex were not associated with poor glycaemic control (Table 3).
Table 3.
Factors predicting poor glycaemic control (HbA1c ≥ 75 mmol/mol [9.0%]) at follow-up study visit
| Adjusted results (N = 182) | |||
|---|---|---|---|
| Variable | Odds ratio | 95% CI | P-value |
| Duration of diabetes at follow-up (years) |
0.9 | 0.7, 1.1 | 0.2856 |
| Age at diagnosis (years) | 0.8 | 0.6, 0.9 | 0.0099 |
| Sex (female vs. male) | 1.1 | 0.5, 2.2 | 0.8582 |
| Race/ethnicity (Hispanic/non-Hispanic black/other vs. non-Hispanic white) |
1.1 | 0.5, 2.4 | 0.8339 |
| HbA1c at baseline (%) | 1.4 | 1.1, 1.7 | 0.0011 |
| Provider at follow-up | 0.0035 | ||
| visit (reference: paediatric) | |||
| Adult care | 4.5 | 1.8, 11.2 | |
| No care | 4.6 | 1.4, 14.6 | |
Model controls for all variables presented in the table plus SEARCH study site. P-values are type 3 chi-square tests of the model’s maximum likelihood estimates.
Discussion
In this geographically and ethnically diverse sample from the SEARCH for Diabetes in Youth study, 57% of young with Type 2 diabetes transferred from paediatric to adult care after age 18, but a substantial proportion (15%) reported receiving no medical care. There were few demographic or clinical characteristics that predicted transfer status or age at transfer. Leaving paediatric for adult care or no care was associated with 4.5 and 4.6 times higher odds of poor glycaemic control, respectively, regardless of baseline control, sex, race/ethnicity, age at diagnosis and duration of diabetes. Poor glycaemic control at baseline predicted poor glycaemic control at follow-up, regardless of transfer status. To our knowledge, this is the first report of healthcare transition trends in youth with Type 2 diabetes, and sheds light on how the transition period influences risk specific to Type 2 diabetes populations.
Although it is encouraging that the majority of young adults transferred to adult care by age 25 years, it is concerning that a large proportion experienced significantly worsened glycaemic control at follow-up. Based upon earlier reports from SEARCH on transition to adult care among young adults with Type 1 diabetes, young adults were similarly more likely to have poor glycaemic control after transfer to adult care [15]. However, the likelihood estimate of worsened glycaemic control was more pronounced in our current study of young adults with Type 2 diabetes [OR 4.5, 95% confidence interval (95% CI) 1.8–11.2] than in the prior report in Type 1 diabetes (OR 2.5, 95% CI 1.09–5.55) [15], highlighting potentially worse outcomes in the young adults Type 2 diabetes population. This is alarming given the increasing burden of youth with Type 2 diabetes on healthcare systems.
Many reasons exist to explain the phenomenon of worsened control observed among young adults who transferred to adult care in our study. Specifically, the transition to adulthood superimposed on the more severe disease process of youth-onset Type 2 diabetes poses particular challenges. Because of competing priorities at this time in life and loss of structural supports in family and school, gaps in medical care could occur after leaving paediatric care in which disease maintenance with medication and self-care decrease, leading to unintended exacerbations [19–21]. Studies have determined that a gap of at least 6 months can significantly influence outcomes during transition [19,22], and might explain why the adult care and no care groups in our study had similarly poor glycaemic profiles. Additionally, general lack of engagement of young adults in their health care due to feelings of invulnerability to long-term complications could lead to decreased frequency of medical visits or inadequate self-care [10,21]. Our data might also represent the natural progression of youth-onset Type 2 diabetes. The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study showed that β-cell pancreatic failure was accelerated in young adults with youth-onset Type 2 diabetes and that acute worsening of glycaemic control occurred despite insulin use [23]. Thus, even with proper access to and use of diabetes care, biological variables may be influential. Lastly, primary care practices, who usually treat adult-onset Type 2 diabetes, may not be adequately trained or prepared to manage the more aggressive disease course of youth-onset Type 2 diabetes [24–26]. Early efforts to train adult specialists and generalists alike on both the influential developmental aspects of the transition to adulthood and management and risk stratification of youth-onset vs. adult-onset Type 2 diabetes would be beneficial and could prevent unnecessary deterioration. Furthermore, recognition of the need for extra psychosocial support for this population would help practices stratify resources for patients and care for these patients more effectively. Anticipatory guidance for patients on self-advocacy for reasonable accommodations in secondary education and/or the workplace would be a feasible pragmatic approach. Additional focus on developmentally appropriate ways to approach and care for these often overweight or obese patients without penalizing them for their lifestyles may also aid in more long-term retention of this vulnerable group in care.
Health system factors related to transfer in care systems also must be considered. Selection of young adults to refer to adult care may ultimately play a significant role in outcomes. In particular, referral of poorly controlled patients and those that have inconsistent follow-up in paediatric care are at particular risk. In addition, differences between paediatric and adult systems care paradigms are often stark. Paediatric systems can accommodate young adults needs with access to social work and psychology resources, and family-centred, team-based approaches to care [27–30], whereas adult systems are often resource-scarce and individual patient-focused [27,29,30]. Healthcare transition literature suggests that young adults struggle with relationships with new adult care teams given longstanding bonds with paediatric providers, and lack of familiarity with adult care approaches [20,29]. Given specific emphasis on behaviour change and lifestyle modification in Type 2 diabetes management [31,32], young adults with this disease need tailored approaches to care, with careful planning of developmentally appropriate strategies [32,33]. Furthermore, adult providers may not be trained, with a recent study of adult endocrinologists reporting feeling ill-prepared to care for young adults [30]. Further education and exposure to young adult patient care paradigms are needed and could be delivered in didactic form in medical school curricula or through various web-based or in-person formats to practitioners by professional medical societies. In addition, there needs to be prioritization of clinical care pathways and health system policies which improve collaboration between paediatric and adult medical systems, and focus on delivering young adults care through a gradual transition process as opposed to an abrupt transfer. Several such programmes exist in Type 1 diabetes and report improved outcomes for this population [34–39].
Lastly, the role of social determinants of health in young adults with Type 2 diabetes should be noted. Although not studied in depth here, determinants of health have been found to be major contributors to poor outcomes in adults with Type 2 diabetes [8,40,41]. The population of young adults studied here was mostly comprised of racial/ethnic minorities, had low parental educational attainment, with a large proportion on public insurance plans. Thus, this population likely experienced access to care issues, or had low enough general or health literacy to impact self-management knowledge, adherence and maintenance of adequate Type 2 diabetes care [40,42]. Expedited social work evaluation in these cases would facilitate timely screening of barriers such as low health literacy/numeracy, food insecurity and cost burdens that could positively impact outcomes if intervened upon early.
There are several limitations to this study. First, due to inclusion criteria definitions and lack of available information on every SEARCH participant with Type 2 diabetes, we had to exclude a significant number of participants. Although future work needs to be done to confirm our findings in larger cohorts, this population is notoriously difficult to capture. As such, our findings may be an underestimation of the actual risk that healthcare transition poses on this population. Second, because most SEARCH sites are a mix of academic centres and private practices, and did not have formalized transition programmes, these centres and practices have varying degrees of support for this unique population, which likely makes it more real-world in nature than in a controlled setting. Third, we could not measure the duration of gaps in care accurately because SEARCH participants were not asked the timeframe of when they transferred to adult care. Further research is needed to determine whether gaps in care are influential because they could potentially be prevented. Fourth, we could not fully examine the impact of factors related to low socio-economic status or racial/ethnic minority status due to sample size limitations. Given the pivotal role of social determinants of health in Type 2 diabetes outcomes, it would be important to examine how these known determinants of health modify healthcare transition. Lastly, examining the difference in outcomes based on primary versus specialty adult diabetes care is of interest. Our data suggest that there was no difference based on care received, but this study was not designed to directly compare specialty and generalist care, and needs to be a focus of future studies.
In summary, this study reveals that healthcare transition is a critical period of worsening glycaemic control and loss to follow-up for young adults with Type 2 diabetes. Although also present in Type 1 diabetes, the deleterious effects of healthcare transition may be more pronounced in young adults with Type 2 diabetes, but further research is required. Our study underscores the need for the development of tailored clinical programmes and healthcare system policies to support the growing population of young adults with youth-onset Type 2 diabetes. Ultimately, increased focus on patient-centred care in youth-onset Type 2 diabetes during this vulnerable period has the potential to attenuate the risk of poor health outcomes in adulthood. Concerted efforts to train endocrinologists and primary care practitioners to incorporate developmentally appropriate approaches to young adult care, implementation of standardized clinical care pathways which bridge paediatric and adult medical systems, and recognition of the need for more ancillary support services, especially social work and psychosocial support, ultimately will be needed to curb this emergent problem.
What’s new?
This is the first report of paediatric to adult healthcare transfer trends in young adults with youth-onset Type 2 diabetes.
This work studies a population-based group of young adults with Type 2 diabetes across a wide geographic and demographic range, who are difficult to capture in research.
Findings reveal substantial worsening of glycaemic control and loss to follow-up during healthcare transfer, highlighting a previously unidentified issue for this vulnerable population.
This research has implications for clinicians and healthcare systems, to focus on tailored approaches and policies for young adults with Type 2 diabetes in transition.
Acknowledgements
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their healthcare providers, whose participation made this study possible.
Funding sources
The SEARCH for Diabetes in Youth Cohort Study (1UC4DK108173-01) is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and supported by the Centers for Disease Control and Prevention. The Population Based Registry of Diabetes in Youth Study (RFP DP15-002) is funded by the Centers for Disease Control and Prevention and supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Sites: Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U18DP006139, U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati’s Children’s Hospital Medical Center (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U18DP006136, U58/CCU019235-4, U01DP000244, and U18DP002710-01), Wake Forest University School of Medicine (U18DP006131,U48/CCU919219, U01 DP000250, and 200-2010-35171).
The authors wish to acknowledge the involvement of the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR001450; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Competing interests
None declared.
References
- 1.Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care 2009; 2014: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagot-Campagna A, Pettitt DJ, Engelgau MM, Burrows NR, Geiss LS, Valdez R et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000; 136: 664–672. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. J Am Med Assoc 2014; 311: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005; 146: 693–700. [DOI] [PubMed] [Google Scholar]
- 5.Al-Saeed AH, Constantino MI, Molyneaux L, D’Souza M, Limacher-Gisler F, Luo C et al. An inverse relationship between age of Type 2 diabetes onset and complication risk and mortality: the impact of youth-onset Type 2 diabetes. Diabetes Care 2016; 39: dc150991. [DOI] [PubMed] [Google Scholar]
- 6.Treatment Options for Type 2 Diabetes in Adolescents and Youth Study Group. Rapid rise in hypertension and neuropathy in youth with Type 2 diabetes: The TODAY clinical trial. Diabetes Care 2013; 36: 1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care 2005; 28: 1219–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med 2012; 29: 453–463. [DOI] [PubMed] [Google Scholar]
- 9.Nadeau KJ, Anderson BJ, Berg EG, Chiang JL, Chou H, Copeland KC et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 2016; 39: 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 2000; 55: 469–480. [PubMed] [Google Scholar]
- 11.Malik FS, Hall M, Mangione-Smith R, Keren R, Mahant S, Shah SS et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr 2016; 171: 104–110. [DOI] [PubMed] [Google Scholar]
- 12.Busse FP, Hiermann P, Galler A, Stumvoll M, Wiessner T, Kiess W et al. Evaluation of patients’ opinion and metabolic control after transfer of young adults with type 1 diabetes from a pediatric diabetes clinic to adult care. Horm Res 2007; 67: 132–138. [DOI] [PubMed] [Google Scholar]
- 13.Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB et al. Clinical and psychological course of diabetes from adolescence to young adulthood. Diabetes Care 2001; 24: 1536–1540. [DOI] [PubMed] [Google Scholar]
- 14.Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HAW. Poor prognosis of young adults with Type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26: 1052–1057. [DOI] [PubMed] [Google Scholar]
- 15.Lotstein DS, Seid M, Klingensmith G, Case D, Lawrence JM, Pihoker C et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics 2013; 131: e1062–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamman RF, Bell RA, Dabelea D, D’Agostino RB, Dolan L, Imperatore G et al. The SEARCH for diabetes in youth study: rationale, findings, and future directions. Diabetes Care 2014; 37: 3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes 2006; 30: 590–594. [DOI] [PubMed] [Google Scholar]
- 18.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of Type 1 diabetes mellitus after 30 years’ duration. Arch Intern Med 2009; 169: 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garvey KC, Foster NC, Agarwal S, DiMeglio LA, Anderson BJ, Corathers SD et al. Health Care transition preparation and experiences in a U.S. national sample of young adults with Type 1 diabetes. Diabetes Care 2017; 40: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilliard ME, Perlus JG, Clark LM, Haynie DL, Plotnick LP, Guttmann-Bauman I et al. Perspectives from before and after the pediatric to adult care transition: a mixed-methods study in type 1 diabetes. Diabetes Care 2014; 37: 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waitzfelder B, Pihoker C, Klingensmith G, Case D, Anderson A, Bell R et al. Adherence to guidelines for youths with diabetes mellitus. Pediatrics 2011; 128: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garvey KC, Wolpert H, Rhodes ET, Laffel LM, Kleinman K, Beste MG et al. Health care transition in patients with type 1 diabetes: young adult experiences and relationship to glycemic control. Diabetes Care 2012; 35: 1716–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treatment Options for Type 2 Diabetes in Adolescents and Youth Study Group. A clinical trial to maintain glycemic control in youth with Type 2 diabetes. N Engl J Med 2012; 366: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt L, Kreiner M, Brody H. The changing face of chronic illness management in primary care: a qualitative study of underlying influences and unintended outcomes. Ann Fam Med 2012; 10:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bongaerts BWC, Müssig K, Wens J, Lang C, Schwarz P, Roden M et al. Effectiveness of chronic care models for the management of type 2 diabetes mellitus in Europe: a systematic review and metaanalysis. BMJ Open 2017; 7: e013076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soley-Bori M, Benzer JK, Burgess JF. Longitudinal analysis of quality of diabetes care and relational climate in primary care. Health Serv Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hargreaves DS, Greaves F, Levay C, Mitchell I, Koch U, Esch T et al. Comparison of health care experience and access between young and older adults in 11 high-income countries. J Adolesc Health 2015; 57: 413–420. [DOI] [PubMed] [Google Scholar]
- 28.Stollon NB, Paine CW, Lucas MS, Brumley LD, Poole ES, Peyton T et al. Transitioning adolescents and young adults with sickle cell disease from pediatric to adult health care. Provider Perspectives 2015; 37: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S, Garvey KC, Raymond JKSM. Perspectives on care for young adults with type 1 diabetes transitioning from pediatric to adult health systems: a national survey of pediatric endocrinologists. Pediatr Diabetes 2017; 18: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garvey KC, Telo GH, Needleman JS, Forbes P, Finklestein JA. Health care transition in young adults with Type 1 diabetes: perspectives of adult endocrinologists in the U.S. Diabetes Care 2016; 39: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Standards of Medical Care in Diabetes. – 2016. Diabetes Care 2016; 39: 86–93. [Google Scholar]
- 32.Sanghani NB, Parchwani DN, Palandurkar KM, Shah AM, Dhanani JV. Impact of lifestyle modification on glycemic control in patients with type 2 diabetes mellitus. Indian J Endocrinol Metab 2013; 17: 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards D, Larkin M, Milaszewski K, Javier E, Casey T, Grey M. Learning needs of youth with type 2 diabetes. Diabetes Educ 2013; 39: 314–319. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal S, Raymond JK, Cardillo SC, Schutta MH, Miller VA. An adult healthcare-based pediatric to adult transition program for emerging adults with Type 1 diabetes. Diabetes Educ 2017; 43: 87–96. [DOI] [PubMed] [Google Scholar]
- 35.Holmes-Walker DJ, Llewellyn AC, Farrell K. A transition care programme which improves diabetes control and reduces hospital admission rates in young adults with type 1 diabetes aged 15–25 years. Diabet Med 2007; 24: 764–769. [DOI] [PubMed] [Google Scholar]
- 36.Cadario F, Prodam F, Bellone S, Trada M, Binotti M, Trada M et al. Transition process of patients with type 1 diabetes (T1DM) from paediatric to the adult health care service: a hospital-based approach. Clin Endocrinol (Oxf) 2009; 71: 346–350. [DOI] [PubMed] [Google Scholar]
- 37.Levy-Shraga Y, Elisha N, Ben-Ami M, Boyko V, Lerner-Geva L, Ziv T et al. Glycemic control and clinic attendance of emerging adults with type 1 diabetes at a transition care clinic. Acta Diabetol 2015; 53: 27–33. [DOI] [PubMed] [Google Scholar]
- 38.Nagra A, McGinnity PM, Davis N, Salmon AP. Implementing transition: Ready Steady Go. Arch Dis Child Educ Pract Ed 2015; 100: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sequeira PA, Pyatak EA, Weigensberg MJ, Vigen CP, Wood JR, Ruelas V et al. Let’s empower and prepare (LEAP): evaluation of a structured transition program for young adults with Type 1 diabetes. Diabetes Care 2015; 38: 1412–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep 2014; 13: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Commission on Social Determinants of Health. Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health. Geneva: World Health Organization, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Chen R, Cheadle A, Johnson D, Duran B. US trends in receipt of appropriate diabetes clinical and self-care from 2001 to 2010 and racial/ethnic disparities in care. Diabetes Educ 2014; 40: 756–766. [DOI] [PubMed] [Google Scholar]