Abstract
Ajwa dates (Phoenix dactylifera), cultivated particularly in Al-Madina Al-Monawarh in Saudi Arabia, and considered as a rich source of natural antioxidants such as anthocyanins, carotenoids and phenolics. It is believed that Ajwa dates have a wide range of protective effects. For this reason, this work aimed to investigate the ameliorative effect of Ajwa dates aqueous extract against ochratoxin A (OTA)-induced testicular lesions in rats. Rats were divided into four groups and treated for 28 days. Group I served as normal control, group II (OTA exposed) treated orally with 289 μg/kg/day. Group III (Ajwa dates aqueous extract) treated orally with 1 g/kg/day. The last group served as the protective group (pretreated with Ajwa dates extract, then OTA). Histological studies revealed that OTA induced lesions in the testicular structure included proliferation of sertoli cells, impairment of some spermatogenesis stages and accumulation of premeiotic germinal cells. However, pretreatment with Ajwa dates extract prevented all the testicular damage and improved spermatogenesis, as well as remarkably enhanced the testicular structure. In conclusion, Ajwa dates aqueous extract has a powerful protective effect and ameliorative influence against OTA- induced testicular damage and may be used to treat sexual impairment and male infertility.
Keywords: Ajwa dates, ochratoxin A, spermatogenesis
INTRODUCTION
Ochratoxin A (OTA) is a mycotoxin occurs naturally in a wide range of agricultural products, including cereal grains, dried fruits, wine and coffee. It is formed by several different fungi including Aspergillus ochraceus, Ascobolus carbonarius, Aspergillus niger and Penicillium verrucosum. Contamination by OTA occurs as a result of poor storage of food products.[1] OTA was noted to occur in coffee in many countries such as the USA, Canada, Europe, Brazil, Dubai and Japan (Joint FAO/WHO Committee On Food Additives.[2] Animal-derived food such as meat and milk are found to have high levels of OTA,[3] as a consequence of cows exposure to large quantities of OTA.[4] OTA is known as a chemically stable compound, for this reason, conventional food processing methods does not significantly eliminate its presence in foods and beverages. It has been well documented that OTA is toxic and carcinogenic in animals. The kidney, in particular, is the main target organ for OTA.[5,6,7] Several studies stated that OTA can cause kidney failure,[8] liver toxicity[9] and immunotoxicity.[10] The incidence of testicular cancer in developed countries has been raised due to the consumption of OTA-contaminated foods, such as pig meat. OTA has been proved to be a testicular toxin causes testicular atrophy and low semen quality,[11,12] as well as inhibition of testosterone secretion in cultured testes interstitial cells of adult gerbils.[13]
Phoenix dactylifera Ajwa dates are soft, round, dark purple colour berries, particularly cultivated in Al-Madina Al-Munawarah, Saudi Arabia. The inspiration for studying the medicinal effects of Ajwa dates derived from Islamic prophetic medicine. Fresh dates contain high levels of antioxidants anthocyanins, carotenoid, phenolics, free and bound phenolic acids.[14] In comparison to other fruit, a very high level of phenolics has been found in Phoenix dactylifera as a result of exposure to high temperature and hot climate.[15] Apigenin, quercetin and luteolin are found to occur in methylated and sulphated forms of flavonoid glycosides in Phoenix dactylifera. Analysis of mass spectral data has reported that sulphates are linked to flavonol glycosides in Phoenix dactylifera. Accordingly, dates are the only fruit that contains flavonoid sulphates.
Anthocyanins exist only in fresh fruit, and carotenoids levels decline rapidly as the fruit ripens.[16] Aqueous date extracts possess potent free radical scavenging activity.[17] It has been shown that 50% of superoxide radicals resulted from photoreduction of riboflavin were scavenged at a concentration of 0.8 mg/ml, and 100% at a concentration of 1.5 mg/ml. Inhibition of lipid peroxidation was measured and 50% inhibition was evidenced at 1.9 mg/ml and100% inhibition at 4.0 mg/ml. Inhibition of protein oxidation was also found to occur at a concentration of 2.3 mg/ml reduced protein oxidation by 50%.[17] Many studies have shown that Phoenix dactylifera possess free radical scavenging, antioxidant,[18] gastroprotective,[19] hepatoprotective[8,20] nephroprotective,[21] as well as have anticancer activity.[22] Ajwa dates have been shown to retain the highest antioxidant activity among other types of dates, suppress lipid peroxidation, prevent cell damage, improve cancer therapeutics and reduce side effects caused by conventional chemotherapy.[23] Interestingly, it has been shown that Phoenix dactylifera water extract contains significantly higher contents of total phenols than alcoholic extract, especially in Ajwa dates.[24] We aimed in this investigation to study the effect of Ajwa dates aqueous extract against OTA testicular toxicity in rats.
MATERIALS AND METHODS
Materials
OTA (Cat. No. 01877) were purchased from Sigma-Aldrich chemical company (USA).
The fruit of Ajwa dates (Phoenix dactylifera L.) was obtained from the west part of the Kingdom of Saudi Arabia (Al-Madina Al-Monawarh).
Experimental animals
Twenty-eight weaning albino male Wistar rats weigh 35–40 g, obtained from the Experimental Animal House Center of King Fahd Research Center, Jeddah, Saudi Arabia. All animals were given food (rat chow) and water ad libitum, and were maintained at a relative humidity of 65%–86%, a temperature of 18°C–20°C. Appropriate care and hygienic conditions were maintained to keep them healthy and free from any infections. Institutional Animal Ethical Committee permission was obtained before experimenting.
OTA was dissolved in 0.5 M NaHCO3 pH 7.4.[25] OTA was given by gavage through a stomach tube (289 μg/kg/day) for 28 days.
Ajwa dates aqueous extract preparation
Ajwa dates (Phoenix dactylifera L.) flesh was extracted ×3 with distilled water (1:3), centrifuged at 4°C for 20 min at 4000 rpm. The supernatant was collected, lyophilised, and stored at −80°C until use. An aqueous extract was selected because most of the components are extracted in water. This method would exclude the interference of lipophilic constituents such as carotenoids and steroids.[17] Dose of Ajwa dates was equivalent to Prophet Mohammed recommendation seven dates/day (1 g/kg/day).
Methods
Animals were distributed randomly into four groups, with seven rats per group. The groups were treated for 4 weeks (5 days/week).
Group 1 – controls treated orally with sodium bicarbonate buffer 0.5 M, pH 7.4
Group 2 – treated orally with OTA (289 μg/kg/day)
Group 3: treated orally with Ajwa dates aqueous extract (1 g/kg/day)
Group 4: treated with Ajwa dates extract and after 2 h treated with OTA.
After 4 weeks of treatment. Animals were killed 24 h after the last dosing by anaesthesia with ether, dissected and the testes were removed and fixed in 10% buffered formalin solution, processed through graded alcohols and xylene and embedded in paraffin blocks. Tissue sections were cut for 3–5 μm and stained with haematoxylin and eosin stain (H and E) for light microscopy examinations.[26]
RESULTS
Light microscopy examination of (H and E)-stained sections of the control and Ajwa dates extract groups revealed normal testicular architecture. The seminiferous tubules characterised by normal density of germ cells and sperms as well as inconspicuous sertoli cells. In addition, normal structure of interstitial tissue and tunica albuginea capsule surrounded the tubules [Figures 1a, c, 2a, c and 3a, c]. However, examination of OTA group revealed extensive degenerative changes included thick tunica albuginea, necrosis, (small size and necrotic spermatogonia), with dilatation of seminiferous tubules, arrested germ cells mitosis and impaired spermatogenesis stages led to the loss of typical tubular architecture [Figures 1b, 2b and 3b]. DNA alteration was quiet distinct in OTA treated group [Figure 3b]. The OTA has markedly injured all cell lineages in most tubules, resulted in disruption of cell division. Moreover, the density of spermatogenic cells in damaged tubules was apparently reduced. The lumen of the seminiferous was filled with eosinophilic material and ill-defined sperms. A marked decrease in sperm number was evident. However, apparently increased number of sertoli cells [Figure 3b], Leydig cells as well as wide interstitial tissue were observed in this group.
Figure 1.
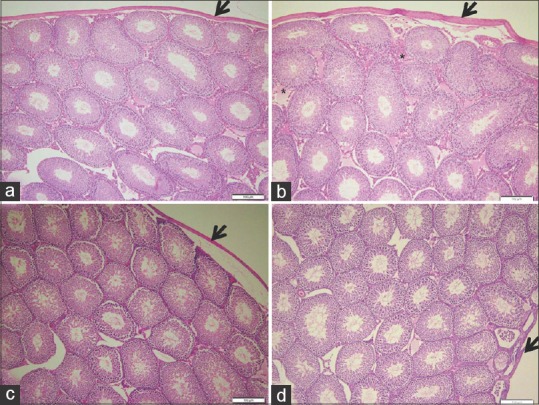
Photomicrographs of rat testis showing (a) control, normal structure of seminiferous tubules separated by interstitial tissue. (b) ochratoxin A, proliferation of interstitial tissue (*) and apparent thickened tunica albuginea capsule (thick arrow). (c) Ajwa dates, typical tubular structure with tightly packed seminiferous tubules and well-defined basal lamina. (d) Ajwa dates and ochratoxin A, normal testis structure with compacted seminiferous tubules. Note decreased capsule thickness in Ajwa dates groups (thick arrow) (H and E ×100)
Figure 2.
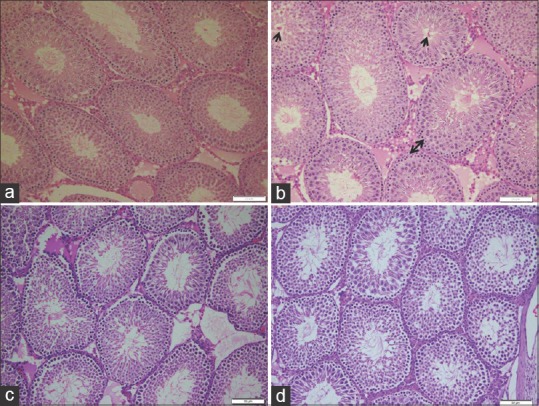
Photomicrographs of rat seminiferous tubules showing (a) control (b) ochratoxin A, disorganized spermatogenic cells with a marked increase of spermatogonia (two heads arrow). Note increased eosinophilic material and clumps of coagulated sperms inside the lumen (short arrow). (c) Ajwa dates, complete spermatogenic series and a well-defined basal lamina. Note the apparent increased number of sperm count inside the lumen. (d) Ajwa date and ochratoxin A, seminiferous tubules with complete stages of spermatogenic cells and apparent increased number of sperms (H and E, ×200)
Figure 3.
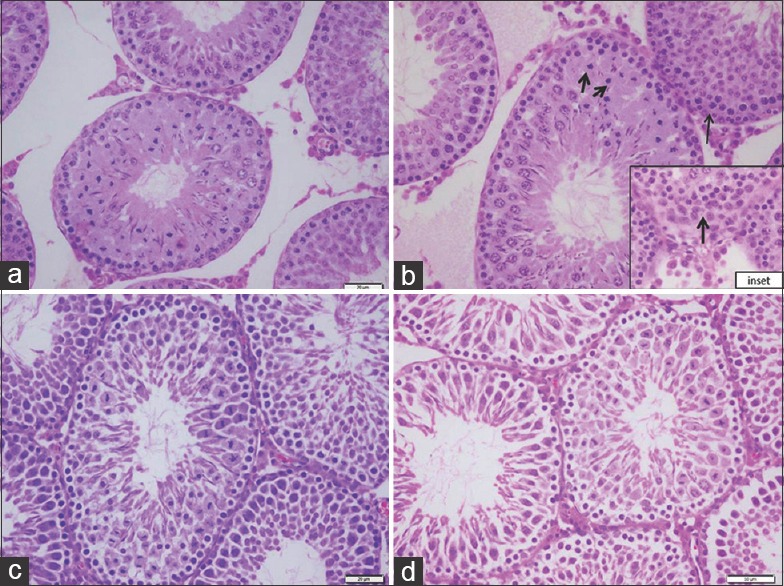
Higher magnification of seminiferous tubules showing (a) control (b) ochratoxin A, degenerative changes with loss of the spermatogenic series (arrows). (inset; base of the same tubule shows degeneration of the basement membrane and proliferation of Sertoli cells (arrow). (c) Ajwa dates, seminiferous tubules with all spermatogenic series. (d) Ajwa dates and ochratoxin A, remarkable improvement, closely packed tubular structure with primary spermatocytes, early and late spermatids as well as a large number of sperms inside the lumen (H and E, ×400)
Histological analysis of the testis from rats pretreated with Ajwa dates aqueous extract, then OTA showed that Ajwa dates extract has ameliorated the histopathological lesions remarkably and highly improved spermatogenesis [Figures 1d, 2d, and 3d]. Cell layers of seminiferous tubules have preserved their normal shape and continuity. Mitotic figures were seen. In addition, the number of sperm cells was apparently increased [Figure 2d]. Pretreatment with Ajwa dates extracts regulated sertoli cells proliferation and attenuated cell damage and OTA toxicity [Figure 3d].
DISCUSSION
The present study highlights the interest to change towards the use of natural medicinal food and declares the protective potentials of the aqueous extract of Saudi Arabian Phoenix dactylifera (Ajwa Dates) on OTA-induced testicular damage in rats.
OTA induces pathological changes in testes structure include an increase of interstitial space[27] and promotes lesions in testicular DNA as well as in male offspring; it also leads to the formation of DNA adducts,[28,29] which predict later tumour development. These lesions may lead to cancer in mice and humans.[12] Furthermore, OTA has adverse effects on germinal cells stages causes a decrease of stages I and VII. Conversely, increase of stages XII and XIII indicating a possible impairment of spermatogenesis.[28] Impaired spermatogenesis has been noticed in the current investigation. In addition, OTA causes significant reductions in sperm motility and longevity on pigs fed OTA even after withdrawal of OTA.[30] A significant increase in defective sperm and a significant decrease in sperm count, from 36 × 104 to 16 × 104 sperm/ml results by the administration of OTA to mice at a level equivalent to the human dietary concentration of l g/kg body weight/day.[31] In the present study, abnormal proliferation of sertoli cells reflects inability to support spermatogenesis and germ-cell differentiation which results in reduced production of spermatozoa. The present investigation is in agreement with Kazanas[32] study that OTA develops testicular hypoplasia of the germinal epithelium in weanling rats. Furthermore, OTA has a strong negative effect on cellular energy (adenosine triphosphate) production,[33] and can cause both apoptotic and necrotic cell death even at nanomolar concentration.[34,35]
The present study is in agreement with Yang et al.[36] that OTA induces disruption of cell division. OTA disruption of cell division and chromosomal instability induces carcinogenesis.[37,38] In human kidney cells, OTA blocks the metaphase/anaphase transition, produces aberrant mitotic formations.[37] Aberrant mitotic figures were seen in the present study in OTA treatment. However, all OTA negative consequences were alleviated and counteracted by Ajwa date aqueous extract pretreatment.
Previously reported that Ajwa dates contain phenols in its constituents.[3,39] Plant polyphenols hold a wide range of effects such as anti-proliferative activity, anti-inflammatory activity, control of the host immune system, adjustment of cytochrome P450 enzymes which are involved in activation of procarcinogens, upregulation of genes producing antioxidant enzymes, in addition to the ability to alter cellular signalling.[40] In this study, the protective effects could be attributed to the high amounts of polyphenolic and flavonoid compounds present in the Ajwa dates aqueous extract. These antioxidant compounds could have played a considerable role in scavenging the reactive oxygen species induced by OTA.
In the current study, Ajwa dates pretreatment is markedly reduced necrosis and normalised the number of the spermatogenic cell layers. This may be attributed to various antioxidant bioactive components in date's extract which can block lipid peroxidation generated by OTA. In addition, Ajwa dates are rich in anthocyanins[14] which have been reported previously to have nutraceutical properties and considered as potent antioxidants. The mechanisms responsible for the protective effects are as follows free-radical scavenging pathway, mitogen-activated protein kinase pathway, cyclooxygenase pathway and inflammatory cytokines signalling.[41] In addition, anthocyanins have anti-cancer properties and antiangiogenic effect.[42] Angiogenesis is an important step for cancer development, where it is necessary for the transition of tumours from a benign state to a malignant one. In cancer prevention, antiangiogenesis is the process that halts the formation of new blood vessels which carry oxygen to the tumour cells. Anthocyanins and flavonoids are potent antiangiogenic agents.[41,42]
The present study findings disagree with the study[43] which stated that the components of the dates extract have the potentials of causing infertility in male rats by decreasing serum testosterone levels, sperm count, sperm motility and sperm morphology. Conversely, in the present study pretreatment of OTA-ingested male rats with Ajwa dates, aqueous extract showed positive effects on the testicular structure.
CONCLUSION
Ajwa dates aqueous extract is very promising and has a potent protective and ameliorative effect against OTA-induced testicular damage and may be used to treat sexual impairment and male infertility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Moss MO. Mode of formation of ochratoxin A. Food Addit Contam. 1996;13(Supp 1):5–9. [PubMed] [Google Scholar]
- 2.Vol. 47. Rome: FAO food and nutrition paper; 74. WHO food additives series; Joint FAO/WHO Expert Committee on Food Additives. Safety evaluation of certain mycotoxins in food / prepared by the fifty-sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) pp. 2007–2001. [Google Scholar]
- 3.Petzinger E, Ziegler K. Ochratoxin A from a toxicological perspective. J Vet Pharmacol Ther. 2000;23:91–8. doi: 10.1046/j.1365-2885.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- 4.Diekman MA, Green ML. Mycotoxins and reproduction in domestic livestock. J Anim Sci. 1992;70:1615–27. doi: 10.2527/1992.7051615x. [DOI] [PubMed] [Google Scholar]
- 5.Duarte SC, Pena A, Lino CM. Human ochratoxin a biomarkers – From exposure to effect. Crit Rev Toxicol. 2011;41:187–212. doi: 10.3109/10408444.2010.529103. [DOI] [PubMed] [Google Scholar]
- 6.Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain veterinary drug residues in food. World Health Organ Tech Rep Ser. 2000;893:i. [PubMed] [Google Scholar]
- 7.Castegnaro M, Canadas D, Vrabcheva T, Petkova-Bocharova T, Chernozemsky IN, Pfohl-Leszkowicz A, et al. Balkan endemic nephropathy: Role of ochratoxins A through biomarkers. Mol Nutr Food Res. 2006;50:519–29. doi: 10.1002/mnfr.200500182. [DOI] [PubMed] [Google Scholar]
- 8.Abdu S, Ali A, Ansari S. Cytotoxic effect of ochratoxin A on the renal corpuscles of rat kidney: Could ochratoxin A cause kidney failure? Histol Histopathol. 2011;26:543–9. doi: 10.14670/HH-26.543. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh BY, Elsaed WM, Samman AH, Ladin AM. Ajwa dates as a protective agent against liver toxicity in rat. Eur Sci J. 2014;3:1857–81. [Google Scholar]
- 10.Bondy GS, Pestka JJ. Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev. 2000;3:109–43. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GG. Hypothesis: Does ochratoxin A cause testicular cancer? Cancer Causes Control. 2002;13:91–100. doi: 10.1023/a:1013973715289. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasa J. Does ochratoxin A (OTA) cause testicular cancer in humans? Acta Med Lituanica. 2011;18:1–3. [Google Scholar]
- 13.Fenske M, Fink-Gremmels J. Effects of fungal metabolites on testosterone secretion in vitro . Arch Toxicol. 1990;64:72–5. doi: 10.1007/BF01973380. [DOI] [PubMed] [Google Scholar]
- 14.Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem. 2005;53:7592–9. doi: 10.1021/jf050579q. [DOI] [PubMed] [Google Scholar]
- 15.Vinson JA, Zubik L, Bose P, Samman N, Proch J. Dried fruits: Excellent in vitro and in vivo antioxidants. J Am Coll Nutr. 2005;24:44–50. doi: 10.1080/07315724.2005.10719442. [DOI] [PubMed] [Google Scholar]
- 16.Hong YJ, Tomas-Barberan FA, Kader AA, Mitchell AE. The flavonoid glycosides and procyanidin composition of deglet noor dates (Phoenix dactylifera) J Agric Food Chem. 2006;54:2405–11. doi: 10.1021/jf0581776. [DOI] [PubMed] [Google Scholar]
- 17.Vayalil PK. Date fruits (Phoenix dactylifera linn): An emerging medicinal food. Crit Rev Food Sci Nutr. 2012;52:249–71. doi: 10.1080/10408398.2010.499824. [DOI] [PubMed] [Google Scholar]
- 18.Rock W, Rosenblat M, Borochov-Neori H, Volkova N, Judeinstein S, Elias M, et al. Effects of date (Phoenix dactylifera L. medjool or hallawi variety) consumption by healthy subjects on serum glucose and lipid levels and on serum oxidative status: A pilot study. J Agric Food Chem. 2009;57:8010–7. doi: 10.1021/jf901559a. [DOI] [PubMed] [Google Scholar]
- 19.Al-Qarawi AA, Abdel-Rahman H, Ali BH, Mousa HM, El-Mougy SA. The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. J Ethnopharmacol. 2005;98:313–7. doi: 10.1016/j.jep.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Saafi EB, Louedi M, Elfeki A, Zakhama A, Najjar MF, Hammami M, et al. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp Toxicol Pathol. 2011;63:433–41. doi: 10.1016/j.etp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Ali A, Abdu S, Alansari S. Biosafty of Ajwa date against biotoxicity of ochratoxin (A) on proximal tubules of male rat. Kidney Res J. 2011;1:1–12. [Google Scholar]
- 22.Khan F, Ahmed F, Pushparaj PN, Abuzenadah A, Kumosani T, Barbour E, et al. Ajwa date (Phoenix dactylifera L.) extract inhibits human breast adenocarcinoma (MCF7) cells in vitro by inducing apoptosis and cell cycle arrest. PLoS One. 2016;11:e0158963. doi: 10.1371/journal.pone.0158963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasin BR, El-Fawal HA, Mousa SA. Date (Phoenix dactylifera) polyphenolics and other bioactive compounds: A traditional islamic Remedy's potential in prevention of cell damage, cancer therapeutics and beyond. Int J Mol Sci. 2015;16:30075–90. doi: 10.3390/ijms161226210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh EA, Tawfik MS, Abu-Tarboush HM. Phenolic contents and antioxidant activity of various date palm (Phoenix dactylifera L.) fruits from Saudi Arabia. Food Nutr Sci. 2011;2:1134. [Google Scholar]
- 25.Atroshi F, Biese I, Saloniemi H, Ali-Vehmas T, Saari S, Rizzo A, et al. Significance of apoptosis and its relationship to antioxidants after ochratoxin A administration in mice. J Pharm Pharm Sci. 2000;3:281–91. [PubMed] [Google Scholar]
- 26.Bancroft JD, Gamble M, editor . Theory and Practice of Histological Techniques. 5th ed. Edinburgh: Churchill Livingstone; 2002. [Google Scholar]
- 27.Akhtar N, Srivastava MK, Raizada RB. Assessment of chlorpyrifos toxicity on certain organs in rat, rattus norvegicus. J Environ Biol. 2009;30:1047–53. [PubMed] [Google Scholar]
- 28.Gharbi A, Trillon O, Betbeder AM, Counord J, Gauret MF, Pfohl-Leszkowicz A, et al. Some effects of ochratoxin A, a mycotoxin contaminating feeds and food, on rat testis. Toxicology. 1993;83:9–18. doi: 10.1016/0300-483x(93)90087-9. [DOI] [PubMed] [Google Scholar]
- 29.Jennings-Gee JE, Tozlovanu M, Manderville R, Miller MS, Pfohl-Leszkowicz A, Schwartz GG, et al. Ochratoxin A: In utero exposure in mice induces adducts in testicular DNA. Toxins (Basel) 2010;2:1428–44. doi: 10.3390/toxins2061428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solti L, Pécsi T, Barna-Vetró I, Szász F, Jr, Biró K, Szabó E. Analysis of serum and seminal plasma after feeding ochratoxin A with breeding boars. Anim Reprod Sci. 1999;56:123–32. doi: 10.1016/s0378-4320(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 31.Bose S, Sinha SP. Modulation of ochratoxin-produced genotoxicity in mice by Vitamin C. Food Chem Toxicol. 1994;32:533–7. doi: 10.1016/0278-6915(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 32.Kazanas N, Ely RW, Fields ML, Erdman JW., Jr Toxic effects of fermented and unfermented sorghum meal diets naturally contaminated with mycotoxins. Appl Environ Microbiol. 1984;47:1118–25. doi: 10.1128/aem.47.5.1118-1125.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poór M, Veres B, Jakus PB, Antus C, Montskó G, Zrínyi Z, et al. Flavonoid diosmetin increases ATP levels in kidney cells and relieves ATP depleting effect of ochratoxin A. J Photochem Photobiol B. 2014;132:1–9. doi: 10.1016/j.jphotobiol.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Ringot D, Chango A, Schneider YJ, Larondelle Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem Biol Interact. 2006;159:18–46. doi: 10.1016/j.cbi.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 35.Sorrenti V, Di Giacomo C, Acquaviva R, Barbagallo I, Bognanno M, Galvano F, et al. Toxicity of ochratoxin a and its modulation by antioxidants: A review. Toxins (Basel) 2013;5:1742–66. doi: 10.3390/toxins5101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Q, He X, Li X, Xu W, Luo Y, Yang X, et al. DNA damage and S phase arrest induced by ochratoxin A in human embryonic kidney cells (HEK 293) Mutat Res. 2014;765:22–31. doi: 10.1016/j.mrfmmm.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Rached E, Pfeiffer E, Dekant W, Mally A. Ochratoxin A: Apoptosis and aberrant exit from mitosis due to perturbation of microtubule dynamics? Toxicol Sci. 2006;92:78–86. doi: 10.1093/toxsci/kfj213. [DOI] [PubMed] [Google Scholar]
- 38.Czakai K, Müller K, Mosesso P, Pepe G, Schulze M, Gohla A, et al. Perturbation of mitosis through inhibition of histone acetyltransferases: The key to ochratoxin a toxicity and carcinogenicity? Toxicol Sci. 2011;122:317–29. doi: 10.1093/toxsci/kfr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Farsi MA, Lee CY. Nutritional and functional properties of dates: A review. Crit Rev Food Sci Nutr. 2008;48:877–87. doi: 10.1080/10408390701724264. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigo R, Libuy M, Feliu F, Hasson D. Polyphenols in disease: From diet to supplements. Curr Pharm Biotechnol. 2014;15:304–17. doi: 10.2174/138920101504140825113815. [DOI] [PubMed] [Google Scholar]
- 41.Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsunaga N, Tsuruma K, Shimazawa M, Yokota S, Hara H. Inhibitory actions of bilberry anthocyanidins on angiogenesis. Phytother Res. 2010;24(Suppl 1):S42–7. doi: 10.1002/ptr.2895. [DOI] [PubMed] [Google Scholar]
- 43.Dibal NI, Hambolu JO, Buraimoh AA. Effects of Phoenix dactylifera on the testes, epididymal sperm pattern and hormonal profiles of male Wistar rats. J Infertil Reprod Biol. 2016;4:45–50. [Google Scholar]


