Abstract
Background
Alzheimer’s and related dementias are on the rise, and older adults and their families are seeking accessible and effective ways to stave off or ameliorate mild cognitive impairment (MCI).
Aim
This pilot clinical trial (ClinicalTrials.gov Identifier: 03069391) examined neuropsychological and neurobiological outcomes of interactive physical and mental exercise.
Participants and methods
Older adults (MCI and caregivers) were enrolled in a 3-month, in-home trial of a portable neuro-exergame (the interactive Physical and Cognitive Exercise System [iPACES™]), in which they pedaled and steered along a virtual bike path to complete a list of errands (Memory Lane™). Neuropsychological function and salivary biomarkers were measured at pre-, mid-, and posttrial. Ten older adults complied with the recommended use of iPACES (complete dose; ≥2×/wk, 67% of the 15 who also had pre- and postevaluation data). Statistical analyses compared change over time and also change among those with a complete dose vs inadequate dose. Correlations between change in neuropsychological and biomarker measures were also examined.
Results
Executive function and verbal memory increased after 3 months (p = 0.01; no significant change was found with an inadequate dose). Change in salivary biomarkers was moderately associated with increasing cognition (cortisol, r = 0.68; IGF-1, r = 0.37).
Conclusion
Further research is needed, but these pilot data provide preliminary indications to suggest neuro-exergaming can impact cognitive function, perhaps via neurobiological mechanisms, and as such may provide an effective and practical way to promote healthy aging.
Keywords: aging, cognition, MCI, exergame, executive function, neurogame, neuro-exergame
Introduction
As our worldwide population ages, the increasing burden of Alzheimer’s and related dementias poses a significant challenge. While cures remain elusive, older adults and their families are seeking accessible, affordable, and effective ways to stave off or ameliorate mild cognitive impairment (MCI). Thus, research continues to explore progressive behavioral methods for combating cognitive decline.1 Currently, dementia cases are estimated at nearly 50 million globally, and the incidence of dementia is predicted to increase by 50% and reach 75 million by 2030.2 These grim statistics have led to increased innovation in clinical research, specifically the exploration of various interventions that might prevent, delay, or ameliorate dementia and MCI.3 Additionally, clinical trials aim to clarify neurobiological mechanisms that might underpin any improvements in cognition or brain health.4 Exercise has long been known to benefit physical health, and more recent meta-analyses of well-controlled trials have identified specific benefits to cognition and brain health, even indicating neurogenesis in older adults.4–7 Exercise is especially promising for those with for Alzheimer’s disease (AD) because of its neuroprotective features and is reported to be more effective early in disease progression,8 and indeed, promising results have been observed for MCI.9–11
Beyond physical activity alone
Despite the well-documented and varied benefits of exercise,6 only a small percentage of older adults engage in the recommended dose of exercise,12 and efforts to increase participation have been explored, including the potential of exergaming, wherein virtual reality or video gaming components may induce proper dosing of exercise.13 Furthermore, some research suggests that combining physical and cognitive intervention components (eg, as in exergaming, dual-task training, or other tandem/sequential offerings) may yield added cognitive benefit, above and beyond traditional exercise alone, perhaps by engaging in both mental and physical exercise yielding a two-for-one or synergistic, compounding effect. For example, in the Cybercycle Study, after 3 months (3M) of exercise, older adults who pedaled along a virtual bike path were found to have increased executive function, whereas those engaged in traditional stationary cycling were yet declining, resulting in a 23% lower rate of conversion to MCI.14 Similarly, other studies have incorporated video game play or virtual scenery to enhance exercise and increase cognitive benefits.15–17
While the research is expanding in this realm of multi-modal interventions wherein physical and mental exercise is mixed, there are a number of factors that may affect outcomes. One consideration is whether it matters if the dual types of exercise are performed separately (in tandem), simultaneously (as in dual-task modalities, but where the outcome of one realm does not affect the other realm), or interactively (as described earlier in exergaming where the two forms of exercise affect one another). Reviews of the literature suggest promise in each variant of multi-modal interventions: tandem18–21 or interactive combinations;22–25 however, it remains unclear what combination of factors are necessary or more powerful in producing added cognitive benefit. Combined/multi-modal interventions have been shown to yield specific cognitive benefits, such as in executive function and memory,17,18,22,24,26–29 as well as in global cognition.30 One study reported contrary evidence, that combined physical and cognitive training did not result in additive effects, but this was a tandem study.31 Another study compared different types of interventions in a randomized clinical trial (RCT) and found that both the simultaneous and interactive conditions yielded better cognitive outcomes than a physical activity-only condition.22,23 More RCTs comparing types of combined interventions are needed to further clarify and confirm possible differential effects.
Physical and mental exercise as components
While multi-modal interventions are gaining some traction in clinical trials, research on the effectiveness of separate components has a longer history with the benefits of physical exercise alone having been well-documented in many high-quality studies;5,32,33 however, the cognitive training literature is more debatable, especially with regards to transfer or generalization of effects.34 One meta-analysis of 52 studies revealed that computerized cognitive training alone does not significantly benefit older adult executive functioning.35 However, other evidence has suggested that cognitive training, specifically cognitively stimulating computer tasks and video game training, may yield cognitive benefits,36–39 with some caveats already noted earlier.34 Additional work has shown immediate positive effects of cognitive training, but these results fail to last long term.37,40,41 It may be that transfer and retention benefits could be enhanced by layering physical activity with or alongside of mental engagement; given their different underlying neurobiological effects, it is conceivable that there could be a useful synergistic or compounding effect.40–42
Synergistic neurobiology
These more refined questions about compounding or enhanced neurobiological effects, in some part, have already started to be investigated; for example, in the tandem category of multi-modal interventions, some research has explored whether the order of or sequencing of mental or physical exercise matters. It has been argued that since physical exercise changes the brain in profound ways, this may “prime” the brain for benefits of cognitive training,40,41 and there is some evidence in animal models to support this.42,43 Alternatively, some have begun to partially test the reverse model in which mental exercise precedes physical activity, and again there is some supportive evidence in animal models,44 as well as a feasibility trial to evaluate this in humans.45 We propose that interactive exercise over time, in humans, may maximize these underlying neurobiological mechanisms and processes promoting both neurogenesis and cell survival via physical and cognitive exercise, but in a maximized way given the constant feedback provided by a naturalistic form of interactive exercise that taps into evolutionarily adaptive survival (eg, locomoting through an environment and accomplishing tasks, akin to foraging and staying alert to cognitive cues that might promote survival such as noticing a food source or detecting a predator43).
The idea of additive, compounding, or synergistic neurobiological/neurophysiological effects has some theoretical and empirical underpinning in the literature exploring mechanisms of action. It may be that there are unique neurobiological contributions of each form of exercise (both physical and mental) that contribute to improved brain health and thus better cognitive outcomes.46 Early indications from the dual-task literature47,48 and also from animal literature43,49,50 have suggested there is merit in the possibility of different neurobiological and neurophysiological mechanisms that may underlie various components of interventions, such as physical and cognitive exercises.50 Other research has investigated neuronal mechanisms behind the benefits reported after exergaming and traditional exercise interventions.15,51,52 The neurobiological mechanism by which enhanced cognition occurs following exercise interventions is still unclear. One suggestion is that aerobic exercise can act in a neuroprotective way, which in turn can increase cognition.53 These increases in cognition are associated with larger hippocampal volumes, increases in global cognition, and increased levels of various biomarkers found in serum or saliva.9,53 Additional research is needed to further clarify the role of physical or cognitive exercise in activating or altering these underlying mechanisms.
Factors affecting cognitive benefit
In addition to examining various outcomes and intermediate neurobiological impacts of multi-modal interventions, more research is needed to evaluate the variety of factors that affect possible cognitive benefit from combined interventions, whether they be in tandem, synchronous/dual-task, or interactive. For example, in a review of the broad multi-modal exercise literature, Tseng et al54 noted that benefit from multi-modal interventions appeared to vary depending upon the premorbid cognitive status of the participants; that is, normative individuals may have been better able to extract benefit while those with cognitive impairment may have found the dual tasks too challenging and thus did not improve much. However, they note this is preliminary speculation based on a formative literature and that more, well-controlled studies are need to confirm this tentative observation. Other research has pointed to the importance of examining the dose of an intervention, whether administered over the longer term 22,27,55 and whether any benefits persisted over time.22,27 Indeed, a review found that length and frequency of these interventions affect the quality of cognitive improvement, suggesting that 1–3 hours a week for 3–4 months were likely necessary for increases in cognition.56
One factor that could be explored further is the “dose” of cognitive challenge offered in multi-modal interventions. While the results of the Cybercycle Study were encouraging, one remaining question has been whether the cognitive benefits of exergaming might be maximized by increasing the “mental exercise” that participants were engaging in during their interactive physical exercise. In a follow-up pilot RCT, the Aerobic and Cognitive Exercise Study (ACES-pilot),57 comparing the cybercycle (virtual bike tour) condition, “exer-tour,” with a more mentally engaging “exer-score” condition, which involved an “off-the-shelf” video game available on the same exergaming bike. In the exer-tour condition, the level of mental exercise was considered “low,” in that participants were observing and minimally interacting with virtual scenery by controlling speed and direction while pedaling and steering; however, there were no consequences to “zoning out” (eg, no crashing or bumping into other riders or obstacles, allowing continuous exercise, passing through “ghost riders,” or riding the curb unaffected). In the exer-score, the level of mental exercise was considered “high,” in that participants’ pedaling and steering were now in a 360-degree off-road environment, with surreal dragons to chase, each valued at increasing points, according to their color and range of difficulty to tag (eg, green was easiest to tag/slowest, whereas blue was more elusive, etc). After 3M, older adults randomly assigned to the exer-score condition were found to improve significantly more on executive function, suggesting that perhaps increasing the degree of mental exercise while exergaming may result in increased cognitive benefit.57
Maximizing the cognitive benefit of exergaming
Subsequently, we launched a grant-funded RCT of ACES to examine the possible differential effect of an increased dose of mental challenge (NCT: 02237560),58 we chose to focus on effect for MCI participants, aiming to replicate and further clarify the role of different levels of mental exercise for any cognitive benefit from exergaming in this vulnerable, target population. However, while we were able to attract over 200 potential participants to the trial, only a fraction were able to comply with traveling to exercise on the specialized exergaming equipment (placed at hospital rehabilitation locations as it was too big and expensive for in-home placement and initially also in monitored locations for safety reasons). In the end, while it was notable that exergaming (with either low or high levels of mental challenge) eventually yielded significant cognitive benefits for those with MCI; however, only 14 participants were able to adhere over 6 months to regular exercise using exergaming equipment located outside of their home. Furthermore, we recognized some of the drawbacks to utilizing an “off-the-shelf” video game as part of exergaming and control conditions in that we were not able to tailor the level of challenge to suit a given participant, and indeed some MCI participants discontinued the trial as the level of challenge was too difficult. Additionally, we were not able to reliably quantify the level of mental engagement, and although we assumed the challenge was “high,” it was observed that some participants approached the game casually, whereas others were strategic and rigorous in their approach. Yet, the final “score” did not necessarily reflect the approach taken by a participant (eg, strategic vs coasting), since scores could be affected by a number of factors.
Further efforts to maximize cognitive benefits of exergaming
Given the above-mentioned limitations of prior research and the “off-the-shelf” exergaming technology used in our own ACES investigations, our lab developed a tailored intervention for the purposes of: 1) improving compliance (providing an affordable, portable, in-home system); 2) increasing precision in measuring behaviors that could quantify “mental exercise;” and 3) allowing flexibility in the adaptability of the game’s challenges to meet the varied cognitive function of participants, as well as allowing the design of mental challenges to target specific cognitive domains (eg, assigning a game-like task that would stretch executive function, specifically). We refer to this approach as the interactive Physical and Cognitive Exercise System (iPACES™; patent pending: US15087351).
We refer to the iPACES as an example of a neuro-exergame,59 since it synergizes exergaming with interactive mental exercise that is specifically designed to maximize brain health and thus, cognitive benefits while exercising. Neuro-exergames such as iPACES could thus utilize many different forms of exercise (eg, treadmill, ergometer, rower) and employ varied mental challenges that strategically tap many varied cognitive domains (eg, memory, executive function, visuospatial skill, etc). To date, the iPACES has been tested using a cycling modality and a tablet-based video game designed to challenge executive function (Figure 1). In a single bout study, older adults were found to have significantly improved executive functioning (ie, generalized to a separate, standardized neuropsychological test).59 In that single bout study, participants pedaled and used a controller to steer along a virtual bike path. A naturalistic game scenario had been designed for the study, a priori, to increase salience and possible transfer of learning,60 and as such a list of errand locations were presented (eg, doctor, pharmacy, grocery), and recall was subsequently challenged as participants were presented with forced-choice forks in the road (eg, florist or doctor; Figure 1). The mental challenge was further amplified when participants complete the list of errands and had to retrace their pathway home, recalling the errand locations in reverse order. The culmination in a working memory task was specifically designed in an effort target and strengthen executive functioning, which is key to maintaining independence in later life.
Figure 1.

The interactive Physical and Cognitive Exercise System (iPACES) shown as used in-home via a portable tablet-laptop paired with a cadence monitor on an under-table elliptical and operated by a joystick equipped with a HR monitor (finger sensor).
Notes: The image in Figure 1 shows the use of iPACES (tablet, pedaler, controller, and HR monitor) in a home environment. The person in Figure 1 has provided written informed consent for his image to be published.
Abbreviation: HR, heart rate.
The present iPACES pilot study extended the above prior RCT and lab-based research, and aimed to address barriers to long-term exercise among those with MCI, by taking this novel neuro-exergame into the home and providing iPACES to co-residing MCI–companion/caregiver pairs. Cognitive function was the primary outcome to be studied, in particular, executive function. Additionally, we planned to examine possible underlying neurobiological mechanisms coinciding with any cognitive changes, and planned to examine changes in: Insulin-like growth factor 1 (IGF-1), brain-derived neurotrophic factor (BDNF), cortisol, and dehydroepiandrosterone sulfate (DHEA-S).
While a major aim of this pilot study was to examine the feasibility of protocol methods in preparation for a larger clinical trial,61 analyses were also conducted to examine any effects of the intervention. It was anticipated that following 3M of prescribed use of iPACES:
- Cognitive function might be affected as follows:
- Hypothesis 1 (a priori/primary): executive function would significantly improve (as evaluated with ratio scores from Digit Span, Color Trails, and Stroop tasks)
- Hypothesis 2 (validity check): verbal memory (as measured with the Alzheimer’s Disease Assessment Scale [ADAS]) was not expected to significantly improve (based on the variability in past literature on exercise effects); however, it was monitored as a validity check given the memory-intensive nature of the first component of the game that precedes the targeted “mental exercise” (reverse-recall) task component (ie, executive function)
- Biomarkers might be affected as follows:
- Hypotheses 3–6 (exploratory): salivary IGF-1, BDNF, and DHEA-S levels would significantly increase, whereas salivary cortisol would significantly decrease
- Cognition and biomarker changes over 3M might be associated:
- Hypotheses 7–10: executive function and memory changes (3M baseline) will be correlated with bio-marker changes (3M baseline)
Participants and methods
This quasi-experimental pilot study was approved by the Human Subjects Review Committee of Union College, and participants were recruited via networking emails (announcing the study to the principal investigator’s academic campus, and regional senior living communities and medical providers), posting of flyers, running periodic local newspaper ads, and holding recruitment sessions at 55+ older adult communities. Notices invited co-residing MCI-caregiver pairs, but enrollment also allowed for “normative” co-residing pairs since sometimes no diagnosis had previously been sought by community dwelling older adults, yet cognitive screening criteria for MCI on the Montreal Cognitive Assessment (MoCA ≤ 26) could be met and utilized for the purposes of the research.
Participants
An initial pilot sample of 10 pairs was sought (vs utilizing a power analysis to estimate sample size), given goals of evaluating methods and establishing feasibility since the iPACES was still in development and thus further iterations were anticipated, before funding for a full randomized clinical trial could be obtained. Volunteers were excluded if they were <50 years of age, could not read a computer screen, had inadequate hearing or speech to be tested, believed they could not pedal an under-desk elliptical, were already exercising at recommended levels (45 minutes of aerobic exercise 5–7×/week), or were not available for regular exercise participation for 3–5×/week for 3M. All participants were screened with the Impaired Decision-Making Capacity structured interview (IDMC62) and provided informed consent (cosigned by a surrogate or legally authorized representative as applicable and/or per the IDMC).
Procedures
After completing the initial intake and baseline cognitive evaluation with saliva sampling (passive drool), participants were trained to use the tablet-laptop video game, Memory Lane™, for a 20-minute single bout (as described earlier, accomplishing a list of errands by choosing correct paths along a virtual reality roadway and then retracing the list in reverse order; no physical exercise/pedaler at this time). Cognitive and biomarker assessments were repeated at the beginning and end of each single bout training session and thus before and after 2-week familiarization windows (game-only and iPACES). These additional assessments were included primarily to washout learning/practice effects63,64 ahead of the major mid-point and final (3M) assessments which were of central, a priori, interest. Single bout and 2-week familiarization results were examined for exploratory and feasibility purposes and reported elsewhere (unpublished thesis by VanBrakle, 2016). Participants were instructed to practice in the game for 20–40 minutes, 3–5×/week (per the American College of Sports Medicine12) for 2 weeks, after which time there was another 20-minute single bout training session to learn to use the complete iPACES.
The iPACES intervention
The iPACES utilized in this study included an under-table elliptical pedaler and a joystick to control the Memory Lane™ gamei installed on a tablet-laptop. When using the iPACES, participants were instructed to observe their heart rate (HR; via a finger or wrist monitor) and maintain it in a target range (calculated using the Karvonen equation).65 Participants were instructed to practice iPACES 20–40 minutes, 3–5×/week for a 2-week familiarization window, and then continue through the end of 3M with cognitive and biomarker assessments mid-trial (6 weeks [6w]) and at the end (3-months).
Measures
The cognitive assessments focused on executive function administered in alternate forms at each evaluation and ratio scores were computed to isolate the executive function component from psychomotor and other effects: Stroop A/C (40-item66), Digit Span B/F67, and Color Trails 1/2.68 These measures of executive functioning were administered several times to washout practice/learning effects, but the a priori focus of analyses was on change from baseline to mid-trial (6w) and end of trial (3M). The ADAS: word list69 was administered as a type of validity check since memory was not expected to improve based on the past literature; however, given the intensive involvement of list-learning involved in the Memory Lane™ game, memory was assessed to verify it was not affected.
The biomarker assessments aimed to measure BDNF, cortisol, DHEA-S, and IGF-1 in saliva. Samples were analyzed via enzyme-linked immunosorbent assays in triplicate. However, not all participants were willing or able to provide saliva samples and some assay results were outside the limit of detection, and as such, on some variables, the missing data from the already small sample of those who achieved a complete dose of iPACES prohibited statistical analyses; pilot results are reported for cortisol and IGF-1.
Analyses
Paired sample t-tests were used to evaluate change in cognitive and biomarker measures from baseline to mid-trial (6w) and end of trial (3M). To evaluate the possible relationship between changes in cognition and biomarkers over time, Pearson correlations were computed using change scores (6w baseline and 3M baseline) for cognitive and biomarker measures.
Results
Potential participants were screened as mentioned earlier (n = 74), and those who were enrolled in the study (n = 31) were primarily co-residing pairs (eg, spouses/partners or adult children/parent pairs; some single older adults were allowed to participate if they were able to complete the exercises, for safety, in a designated common area at their senior living facility; see Figure 2 for CONSORT flow diagram detailing participant enrollment and progress in the trial). The average age of enrollees was 76.1 years (SD = 10.4), and the average years of education was high (16.6; SD = 2.5). There were 18 female and 13 male enrollees; all indicated Caucasian/nonminority status. Overall cognitive function was assessed at baseline using the Montreal Cognitive Assessment (MoCA; a brief screening tool that samples multiple cognitive domains; average = 24; SD = 4.1). The participants that were compliant with the iPACES intervention as prescribed were characterized as having received a “complete dose” (≥2×wk; n = 10; the minimum intervention dose of ≥2 sessions per week based on prior literature,17 allowing for up to 2 weeks off due to illness, travel, or technical difficulties). Those participants who were not fully compliant with the recommended dose, but yet also completed baseline, midpoint, and final assessments, were characterized as having received an “inadequate dose” (<1×/wk; n = 5) were included in some analyses below as a quasi-control group serving as a point of comparison. Two other participants were excluded from analyses due to receiving an ambiguous dose as they averaged between 1 and 2×/wk). No significant differences were found between participants with a complete (≥2×/wk) vs inadequate (<1×/wk) dose at baseline (eg, age, education, MoCA, and cognitive variables; Table 1).
Figure 2.
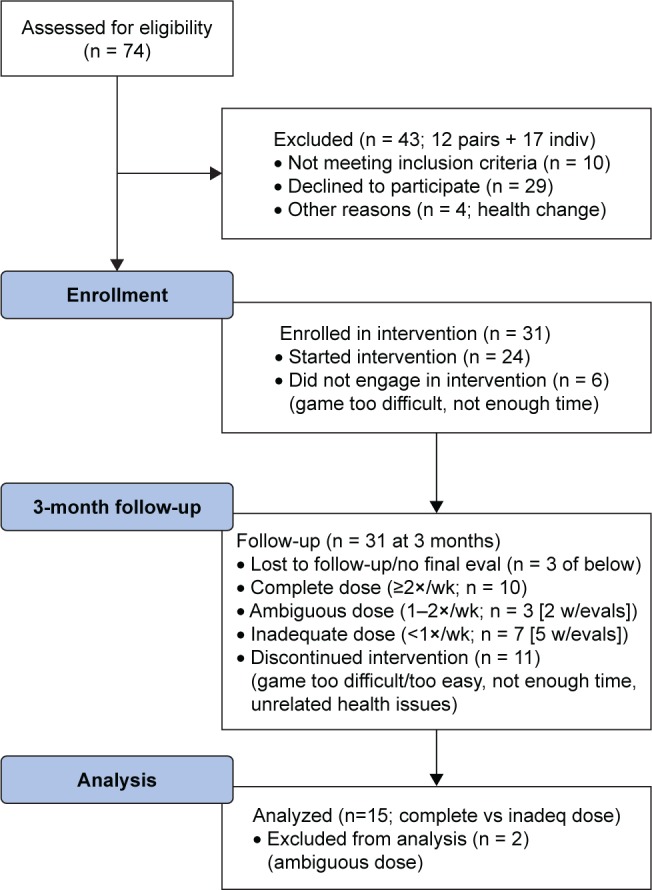
CONSORT flow diagram showing enrollment and progress through trial.
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; eval, evaluate; wk, week; indiv, individual; inadeq, inadequate.
Table 1.
Participants with complete dose of iPACES™ vs inadequate dose
| Timepoint | Variable | Complete dose (≤2×/wk) |
Inadequate dose (≤2×/wk) |
p-value | ||
|---|---|---|---|---|---|---|
| Ave | SD | Ave | SD | |||
| Demographics | Age | 69.40 | 13.81 | 61.60 | 13.05 | 0.31 |
| Education (years) | 15.80 | 2.39 | 14.80 | 2.28 | 0.45 | |
| MoCA | 24.70 | 3.56 | 23.20 | 4.97 | 0.51 | |
| Pretrial | Color Trails ratio 1/2 | 0.45 | 0.16 | 0.45 | 0.03 | 1.00 |
| Digit Span ratio F/B | 0.65 | 0.17 | 0.69 | 0.21 | 0.70 | |
| Stroop ratio A/C | 0.49 | 0.10 | 0.38 | 0.16 | 0.11 | |
| Word recall (ADAS delayed) | 6.83 | 1.17 | 6.00 | 3.54 | 0.60 | |
| Midtrial (6 weeks) | Color Trails ratio 1/2 | 0.50 | 0.14 | 0.41 | 0.05 | 0.20 |
| Digit Span ratio F/B | 0.71 | 0.24 | 0.67 | 0.18 | 0.84 | |
| Stroop ratio A/C | 0.49 | 0.11 | 0.43 | 0.24 | 0.50 | |
| Word recall (ADAS delayed) | 8.00 | 2.76 | 6.40 | 3.78 | 0.44 | |
| Posttrial (3 months) | Color Trails ratio 1/2 | 0.44 | 0.11 | 0.36 | 0.11 | 0.19 |
| Digit Span ratio F/B | 0.62 | 0.09 | 0.53 | 0.24 | 0.34 | |
| Stroop ratio A/C | 0.53 | 0.12 | 0.41 | 0.14 | 0.10 | |
| Word recall (ADAS delayed) | 8.83 | 0.98 | 6.20 | 3.90 | 0.14 | |
Abbreviations: iPACES, interactive Physical and Cognitive Exercise System; MoCA, Montreal Cognitive Assessment; ADAS, Alzheimer’s Disease Assessment Scale; Ave, average; wk, week.
Cognitive function
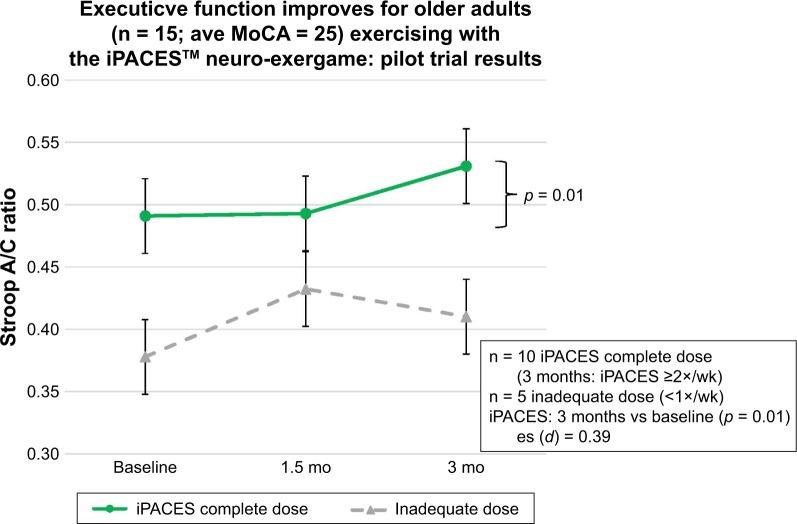
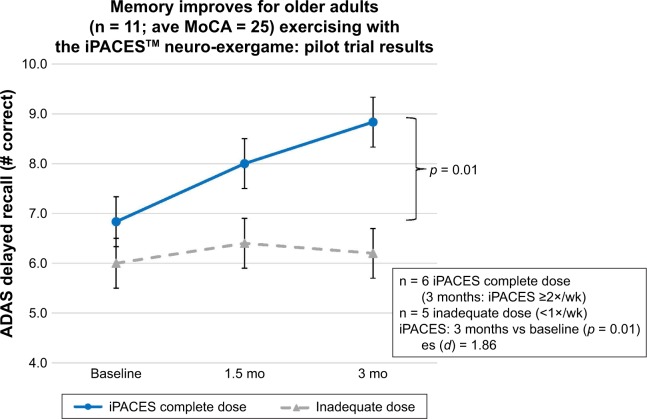
Cognitive function was assessed for change over time. Cognitive performance after 3M of in-home use of the iPACES was compared at mid-trial (6w) and post-trial (3M) with baseline performance for those participants who achieved a “complete dose.” Significant increases were found after 3M for one measure of executive function (Stroop A/C; p = 0.01; Hypothesis 1; Figure 3) and delayed verbal memory (ADAS; p = 0.01; Hypothesis 2; Figure 4).
Figure 3.
Executive function improves after 3 months pedaling and playing the interactive Physical and Cognitive Exercise System (iPACES) for community-dwelling older adults (MCI and caregiver/companions).
Abbreviations: MoCA, Montreal Cognitive Assessment; wk, week; es, effect size.
Figure 4.
Verbal memory improves after 3 months pedaling and playing the interactive Physical and Cognitive Exercise System (iPACES) for community-dwelling older adults (MCI and caregiver/companions).
Abbreviations: ADAS, Alzheimer’s Disease Assessment Scale; MoCA, Montreal Cognitive Assessment; wk, week; es, effect size.
Further probing of the data was pursued to aid in interpretation of the above-mentioned cognitive hypotheses and their results. No significant differences were found at mid-trial. In order to rule out practice effects as a sole cause of improvements and as a point of comparison, the trajectory of participants who did not achieve an inadequate dose of iPACES (<1×/wk), has been also been displayed (n = 5), and as expected, no significant changes in executive function or memory were found.
Biomarkers
Biomarkers were available for some participants (n = 9) and exploratory pilot analyses were undertaken. Given some limitations noted earlier in collecting and processing salivary biomarker, leading to fewer than five cases with useable data for some analytes, some planned hypotheses could not be conducted (ie, BDNF and DHEA-S, Hypotheses 4 and 5, were dropped from analyses). Among those who had achieved a complete dose of iPACES and who also had useable biomarkers (n = 5), no significant changes were found from baseline to 3M for IGF-1 (Hypothesis 3) or cortisol (Hypothesis 6).
Pearson correlations were computed to compare change at mid-point and over 3M in cortisol and IGF-1 with change in the two cognitive outcomes found to significantly improve (ie, Stroop A/C and ADAS delayed memory). An increase in cortisol by mid-trial was moderately correlated with posttrial improvement in executive function (Stroop A/C; r = 0.68) and similarly an increase in IGF-1 by posttrial was moderately associated with final improvement in delayed verbal memory (ADAS; r = 0.37).
Discussion
Given the rise in the incidence of Alzheimer’s and related dementias, and a resultant desire to prevent or ameliorate cognitive decline in aging, this pilot study was conducted to evaluate the feasibility and outcomes of long-term, in-home use of a neuro-exergame for older adults. More specifically, this pilot trial with MCI and caregiver/companions suggests some encouraging neuropsychological benefits and concomitant neurobiological changes after 3M of using the iPACES. Ten older adults who were compliant over 3M with the prescribed dose of iPACES pedaled and steered in a video game, Memory Lane™, wherein executive function was challenged as they traveled along a virtual bike path completing errands by discriminating forced-choice options presented as forks in the road, and then returning home by again recalling the correct path in reverse order. This neuro-exergame, iPACES, engaged the older adult with interactive aerobic physical activity, wherein pedaling and steering controlled a set of mental challenges that were a priori designed to target executive functions (needed for maintaining independence in later life). Among those achieving a complete dose in this pilot study, significant improvements in executive function and verbal memory were found after 3M, while no significant change was observed among participants who did not complete the recommended dose. Among a subset of participants for whom saliva samples were available, exploratory analyses suggest that changes from baseline to 3M in cortisol and IGF-1 were positively and moderately associated with the significant cognitive improvements.
Feasibility of methods was demonstrated in that the equipment and assessments worked well overall, with some fine-tuning needed (eg, trouble-shooting over the phone if a game/tablet “crashed” and the participant needed help to adjust settings or reboot, etc). Exit interview data revealed some attrition from the trial was due to the game not always being well-matched to the participant’s ability level (eg, participants reported discontinuing due to dissatisfaction with the game, citing it was either too hard or too easy/boring). Future iterations of the game will address this issue by increasing the responsiveness of the game to a participant’s ability level, for example, ensuring the game adapts to decrease or increase the level of challenge at a pace suitable for a given participant.
The results of this pilot study partially replicate and extend results from a prior study with the iPACES which found an improvement in executive function for older adults who pedaled and steered through the Memory Lane™ for a single bout in the lab.57 The change in executive function noted here after 3M of neuro-exergaming yielded similar results as did past clinical trials, such as the Cybercycle Study14 and ACES-pilot57 which found 3M of exergaming for older adults yielded increased executive function. This pilot demonstrated similar feasibility to that of Chew et al,31 by successfully implementing an in-home, multi-modal intervention for both caregivers and those with MCI. Additionally, this pilot trial yielded promising significant improvements from baseline to 3M, perhaps indicating that the interactivity is a key, salient component of the intervention (whereas another study,31 which evaluated a multi-modal, but tandem intervention, did not find any significant gains). Nevertheless, the findings reported herein should be interpreted tentatively given that the sample is small and only from a pilot trial, compared with other studies that are fully powered with large samples and randomized designs.
The neurobiological outcomes of this study are preliminary and interpreted cautiously given the small sample size for which there were analyzable salivary biomarker results. The relationship between increasing IGF-1 and cognitive outcomes following exercise is corroborated in past research. However, the finding of increasing cortisol coinciding with increasing cognition following exercise was unexpected as it seems most prior literature on exercise finds a decrease in inflammatory markers such as cortisol. However, there are some studies that have reported an increase in cortisol with exercise, particularly among older adults.
This pilot study has a number of strengths that may be useful to carry forward in future work. First, the tablet and pedaler were portable and could be readily placed in the homes of the co-residing pairs (eg, under the kitchen table or in the living room). This made it easier for the pairs to exercise regularly because they did not have to wrestle with transportation logistics or weather considerations. The Memory Lane™ game provided some distraction to capture participants’ attention during otherwise mundane and potentially tiring pedaling exercise. Prior research has found that in dual-task scenarios (walking and memorizing), older adults prioritize physical over cognitive performance,70 but one strength of this study and intervention is that seated pedaling can be carried out with minimal allocation of resources which increases safety and possibly maximizes the effect of mental exercise. Additionally, the game was adaptive to the participant’s level of ability, recalling where the exerciser left off and restarting the list of errand locations if the participant could not accurately trace the path after two tries. Similarly, participants also experienced adaptive, increasing challenge with repeated successes, for example, mastering recall of 10 errand locations (both “forward” and in “reverse”) while retracing the bike path home.
However, despite the enticements of three-dimensional scenery and a mental challenge to attract one’s attention and reinforce engagement, participants did not universally achieve the recommended number of “rides” of at least 3–5 times a week. Some participants found the mental challenge too difficult; learning and recalling a list of three errands was too much for some participants, whereas others found it too easy (quickly maxing out the length of errand list, adding distracters such as birds flying across the scene, or layering in another cognitive task as in the dual n-back). Thus, future work should investigate different ways to motivate participants to exercise at recommended levels. It is possible that further enhancements to the Memory Lane™ game would increase engagement and motivation (eg, adding more compelling graphics, audio reinforcers, enhanced scoring perhaps for sequential consistency, etc). Also, by integrating alternate storyboards, the iPACES could increase interest and be a challenge for more capable users (eg, normative caregivers), for example, pedaling to various tourist destinations with a given state (vs to errand locations). Perhaps taking advantage of additional technologies (eg, portable virtual reality such as Oculus Rift) that deepen the experience of a naturalistic task may increase motivation and may also garner additional neurobiological synergy by deepening activation of possible evolutionarily adaptive processes noted earlier. Similarly, for less capable participants, it could be useful to have games integrated into iPACES that target certain cognitive functions based on specific cognitive challenges (eg, memory for family members’ names might be reinforced when the correct fork in the road is taken to pair a name with a face, or social skills/emotion recognition is reinforced when the correct fork in the road is taken to pair a stated emotion with a facial expression).
Limitations of this pilot study include a high dropout rate (roughly half of participants). There were a few reported reasons for this, which include unrelated health problems, but more often participants realized that they could not commit to exercising 3–5 times a week after enrolling in the study or found the game or the technology/tablet too difficult to use. The resulting small sample size put the pilot at risk of being underpowered, and indeed just one of the three executive function measures showed significant change. However, that might be due to varied components of executive function captured by each measure, and it is furthermore encouraging that a significant change was identified even with a small sample, indicating a potent/measurable effect (as also evidenced in the medium effect size). Clearly, this pilot is only a preliminary step that needs to be followed up with a controlled trial (perhaps comparing an active control condition) with a larger sample, such that comparisons between conditions might be made as well as salient factors covaried in analyses (eg, age, education, sex, etc) and other variables examined for possible mediating or moderating roles (eg, miles pedaled, gains in score/word list length forward and backward, etc). For example, being able to balance, homogenize on, or at least measure/control for sex could affect results since aerobic exercise interventions, resistance exercise interventions, and combined aerobic and resistance training interventions all had more pronounced effects of cognition when study samples included more women than men.71 Furthermore, the underlying neurobiological mechanisms through proteins like BDNF as well as steroid hormones that differ between men and women may account for these differences.71
Despite these limitations, this pilot study’s successes suggest that further research on this type of interactive mental and physical exercise should be pursued, given the feasibility of a long-term, in-home intervention. Future work should include larger cohorts and preferably have a game interface that is even easier and more enticing for an older adult (especially those with MCI) to use; an appealing video game and feedback from the exergame can increase older adults’ compliance with a sufficient dose of exercise.16 Additionally, future studies might focus on making the Memory Lane™ game an app that people could download on their smart phones or tablets so that they could use an interface or device that they are already comfortable using.
Future studies should also explore transfer of cognitive changes and generalizability of impacts to ecologically valid measures of everyday function, subject experience of cognitive function, or overall well-being, such markers might further clarify the possible clinical significance of this type of intervention.57,60,72,73 Follow-up research might also examine additional biomarkers that may mediate the relationship between exergaming interventions and increases in cognitive functioning. For instance, recent research has explored how physical and mental training paradigms affect brain network configurations.74 Also, it would be useful to extend research, by teasing apart and comparing component parts of the iPACES intervention, to explore how physical and mental exercise may differentially affect neural plasticity and neurogenesis,43 as well as how different types of exercise (eg, aerobic, strengthening, coordinated motions – be they scattered, sequential, etc) may influence cognition via alternate pathways.21
The current pilot study contributes to the evolving literature on exergaming, cognition, and biomarkers. It is hoped that future research will continue to evaluate feasibility, effectiveness, and relative efficacy of combined, especially interactive, physical, and cognitive exercise interventions. Delineating more specific outcomes from specific interventions would allow healthcare providers to make clearer exercise recommendations to their older adult patients, and such results will also encourage senior living communities to offer appropriate classes or equipment that promote healthy aging to delay and prevent costly cognitive decline.16 Such goals are increasingly salient given the expanding older adult population, and thus, both innovation and research are needed to develop and identify efficacious methods for addressing cognitive decline.
Conclusion
An in-home pilot study of a portable tablet-based neuro-exergame for older adults (MCI and co-residing partners) revealed the feasibility of trial methods and potential for executive function and memory to be affected by 3M of regular interactive physical and cognitive exercise (iPACES). Further research is needed to replicate the findings in a fully powered RCT, but if confirmed, the implication is that older adults, including those with MCI, may benefit from interactive (rather than tandem) physical and cognitive exercise, which is feasible to integrate into their daily life and home environment.
Acknowledgments
The study received funding from NIH/NIA (Grant No R15AG042109). The Memory Lane™ game name comes from more recent collaboration with first Playable Productions, especially Dr Tobi Saulnier and Elizabeth McLaren (assisting with subsequent refinements in iPACES v2.0). We extend our thanks to the dedicated participants and staff at retirement communities where some participants resided and exercised during the study, including Schaffer Heights and Shaker Pointe. This study would not have been possible without them, and we hope your efforts will yield fruit in the good cause of preventing or slowing cognitive decline in later life. We also thank Chris Avanessian, research assistant, for assistance in preparing this manuscript for publication. The image in Figure 1 shows the use of iPACES (tablet, pedaler, controller, and HR monitor) in a home environment. The participant in Figure 1 has provided written informed consent for his image to be published.
Footnotes
Disclosure
The authors report no conflicts of interest in the conduct or report of this research.
References
- 1.Eshkoor S, Hamid T, Mun C, Ng C. Mild cognitive impairment and its management in older people. Clin Interv Aging. 2015;10:687–693. doi: 10.2147/CIA.S73922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer Report 2015–The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 3.Klimova B, Valis M, Kuca K. Cognitive decline in normal aging and its prevention: a review on non-pharmacological lifestyle strategies. Clin Interv Aging. 2017;12:903–910. doi: 10.2147/CIA.S132963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirk-Sanchez N, McGough E. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colcombe S, Kramer A. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 6.Hillman C, Erickson K, Kramer A. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 7.Kramer A, Hahn S, Gopher D. Task coordination and aging: explorations of executive control processes in the task switching paradigm. Acta Psychol. 1999;101(2–3):339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 8.Phillips C, Baktir M, Das D, Lin B, Salehi A. The link between physical activity and cognitive dysfunction in Alzheimer disease. Phys Ther. 2015;95(7):1046–1060. doi: 10.2522/ptj.20140212. [DOI] [PubMed] [Google Scholar]
- 9.Baker L, Frank L, Craft S, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gates N, Fiatarone Singh M, Sachdev P, Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Int J Geriatr Psychiatry. 2013;21(11):1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Yin S, Lang M, He R, Li J. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res Rev. 2016;31:67–79. doi: 10.1016/j.arr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Chodzko-Zajko W, Proctor D, Skinner J, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 13.Read J, Shortell S. Interactive games to promote behavior change in prevention and treatment. JAMA. 2011;305(16):1704–1705. doi: 10.1001/jama.2011.408. [DOI] [PubMed] [Google Scholar]
- 14.Anderson-Hanley C, Arciero P, Zimmerman E, et al. Exergaming and older adult cognition: a cluster randomized clinical trial. Am J Prev Med. 2012;42(2):109–119. doi: 10.1016/j.amepre.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Bamidis P, Vivas A, Papageorgiou S, et al. A review of physical and cognitive interventions in aging. Neurosci Biobehav Rev. 2014;44:206–220. doi: 10.1016/j.neubiorev.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Chao YY, Scherer YK, Montgomery CA. Effects of using Nintendo Wii™ exergames in older adults: a review of the literature. J Aging Health. 2014;27(3):379–402. doi: 10.1177/0898264314551171. [DOI] [PubMed] [Google Scholar]
- 17.Maillot P, Perrot A, Hartley A. Effects of interactive physical-activity video-game training on physical and cognitive function in older adults. Psychol Aging. 2012;27(3):589–600. doi: 10.1037/a0026268. [DOI] [PubMed] [Google Scholar]
- 18.Lipardo D, Aseron A, Kwan M, Tsang W. Effect of exercise and cognitive training on falls and fall-related factors in older adults with mild cognitive impairment: a systematic review. Arch Phys Med Rehabil. 2017;98(10):2079–2096. doi: 10.1016/j.apmr.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Karssemeijer E, Aaronson J, Bossers W, Smits T, Olde Rikkert M, Kessels R. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: a meta-analysis. Ageing Res Rev. 2017;40:75–83. doi: 10.1016/j.arr.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Desjardins-Crépeau L, Berryman N, Bherer L, et al. Effects of combined physical and cognitive training on fitness and neuropsycho-logical outcomes in healthy older adults. Clin Interv Aging. 2016;11:1287–1299. doi: 10.2147/CIA.S115711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forte R, Boreham C, Pesce C, et al. Enhancing cognitive functioning in the elderly: multicomponent vs resistance training. Clin Interv Aging. 2013;8:19–27. doi: 10.2147/CIA.S36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggenberger P, Schumacher V, Angst M, Theill N, de Bruin E. Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clin Interv Aging. 2015;10:1335–1349. doi: 10.2147/CIA.S87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggenberger P, Theill N, Holenstein S, Schumacher V, de Bruin E. Multicomponent physical exercise with simultaneous cognitive training to enhance dual-task walking of older adults: a secondary analysis of a 6-month randomized controlled trial with 1-year follow-up. Clin Interv Aging. 2015;10:1711–1732. doi: 10.2147/CIA.S91997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanmore E, Stubbs B, Vancampfort D, de Bruin E, Firth J. The effect of active video games on cognitive functioning in clinical and non-clinical populations: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2017;78:34–43. doi: 10.1016/j.neubiorev.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Yu J, Wang H, Tan C, Meng X, Tan L. Non-pharmacological interventions for patients with mild cognitive impairment: a meta-analysis of randomized controlled trials of cognition-based and exercise interventions. J Alzheimer’s Dis. 2014;42(2):663–678. doi: 10.3233/JAD-140660. [DOI] [PubMed] [Google Scholar]
- 26.Bherer L. Cognitive plasticity in older adults: effects of cognitive training and physical exercise. Ann NY Aca Sci. 2015;1337:1–6. doi: 10.1111/nyas.12682. [DOI] [PubMed] [Google Scholar]
- 27.Kayama H, Okamoto K, Nishiguchi S, Yamada M, Kuroda T, Aoyama T. Effect of a Kinect-based exercise game on improving executive cognitive performance in community-dwelling elderly: case control study. J Med Internet Res. 2014;16(2):e61. doi: 10.2196/jmir.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiguchi S, Yamada M, Tsuboyama T, et al. A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: a randomized controlled trial. J Am Geriatr Soc. 2015;63(7):1355–1363. doi: 10.1111/jgs.13481. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa E, You T, Leveille S. Potential benefits of exergaming for cognition and dual-task function in older adults: a systematic review. J Aging Phys Act. 2016;24(2):332–336. doi: 10.1123/japa.2014-0267. [DOI] [PubMed] [Google Scholar]
- 30.Bamidis P, Fissler P, Kolassa I, et al. Gains in cognition through combined cognitive and physical training: the role of training dosage and severity of neurocognitive disorder. Front Aging Neurosci. 2015;7:152. doi: 10.3389/fnagi.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chew J, Chong M, Fong Y, Tay L. Outcomes of a multimodal cognitive and physical rehabilitation program for persons with mild dementia and their caregivers: a goal-oriented approach. Clin Interv Aging. 2015;10:1687–1694. doi: 10.2147/CIA.S93914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly M, Loughrey D, Lawlor B, Robertson I, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12–31. doi: 10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Groot C, Hooghiemstra A, Ossenkoppele R, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Simons D, Boot W, Stine-Morrow E, et al. Do “Brain-Training” programs work? Psychol Sci Public Interest. 2016;17(3):103–186. doi: 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- 35.Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11(11):e1001756. doi: 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anguera J, Boccanfuso J, Gazzaley A, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballesteros S, Prieto A, Waterworth J, et al. Brain training with non-action video games enhances aspects of cognition in older adults: a randomized controlled trial. Front Aging Neurosci. 2014;6:277. doi: 10.3389/fnagi.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbett A, Owen A, Ballard C, et al. The effect of an online cognitive training package in healthy older adults: an online randomized controlled trial. J Am Med Dir Assoc. 2015;16(11):990–997. doi: 10.1016/j.jamda.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Mowszowski L, Lampit A, Walton C, Naismith S. Strategy-based cognitive training for improving executive functions in older adults: a systematic review. Neuropsychol Rev. 2016;26(3):252–270. doi: 10.1007/s11065-016-9329-x. [DOI] [PubMed] [Google Scholar]
- 40.Ballesteros S, Mayas J, Waterworth J, et al. A randomized controlled trial of brain training with non-action video games in older adults: results of the 3-month follow-up. Front Aging Neurosci. 2015;7:45. doi: 10.3389/fnagi.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chodzko-Zajko W, Kramer A, Poon L. Enhancing Cognitive Functioning and Brain Plasticity [e-book] Champaign: Human Kinetics; 2009. [Google Scholar]
- 42.Fabel K, Wolf S, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kempermann G, Fabel K, Wolf S, et al. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiFeo G, Shors T. Mental and physical skill training increases neurogenesis via cell survival in the adolescent hippocampus. Brain Res. 2017;1654(Part B):95–101. doi: 10.1016/j.brainres.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Shors T, Olson R, Bates M, Selby E, Alderman B. Mental and Physical (MAP) Training: a neurogenesis-inspired intervention that enhances health in humans. Neurobio of Learn Mem. 2014;115:3–9. doi: 10.1016/j.nlm.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erickson KI, Hillman C, Kramer AF. Physical activity, brain, and cognition. Curr Opin Behav Sci. 2015;4:27–32. [Google Scholar]
- 47.Kramer A, Larish J, Strayer D. Training for attentional control in dual task settings: a comparison of young and old adults. J Exp Psychol App. 1995;1(1):50–76. [Google Scholar]
- 48.Kramer A, Hahn S, Colcombe A, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 49.van Praag H, Shubert T, Zhao C, Gage F. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 51.Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37(9, Part B):2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Voss M, Prakash R, Kramer A, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. pii:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahlskog J, Geda Y, Graff-Radford N, Petersen R. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tseng C, Gau B, Lou M. The effectiveness of exercise on improving cognitive function in older people: a systematic review. J Nurs Res. 2011;19(2):119–131. doi: 10.1097/JNR.0b013e3182198837. [DOI] [PubMed] [Google Scholar]
- 55.Eggenberger P, Wolf M, Schumann M, de Bruin E. Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Front Aging Neurosci. 2016;8:66. doi: 10.3389/fnagi.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lauenroth A, Ioannidis A, Teichmann B. Influence of combined physical and cognitive training on cognition: a systematic review. BMC Geriat. 2016;16:141. doi: 10.1186/s12877-016-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barcelos N, Shah N, Anderson-Hanley C, et al. Aerobic and cognitive exercise (ACE) pilot study for older adults: executive function improves with cognitive challenge while exergaming. J Int Neuropsychol Soc. 2015;21(10):768–779. doi: 10.1017/S1355617715001083. [DOI] [PubMed] [Google Scholar]
- 58.Anderson-Hanley C, Barcelos NM, Zimmerman EA, et al. The Aerobic and Cognitive Exercise Study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front Aging Neurosci. 2018;10:76. doi: 10.3389/fnagi.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson-Hanley C, Maloney M, Barcelos N, Striegnitz K, Kramer A. Neuropsychological benefits of neuro-exergaming for older adults: a pilot study of an interactive Physical and Cognitive Exercise System (iPACES) J Aging Phys Act. 2017;25(1):73–83. doi: 10.1123/japa.2015-0261. [DOI] [PubMed] [Google Scholar]
- 60.Baniqued P, Kranz M, Kramer A, et al. Cognitive training with casual video games: points to consider. Front Psychol. 2014;4:1010. doi: 10.3389/fpsyg.2013.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veterans Health Administration . Veterans Health Administration Handbook (120005) Washington, DC: Department of Veterans Affairs; 2008. Impaired decision making capacity screening form. [Google Scholar]
- 63.Duff K, Beglinger L, Moser D, Paulsen J, Schultz S, Arndt S. Predicting cognitive change in older adults: the relative contribution of practice effects. Arch Clin Neuropsychol. 2010;25(2):81–88. doi: 10.1093/arclin/acp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duff K, Atkinson T, Suhrie K, Dalley B, Schaefer S, Hammers D. Short-term practice effects in mild cognitive impairment: evaluating different methods of change. J Clin Exp Neuropsychol. 2017;39(4):396–407. doi: 10.1080/13803395.2016.1230596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McAuley E, Szabo A, Kramer A, et al. Non-exercise estimated cardiorespiratory fitness: associations with brain structure, cognition, and memory complaints in older adults. Ment Health Phys Act. 2011;4(1):5–11. doi: 10.1016/j.mhpa.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van der Elst W, Van Boxtel M, Van Breukelen G, Jolles J. The Stroop color-word test: influence of age, sex, and education, and normative data for a large sample across the adult age range. Assessment. 2006;13(1):62–79. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- 67.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 68.D’Elia LG, Satz P, Uchiyama CL, White T. Color Trails Test: Professional Manual. Lutz: Psychological Assessment Resources; 1996. [Google Scholar]
- 69.Harrison J. Measuring cognitive change in Alzheimer’s disease clinical drug trials. J Nutr Health Aging. 2007;11(4):327–329. [PubMed] [Google Scholar]
- 70.Li K, Lindenberger U, Freund A, Baltes P. Walking while memorizing: age-related differences in compensatory behavior. Psychol Sci. 2001;12(3):230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- 71.Barha C, Davis J, Falck R, Nagamatsu L, Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol. 2017;46:71–85. doi: 10.1016/j.yfrne.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Gates N, Valenzuela M, Sachdev P, Singh M. Psychological well-being in individuals with mild cognitive impairment. Clin Interv Aging. 2014;9:779–792. doi: 10.2147/CIA.S58866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kalisch T, Richter J, Dinse H, et al. Questionnaire-based evaluation of everyday competence in older adults. Clin Interv Aging. 2011;6:37–46. doi: 10.2147/CIA.S15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster P. Role of physical and mental training in brain network configuration. Front Aging Neurosci. 2015;7:117. doi: 10.3389/fnagi.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]