Abstract
Background/objective
Alzheimer’s disease (AD) is mainly characterized by decline of cognitive functions such as memory and learning, which has a high prevalence and poor drug efficacy in treatment regimes. A systematic review and meta-analysis of randomized controlled trials (RCTs) were conducted to evaluate the effectiveness of exercise on cognitive function in patients diagnosed with AD.
Methods
The bibliographic databases (PubMed, Cochrane Library and Embase, and Web of Science) and four Chinese databases (Wanfang data, CBM, CNKI, and VIP) were searched to identify RCTs published in any language between January 1, 1960, and January 1, 2018. Only peer-reviewed articles and RCTs were included. The collected data were analyzed by Review Manager (5.3).
Results
Overall, 869 patients diagnosed with AD were included from 13 RCTs. Patients in the intervention group received pure exercise interventions and a cognitive test. Although there was heterogeneity in intervention methods and cognitive measures among studies, meta-analysis (seven studies) supports positive effects of physical activity on cognitive function of patients with AD (mean difference [MD] =2.53, the 95% CI=0.84 to 4.22, test for overall effect: Z=2.93 [P=0.003]). Eight studies demonstrated that exercise improves cognitive function for individuals with AD. However, the remaining five studies did not display a beneficial effect of exercise on cognitive function in patients with AD.
Conclusion
This meta-analysis and systematic review indicated that exercise intervention might improve the cognitive function of AD or slow down the decline of cognition; however, this relationship was not always true across studies. RCTs with clear intervention criteria, large samples, and long-term follow-up are needed in the future to demonstrate the benefits of exercise for cognitive function in AD patients.
Keywords: Alzheimer’s disease, exercise, cognitive function, randomized controlled trial
Introduction
Alzheimer’s disease (AD) is an insidious and progressive neurodegenerative disease characterized by cognitive dysfunction. It has a high prevalence,1 and there exists no effective treatment, resulting in a burden to family and society. AD is the most common form of dementia, accounting for 60%–70% of patients with dementia.2 Dementia is a chronic and progressive neurological disease, which is clinically char-acterized by the slow onset of memory deficits, accompanied by varying degrees of personality changes. It is a group of clinical syndromes rather than an independent disease. Currently, there are ∼47 million people with dementia worldwide,3 and the number is expected to triple by 2050.4 Dementia significantly erodes the function and quality of life, impedes economic and social development, and brings burdens and pressures to families. Cost associated with dementia exceed that of heart disease and cancer and is usually paid directly by the family.5 Other types of dementia include frontal–temporal dementia, dementia with Lewy bodies, Parkinson’s disease, Huntington’s disease, and vascular dementia. Treatment of dementia should be directed toward the root cause of the disease. However, there currently exists no specific drug for the treatment of neurodegenerative diseases. Treatment is primarily aimed at improving cognition and relieving symptoms. Traditional treatments are mainly pharmacotherapy; however, pharmacotherapies only relieve symptoms and might have side effects.6 Exercise can be used as an adjuvant therapy before effective new drugs are developed and has thus emerged as a treatment option. An update of the 2001 American Academy of Neurology of guidelines on mild cognitive impairment (MCI) demonstrated that exercise training (6 months) might improve cognitive function in MCI patients.7 Several studies have demonstrated that physical exercise can slow down the progression of cognitive declines. Studies have demonstrated that exercise reduces the risk of dementia in older adults who are cognitively normal.8,9 Kemoun et al10 conducted a 15-week physical activity program and demonstrated that subjects in the intervention group (IG) had improved overall Rapid Evaluation of Cognitive Function (ERFC) scores (P<0.01), while the control group (CG) ERFC scores declined. Yang et al11 conducted a 3-month randomized controlled trial (RCT) with 50 patients with AD, and they demonstrated that moderate intensity aerobic exercise can improve cognitive function in patients with AD. Yágüez et al12 carried out a study which indicated that a short course of nonaerobic movement-based exercise is effective at improving some aspects of cognitive function in patients with AD. In addition, many systematic reviews and meta-analyses show a positive effect of physical activity as an intervention therapy. A systematic review by Farina et al13 demonstrated that exercise generally had a positive effect on the rate of cognitive decline in AD. Similarly, a meta-analysis by Heyn et al14 involving 2,020 participants demonstrated that exercise training increased cognitive function in people with dementia and related cognitive impairments. Scherder et al15 carried out a systematic review and meta-analysis, which indicated a positive finding that participants leading a sedentary life might prevent or delay declines in executive function when following a walking program. However, these conclusions are not always observed, most studies vary in terms of research design, nonstandardized interventions, and research type. Although studies that emphasize the importance of exercise exist, the effects of exercise on cognitive functions in Alzheimer’s patients are mixed as some studies have observed no positive effect of physical activity on cognitive function in people with AD. An updated review in 2013 found no evidence of cognitive benefits in patients with AD.16 A systematic review of Littbrand et al17 demonstrated that whether physical exercise can improve cognitive functions among people with dementia remains unclear. Öhman et al18 conducted a systematic review of RCTs on the cognition of older patients with dementia and found that most studies demonstrated that exercise has no effect on the cognitive function.
Stronger evidence is needed to confirm the role of exercise in cognitive function in patients with AD. The purpose of this meta-analysis and systematic review was to review research that demonstrates the efficacy of exercise on cognitive function of AD. Furthermore, in order to reduce the heterogeneity among studies resulting from inherent study differences, we included only RCTs for analysis, to gain a greater understanding of this relationship and to provide a more informed suggestion by analyzing the evidence.
Materials and methods
Study selection strategy
The bibliographic databases (PubMed, Cochrane Library and Embase, and Web of Science) and four Chinese databases (Wanfang data, CBM, CNKI, and VIP) were searched to identify RCTs published in any language between January 1, 1960, and January 1, 2018. At first, Medical Subject Headings (MeSH) of exercise were identified for AD and cognition by searching the Chinese Medical Subject Headings (CMeSH) on SinoMed. This was followed by using “MeSH” to identify individual entry terms that were used as keywords in subsequent searches. Finally, we retrieved articles using the search builder of MeSH terms and various combinations of the entry terms “exercise(s)”, “physical activity”, “isometric exercise”, “resistance training”, “aerobic exercise”, “running”, “jogging”, “swimming”, “walking”, “stair climbing”, “Muscle Stretching Exercises”, “yoga”, “tai chi”, with AD terms “Alzheimer(’s) disease” or “dementia, Alzheimer type” or “Alzheimer syndrome” or “AD”, as well as the cognition terms “cognition(s)” or “cognitive function(s)”. (see Table S1 for search strategies in detail) Only peer-reviewed articles and RCTs were included, and animal studies were excluded. More relevant studies were retrieved from the meta-analysis and the list of references of related studies. We used citation search to check the results of the search by searching relevant systematic reviews.
Study design
Based on the following criteria, we selected eligible studies for our meta-analysis from the initial search: 1) participants: trials enrolled patients with clinically diagnosed AD. There is no limit to the severity of AD. We excluded studies that included patients who might have a disease that affects cognitive function (such as stroke, cancer, Parkinson’s disease, and traumatic brain injury). Participants with Lewy body dementia, frontotemporal dementia, vascular dementia, or other rare forms of dementia were also excluded; 2) interventions: the research must have physical exercise intervention, such as aerobic fitness, strength training, balance and flexibility training, cycling training, walking, and stamina. Trials of multimodal interventions such as exercise accompanied by music therapy or cognitive therapy were excluded. There was no restriction on the type, intensity, and frequency of physical activity; 3) outcome measures: the trial must have a measured cognitive outcome for patients with AD, including the use of scales or other cognitive measurement tools; and 4) the study must be an RCT.
Data extraction and quality assessment
Two investigators independently reviewed the title and abstract of search results and screened the full text of references that might be eligible. When differences occurred in eligibility of inclusion, exclusion, or data extraction, a third reviewer participated in the discussion. Physical activity interventions of all sample sizes were included. Author, publication year, country, age, sample size, intervention characteristics, duration, measurement instrument, results, and dropouts were included for all eligible studies. Two researchers assessed the risk of bias independently using the Cochrane handbook (5.1.0).19
Quantitative data synthesis
Review Manager (5.3) was used to estimate the overall effect of physical therapy in each study. The intervention effect was described as mean±SD with 95% CI of cognitive scores of postintervention between the IG and CG, respectively. According to the Cochrane Collaboration handbook for systematic reviews of interventions, selection of fixed or random effects meta-analysis should be based on the potential real effect of intervention on outcome measures.19 Considering the heterogeneity of intervention type, result measurement, and sample characteristics, we chose to use a random effects model as a matter of priority.20 Statistical heterogeneity was revealed by I2 statistics. When I2 is >50%, the result is considered heterogeneous.21 In addition, it is said that because the random effects model does not provide a quantitative measure of heterogeneity, it was evaluated by visual inspection of the funnel plot.22 Therefore, both methods were used to assess heterogeneity. P-value <0.05 was considered statistically significant.
Results
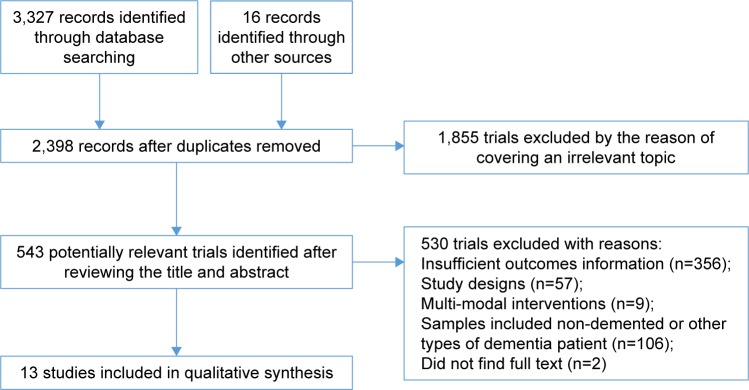
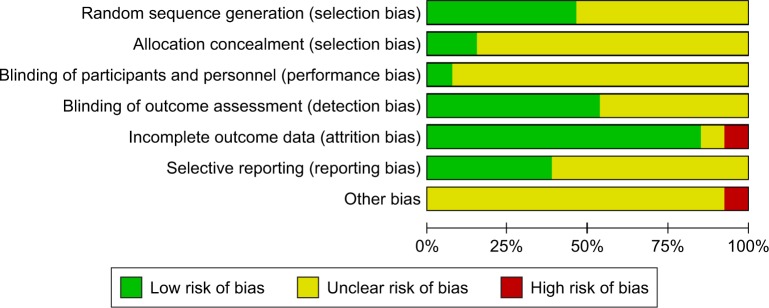
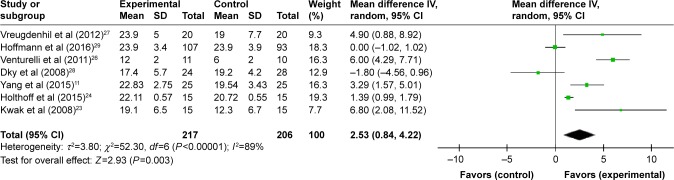
Initially, 3,327 articles were obtained through database search, and 16 articles were retrieved by performing manual searches of the selected key references. After removing duplicates, there were 2,398 articles remaining. Furthermore, 1,855 trials were excluded as they covered an irrelevant topic. After reviewing the title and abstract, 530 trials were excluded due to the following reasons: insufficient or irrelevant outcome information (n=356), study designs (n=57), multi-modal interventions (n=9), inclusion of nondemented patients (n=106), and unlocated full text (n=2). As a result of the screening process, 13 RCT articles were selected,10–12,23–32 which included 659 patients who were diagnosed with AD as presented in Figure 1. Patients were split into control (319) or patients receiving intervention treatment (340). Sample sizes of studies ranged from 21 to 200, and studies were published between 2002 and 2017. Cognitive result measurements usually included Mini-Mental State Examination (MMSE) (nine studies)11,23–30 or other cognitive function tests such as Alzheimer’s Disease Assessment Scale – Cognitive Sub-Scale,27–29 verbal fluency,29 clock-drawing test,30 ERFC,10 Cambridge Neuropsychological Test Automated Battery-Expedio,12 test of phonemic verbal fluency,24 Stroop Color and Word Test,29 Frontal Assessment Battery,30 and Functional Assessment of Communication Skills Mental Sub-scale.31 More information or characteristics of the included studies are summarized in Table 1. An assessment of bias in each field is presented in Figures 2 and 3. A meta-analysis was conducted on seven articles that had available data for MMSE as an observation index, and a comparison of the experimental group and the CG is presented in Figure 4. The heterogeneity of the included articles is shown in Figure 5.
Figure 1.
Flow diagram of the study identification process.
Table 1.
Patient characteristics of included studies
| Study | Publication year | Location | Age (y), mean ± SD; median (range) | Sample | Type of intervention/frequency of exercise
|
Program duration | Measurement instrument | |
|---|---|---|---|---|---|---|---|---|
| IG | CG | |||||||
| Vreugdenhil et al27 | 2012 | Australia | IG: 73.5 (51–83) CG: 74.7 (58–89) |
IG=20 CG=20 |
Exercise plus usual treatment, daily exercises and walking | Usual treatment | 4 mo | ADAS-Cog; MMSE |
| Kwak et al23 | 2008 | Republic of Korea | IG: 79.67±6.64 CG: 82.27±7.09 |
IG=15 CG=15 |
Exercise intensity was gradually increased from 30% to 60% of expected maximal oxygen consumption; 30–60 minutes per day, 2–3 times/w | No intervention | 12 mo | MMSE |
| Kemoun et al10 | 2010 | France | IG: 82.0±5.8 CG: 81.7±5.1 |
IG=20 CG=18 |
The first 2 w: prepared for physical activity; the next 13 w: walking, equilibrium, and stamina | Did not practice any physical activity | 15 w | The French ERFC |
| Holthoff et al24 | 2015 | Germany | IG: 72.40±4.34 CG: 70.67±5.41 |
IG=15 CG=15 |
PA intervention program: 30 minutes/day, 3 times/w, with at least 1 day without training in between 2 training days | Usual care group | 12 w | MMSE, FAS test |
| Steinberg et al25 | 2009 | USA | IG: 76.5±3.9 CG: 74.0±8.1 |
IG=14 CG=13 |
A daily exercise program: aerobic fitness, strength training, and balance and flexibility training | A home safety assessment | 12 w | MMSE, BNT, HVLT |
| Venturelli et al26 | 2011 | Italy | IG: 83±6 CG: 85±5 |
IG=12 CG=12 |
A minimum of 30 minutes of moderate exercise (walking) 4 times a week | Daily organized activities like bingo, patchwork sewing, and music therapy | 6 mo | MMSE |
| Yang et al11 | 2015 | China | IG: 72.00±6.69 CG: 71.92±7.28 |
IG=25 CG=25 |
Cycling training: 70% maximum HR, 3 days/w, 40 minutes/day | Health education | 3 mo | MMSE |
| Yágüez et al12 | 2011 | UK | IG: 70.5±8 CG: 75.7±6.90 |
IG=15 CG=12 |
Brain gym training, with weekly sessions of 2 hour and a 30 minute break | Standard care group | 6 w | (CANTAB)- Expedio |
| Pedroso et al30 | 2012 | Brazil | IG: 78.3±7.4 CG: 77.45±6.9 |
IG=10 CG=11 |
Coordination, aerobic resistance, flexibility, balance and agility, and, at the same time, the performance of a cognitive task | Do not engage in regular practice of physical activity; 3/w, each session lasted 60 minutes | 4 mo | MMSE, CDT, FAB |
| Hoffmann et al29 | 2016 | Denmark | IG: 69.8±7.4 CG: 71.3±7.3 |
IG=107 CG=93 | The first 4 w of exercise (adaption): getting used to exercising, building up strength, aerobic exercise (once weekly); the remaining 12 w: aerobic exercise of moderate to high intensity (in total 3×10 minutes on an ergometer bicycle, cross trainer, and treadmill with 2–5 minute rest between); the target intensity was 70%–80% of maximal HR | Received treatment as usual | Intervention: 16 w; follow-up: 2.5 y | ADAS-Cog, MMSE, Stroop, VF |
| Vidoni et al32 | 2017 | America | IG: 74.1±6.8 CG: 71.1±8.8 |
IG=33 CG=32 |
60 minutes of aerobic exercise in week 1 and increased 21 minutes/w until they achieved 150 minutes/w; HR zones were gradually increased from 40%–55% to 60%–75% of HR | Nonaerobic exercises that rotated weekly (core strengthening, resistance bands, modified tai chi, and modified yoga); HR below 100 bpm | 26 w | DAD, a standard cognitive battery |
| Dky e t al28 | 2008 | China, Hong Kong | IG: 75±7 CG: 78±6 |
IG=24a CG=28a |
Treadmill, bicycle and arm ergometry, and 10-minute flexibility training; 1 hour twice a week | Conventional medical treatment | Intervention: 12 w; follow-up: 12 mo | MMSE, ADAS- Cog |
| Cott et al31 | 2002 | Canada | IG: 83.23±8.34 CG: 79.78±8.30 |
IG=30 CG=25 |
Walk-and-talk group (30 minutes, 5 times/w for 16 w, walking/talking in pairs) | No intervention | 16 w | FACSM |
Notes:
The original sample contained dementia patients who did not meet the criteria, and only the separate data on AD patients were used. FAS, test of phonemic verbal fluency.
Abbreviations: AD, Alzheimer’s disease; ADAS-Cog, Alzheimer’s Disease Assessment Scale – Cognitive Sub-Scale; BNT, Boston Naming Test; bpm, beats per minute; (CANTAB)-Expedio, The Cambridge Neuropsychological Test Automated Battery; CDR, Clinical Dementia Rating Scale; CDT, clock-drawing test; CG, control group; DAD, Disability Assessment for Dementia; ERFC, Rapid Evaluation of Cognitive Function; FAB, Frontal Assessment Battery; FACSM, Functional Assessment of Communication Skills Mental Subscale; HVLT, Hopkins Verbal Learning Test; IG, intervention group; MMSE, Mini-Mental State Examination (/30); mo, months; PA, physical activity; Stroop, Stroop Color and Word Test; VF, verbal fluency; w, week(s); y, years.
Figure 2.
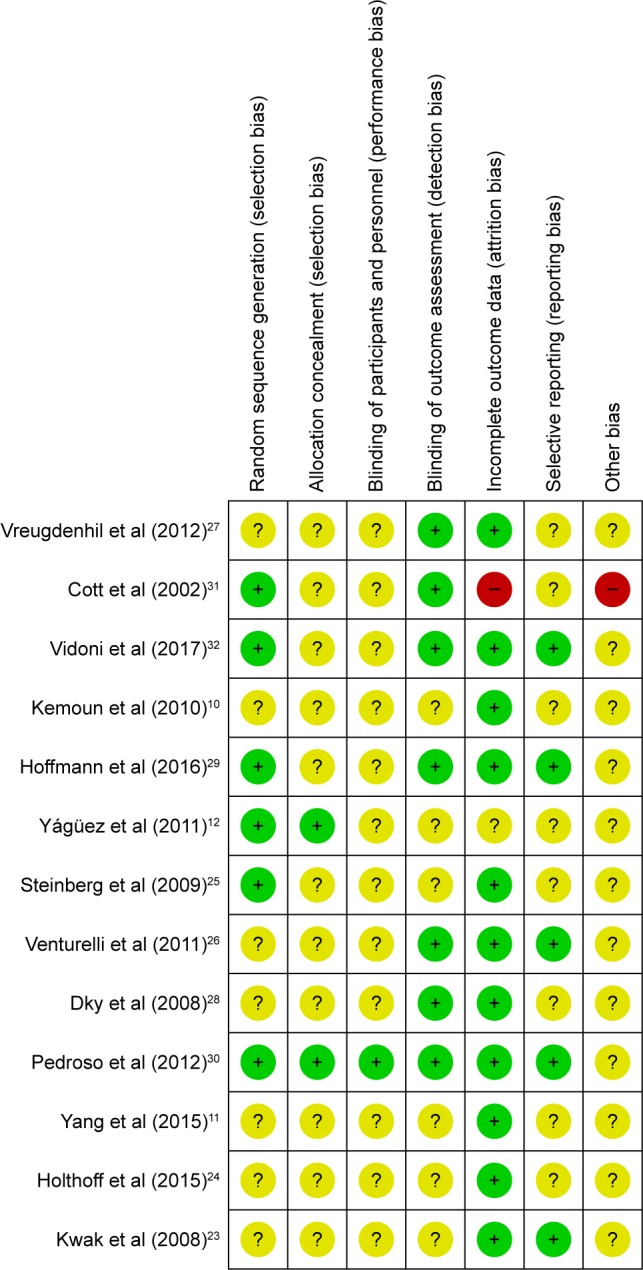
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Figure 3.
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Figure 4.
A forest plot shows the results of a meta-analysis of six RCTs measuring MMSEoutcome for individuals with AD.
Note: Exercise intervention had a positive effect on MMSEresults.
Abbreviations: AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination; RCT, randomized controlled trial.
Figure 5.
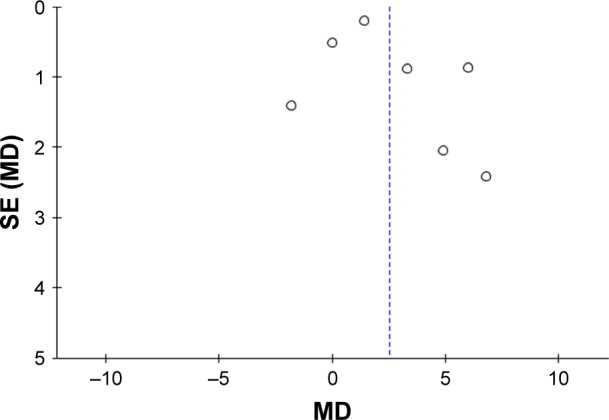
A funnel plot of the meta-analysis of six studies.
Notes: SE indicates the accuracy of the estimated value of the treatment effect, and the smaller the SE, the more accurate it is. The MD measures the treatment effect size.
Abbreviations: MD, mean difference; SE, standard error.
Risk of bias assessment
In order to avoid false findings in the comprehensive analysis and to ensure reliability of results, quality of selected studies was assessed for risk of bias. The risk of bias graph and the authors’ judgment about each risk of bias item are presented in Figure 2. Figure 2 indicates the quality details of each study and demonstrates that only one research has a high-risk quality assessment.31 In one trial, individuals had a different baseline in MMSE.25 In addition, the study carried out by Cott et al31 had a high risk for incomplete outcome data (attrition bias) and considerable individual variability in MMSE scores, and it did not use intervention-to- to treat to avoid the occurrence of bias. The authors’ judgments of each risk of bias item are presented in Figure 3. In general, the proportion of high-risk bias is small.
Meta-analysis of the seven studies supports exercise as a treatment option to help improve cognition of AD patients
In the meta-analysis of the seven articles,11,23,24,26–29 results demonstrated that studies were heterogeneous (P<0.001, I2=89%), therefore a random effects model was chosen for analysis. Figure 4 demonstrates that exercise can improve cognition of AD patients and that total outcome: MD=2.53, 95% CI=0.84 to 4.22, and Z=2.93 (P=0.003). Results of five of the included articles are consistent with overall results of this meta-analysis and support exercise as a treatment option in improving the cognition of patients with AD.11,23,24,26,27 Although results from two articles do not support the findings of this study, the 95% CI of both studies covered the invalid vertical line, suggesting that the study was not statistically significant.28,29 The risk of bias of this meta-analysis is presented in Figure 5. Scatter points of each study are distributed at the top of the funnel and are symmetrically distributed, suggesting a small bias of those research studies (Figure 5).
Review – complete data set (n=13)
Six articles had no associated MMSE data and were not included in the meta-analysis. Eight articles demonstrated that exercise improves cognitive function for individuals with AD and supports physical activity as a treatment option for patients with AD.10–12,23,24,26,27,30 Kemoun et al10 found that physical activity programs slow cognitive decline: postintervention cognitive scores: intervention: 30.4±7.7, control: 23.2±8.4; overall ERFC score of the subjects from the IG improved (P<0.01), while that of the CG decreased. Yang et al11 recruited 50 elderly people with mild AD and found that MMSE score significantly increased (P<0.05) in aerobic groups before and after 3 months, while for the CG, MMSE scores decreased significantly after 3 months (P<0.05). These results provide strong evidence that aerobic exercise can improve cognition of AD patients.11 Yágüez et al12 demonstrated significant improvements in sustained attention, visual memory, and working memory in the exercise group when compared with the CG after a 6-week training regime. Kwak et al’s23 findings demonstrated that MMSE score increases 20% (pre: 14.53±5.34, P<0.05) and 30% (post: 17.47±6.90, P<0.01) at 6 and 12 months. Venturelli et al26 aimed to compare the effect of a walking program on cognitive decline of elderly nursing home residents in the later stages of AD. They found that the walking group did not show a significant change in MMSE scores before and after the training period, whereas the CG group showed a decrease in MMSE scores during the same period. Vreugdenhil et al27 examined the effect of community-based exercise programs on AD patients. After 4 months, patients who exercised had improved cognition when compared with control (increased MMSE scores by 2.6 points, P<0.001). Pedroso et al30 studied 21 older patients with probable AD and detected a positive interaction between groups and moments (P=0.000479), confirming the effect of physical activity on global cognitive function. In addition, the outcomes of MMSE, FAB, and CDT of IG were all positive with P<0.05.30 However, the remaining five studies did not display a beneficial effect of exercise on cognitive function in patients with AD.25,28,29,31,32 It is important to note that one study observed that the effects of exercise on cognitive function in patients with AD differed from each other when different observational indicators were used.32
Discussion
The prevalence of AD has increased over recent years, resulting in a heavy burden to families, economies, and society. At present, there are no effective therapeutic drugs. Exercise is a preferred nonpharmacotherapy treatment option due to its wide range of positive effects, fewer side effects, and low economic burden. However, to date, results of studies have been inconsistent regarding the effectiveness of exercise in improving cognitive function in AD patients. Therefore, literature was systematically reviewed to investigate the effect of exercise on AD patients. Initially, 3,343 articles were retrieved; however, as a result of the screening process, 13 articles were selected. This study presents evidence supporting exercise as an effective intervention option for cognitive function in patients with AD, although the methodological heterogeneity in the literature limits our conclusion. Eight studies demonstrated that exercise has a positive effect on cognitive function in people with AD.10–12,23,24,26,27,30 The remaining articles had mixed results or did not demonstrate statistical differences.25,28,29,31,32 Additionally, types of physical activity are different. Nine studies had an intervention period between 12 and 16 weeks,10,11,24,25,27–31 while other trials had an intervention period from 1.5 to 12 months.12,23,26,32 In eight studies, the CG had patients with no exercise intervention or treatment as usual,10,12,23,24,27–29,31 in one trial patients in the CG performed nonaerobic exercises,32 and in one trial, patients in the CG participated in talk only visits.31 It is important to note that selected studies used several cognitive tests, and MMSE was the most commonly used cognition detection method. Nine studies used MMSE as a measure of cognitive ability,11,23–30 nine studies applied various other monitoring indicators,10,12,24,25,27–31 and seven studies identified cognition by means of multiple outcome measures.24,25,27–30,32 One study presented inconsistent results in different outcome measures for the same study.32 It is therefore difficult to make direct comparisons. In addition, it was hard to determine whether exercise is invalid or the cognitive measurement tools are not properly selected when there are no significant differences between the two groups. Because the selected studies demonstrated great heterogeneity in type of intervention, duration, intensity, and frequency of exercise, there is a need to develop uniform research criteria, including the characteristics of the participants, research designs, intervention methods, selection of cognition measurements, and so on, in order to conduct large sample studies with uniform standards, which can provide a reliable basis for clinical treatment application.
It is difficult to achieve high-frequency and continuous intervention for AD patients in real-world studies. In addition, it is difficult to perform exercise-intervened RCT in AD patients. These results are inconsistent and controversial. Exercise conditions are usually self-reported data with uncertain accuracy, and it is difficult to exclude the impact of other factors, such as diet, environment, social support, and so on, on the results of exercise intervention; for the elderly, especially those with moderate to severe dementia, exercise intervention is potentially dangerous. Therefore, exercise interventions have limitations. Well-designed research is needed to solve these problems in the future. Due to the inconsistency in monitoring indicators, meta-analysis can only be conducted on studies with MMSE as an outcome measure. Due to the unavailability of MMSE outcome data for two studies,25,30 this study conducted a meta-analysis of seven studies.11,23,24,26–29 Overall, results of this meta-analysis support exercise to improve cognitive function in people with AD, which is in line with our expectation. In the quantitative meta-analysis, research types and result measurement tools included are the same, greatly improving the strength of the evidence and adding much more authority to the evidence. Of the six studies that were not included in this meta-analysis, although three studies failed to achieve positive results, lack of results did not mean that exercise was not effective. In one study, no significant differences in the outcomes measured posttest were found. In this study, participants in the CG received home safety training.25 In one study, patients in the CG participated in talk only visits.31 In one study, participants in the CG were asked to do stretching and toning, and the Disability Assessment for Dementia total functional independence was associated with changes in memory-specific cognitive function (r=0.26, 95% CI=0.06 to ∞, P=.017) but not executive function (r=0.18, 95% CI=−0.03 to ∞, P=.07).32 Possible explanations for the lack of cognitive benefits in exercise include the following: 1) training activities and social participation in the CG might have produced cognitive benefits; 2) the short period of intervention might not be enough to produce cognitive benefits; and 3) the level (intervention type, time, intensity, and frequency) of physical activity might not be sufficient to produce cognitive benefits. Therefore, we believe that physical activity can improve the cognitive function of patients with AD and that some studies have not had long enough times of intervention or sufficient power to demonstrate the effectiveness of exercise. In order to achieve this positive result, it is necessary to start the exercise intervention in the early stage and to persevere in the long run as a daily habit.
There are three mechanisms for exercise to improve cognition. First, animal experiments demonstrated that exercise can promote neural plasticity33,34 and induce increases in neurogenesis in the hippocampus, and35,36 aerobic fitness promotes blood flow, glucose utilization, and oxygen extraction and improves functional and structural brain reserves.37 Second, exercise can promote the secretion of brain-derived neurotrophic factor (BDNF),38–41 which is related to learning and memory. Third, the indirect effects of exercise on the brain cannot be ignored. Exercise can reduce certain chronic diseases (such as cardiovascular diseases, obesity, diabetes, and so on) and the risk factors that have been associated with dementia and other cognitive dysfunctions.42
This study only included RCTs, which unified the study types, reduced bias, and provided strong evidence. In addition, the meta-analysis included seven new RCTs. The overall outcome demonstrates that physical activity benefits the cognitive function of AD patients. Although strength of validity evidence was not significant, physical activity as a positive lifestyle choice can improve patient’s cognitive function, reduce risk of multiple diseases, and improve patients’ physical function. Moreover, physical activity has less side effects and less economic burden, which has more extensive benefits than drug therapy.
Our review also has several limitations. Although there are no language restrictions in the search strategy, the studies eventually included were in English and might have had language bias. Most trials have small sample sizes, and the intervention methods such as type of intervention, intensity, duration, and frequency are heterogeneous. The follow-up period was not long enough and measures of cognitive function were different, while some trials used several cognitive measurements. In this study, although some results were not statistically different, it is difficult to determine whether the power of exercise intervention is insufficient or the exercise is ineffective. The research protocol could not always be found; therefore, there might be some publication bias.
Conclusion
The purpose of this review was to reveal the effects of physical activity on cognitive function in patients with AD. RCTs of exercise and cognitive function in patients with AD were reviewed, and a positive effect was found through meta-analysis and some other research outcomes. However, the heterogeneity of intervention type, time, intensity, frequency of physical activity, cognitive tests, follow-up time, control conditions as well as small sample trials do not allow us to make a very definitive conclusion about the effects of exercise intervention. In clinical practice, it is strongly recommended to use exercise therapy to prevent or control cardiovascular diseases, obesity, and so on. Therefore, physical activity should continue to be encouraged. Targeted multimodal interventions may be more effective for different treatment goals. In addition, future trials call for standardized intervention characteristics and large-scale, long-term follow-up to generate positive outcomes that support the ability of exercise to improve cognition of AD patients.
Supplementary material
Table S1.
The complete bibliographic strategy for PubMed
| Search | Query | Items found |
|---|---|---|
| #1 | Search (((((((((((((((((((((((((((((((((((Alzheimer’s Disease[Title/Abstract]) OR Dementia, Senile[Title/Abstract]) OR Senile Dementia[Title/Abstract]) OR Dementia, Alzheimer Type[Title/Abstract]) OR Alzheimer Type Dementia[Title/Abstract]) OR Alzheimer-Type Dementia (ATD)[Title/Abstract]) OR Alzheimer Type Dementia (ATD)[Title/Abstract]) OR Dementia, Alzheimer-Type (ATD)[Title/Abstract]) OR Alzheimer Type Senile Dementia[Title/Abstract]) OR Primary Senile Degenerative Dementia[Title/Abstract]) OR Dementia, Primary Senile Degenerative[Title/Abstract]) OR Alzheimer Sclerosis[Title/Abstract]) OR Sclerosis, Alzheimer[Title/Abstract]) OR Alzheimer Syndrome[Title/Abstract]) OR Alzheimer Dementia[Title/Abstract]) OR Alzheimer Dementias[Title/Abstract]) OR Dementia, Alzheimer[Title/Abstract]) OR Dementias, Alzheimer[Title/Abstract]) OR Senile Dementia, Alzheimer Type[Title/Abstract]) OR Acute Confusional Senile Dementia[Title/Abstract]) OR Senile Dementia, Acute Confusional[Title/Abstract]) OR Dementia, Presenile[Title/Abstract]) OR Presenile Dementia[Title/Abstract]) OR Alzheimer Disease, Late Onset[Title/Abstract]) OR Late Onset Alzheimer Disease[Title/Abstract]) OR Alzheimer’s Disease, Focal Onset[Title/Abstract]) OR Focal Onset Alzheimer’s Disease[Title/Abstract]) OR Familial Alzheimer Disease (FAD)[Title/Abstract]) OR Alzheimer Disease, Familial (FAD) [Title/Abstract]) OR Alzheimer Diseases, Familial (FAD)[Title/Abstract]) OR Familial Alzheimer Diseases (FAD)[Title/Abstract]) OR Alzheimer Disease, Early Onset[Title/Abstract]) OR Early Onset Alzheimer Disease[Title/Abstract]) OR Presenile Alzheimer Dementia[Title/Abstract]) OR Alzheimer Disease[Title/Abstract]) OR AD[Title/Abstract] | 126045 |
| #2 | Search “Alzheimer Disease”[Mesh] | 82670 |
| #3 | #1 or #2 | 164728 |
| #4 | Search Exercise[Title/Abstract] OR Exercises[Title/Abstract] OR Physical Activity[Title/Abstract] OR Activities, Physical[Title/Abstract] OR Activity, Physical[Title/Abstract] OR Physical Activities[Title/Abstract] OR Exercise, Physical[Title/Abstract] OR Exercises, Physical[Title/Abstract] OR Physical Exercise[Title/Abstract] OR Physical Exercises[Title/Abstract] OR Acute Exercise[Title/Abstract] OR Acute Exercises[Title/Abstract] OR Exercise, Acute[Title/Abstract] OR Exercises, Acute[Title/Abstract] OR Exercise, Isometric[Title/Abstract] OR Exercises, Isometric[Title/Abstract] OR Isometric Exercises[Title/Abstract] OR Isometric Exercise[Title/Abstract] OR Exercise, Aerobic[Title/Abstract] OR Aerobic Exercise[Title/Abstract] OR Aerobic Exercises[Title/Abstract] OR Exercises, Aerobic[Title/Abstract] OR Exercise Training[Title/Abstract] OR Exercise Trainings[Title/Abstract] OR Training, Exercise[Title/Abstract] OR Trainings, Exercise[Title/Abstract] OR Running[Title/Abstract] OR runnings[Title/Abstract] OR jogging[Title/Abstract] OR joggings[Title/Abstract] OR Swimming[Title/Abstract] OR Walking[Title/Abstract] OR Ambulation[Title/Abstract] OR Stair Climbing[Title/Abstract] OR Climbing, Stair[Title/Abstract] OR Stair Navigation[Title/Abstract] OR Navigation, Stair[Title/Abstract] OR Muscle Stretching Exercises[Title/Abstract] OR yoga[Title/Abstract] OR tai chi[Title/Abstract] OR Tai-ji[Title/Abstract] OR Tai Chi[Title/Abstract] OR Chi, Tai[Title/Abstract] OR Tai Ji Quan[Title/Abstract] OR Ji Quan, Tai[Title/Abstract] OR Quan, Tai Ji[Title/Abstract] OR Taiji[Title/Abstract] OR Taijiquan[Title/Abstract] OR T’ai Chi[Title/Abstract] OR Tai Chi Chuan[Title/Abstract] OR Muscle Stretching Exercises[Title/Abstract] OR Exercise, Muscle Stretching[Title/Abstract] OR Exercises, Muscle Stretching[Title/Abstract] | 451829 |
| #5 | Search “Exercise”[Mesh] | 165076 |
| #6 | #4 or #5 | 482666 |
| #7 | Search (((((cognition[Title/Abstract]) OR cognitions[Title/Abstract]) OR cognitive function[Title/Abstract]) OR cognition functions[Title/Abstract]) OR Function, Cognitive[Title/Abstract]) OR Functions, Cognitive[Title/Abstract] | 89176 |
| #8 | Search “Cognition”[Mesh] | 139349 |
| #9 | #7 or #8 | 202199 |
| #10 | #3 and #6 and #9 | 478 |
Notes: Sort by: most recent. Date: 20/05/2018.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (number 81570491).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alzheimer’s Association 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT, Yt W. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta-analysis. Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Kelley AS, Mcgarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163(10):729–736. doi: 10.7326/M15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang T, Yu JT, Tan L. Novel disease-modifying therapies for Alzheimer’s disease. J Alzheimers Dis. 2012;31(3):475–492. doi: 10.3233/JAD-2012-120640. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292(12):1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 9.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemoun G, Thibaud M, Roumagne N, et al. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn Disord. 2010;29(2):109–114. doi: 10.1159/000272435. [DOI] [PubMed] [Google Scholar]
- 11.Yang SY, Shan CL, Qing H, et al. The effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol Disord Drug Targets. 2015;14(10):1292–1297. doi: 10.2174/1871527315666151111123319. [DOI] [PubMed] [Google Scholar]
- 12.Yágüez L, Shaw KN, Morris R, Matthews D. The effects on cognitive functions of a movement-based intervention in patients with Alzheimer’s type dementia: a pilot study. Int J Geriatr Psychiatry. 2011;26(2):173–181. doi: 10.1002/gps.2510. [DOI] [PubMed] [Google Scholar]
- 13.Farina N, Rusted J, Tabet N. The effect of exercise interventions on cognitive outcome in Alzheimer’s disease: a systematic review. Int Psychogeriatr. 2014;26(1):9–18. doi: 10.1017/S1041610213001385. [DOI] [PubMed] [Google Scholar]
- 14.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Scherder E, Scherder R, Verburgh L, et al. Executive functions of sedentary elderly may benefit from walking: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2014;22(8):782–791. doi: 10.1016/j.jagp.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4:CD006489. doi: 10.1002/14651858.CD006489.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littbrand H, Stenvall M, Rosendahl E. Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: a systematic review. Am J Phys Med Rehabil. 2011;90(6):495–518. doi: 10.1097/PHM.0b013e318214de26. [DOI] [PubMed] [Google Scholar]
- 18.Öhman H, Savikko N, Strandberg TE, Pitkälä KH. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement GeriatrCogn Disord. 2014;38:347–365. doi: 10.1159/000365388. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; 2011. updated March 2011. [Google Scholar]
- 20.Field AP, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol. 2010;63(Pt 3):665–694. doi: 10.1348/000711010X502733. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwak YS, Um SY, Son TG, Kim DJ. Effect of regular exercise on senile dementia patients. Int J Sports Med. 2008;29(6):471–474. doi: 10.1055/s-2007-964853. [DOI] [PubMed] [Google Scholar]
- 24.Holthoff VA, Marschner K, Scharf M, et al. Effects of physical activity training in patients with Alzheimer’s dementia: results of a pilot RCT study. PLoS One. 2015;10(4):e0121478. doi: 10.1371/journal.pone.0121478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg M, Leoutsakos JM, Podewils LJ, Lyketsos CG. Evaluation of a home-based exercise program in the treatment of Alzheimer’s disease: the maximizing independence in dementia (MIND) study. Int J Geriatr Psychiatry. 2009;24(7):680–685. doi: 10.1002/gps.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26(5):381–388. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci. 2012;26(1):12–19. doi: 10.1111/j.1471-6712.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 28.Dky M, Szeto SL, Mak YF. A randomised controlled trial on the effect of exercise on physical, cognitive and affective function in dementia subjects. Asian J Gerontol Geriatr. 2008;3(1):8–16. [Google Scholar]
- 29.Hoffmann K, Sobol NA, Frederiksen KS, et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J Alzheimers Dis. 2016;50(2):443–453. doi: 10.3233/JAD-150817. [DOI] [PubMed] [Google Scholar]
- 30.Pedroso RV, Coelho FG, Santos-Galduróz RF, Costa JL, Gobbi S, Stella F. Balance, executive functions and falls in elderly with Alzheimer’s disease (AD): a longitudinal study. Arch Gerontol Geriatr. 2012;54(2):348–351. doi: 10.1016/j.archger.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 31.Cott CA, Dawson P, Sidani S, Wells D. The effects of a walking/talking program on communication, ambulation, and functional status in residents with Alzheimer disease. Alzheimer Dis Assoc Disord. 2002;16(2):81–87. doi: 10.1097/00002093-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Vidoni ED, Perales J, Alshehri M, Giles AM, Siengsukon CF, Burns JM. Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer disease. J Geriatr Phys Ther. 2017;1 doi: 10.1519/JPT.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Keefe J, Nadel L, Keightley S, Kill D. Fornix lesions selectively abolish place learning in the rat. Exp Neurol. 1975;48(1):152–166. doi: 10.1016/0014-4886(75)90230-7. [DOI] [PubMed] [Google Scholar]
- 35.Sahay A, Scobie KN, Hill AS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 37.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 38.Belarbi K, Burnouf S, Fernandez-Gomez FJ, et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol Dis. 2011;43(2):486–494. doi: 10.1016/j.nbd.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5(4):287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement. 2007;3(2 Suppl):S30–S37. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20(10):2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
The complete bibliographic strategy for PubMed
| Search | Query | Items found |
|---|---|---|
| #1 | Search (((((((((((((((((((((((((((((((((((Alzheimer’s Disease[Title/Abstract]) OR Dementia, Senile[Title/Abstract]) OR Senile Dementia[Title/Abstract]) OR Dementia, Alzheimer Type[Title/Abstract]) OR Alzheimer Type Dementia[Title/Abstract]) OR Alzheimer-Type Dementia (ATD)[Title/Abstract]) OR Alzheimer Type Dementia (ATD)[Title/Abstract]) OR Dementia, Alzheimer-Type (ATD)[Title/Abstract]) OR Alzheimer Type Senile Dementia[Title/Abstract]) OR Primary Senile Degenerative Dementia[Title/Abstract]) OR Dementia, Primary Senile Degenerative[Title/Abstract]) OR Alzheimer Sclerosis[Title/Abstract]) OR Sclerosis, Alzheimer[Title/Abstract]) OR Alzheimer Syndrome[Title/Abstract]) OR Alzheimer Dementia[Title/Abstract]) OR Alzheimer Dementias[Title/Abstract]) OR Dementia, Alzheimer[Title/Abstract]) OR Dementias, Alzheimer[Title/Abstract]) OR Senile Dementia, Alzheimer Type[Title/Abstract]) OR Acute Confusional Senile Dementia[Title/Abstract]) OR Senile Dementia, Acute Confusional[Title/Abstract]) OR Dementia, Presenile[Title/Abstract]) OR Presenile Dementia[Title/Abstract]) OR Alzheimer Disease, Late Onset[Title/Abstract]) OR Late Onset Alzheimer Disease[Title/Abstract]) OR Alzheimer’s Disease, Focal Onset[Title/Abstract]) OR Focal Onset Alzheimer’s Disease[Title/Abstract]) OR Familial Alzheimer Disease (FAD)[Title/Abstract]) OR Alzheimer Disease, Familial (FAD) [Title/Abstract]) OR Alzheimer Diseases, Familial (FAD)[Title/Abstract]) OR Familial Alzheimer Diseases (FAD)[Title/Abstract]) OR Alzheimer Disease, Early Onset[Title/Abstract]) OR Early Onset Alzheimer Disease[Title/Abstract]) OR Presenile Alzheimer Dementia[Title/Abstract]) OR Alzheimer Disease[Title/Abstract]) OR AD[Title/Abstract] | 126045 |
| #2 | Search “Alzheimer Disease”[Mesh] | 82670 |
| #3 | #1 or #2 | 164728 |
| #4 | Search Exercise[Title/Abstract] OR Exercises[Title/Abstract] OR Physical Activity[Title/Abstract] OR Activities, Physical[Title/Abstract] OR Activity, Physical[Title/Abstract] OR Physical Activities[Title/Abstract] OR Exercise, Physical[Title/Abstract] OR Exercises, Physical[Title/Abstract] OR Physical Exercise[Title/Abstract] OR Physical Exercises[Title/Abstract] OR Acute Exercise[Title/Abstract] OR Acute Exercises[Title/Abstract] OR Exercise, Acute[Title/Abstract] OR Exercises, Acute[Title/Abstract] OR Exercise, Isometric[Title/Abstract] OR Exercises, Isometric[Title/Abstract] OR Isometric Exercises[Title/Abstract] OR Isometric Exercise[Title/Abstract] OR Exercise, Aerobic[Title/Abstract] OR Aerobic Exercise[Title/Abstract] OR Aerobic Exercises[Title/Abstract] OR Exercises, Aerobic[Title/Abstract] OR Exercise Training[Title/Abstract] OR Exercise Trainings[Title/Abstract] OR Training, Exercise[Title/Abstract] OR Trainings, Exercise[Title/Abstract] OR Running[Title/Abstract] OR runnings[Title/Abstract] OR jogging[Title/Abstract] OR joggings[Title/Abstract] OR Swimming[Title/Abstract] OR Walking[Title/Abstract] OR Ambulation[Title/Abstract] OR Stair Climbing[Title/Abstract] OR Climbing, Stair[Title/Abstract] OR Stair Navigation[Title/Abstract] OR Navigation, Stair[Title/Abstract] OR Muscle Stretching Exercises[Title/Abstract] OR yoga[Title/Abstract] OR tai chi[Title/Abstract] OR Tai-ji[Title/Abstract] OR Tai Chi[Title/Abstract] OR Chi, Tai[Title/Abstract] OR Tai Ji Quan[Title/Abstract] OR Ji Quan, Tai[Title/Abstract] OR Quan, Tai Ji[Title/Abstract] OR Taiji[Title/Abstract] OR Taijiquan[Title/Abstract] OR T’ai Chi[Title/Abstract] OR Tai Chi Chuan[Title/Abstract] OR Muscle Stretching Exercises[Title/Abstract] OR Exercise, Muscle Stretching[Title/Abstract] OR Exercises, Muscle Stretching[Title/Abstract] | 451829 |
| #5 | Search “Exercise”[Mesh] | 165076 |
| #6 | #4 or #5 | 482666 |
| #7 | Search (((((cognition[Title/Abstract]) OR cognitions[Title/Abstract]) OR cognitive function[Title/Abstract]) OR cognition functions[Title/Abstract]) OR Function, Cognitive[Title/Abstract]) OR Functions, Cognitive[Title/Abstract] | 89176 |
| #8 | Search “Cognition”[Mesh] | 139349 |
| #9 | #7 or #8 | 202199 |
| #10 | #3 and #6 and #9 | 478 |
Notes: Sort by: most recent. Date: 20/05/2018.