Abstract
Background
As newly available antidiabetic drugs (ADs) are used more commonly as initial hypoglycemic choice for early stage diabetes patients, there is an urgent need to investigate how these agents may differ in treatment durability relative to metformin. This study aimed to investigate the incidence and risk of treatment adjustment among newly treated type 2 diabetes mellitus (T2DM) patients receiving an oral AD as initial monotherapy.
Methods
T2DM patients registered in the National Health Insurance Program who were newly prescribed an oral AD were identified. Time to treatment addition or switch to alternative antidiabetic therapy was determined using the Kaplan–Meier survival analysis. Cox proportional hazards regression was performed to estimate the hazard ratio (HR) after adjusting for potential confounding factors.
Results
The median time to treatment adjustment was shorter for sulfonylureas (SUs), dipeptidyl peptidase-4 (DPP-4) inhibitors, alphaglucosidase (AG) inhibitors, and thiazolidinediones (TZDs) compared to that for metformin. Initiation of therapy with SUs or DPP-4 inhibitors was associated with a significantly higher risk of both treatment addition and switching than with metformin (HR 1.49 versus 1.47 for overall treatment adjustment, respectively). In contrast, among incident users of AG inhibitors or TZDs, only the hazard of switch was substantially increased compared to metformin starters (6.19, 95% confidence interval [CI] 5.77–6.64 and 7.31, 95% CI 6.35–8.42, respectively). When addition and switch events were collectively assessed, the risk of treatment adjustment was significantly elevated in all non-metformin cohorts.
Conclusion
Our results demonstrated that the durability of metformin as an initial monotherapy was superior to that of other ADs, including newer classes of antidiabetics, and appeared to be more effective in delaying treatment adjustment in real-world clinical practice.
Keywords: drug utilization patterns, antidiabetic drugs, type 2 diabetes
Introduction
Clinical guidelines for diabetes management advocate comprehensive glycemic control by using multifactorial risk-reduction strategies with pharmacologic glucose-lowering therapy as a pivotal intervention.1,2 Thanks to several novel hypoglycemic agents newly approved for clinical use over the last decades, prescribers and patients now have a wide variety of therapeutic options to choose from for treatment escalation or switching.3 Despite the accumulating evidence on the efficacy and safety of newly available antidiabetic drugs (ADs), clinical trials in the past were conducted primarily focusing on intermediate physiological outcomes, such as hemoglobin A1c, with cardiovascular (CV) outcomes under investigation only recently.4–6
Evaluation of the durability of drug therapy as a composite measure to investigate important attributes, incorporating efficacy, safety, and tolerability simultaneously, has been performed in an attempt to better understand the impact of the respective therapy in the context of real-world clinical practice. In a randomized controlled trial referred to as A Diabetes Outcome Progression Trial (ADOPT), the glycemic durability of rosiglitazone, a thiazolidinedione (TZD) whose prescription rates were affected to varying degrees around the world following controversies over its CV safety, has been investigated as compared with metformin or glyburide monotherapy in treatment-naïve patients with type 2 diabetes mellitus (T2DM).7 The trial was designed to maximize internal validity by enrolling only highly selected patients who met restrictive eligibility criteria in the study; hence, the study participants may not represent the diabetes patient population in real-world clinical settings. There have been other observational studies conducted to evaluate the differential durability of glycemic control or therapy persistence among different ADs, but the comparison was primarily carried out between metformin and sulfonylureas (SUs).8–10 As newly approved ADs, most importantly dipeptidyl peptidase-4 (DPP-4) inhibitors, are used more frequently as initial monotherapy, there is an urgent need to investigate how these agents may differ in terms of treatment durability as compared with metformin, the guideline-recommended first-line therapy.
The primary aim of this nationwide cohort study was to evaluate the effect of initial choice of oral antidiabetic monotherapy including newly approved agents on subsequent regimen change and time to treatment adjustment in patients with T2DM in real-world clinical practice by analyzing claims data of Korean National Health Insurance.
Methods
Data source
This retrospective cohort study was performed using the Korean Health Insurance Review and Assessment Service (HIRA) database, which encompasses the records of entire health care institutions for medical claim reimbursement. The HIRA data enclose information on patient demographics, diagnoses, health care institution types, medical procedures and services and medical utilization information relating to inpatient hospitalization and outpatient medical episodes including prescription records. The protocol of the present study was approved by the institutional review board of Ajou University (No 201706-HB-EX-001). No further ethics approval was required, because the researchers are authorized to analyze de-identified patient data provided by the HIRA for research purposes.
Study population and ADs
T2DM patients were identified if they had a medical insurance claim submitted for T2DM based on the International Classification of Diseases, Tenth Revision (ICD-10) code E11 and received at least one prescription for antidiabetic therapy from January 1, 2007, to December 31, 2015. To capture only treatment-naïve patients, we excluded those who were prescribed any ADs during the pre-index period till January 1, 2011. The year 2011 was chosen as the start of the index period considering a series of safety alerts regarding rosiglitazone’s CV risks issued from 2007 onward until the heavy access restrictions imposed on the TZD agent in late 2010, which substantially affected the use rates of TZDs.3 To ensure that we selected the patients tolerating their initial antidiabetic therapy, we restricted our analyses to only those with initial treatment duration of at least 90 consecutive days. Patients with duration of antidiabetic therapy <365 days since the commencement of their initial therapy were excluded. Because of the small number of patients initiated with meglitinides, sodium-glucose co-transporter-2 inhibitors, or glucagon-like peptide-1 (GLP-1) analogs, our analysis was limited to patients started with oral ADs belonging to the following five therapeutic categories: biguanides (metformin), SUs, DPP-4 inhibitors, alpha-glucosidase (AG) inhibitors, and TZDs. Patients initiating therapy with insulin or more than one AD concurrently (including combination products) were excluded in order to ascertain that we selected only treatment-naïve early stage T2DM patients and to improve the homogeneity of the study cohorts. We further excluded the subjects who had a second AD addition within 30 days following treatment initiation with their first drug. The index date was defined as the date of the first eligible prescription of individual patients. We followed the included patients from their index date till the occurrence of treatment adjustment (the primary outcome of interest), death, or the end of the study period (December 31, 2015), whichever came first, allowing a maximum follow-up period of 5 years.
Study end point: treatment adjustment (addition and switching)
To investigate the persistence of initial oral antidiabetic therapy, the first encounter of treatment adjustment in each patient was tracked and analyzed. Addition of therapy was defined as subjects being prescribed a new AD from a different therapeutic class while continuing to receive their initial therapy at least for 90 days. Switching therapy was defined as starting a new class of AD including injectable agents within 90 days before or after the discontinuation of the initial oral therapy. When the days of the first and second prescriptions were not overlapping, a predetermined grace period was applied; if patients received the first prescription for the subsequent therapy by the date of the last prescription for the preceding therapy plus 1.5 times the prescribed days’ supply, then their therapy was classified as persistent.11 Discontinuation and restart of the initial AD was defined as the existence of a long treatment gap (.180 days) between the last date of the prior treatment and the start date of the following treatment. A different class of AD for adding or switching therapy was only considered for the analysis if it had not been prescribed within 180 days prior to the start date of the respective treatment and if at least a 30-day supply was prescribed at a time. We also evaluated time to treatment addition or switching separately and collectively as treatment adjustment. The time to either addition or switch was defined as the duration of the initial therapy between the index date and the date of a prescription for a second class of AD. Patients were censored at the end of the follow-up period if no treatment adjustment occurred.
Statistical analysis
We took into account several factors that may have influenced the receipt of the initial AD, including age, sex, and the presence of comorbidities identified using ICD-10 codes (hypertension, congestive heart failure, CV disease, and chronic renal disease). We calculated a comorbidity score with the Charlson Comorbidity Index (CCI) and additionally a multinomial propensity score based on a set of relevant variables to predict the probability that the first AD a patient received would be metformin (the index drug).12 First, we estimated the propensity scores without reference to the outcomes via multiple logistic regression analysis using the following covariates: age category, sex, CCI category, and comorbidity. Then, a stratification method was used on the basis of individual propensity scores for further analysis. Cox proportional hazards regression analysis was used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) of treatment adjustment (addition and switching) with the aforementioned covariates in the study groups post propensity score-based stratification, and to examine differences in time to treatment adjustment for those initiated on the four study AD classes, compared with metformin. Kaplan–Meier curves were plotted to describe the time till the first treatment adjustment (addition and switching combined and separately) and to compare differences among the study cohorts using the logrank test. P-values were two tailed and considered as statistically significant when below 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of study patients and distribution of initial antidiabetic therapy
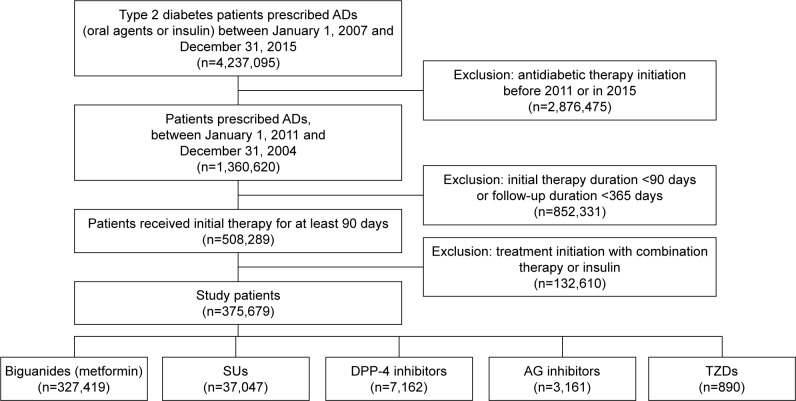
A total of 375,679 patients with T2DM met the inclusion criteria as the first-time users of oral ADs during the subject recruitment period from January 1, 2011, to December 31, 2014 (Figure 1). The distribution of baseline characteristics of the included patients is summarized by drug classes in Table 1.
Figure 1.
Flow chart of the process of identifying and selecting study patients: type 2 diabetes patients initiated oral antidiabetic therapy from 2011 up until 2015.
Abbreviations: AD, antidiabetic drug; SU, sulfonylurea; DPP-4, dipeptidyl peptidase-4; AG, α-glucosidase; TZD, thiazolidinedione.
Table 1.
Baseline characteristics of the study patients and treatment initiation with oral ADs
| Characteristics | Biguanides (metformin)
|
SUs
|
DPP-4 inhibitors
|
AG inhibitors
|
TZDs
|
|---|---|---|---|---|---|
| n=327,419 | n=37,047 | n=7,162 | n=3,161 | n=890 | |
| Male, n (%) | 170,151 (52.0) | 20,023 (54.1) | 3,697 (51.6) | 1,538 (48.7) | 476 (53.5) |
| Age (years), n (%) | |||||
| Mean±SD | 58.5±12.0 | 60.0±12.4 | 61.1±12.6 | 64.0±12.5 | 60.0±12.4 |
| <18 | 778 (0.2) | 24 (0.1) | 3 (0.0) | 3 (0.1) | 2 (0.2) |
| 18–40 | 16,458 (5.0) | 1,509 (4.1) | 309 (4.3) | 103 (3.3) | 40 (4.5) |
| 41–64 | 206,484 (63.1) | 20,972 (56.6) | 3,926 (54.8) | 1,558 (49.3) | 511 (57.4) |
| 65–74 | 72,742 (22.2) | 9,221 (24.9) | 1,794 (25.1) | 859 (27.2) | 222 (24.9) |
| ≥75 | 30,957 (9.5) | 5,321 (14.4) | 1,130 (15.8) | 638 (20.2) | 115 (12.9) |
| CCI, mean±SD | 1.9±1.3 | 2.2±1.6 | 2.0±1.5 | 2.4±1.7 | 1.9±1.5 |
| Comorbidities, n (%) | |||||
| Hypertension | 211,883 (64.7) | 25,878 (69.9) | 4,764 (66.5) | 2,153 (68.1) | 536 (60.2) |
| Congestive heart failure | 11,035 (3.4) | 1,957 (5.2) | 351 (4.9) | 217 (6.8) | 28 (3.2) |
| CV disease | 28,182 (8.6) | 4,286 (11.6) | 722 (10.1) | 456 (14.4) | 111 (12.5) |
| Chronic renal disease | 4,249 (1.3) | 1,309 (3.5) | 427 (5.9) | 147 (4.7) | 23 (2.6) |
Abbreviations: AD, antidiabetic drug; SU, sulfonylurea; DPP-4, dipeptidyl peptidase-4; AG, α-glucosidase; TZD, thiazolidinedione; SD, standard deviation; CCI, Charlson comorbidity index; CV, cardiovascular.
Treatment adjustment (addition and switching)
Among metformin starters, 139,133 (42.5%) experienced treatment adjustment. The frequent additions that the metformin group patients received were DPP-4 inhibitors (56.6%) and SUs (33.3%). In only 0.6% of metformin starters, insulin was added as a second AD, and 1.4% of metformin starters were prescribed more than two classes of ADs as add-on simultaneously. Although the proportion of switches was lower (only 4.4%) compared to other cohorts, the most frequent switches among metformin starters were switches to DPP-4 inhibitors (43.9%) and SUs (33.8%).
Of subjects initiated on SUs, 21,604 (58.3%) patients encountered treatment adjustment. Regarding treatment addition, a significantly large proportion of SU starters were prescribed metformin as add-on (74.2%). Following metformin, DPP-4 inhibitors (8.7%) and more than two classes of ADs (10.1%) were the next common add-on treatments. In more than a half of patients (57.9%) who underwent treatment switching, metformin was the agent that replaced their initial therapy. A DPP-4 inhibitor was the next most frequent AD to be switched to as a single entity (12.3%), but a larger proportion of patients (24.4%) were switched to more than two classes of ADs simultaneously.
Following metformin and SUs, a substantial minority of patients were initiated on DPP-4 inhibitor therapy (1.9%). The most common second AD added to the initial monotherapy was metformin (73.0%), followed by an SU (14.9%). Switches most commonly were to metformin (58.7%), with more than two classes of ADs (14.1%) and an SU (12.1%) the next most frequent treatment options to switch to. On the other hand, only a minority of patients were prescribed an AG inhibitor (0.8%) or a TZD (0.2%) on treatment initiation. Notably, switches occurred more frequently than addition in these two study cohorts, whereas opposite trends were observed in metformin, SU, and DPP-4 inhibitor cohorts.
Time to treatment adjustment (addition and switching)
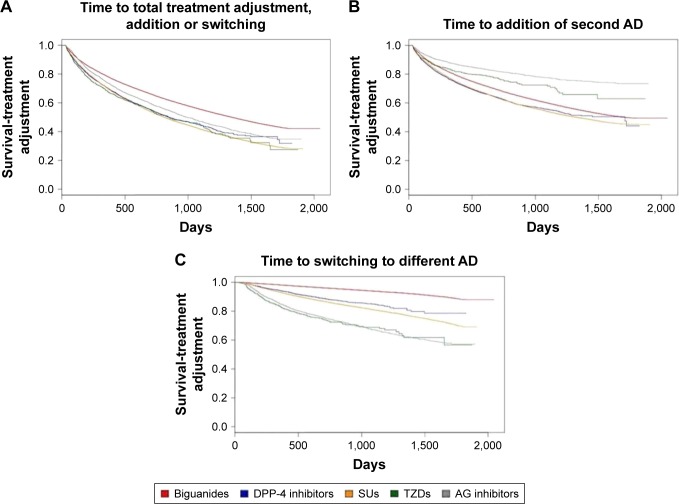
The median time to treatment adjustment was shorter in the DPP-4 inhibitor group (246 days) and TZD group (223 days) compared to that of the metformin group (353 days), which was the longest among the five cohorts (Table 2). The likelihood of treatment adjustment was assessed using Cox regression models with relevant variables, such as age, sex, comorbidities, and propensity scores, controlled for the analyses (Table 3). The likelihood of receiving treatment addition was statistically significantly higher in SU and DPP-4 inhibitor groups (HR 1.21 versus HR 1.26, respectively); on the contrary, the risk was lower with statistical significance in AG inhibitor and TZD groups (HR 0.54 versus HR 0.78, respectively). As for switches, initiating antidiabetic therapy with non-metformin agents were all associated with a significantly increased risk of undergoing treatment switching: the HR ranged from 2.87 for DPP-4 inhibitors to 7.31 for TZDs. As confirmed in Kaplan–Meier survival curves for the cumulative risk, the distribution of treatment addition and switching, both combined and separately, was distinct between drug classes (logrank test, P<0.001 for all comparisons; Figure 2). Patients initiated with SUs or DPP-4 inhibitors tended to add a second agent sooner than other AD starters. Patients on any AD therapy other than metformin on treatment initiation were likely to switch sooner to alternative therapy than were metformin starters.
Table 2.
Patterns of the first treatment adjustment (addition and switching) following treatment initiation by study cohorts
| Study end point | Biguanides (metformin)
|
SUs
|
DPP-4 inhibitors
|
AG inhibitors
|
TZDs
|
|---|---|---|---|---|---|
| n=327,419 | n=37,047 | n=7,162 | n=3,161 | n=890 | |
| Time to treatment adjustment, median | 353 (144–684) | 352 (150–688) | 246 (110–455) | 347 (159–690) | 223 (98–442) |
| (IQR) in days | |||||
| Total treatment adjustment, n (%) | 139,133 (42.5) | 21,604 (58.3) | 3,285 (45.9) | 1,578 (49.9) | 432 (48.5) |
| Addition, n (%) | 121,766 (37.2) | 15,430 (41.7) | 2,530 (35.3) | 576 (18.2) | 193 (21.7) |
| Biguanides (metformin) | 11,444 (74.2) | 1,847 (73.0) | 299 (51.9) | 102 (52.8) | |
| SUs | 40,595 (33.3) | 378 (14.9) | 157 (27.3) | 32 (16.6) | |
| DPP-4 inhibitors | 68,930 (56.6) | 1,338 (8.7) | 21 (3.6) | 20 (10.4) | |
| AG inhibitors | 1,830 (1.5) | 397 (2.6) | 13 (0.5) | 1 (0.5) | |
| TZDs | 5,557 (4.6) | 391 (2.5) | 79 (3.1) | 7 (1.2) | |
| Meglitinides | 503 (0.4) | 66 (0.4) | 5 (0.2) | 16 (2.8) | 1 (0.5) |
| SGLT-2 inhibitors | 1,836 (1.5) | 51 (0.3) | 10 (0.4) | 2 (0.3) | 0 |
| Insulin | 766 (0.6) | 180 (1.2) | 22 (0.9) | 14 (2.4) | 0 |
| More than two ADs | 1,749 (1.4) | 1,563 (10.1) | 176 (7.0) | 60 (10.4) | 37 (19.2) |
| Switching, n (%) | 14,528 (4.4) | 5,171 (14.0) | 629 (8.8) | 833 (26.4) | 210 (23.6) |
| Biguanides (metformin) | 2,992 (57.9) | 369 (58.7) | 442 (53.1) | 92 (43.8) | |
| SUs | 4,914 (33.8) | 76 (12.1) | 77 (9.2) | 22 (10.5) | |
| DPP-4 inhibitors | 6,379 (43.9) | 638 (12.3) | 106 (12.7) | 42 (20.0) | |
| AG inhibitors | 450 (3.1) | 69 (1.3) | 3 (0.5) | 2 (1.0) | |
| TZDs | 883 (6.1) | 79 (1.5) | 45 (7.2) | 8 (1.0) | |
| Meglitinides | 131 (0.9) | 38 (0.7) | 5 (0.8) | 25 (3.0) | 1 (0.5) |
| SGLT-2 inhibitors | 602 (4.1) | 37 (0.7) | 38 (6.0) | 9 (1.1) | 6 (2.9) |
| Insulin | 200 (1.4) | 56 (1.1) | 4 (0.6) | 9 (1.1) | 0 |
| More than two ADs | 969 (6.7) | 1,262 (24.4) | 89 (14.1) | 157 (18.8) | 45 (21.4) |
| Discontinuation and restart, n (%) | 2,839 (0.9) | 1,003 (2.7) | 126 (1.8) | 169 (5.3) | 29 (3.3) |
| No treatment adjustment, n (%) | 188,286 (57.5) | 15,443 (41.7) | 3,877 (54.1) | 1,583 (50.1) | 458 (51.5) |
Abbreviations: SU, sulfonylurea; DPP-4, dipeptidyl peptidase-4; AG, α-glucosidase; TZD, thiazolidinedione; IQR, interquartile range; SGLT-2, sodium-glucose co-transporter-2; AD, antidiabetic drug.
Table 3.
HR with 95% CI for the first treatment adjustment (addition and switching) as compared with metformin
| Study end point | Biguanides (metformin) | SUs | DPP-4 inhibitors | AG inhibitors | TZDs |
|---|---|---|---|---|---|
| Treatment adjustment | 1 (reference) | 1.49 (1.46–1.51) | 1.47 (1.41–1.52) | 1.30 (1.23–1.36) | 1.51 (1.37–1.66) |
| Addition | 1 (reference) | 1.21 (1.19–1.24) | 1.26 (1.21–1.31) | 0.54 (0.50–0.59) | 0.78 (0.67–0.90) |
| Switching | 1 (reference) | 3.31 (3.20–3.42) | 2.87 (2.65–3.11) | 6.19 (5.77–6.64) | 7.31 (6.35–8.42) |
Abbreviations: HR, hazard ratio; CI, confidence interval; SU, sulfonylurea; DPP-4, dipeptidyl peptidase-4; AG, α-glucosidase; TZD, thiazolidinedione.
Figure 2.
Kaplan–Meier curves for cumulative hazard of treatment adjustment (addition and switching) by study cohorts.
Notes: (A) Describes the time till the first treatment adjustment (addition and switching combined) and compares differences among the study cohorts (B) for the addition component and (C) for the switching component separately. Logrank test, P,0.001 for all comparisons.
Abbreviations: AG, α-glucosidase; TZD, thiazolidinedione; SU, sulfonylurea; DPP-4, dipeptidyl peptidase-4; AD, antidiabetic drug.
Discussion
In this nationwide cohort study, we investigated prescribing patterns and the durability of initial AD therapy and its impact on subsequent treatment adjustment in newly treated T2DM patients. Although metformin, the guideline-recommended first-line therapy, was widely prescribed on treatment initiation, a considerable minority of incident AD users were initiated on alternative glucose-lowering therapy, of which DPP-4 inhibitors accounted for the largest portion of the remaining patients. In this study, we found that almost half of the newly treated T2DM patients experienced treatment adjustment when followed up for up to 5 years.
Compared with patients initiated on alternative oral antidiabetic therapy (including SUs, DPP-4 inhibitors, AG inhibitors, and TZDs), metformin starters were significantly less likely to encounter treatment adjustment (Table 3). Non-metformin ADs by drug class were all assessed as a strong determinant of treatment adjustment risk associated with increased HRs and statistically significant 95% CIs. Another notable finding of this study was that time to the first treatment adjustment was delayed among incident users of metformin relative to those initially treated with alternative oral monotherapy (Figure 2). Additionally, 57.5% of metformin starters (the highest proportion among the five study cohorts) were persistent with their first-line therapy with no treatment adjustment over the follow-up years. The low incidence and risk of treatment adjustment suggests that starting antidiabetic therapy with metformin seems beneficial in reducing the risk of suboptimal glycemic control or adverse drug events and as a result would have been less likely to result in outcome episodes than do other classes of ADs.
Interestingly, despite current guideline recommendations regarding a universal use of metformin as the first-line hypoglycemic therapy unless clinically contraindicated, a sizable proportion of elderly patients aged 65 years or older received an alternative oral agent as an initial choice rather than metformin (Table 1). These drug utilization patterns are in line with the results shown in a previous study,9 where older patients were found to be more likely to receive an SU as the first-line treatment. One explanation for this trend is that as older adults with impaired renal function are generally considered susceptible to metformin-induced lactic acidosis,13 clinicians may have been reluctant to prescribe metformin for these patients. On the downside, however, SUs have been associated with a high risk of hypoglycemia, and elderly patients are more vulnerable to such episodes. Furthermore, previous studies noted that their use may increase the risk of CV diseases.14,15 Hence, comprehensive risk versus benefit analysis and multifactorial assessment to identify patients’ individual risk factors for drug-induced adverse events should be incorporated into the decision-making process of selecting the best option for initial treatment in this age group.
The Kaplan–Meier analysis in this study revealed a distinct pattern of treatment addition versus switching (Figure 2), and the significant increase in the risk of overall treatment adjustment appeared to be predominantly attributable to the switching component of the analysis, which showed substantially high HRs (Table 3). Overall, treatment addition occurred significantly sooner with SUs and DPP-4 inhibitors than with metformin (HR 1.21 versus HR 1.26, respectively), and similar trends were observed for treatment switching (HR 3.31 versus HR 2.87, respectively). In contrast, incident users of AG inhibitors and TZDs were significantly less likely than metformin starters to add a second AD (HR 0.54 versus HR 0.78, respectively), whereas they appear to have a substantially elevated risk of switching to alternative therapy (HR 6.19 versus HR 7.31, respectively). These trends can be explained in terms of insufficient glycemic control or intolerance associated with the initially chosen monotherapy. With regard to SUs and TZDs with relatively well-established glucose-lowering efficacy, clinicians’ decisions to modify therapy might have been primarily affected by the safety profile of the respective agents: SUs are associated with a higher incidence of hypoglycemia and weight gain,16 whereas TZDs may increase the risk of edema and chronic heart failure.17 On the other hand, DPP-4 inhibitors and AG inhibitors are known for their intermediate glucose-lowering efficacy,18,19 which may negatively influence the durability of the respective therapy and necessitate earlier treatment intensification for tighter glycemic control by adding a second AD.
Concerning TZD monotherapy on treatment initiation, different trends were observed in a previous study, where it was associated with an increased risk of treatment intensification (1.61, 95% CI 1.43–1.80).14 In contrast, in the ADOPT study, rosiglitazone was assessed as superior to metformin and glyburide in terms of glycemic durability when used as initial monotherapy: rosiglitazone was associated with a risk reduction of 32% compared to metformin and 63% compared to glyburide (P<0.001).7 However, the study came under criticism for its design where the primary outcome measure was based on fasting glucose levels and not glycated hemoglobin levels, which are deemed superior to the former as a strong predictor of diabetes complications.20 Of note is that TZD agents analyzed in our study were primarily pioglitazone as rosiglitazone’s use became almost nonexistent post the severe access restrictions on the latter TZD agent in late 2010 in Korea due to increased, albeit controversial, CV risk.3 TZD therapy may improve insulin sensitivity and reduce the rate of decline in β-cell function, and these features might be effective in delaying monotherapy failure by positively influencing the durability of the therapeutic effect.7,21 Nevertheless, the findings in the present study did not support the durability of TZD-based initial monotherapy as demonstrated in the previous study. One explanation for the trends observed in our study is that, with its strong glucose-lowering potency, treatment initiation with a TZD agent may provide effective blood glucose control without necessitating add-on therapy for an extended period, but treatment switches occurred early, possibly due to adverse drug events or to avoid such events in high-risk patients. Additionally, although we minimized the confounding effects of the 2010 regulatory actions for rosiglitazone by excluding subjects prescribed AD therapy prior to January 1, 2011, from our analysis, prescribers’ decision to modify therapy thereafter might still have been influenced by multiple factors surrounding the highly publicized controversy over rosiglitazone safety, such as patient safety and preference along with changes in insurance coverage and reimbursement policies.
Over the last decade, DPP-4 inhibitors have achieved a substantial uptake in prescription volume, and they have overtaken SUs as the most preferred add-on to metformin since 2014 in Korea.3 In the current analysis, among incident users of DPP-4 inhibitors, a tendency more toward treatment addition rather than switches to alternative therapy was observed, which could be understood in the context of their favorable safety and tolerability profile. Despite the growing preference for DPP-4 inhibitors in clinical practice, in our analysis, they were evaluated as inferior to metformin in delaying the first-treatment adjustment when used as initial monotherapy. Although current guidelines in Korea recommend DPP-4 inhibitor therapy as one of the second-line anti-hyperglycemic treatments to add in the settings of suboptimal glycemic control or disease progression, it is not suggested as an initial treatment. The long-term benefits and risks of newer agents, including DPP-4 inhibitors, have not been fully explored prior to clinical use, leaving prescribers with insufficient information on which to base their treatment decisions for diabetes patients. Hence, further postmarketing surveillance studies are of critical importance in order to aid in driving future treatment guideline recommendations and quality improvement initiatives.
As for an agent to add or switch to, metformin was by far the most preferred choice among patients initiated with alternative therapy. Interestingly, these findings imply that those incident users of non-metformin ADs did not have a contraindication to metformin on treatment initiation. Similar patterns were also noted in previous studies.9,14 Such a practice was deemed inconsistent with the current guideline recommendations concerning first-line treatment. Although the reason for initial AD selection in individual patients was not available, these drug utilization patterns indicate a need for better understanding of the value of guideline-recommended therapeutic interventions and potential risks versus benefits as well as patient- and drug-related factors, such as comorbidity and tolerability profile, associated with each treatment option.
Overall, we found that therapy choice for second-line or alternative agents was largely concentrated on metformin, DPP-4 inhibitors, and SUs. Across the study cohorts, only a minority of patients received insulin for treatment addition or switching, suggesting that the included patients were generally early-stage diabetes patients. The use rate of insulin in combination with oral ADs appeared to be <1% in our analysis, as opposed to 3%–5% observed in a European study.9 Additionally, a US study showed that GLP-1 receptor agonists were selected as add-on therapy in 4.0%–8.6% of patients.14 However, they were rarely used in Korea, possibly because of their cost, injection route, and patient preference; hence, only a negligible number of patients received them for treatment adjustment.
Evaluation of treatment adjustment in the context of diabetes management can be a critical indicator of overall efficacy, safety, and tolerability of drug therapy in real-world clinical practice. The findings in our study support the guidelines recommendation that metformin should be used as the first-line therapy for T2DM patients. Metformin monotherapy was evaluated as most effective in delaying treatment adjustment in incident users of oral glucose-lowering agents. This attribute may also have beneficial effects on patient perception and treatment adherence because treatment intensification, particularly insulin initiation, tends to be perceived as disease progression and greater financial challenges, by patients.22 The complexity of drug regimen is likely to grow as the disease progresses; thus, delaying treatment intensification by selecting the most effective initial choice of glucose-lowering therapy would have a significant bearing on long-term patient outcomes and quality of life.15,23–25
To our knowledge, this is the first nationwide population-based study that investigated the durability of different classes of oral ADs used as initial monotherapy in usual clinical practice, including metformin, SUs, DPP-4 inhibitors, TZDs, and AG inhibitors, and assessed the patterns not only for treatment addition but also for switching following treatment initiation. The results of this study reflect real-world clinical practice and have clinical implications for improving antidiabetic treatment in patients with T2DM. This study highlights the importance of the optimal provision of glucose-lowering treatment for patients, especially the use of metformin as the first-line treatment in accordance with the guideline recommendations.
Our study is subject to several limitations inherent to observational studies based on administrative data. First, information in the claims database is restricted to that required for reimbursement and does not incorporate information on glycemic control levels and potential confounders, including severity of condition, patients’ overall health, diet and lifestyle modifications, and reasons for initial AD selection and subsequent treatment change. Second, we assumed that all prescriptions were dispensed and the entire day’s supply was completed by patients, although individual patient adherence cannot be ascertained. Furthermore, this study was not designed to investigate whether better glycemic control with initial monotherapy or delayed treatment escalation contributes to reducing long-term CV complications in diabetes patients, and this remains to be addressed in future research.
Conclusion
T2DM patients initiated on therapy with metformin appeared less likely to have therapy adjustment than those initiated on other classes of oral ADs, including SUs, DPP-4 inhibitors, TZDs, and AG inhibitors. Metformin was widely used as the first-line antidiabetic therapy, but in a considerable minority of patients, the provision of initial treatment appeared not in accordance with the current guidelines.
Acknowledgments
This study was supported by the Ajou University Research Fund (No S-2017-G0001-00211), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No 2017R1C1B5015912), and the Bio and Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science, Information and Communications Technology, and Future Planning, Republic of Korea (No. 2013M3A9B5075838). The national patient data for the analysis were provided by the HIRA. The contents of this research do not represent the official views of the HIRA.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Korean Diabetes Association [webpage on the Internet] Korean Treatment Guideline for Diabetes. 2015. [Accessed January 8, 2018]. Available from: http://www.diabetes.or.kr/pro/publish/guide.php?mode=list.
- 2.American Diabetes Association Standards of medical care in diabetes-2017. Diabetes Care. 2017;40(suppl 1):S1–S132. doi: 10.2337/dc17-0299. [DOI] [PubMed] [Google Scholar]
- 3.Noh Y, Kang DR, Kim DJ, Lee KJ, Lee S, Shin S. Impact of clinical evidence communications and drug regulation changes concerning rosiglitazone on prescribing patterns of antidiabetic therapies. Pharmacoepidemiol Drug Saf. 2017;26(11):1338–1346. doi: 10.1002/pds.4262. [DOI] [PubMed] [Google Scholar]
- 4.Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(5):442–451. doi: 10.1111/j.1463-1326.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 5.Aschner P, Katzeff HL, Guo H, et al. Sitagliptin Study 049 Group Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(3):252–261. doi: 10.1111/j.1463-1326.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 6.Shin S, Kim H. The effect of sitagliptin on cardiovascular risk profile in Korean patients with type 2 diabetes mellitus: a retrospective cohort study. Ther Clin Risk Manag. 2016;12:435–444. doi: 10.2147/TCRM.S105285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 8.Lamberts EJ, Nijpels G, Welschen LM, et al. Long term patterns of use after initiation of oral antidiabetic drug therapy. Pharmacoepidemiol Drug Saf. 2011;20(4):351–358. doi: 10.1002/pds.2089. [DOI] [PubMed] [Google Scholar]
- 9.Grimes RT, Bennett K, Tilson L, Usher C, Smith SM, Henman MC. Initial therapy, persistence and regimen change in a cohort of newly treated type 2 diabetes patients. Br J Clin Pharmacol. 2015;79(6):1000–1009. doi: 10.1111/bcp.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekstrom N, Svensson AM, Miftaraj M, et al. Durability of oral hypoglycemic agents in drug naive patients with type 2 diabetes: report from the Swedish National Diabetes Register (NDR) BMJ Open Diabetes Res Care. 2015;3(1):e000059. doi: 10.1136/bmjdrc-2014-000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–457. [PubMed] [Google Scholar]
- 12.Spreeuwenberg MD, Bartak A, Croon MA, et al. The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Med Care. 2010;48(2):166–174. doi: 10.1097/MLR.0b013e3181c1328f. [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312(24):2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkowitz SA, Krumme AA, Avorn J, et al. Initial choice of oral glucose-lowering medication for diabetes mellitus: a patient-centered comparative effectiveness study. JAMA Intern Med. 2014;174(12):1955–1962. doi: 10.1001/jamainternmed.2014.5294. [DOI] [PubMed] [Google Scholar]
- 15.Phung OJ, Schwartzman E, Allen RW, Engel SS, Rajpathak SN. Sulphonylureas and risk of cardiovascular disease: systematic review and meta-analysis. Diabet Med. 2013;30(10):1160–1171. doi: 10.1111/dme.12232. [DOI] [PubMed] [Google Scholar]
- 16.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303(14):1410–1418. doi: 10.1001/jama.2010.405. [DOI] [PubMed] [Google Scholar]
- 17.Kung J, Henry RR. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11(4):565–579. doi: 10.1517/14740338.2012.691963. [DOI] [PubMed] [Google Scholar]
- 18.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis TME. Beyond metformin: selecting a second therapy. Medicographia. 2016;38:37–44. [Google Scholar]
- 20.Nathan DM. Thiazolidinediones for initial treatment of type 2 diabetes? N Engl J Med. 2006;355(23):2477–2480. doi: 10.1056/NEJMe068264. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87(6):2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 22.Grant RW, Pabon-Nau L, Ross KM, Youatt EJ, Pandiscio JC, Park ER. Diabetes oral medication initiation and intensification: patient views compared with current treatment guidelines. Diabetes Educ. 2011;37(1):78–84. doi: 10.1177/0145721710388427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SE, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and non-vulnerable older patients. J Am Geriatr Soc. 2008;56(7):1183–1190. doi: 10.1111/j.1532-5415.2008.01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toh MR, Teo V, Kwan YH, Raaj S, Tan SY, Tan JZ. Association between number of doses per day, number of medications and patient’s non-compliance, and frequency of readmissions in a multi-ethnic Asian population. Prev Med Rep. 2014;1:43–47. doi: 10.1016/j.pmedr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wimmer BC, Dent E, Bell JS, et al. Medication regimen complexity and unplanned hospital readmissions in older people. Ann Pharmacother. 2014;48(9):1120–1128. doi: 10.1177/1060028014537469. [DOI] [PubMed] [Google Scholar]