ABSTRACT
Objective:
To investigate the diagnostic value of α-enolase (ENO1) and serum ENO1 autoantibody levels in lung cancer.
Methods:
Immunohistochemistry staining and ELISA were performed to detect ENO1 expression in lung tissue and serum ENO1 autoantibody levels, respectively.
Results:
The expression of ENO1 was higher in lung cancer tissues than in benign lung disease tissues (p < 0.001). The proportion of lung cancer samples expressing ENO1 was not significantly different among the various pathological classification groups. The proportion of samples expressing ENO1 was higher in lung cancer patients in stages I/II than in those in stages III/IV (ℳ2 = 5.445; p = 0.018). The expression of ENO1 in lung cancer tissues was not associated with age, gender, or smoking history. Serum ENO1 antibody levels were significantly higher in the lung cancer group than in the benign lung disease and control groups (p < 0.001). The differences among the pathological classification groups were not statistically significant. Serum ENO1 antibody levels were also in lung cancer patients in stages I/II than in those in stages III/IV (p < 0.01). Serum ENO1 antibody levels were not associated with age, gender, or smoking history in lung cancer patients. The ROC curve representing the diagnosis of lung cancer based on ENO1 antibody levels had an area under the curve of 0.806.
Conclusions:
Our results suggest that high levels of ENO1 are associated with the clinical stage of lung cancer and that ENO1 expression and its serum autoantibody levels show diagnostic value in lung cancer.
Keywords: Phosphopyruvate hydratase/analysis, Enzyme-linked immunosorbent assay, Immunohistochemistry, Lung neoplasms
RESUMO
Objetivo:
Investigar o valor diagnóstico da α-enolase (ENO1) e dos níveis séricos de autoanticorpos contra ENO1 no câncer de pulmão.
Métodos:
Marcação imuno-histoquímica e ELISA foram realizados para detectar a expressão de ENO1 no tecido pulmonar e os níveis séricos de autoanticorpos contra ENO1, respectivamente.
Resultados:
A expressão de ENO1 foi maior nos tecidos de câncer de pulmão que nos tecidos de doença pulmonar benigna (p < 0,001). Não houve diferença significativa entre os diversos grupos de classificação patológica quanto à proporção de amostras de câncer de pulmão que expressaram ENO1. A proporção de amostras que expressaram ENO1 foi maior nos pacientes com câncer de pulmão nos estágios I/II que naqueles com câncer de pulmão nos estágios III/IV (χ2 = 5,445; p = 0,018). Não houve relação entre a expressão de ENO1 em tecidos de câncer de pulmão e idade, sexo ou histórico de tabagismo. Os níveis séricos de anticorpos contra ENO1 foram significativamente maiores no grupo câncer de pulmão que nos grupos doença pulmonar benigna e controle (p < 0,001). As diferenças entre os grupos de classificação patológica não foram estatisticamente significativas. Os níveis séricos de anticorpos contra ENO1 foram também significativamente maiores nos pacientes com câncer de pulmão nos estágios I/II que naqueles com câncer de pulmão nos estágios III/IV (p < 0,01). Nos pacientes com câncer de pulmão, não houve relação entre os níveis séricos de anticorpos contra ENO1 e idade, sexo ou histórico de tabagismo. A curva ROC do diagnóstico de câncer de pulmão baseado nos níveis de anticorpos contra ENO1 apresentou área sob a curva = 0,806.
Conclusões:
Nossos resultados sugerem que há relação entre níveis elevados de ENO1 e o estágio clínico do câncer de pulmão e que a expressão de ENO1 e os níveis séricos de autoanticorpos contra ENO1 têm valor diagnóstico no câncer de pulmão.
Descritores: Fosfopiruvato hidratase/análise, Ensaio de imunoadsorção enzimática, Imuno-histoquímica, Neoplasias pulmonares
INTRODUCTION
Lung cancer is one of the most common malignant tumors. The morbidity and mortality of lung cancer are very high; therefore, an early, accurate diagnosis is key for increasing treatment efficacy. 1 However, early-stage lung cancer is very insidious, and the disease progresses quickly. Currently, there is no effective diagnostic method or indicator for early-stage lung cancer. When the disease is confirmed, more than 70% of patients with lung cancer have already progressed past the ideal time for treatment. Therefore, identifying early-diagnosis markers for lung cancer has important clinical value. One of three subtypes of enolase is α-enolase (ENO1). Researchers have previously detected high levels of ENO1 protein in tumor tissues and peripheral blood in patients with lung cancer, 2 suggesting that ENO1 could be used as a lung cancer marker. However, the mechanism underlying the effects of ENO1 on the occurrence and development of lung cancer remains unclear, and ENO1 expression and its autoantibody levels for the diagnosis of lung cancer have yet to be clarified. The objective of the present study was to investigate the diagnostic value of ENO1 expression and serum ENO1 autoantibody levels in lung cancer in order to determine whether there is a possibility of using those as lung cancer markers.
METHODS
In the present study, tissue and blood samples were collected from untreated patients with suspected lung cancer. We initially determined the expression of ENO1 in tumor tissues and serum ENO1 autoantibody levels in lung cancer patients in a pairwise fashion and analyzed the correlation between serum ENO1 antibody levels and tissue ENO1 expression.
Pathological tissue specimens
A total of 132 pathological tissue samples from 132 patients with suspected lung cancer were collected in our hospital between January of 2012 and May of 2013. Pathological diagnosis confirmed that there were 72 cases of lung cancer and 60 cases of benign lung diseases (chronic inflammation, bullous lung disease, inflammatory pseudotumor, atypical hyperplasia, and fibroma). Given that palliative radiotherapy and chemotherapy are the major types of treatment for advanced lung cancer patients, there were only 21 patients (29.2%) with confirmed lung cancer in stages III and IV (Table 1).
Table 1. Clinical data of the specimens.
| Parameter | Tissue specimens | Serum specimens | |||
|---|---|---|---|---|---|
| Group | |||||
| Lung cancer | Benign lung disease | Lung cancer | Benign lung disease | ||
| (n = 72) | (n = 60) | (n = 72) | (n = 69) | ||
| Gender | |||||
| Male | 46 | 35 | 46 | 40 | |
| Female | 26 | 25 | 26 | 29 | |
| Age, years | |||||
| Median | 64 | 58 | 64 | 58 | |
| Interquartile range | 37-82 | 28-85 | 37-82 | 26-87 | |
| Smoking history | |||||
| Yes | 38 | 38 | |||
| No | 34 | 34 | |||
| Pathological type | |||||
| Lung adenocarcinoma | 38 | 38 | |||
| Lung squamous cell carcinoma | 24 | 24 | |||
| Small cell lung cancer | 4 | 4 | |||
| Bronchoalveolar carcinoma | 4 | 4 | |||
| Small cell lung cancer + adenocarcinoma | 1 | 1 | |||
| Small cell lung cancer + squamous cell carcinoma | 1 | 1 | |||
| Clinical stage | |||||
| I/II | 51 | 51 | |||
| III/IV | 21 | 21 | |||
Serum specimens
A total of 141 serum specimens from 72 patients with lung cancer and 69 patients with benign lung diseases were collected. In addition, 70 serum samples from healthy individuals who underwent physical examination during the study period were used as a control group (Table 1).
Sample collection and storage
Tissue specimens were fixed in 10% neutral formalin solution, embedded in paraffin, sectioned into 4-µm sections, and stored at 4°C. Peripheral blood samples (3 mL) were collected and centrifuged at 3,500 rpm for 5 min, and serum samples were collected, aliquoted, and stored at −20°C.
Reagents
The reagents used were rabbit anti-ENO1 monoclonal antibody (Abcam Biotechnology Co. Ltd., Cambridge, UK), an immunohistochemistry reagent kit (Maixin, Fuzhou, China) and an ENO1 antibody ELISA reagent kit (HuaAn, Hangzhou, China).
Detection of tissue ENO1
Immunohistochemistry using the streptavidin-peroxidase staining method was performed according to the manufacturer’s instruction manual. For the interpretation of the results, five random fields from each section were selected and examined under a high-power microscope, 100 tumor cells being counted per field. Cells were considered to be positive when ENO1 was localized in the cytoplasm, cell membrane, or cell nucleus as yellow or brownish yellow granules. Samples with a proportion of positive cells ≥ 5% were considered to be positive, whereas those with a proportion < 5% were considered negative. 3
Detection of serum ENO1 antibody
The ELISA method was performed in accordance with the manufacturer’s instruction manual. The concentration of standards provided by the reagent kit and the detected optical density values were used to plot a standard curve. The optical density values of the samples were introduced into the equation to calculate the concentration of samples and to calibrate differences among the plates.
Statistical methods
Data were processed using the IBM SPSS Statistics software package, version 19.0 (IBM Corporation, Armonk, NY, USA) for statistical analysis. Categorical variables were described as absolute and relative frequencies, whereas continuous variables were presented as median and interquartile range. We used the nonparametric Mann-Whitney U test to determine differences between two groups and the Kruskal-Wallis test to determine differences among three groups. We used the chi-square test for comparisons of proportions. Statistical significance was set at p < 0.05.
RESULTS
The expression of ENO1 in lung cancer tissue samples was mainly distributed in the cytoplasm and occasionally in the cell membrane or the nucleus. Positive signs are visualized as yellow or brownish yellow granules. No expression of ENO1 was found in the majority of the samples in the benign lung disease group (Figure 1). The present study analyzed tumor tissue samples from 72 patients with lung cancer. The patients were grouped according to pathological type, clinical stage, age, gender, and smoking history. Comparisons of differences in expression in each group were performed using the chi-square test (Table 2). Table 2 shows that ENO1 expression was significantly higher in the lung cancer group (50.0%) than that in the benign lung disease group (10.0%), the difference being statistically significant (χ2 = 24.137; p < 0.001). However, ENO1 expression among the groups of patients with adenocarcinoma, squamous cell carcinoma, and other types of cancer was not significantly different (p > 0.05), suggesting that ENO1 expression in lung cancer tissues was not associated with the pathological types. In addition, the proportion of ENO1-positive samples was higher in the group of patients in stages I and II than in that of those in stages III and IV. This difference was statistically significant (χ2 = 5.445; p = 0.018), suggesting that ENO1 expression in lung cancer tissues was associated with the clinical stage of the disease. However, ENO1 expression was not associated with age, gender, or smoking history (p > 0.05).
Figure 1. Photomicrographs showing α-enolase (ENO1) expression in lung tissue samples (immunohistochemistry using the streptavidin-peroxidase staining method). Positive signs are visualized as yellow or brownish yellow granules. In A and B, adenocarcinoma tissue samples showing positive ENO1 expression (magnification, ×400 and ×100, respectively). In C and D, squamous cell carcinoma tissue samples showing positive ENO1 expression (magnification, ×400 and ×100, respectively). In E and F, pulmonary inflammatory pseudotumor tissue samples showing negative ENO1 expression (magnification, ×400 and ×100, respectively).
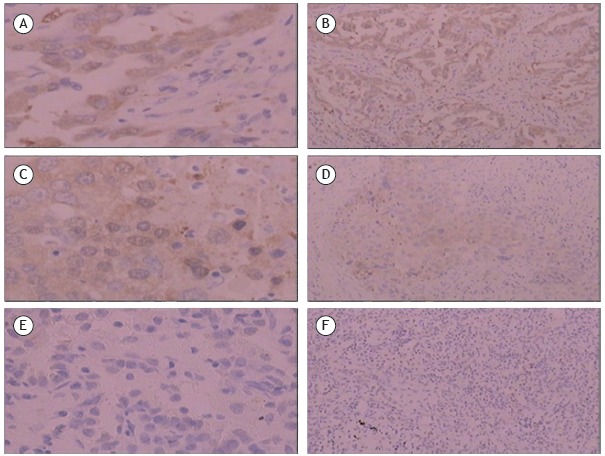
Table 2. α-enolase expression in pathological tissue samples.
| Group | Result | χ2 | p | ||||
|---|---|---|---|---|---|---|---|
| n | Positive | Negative | Positive, % | ||||
| Benign disease tissue sample | 60 | 6 | 54 | 10.0 | 24.137 | < 0.001 | |
| Lung cancer tissue sample | 72 | 36 | 36 | 50.0 | |||
| Pathological type | |||||||
| Adenocarcinoma | 38 | 20 | 18 | 52.6 | |||
| Squamous cell carcinoma | 24 | 12 | 12 | 50.0 | |||
| Other types | 10 | 4 | 6 | 40.0 | |||
| Clinical stage | |||||||
| I/II | 51 | 30 | 21 | 58.8 | 5.445 | 0.018 | |
| III/IV | 21 | 6 | 15 | 28.6 | |||
| Age, years | |||||||
| > 60 | 50 | 27 | 23 | 54.0 | 1.047 | 0.443 | |
| ≤ 60 | 22 | 9 | 13 | 40.9 | |||
| Gender | |||||||
| Male | 46 | 21 | 25 | 45.7 | 0.963 | 0.462 | |
| Female | 26 | 15 | 11 | 57.7 | |||
| Smoking history | |||||||
| Yes | 38 | 23 | 15 | 60.5 | 3.567 | 0.098 | |
| No | 34 | 13 | 21 | 38.2 | |||
The data regarding serum ENO1 antibody levels in the three studied groups all showed skewed distributions. The median (interquartile range) was used in order to represent these levels, and the nonparametric Mann-Whitney U test and the Kruskal-Wallis test were used in order to compare the differences among the groups (Table 3).
Table 3. Comparison of serum α-enolase antibody levels among the three groups studied.
| Group | n | α-enolase antibody, ng/mLa | p | ||
|---|---|---|---|---|---|
| Control | 70 | 16.5 (10.3-19.6) | < 0.001 | ||
| Benign lung disease | 69 | 17.5 (15.0-21.3) | |||
| Lung cancer | 72 | 22.8 (19.9-25.1) | |||
| Pathological type | |||||
| Squamous cell carcinoma | 24 | 22.8 (20.7-27.2) | 0.571 | ||
| Adenocarcinoma | 38 | 23.5 (20.0-25.0) | |||
| Other types | 10 | 21.2 (18.0 -24.7) | |||
| Clinical stage | |||||
| I/II | 51 | 24.2 (20.5-27.3) | 0.006 | ||
| III/IV | 21 | 21.3 (17.5-22.8) | |||
| Age, years | |||||
| > 60 | 50 | 22.9 (20.7-25.0) | 0.456 | ||
| ≤ 60 | 22 | 22.3 (17.5-29.2) | |||
| Gender | |||||
| Male | 46 | 22.9 (20.0-27.3) | 0.376 | ||
| Female | 26 | 22.4 (19.6-24.5) | |||
| Smoking history | |||||
| Yes | 38 | 22.9 (19.8-27.7) | 0.573 | ||
| No | 34 | 22.7 (19.9-24.7) | |||
Values expressed as median (interquartile range).
Table 3 shows that serum ENO1 antibody levels were significantly higher in the lung cancer group than those in the control and in the benign lung disease groups, the differences being statistically significant (p < 0.001). Moreover, ENO1 antibody levels in the benign lung disease group were higher than were those in the control group (p < 0.05). Among the groups with squamous cell carcinoma, adenocarcinoma, and other types of cancer, ENO1 antibody levels were not significantly different (p > 0.05), which suggested that serum ENO1 antibody levels were not associated with the pathological type of lung cancer. In addition, ENO1 antibody levels in the patients in stages I and II were higher than were those in the patients in stages III and IV (p < 0.01), suggesting that serum ENO1 antibody levels might be associated with the clinical stage of lung cancer. However, ENO1 antibody levels were not associated with age, gender, or smoking history (p > 0.05).
Differences in serum ENO1 antibody levels in patients with positive or negative ENO1 expression in lung cancer tissues were analyzed (Table 4). The results demonstrated that serum ENO1 antibody levels were significantly higher in the lung cancer patients with positive ENO1 expression than in those with negative ENO1 expression (p = 0.019). Therefore, there is a correlation between serum ENO1 antibody levels and ENO1 expression in tissue samples.
Table 4. Correlation between α-enolase expression and serum α-enolase antibody levels in the lung cancer group.
| α-enolase expression | n | α-enolase antibody, ng/mLa | p |
|---|---|---|---|
| Positive | 36 | 24.1 (21.3-33.3) | 0.019 |
| Negative | 36 | 21.7 (18.9-24.6) |
Values expressed as median (interquartile range).
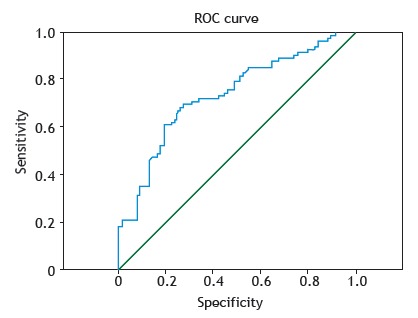
The ROC curve representing the diagnosis of lung cancer based on ENO1 antibody levels was plotted, and the area under the curve was 0.806. The maximum value of the Youden index (0.533) was selected as the best cut-off point (19.62 ng/mL) for screening. The results showed that the diagnostic sensitivity and specificity of the test were 80.6% and 72.7%, respectively (Figure 2).
Figure 2. ROC curve showing the sensitivity and specificity of serum α-enolase antibody levels for the diagnosis of lung cancer.
DISCUSSION
Enolase is an important metabolic enzyme in the glycolysis pathway. There are three subtypes in mammalian cells: ENO1, β-enolase (ENO3), and γ-enolase (ENO2). ENO1 is extensively distributed in various tissues in the human body, whereas ENO2 and ENO3 expression is tissue specific. ENO2 is also called neuron-specific enolase and is primarily distributed in neurons and neuroendocrine tissues. ENO3 is also called muscle-specific enolase and is mainly present in muscle tissues. ENO1 mainly localizes to the cytoplasm. During glycolysis, ENO1 converts 2-phosphoglycerate into phosphoenolpyruvate. In recent years, in addition to glycolysis, it was discovered that ENO1 has various biological functions. The association of ENO1 with malignant tumors has received increasing attention, and ENO1 has bidirectional functions in the occurrence and development of tumors. 4 ENO1 localized in the nucleus is also called c-myc promoter-binding protein 1 and inhibits the transcription of c-myc to inhibit tumor growth. 5 ENO1 localized on the surface of the cell can be used as a receptor for tissue plasminogen activator and plays a role in the invasion and metastasis of tumor cells. ENO1 also strengthens the infiltration ability of monocytes and macrophages, and it can participate in tumor formation by controlling the expression of the c-myc oncoprotein through the Notch signaling pathway. 6 , 7
In the present study, immunohistochemistry with the streptavidin-peroxidase staining method was used in order to determine the expression of ENO1 in lung cancer tissues and in benign lung disease tissues. The results showed that the proportion of lung cancer tissues expressing ENO1 (50%) was significantly higher than that of benign lung disease tissues (10%), which is consistent with a previous study. 4 Differences in ENO1 expression among the groups of patients with different cancer types were not statistically significant, suggesting that ENO1 expression was not associated with the pathological type of lung cancer. However, in the subjects included in the present study, adenocarcinoma accounted for 52.8% of the cases, whereas squamous cell carcinoma and other lung cancer types only accounted for 33.3% and 13.9% of the cases, respectively. Therefore, this conclusion should be further validated with an increased number of samples of other pathological types, such as squamous cell carcinoma and small cell lung cancer. The proportion of positive ENO1 expression was higher in patients in stages I and II (58.8%) than in those in stages III and IV (28.6%). It is possible that ENO1 plays different roles in different stages of tumor growth and that it possesses a more active role in energy metabolism processes at an early stage; however, the specific mechanism of action remains unclear. Cheng et al. 3 ) studied the ENO1 expression in nasopharyngeal cancer tissues and showed that the proportion of ENO1-positive samples exhibited a decreasing trend with an increase in the clinical stage of nasopharyngeal cancer. Other researchers also noted that the proportion of samples with positive ENO1 expression was higher in the early stages of colon cancer than in advanced stages of colon cancer. 3 These results are all consistent with the results in our study. However, the subjects in the present study were all patients who had undergone lung cancer surgery; thus, the proportion of patients in advanced stages of lung cancer was lower, and patients in stages III and IV accounting for only 29.2%. Therefore, this conclusion should be further validated by means of studies involving a greater number of samples and improved detection methods. We also analyzed the association of ENO1 expression in lung cancer tissues with some risk factors for lung cancer, such as age, gender, and smoking history. The results showed that ENO1 expression in lung cancer tissues was not associated with these clinical features, which is in accordance with the study of Hsiao et al. 8
We used the ELISA method to detect serum ENO1 antibody levels in patients with lung cancer, in those with benign lung disease, and in healthy control individuals. The results showed that ENO1 antibody levels in the lung cancer group were significantly higher than were those in the other two groups. In addition, ENO1 antibody levels in the benign lung disease group were higher than were those in the control group, which might due to an increase in ENO1 antibody levels in patients with diseases such as chronic inflammation. 9 Pairwise comparisons between serum ENO1 antibody levels and pathological subtypes in the groups of patients with adenocarcinoma, squamous cell carcinoma, and other types of lung cancer showed that ENO1 antibody levels were not significantly different among these groups, suggesting that serum ENO1 antibody levels were not associated with the pathological type of lung cancer. However, these levels were higher in the patients in stages I and II of lung cancer than in those in stages III and IV, suggesting that serum ENO1 antibody levels might be associated with the clinical stage of lung cancer. This might be due to the early stage of tumor development, in which the immune surveillance function of the body activates antigen-specific destruction of tumor cells to induce immune responses that produce high titers of autoantibodies. As the tumor progresses, tumor cells and host immune cells interact in the tumor microenvironment to establish an immunosuppressive network, including the transduction of inhibitory signals and the production of immunosuppressive cells to inhibit tumor cells, causing a reduction in autoantibody levels. 9 We also studied the association of serum ENO1 antibody levels with age, gender, and smoking history. The results showed that ENO1 antibody levels were not associated with these risk factors for lung cancer. Furthermore, the analysis of the relationship between serum ENO1 antibody levels and ENO1 expression demonstrated a positive correlation between them. Therefore, the quantification of serum ENO1 antibodies, which is a simple, efficient, and minimally invasive method, might be of great clinical significance in the diagnosis of lung cancer.
The ROC curve of the diagnosis of lung cancer based on ENO1 antibody levels was plotted, and the maximum value of the Youden index was used as the best cut-off point for screening. The results showed that the sensitivity of the method for the diagnosis of lung cancer was 80.6%, and its specificity was 72.7%. Various studies have confirmed that tumor markers, such as CYFRA21-1, ENO2, CEA, and SCC, play an important role in the diagnosis of lung cancer. 10 , 11 However, these markers usually have low sensitivity, and their positive frequencies vary greatly in different pathological types of lung cancer, which can cause misdiagnoses and might not be conducive to an early diagnosis of lung cancer. 10 , 11 The ENO1 autoantibody presents a more stable titer in the peripheral blood of early-stage lung cancer patients, which points to its value for the early diagnosis of lung cancer.
In summary, our data support that ENO1 has an important value in the diagnosis of lung cancer and can be used as a lung cancer marker. The quantification of serum ENO1 antibody has as advantages the easiness of sample collection and minimal invasiveness, and it seems to have a high value in the early diagnosis of lung cancer. Further studies should be carried out in order to confirm our conclusion.
Study carried out in the Department of Clinical Laboratory Center, Shaoxing People’s Hospital, Shaoxing Hospital at Zhejiang University, Shaoxing, China.
Financial support: This study received financial support from the Medicine and Health Project of Zhejiang Province (2016KYB302) and the Medicine Health Platform Project of Zhejiang Province (2017RC030).
REFERENCES
- 1.Blanco-Prieto S, Vázquez-Iglesias L, Rodríguez-Girondo M, Barcia-Castro L, Fernández-Villar A, Botana-Rial MI. Serum calprotectin, CD26 and EGF to establish a panel for the diagnosis of lung cancer. PloS One. 2015;10(5):e0127318. doi: 10.1371/journal.pone.0127318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Li M, Liu Y, Han NJ, Zhang KT, Xiao T. ENO1 protein levels in the tumor tissues and circulating plasma samples of non-small cell lung cancer patients [Article in Chinese] Chinese J Lung. Cancer. 2011;13:1089–1093. doi: 10.3779/j.issn.1009-3419.2010.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng C, Long X, Li X, Xie M, Guo M. The expressions of alpha-enolase in the nasopharyngeal cancer tissue [Article in Chinese] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za. Zhi. 2011;25(12):554–556. [PubMed] [Google Scholar]
- 4.Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong SW. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol. 2015;8:22–22. doi: 10.1186/s13045-015-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu KW, Hsieh RH, Lee YH, Chao CH, Wu KJ, Tseng MJ. The activated Notch1 receptor cooperates with alpha-enolase and MBP-1 in modulating c-myc activity. Mol Cell Biol. 2008;28(15):4829–4842. doi: 10.1128/MCB.00175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiao KC, Shih NY, Fang HL, Huang TS, Kuo CC, Chu PY. Surface -enolase promotes extracellular matrix degradation and tumor metastasis and represents a new therapeutic target. PloS One. 2013;8(7):e69354. doi: 10.1371/journal.pone.0069354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Zhang Y, Han N, Guo SP, Xiao T, Cheng SJ. α-enolase (ENO1) inhibits epithelial-mesenchymal transition in the A549 cell line by suppressing ERK1/2 phosphorylation [Article in Chinese] Chinese J Lung Cancer. 2013;16:221–226. doi: 10.3779/j.issn.1009-3419.2013.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao KC, Shih NY, Chu PY, Hung YM, Liao JY, Chou SW. Anti- α-enolase is a prognostic marker in postoperative lung cancer patients. Oncotarget. 2015;6(33):35073–35086. doi: 10.18632/oncotarget.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih NY, Lai HL, Chang GC, Lin HC, Wu YC, Liu JM. Anti-alpha-enolase autoantibodies are down-regulated in advanced cancer patients. Jpn J Clin Oncol. 2010;40(7):663–669. doi: 10.1093/jjco/hyq028. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Wang M. Clinical utility of serum tumor markers in lung cancer. Chinese J Lung Cancer [Article in Chinese] 2011;14:286–291. doi: 10.3779/j.issn.1009-3419.2011.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastawisy AE, Azzouny ME, Mohammed G, Allah AA, Behiry E. Serum cytokeratin 19 fragment in advanced lung cancer could we eventually have a serum tumor marker. Ecancermedicalscience. 2014;8:394–394. doi: 10.3332/ecancer.2014.394. [DOI] [PMC free article] [PubMed] [Google Scholar]


