Significance
Global degradation of coral reefs and macroalgal beds can have ecosystem-wide implications for biodiversity, ecological functioning, and ocean resources. However, recent studies in warm temperate zones have documented community shifts from macroalgae to corals, signaling a potential mechanism for coral conservation under climate warming. Here, we present evidence that warming, aided by the dominant poleward-flowing current system, is facilitating the expansion of tropical corals and herbivorous fishes into existing temperate Japanese macroalgae communities, which are contracting faster than they are expanding. Furthermore, our results suggest future climate change may exacerbate this process, potentially compromising the long-term stability of these communities. Future conservation of these communities might require of a more proactive management toward climate adaptation.
Keywords: climate velocity, coastal tropicalization, community phase shifts, global change, range shifts
Abstract
Coral and macroalgal communities are threatened by global stressors. However, recently reported community shifts from temperate macroalgae to tropical corals offer conservation potential for corals at the expense of macroalgae under climate warming. Although such community shifts are expanding geographically, our understanding of the driving processes is still limited. Here, we reconstruct long-term climate-driven range shifts in 45 species of macroalgae, corals, and herbivorous fishes from over 60 years of records (mainly 1950–2015), stretching across 3,000 km of the Japanese archipelago from tropical to subarctic zones. Based on a revised coastal version of climate velocity trajectories, we found that prediction models combining the effects of climate and ocean currents consistently explained observed community shifts significantly better than those relying on climate alone. Corals and herbivorous fishes performed better at exploiting opportunities offered by this interaction. The contrasting range dynamics for these taxa suggest that ocean warming is promoting macroalgal-to-coral shifts both directly by increased competition from the expansion of tropical corals into the contracting temperate macroalgae, and indirectly via deforestation by the expansion of tropical herbivorous fish. Beyond individual species’ effects, our results provide evidence on the important role that the interaction between climate warming and external forces conditioning the dispersal of organisms, such as ocean currents, can have in shaping community-level responses, with concomitant changes to ecosystem structure and functioning. Furthermore, we found that community shifts from macroalgae to corals might accelerate with future climate warming, highlighting the complexity of managing these evolving communities under future climate change.
Range shifts in foundation species induced by global warming (1–4) are likely to have ecosystem-wide implications for biodiversity, ecological functioning, biogeochemical cycling, and resources used by humans (5–8). Corals and macroalgae are habitat-forming species in coastal ecosystems that cooccur and compete for space, which often result in changes in dominance from tropical corals to macroalgae promoted by climate warming (9–11). However, recent studies from different geographical locations have documented community shifts from macroalgae to corals resulting from the expansion of corals into temperate latitudes as ocean waters become warmer (Fig. 1A) (12–16). In managing the global resilience of corals and macroalgal communities, such biogeographical transition zones between temperate and tropical waters offer potential for conservation during periods of climate change (1, 17).
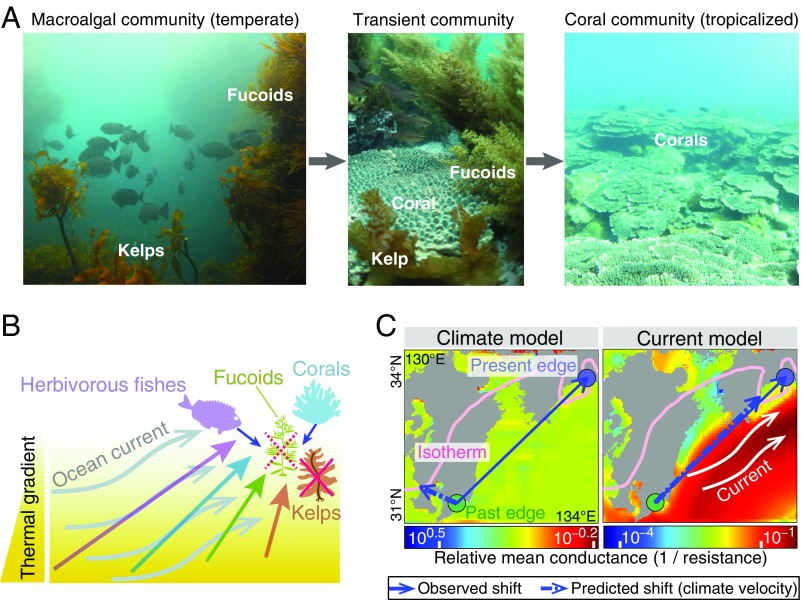
Fig. 1.
Underwater photographs, hypothesized effects, and prediction models for range shifts. (A) Kelps and fucoids are dominant in Japanese coastal communities, but are gradually being replaced by expanding tropical corals with which they compete. (B) We expect that temperate macroalgal community will be more susceptible to deforestation by herbivorous fishes and be replaced by tropical corals (line colors indicate taxa) under strong ocean current flowing poleward (warm currents), because differences among taxa in adult mobility and larval/spore duration (from hours for kelps to a month for fishes) will influence shift rates that will be amplified under increasing strength of current transport. (C) Predictions of species range shifts are based on finding the least-cost path connecting past and present coastal locations of the isotherms corresponding to the temperature found at the initial location for each shift. These trajectories are based on conductance matrices constructed via weight optimization of the magnitude and direction of spatial thermal gradients (climate effect on dispersal) and ocean currents (current effect on dispersal) (see Materials and Methods for details). (Photos courtesy of N.H.K.)
Declines of temperate macroalgae have been attributed to climate-driven thermal stress and associated to an increase in herbivory (4, 18–21). However, increased temperature and herbivory cannot fully explain shifts to coral dominance (11). Indeed, range-expanding corals can colonize macroalgal communities (12, 22) (Fig. 1A). Tropical herbivorous fishes intruding into the tropical–temperate transition zone can also deforest whole primary production of macroalgae unlike their temperate counterparts (19, 20), thus having the potential to release space for colonization of other benthic organisms, including corals (19).
An important but untested factor that could drive these community shifts is the interaction between a warming ocean and the transport imposed by ocean currents (23–25). Both macroalgae and corals disperse passively via planktonic (propagule or larval) stages (1, 17, 26) and adult drift (26), while fishes also benefit from current-driven transport (27) (Fig. 1B). Indeed, a recent global metanalysis found highly dispersive taxa like fish or plankton expand their range faster when warming and ocean currents match in direction (25). However, other than the agreement in direction, current strength is likely to have a key role in driving range expansions under climate warming. Indeed, expansions of tropical corals and herbivorous fishes are especially evident where strong boundary currents flow poleward (1, 17, 27). Moreover, tropical water brought by such current systems can lead to significantly faster warming (28), which may also exacerbate range contractions of temperate macroalgal communities (27). Hence, a community-level analysis incorporating the joint effect of warming and ocean currents (strength and direction) in driving compound range shifts among species can offer important insight.
Japanese coastal marine communities provide a unique opportunity for this purpose. Stretching from tropical to subarctic waters, these coasts host many species, often including the leading or trailing edges of their distributions. Furthermore, the main current systems encircling the Japanese archipelago (SI Appendix, Fig. S1) flow with a strong latitudinal component, yet the directional alignment with existing thermal spatial gradients is contrastingly different between the west and east coasts. This provides ideal test conditions for disentangling the effect of currents and climate changes on range shifts.
Here, we hypothesized that where ocean currents match the direction of warming, they should facilitate the successful expansion (i.e., dispersal and settlement) of tropical corals into temperate macroalgal communities increasingly stressed from the rising temperatures, inducing an eventual change in dominance; an effect that should be proportional to the strength of the current (Fig. 1B). This process should be further exacerbated by the expansion of tropical fishes (19, 20), often concomitant to that of corals (1). We expect this process to be largely dependent on species dispersal traits (29), where higher adult mobility and longer larval/spore duration (from kelps to herbivorous fish) should further facilitate expansion rates under current transport (Fig. 1 B and C). In contrast, we expect range contractions, which are not directly dependent on migration processes, to be weakly related to currents and mainly driven by the effects of climate warming and species interactions via the extinction of local populations (Fig. 1C).
To disentangle the combined effect of climate and currents in driving climate-mediated range shifts, we used a revised version of climate-velocity trajectories (30). Our approach couples the speed and direction of both thermal gradients and ocean currents (Materials and Methods, Fig. 1C, and SI Appendix, SI Methods), using a modeling framework that accounts for the relative weight of both parameters. This model represents a clear improvement from the simple directional agreement metric used in ref. 25, which did not take into consideration current strength and is adequate for a global metaanalysis but becomes too simplistic for regional fine-scale analyses. We tested our hypothesis against observed range shifts inferred from a unique dataset comprising long-term (mainly 1950–2015) distribution records for 8 kelp, 22 fucoid, and 12 coral species, as well as existing records documenting fish-induced deforestation of temperate macroalgal communities by three herbivorous fish species across Japan (Fig. 2 A–C, SI Appendix, Fig. S2 and Table S1, and Dataset S1).
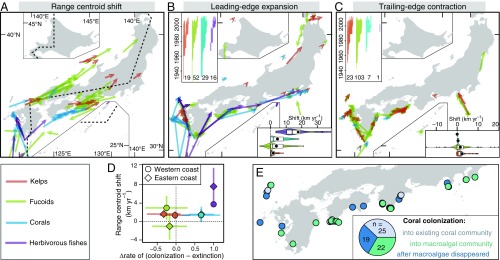
Fig. 2.
Observed range shifts for kelps, fucoids, corals, and deforestation by herbivorous fishes. (A–C) Location, direction, and magnitude (arrow length) of observed shifts for (A) range centroid shifts (n = 82), (B) leading-edge expansions (n = 116), and (C) trailing-edge contractions (n = 134). (A) Shifts are shown for each of the 45 study species from the 1970s to the 2000s divided into western and eastern coasts (dashed line), estimated by multiresponse linear models (SI Appendix, SI Methods). Inset violin plots in B and C represent summaries of shift rates with mean values in circles. Inset bar plots in B and C represent year range of each shift, with the number of shifts for each taxa. (D) Relationship between centroid shifts and differences in the rates of colonization against local extinction (mean ± SD). (E) Processes of community shifts by corals with the number of each type of the processes.
Results and Discussion
The direction and rate of the observed range shifts calculated for each species and coast during the study period showed considerable variation in both distribution centroids and range edges (Fig. 2 A–D and SI Appendix, Table S2). Expansions were scarcer than contractions in macroalgae, whereas fishes and corals experienced expansions much more frequently than contractions (Fig. 2 B and C). Many of these expansions and contractions occurred along southeastern to southwestern areas of the Japanese main islands, coincident with both the northern range limits for corals (except for one inconspicuous species: Oulastrea crispata) and herbivorous fishes, and the southern range limits for many temperate macroalgae (Fig. 2 B and C and SI Appendix, Fig. S2). Expansions were fastest in fishes, followed by corals, fucoids, and kelps, whereas macroalgae contracted their range faster than corals and fishes (Fig. 2 B and C and SI Appendix, Table S2). The speeds and directions of observed shifts were otherwise comparable to those reported for similar species in previous studies (1–4). Expansions of fucoids and corals were 2.6 and 10 times faster, respectively, than their contractions (SI Appendix, Table S2), highlighting the differential processes governing range dynamics at distribution edges (31). Centroid shift rates and occupancy changes were significantly different among taxa and between coasts (Fig. 2D) (two-way multivariate ANOVA, coast, taxa, and interaction: P < 0.01), with centroid shifts in kelps and fucoids driven mainly by local extinctions (range contractions) and those in corals and herbivorous fishes by colonization (expansions), as expected based on the regional biogeography of the species. Corals colonized into three types of communities with equal frequency (Fig. 2E): existing coral community, currently macroalgal-dominated community, and areas from which macroalgae have recently contracted. Despite previous evidence emphasizing the role of herbivory in clearing macroalgal beds and facilitating the colonization of corals through competition release (16, 19), this pattern indicates that direct competition resulting from the expansion of corals into existing macroalgal communities is also a possible important process facilitating macroalgal-to-coral shifts.
Our prediction of range shifts used a climate-velocity trajectory model based on a least-cost path algorithm that determined the minimum-cost route linking the past and current locations of isotherms corresponding to the temperatures found at the initial sites from which each of the observed shift had been detected (Materials and Methods and Fig. 1C). The algorithm was informed by a conductance layer built on two components: the spatial thermal gradient (climate-based movement expectations) and ocean currents (flow-based movement expectations). Optimization of the model was made via the relative weights given to each of these two components when constructing the conductance matrix. Therefore, our reported climate-velocity estimates varied depending on both the temperature parameter [annual mean, minimum or maximum monthly sea surface temperature (SST)] and the model used (SI Appendix, Fig. S3). Although overall differences between a model built only on a conductance matrix informed by the spatial thermal gradient (i.e., the custom approach for calculating climate velocities), and our compound model were small, differences were still large at some locations, particularly around the southern coast (SI Appendix, Fig. S3 G–I), matching the location of many of the observed range expansions and contractions (Fig. 2 B and C).
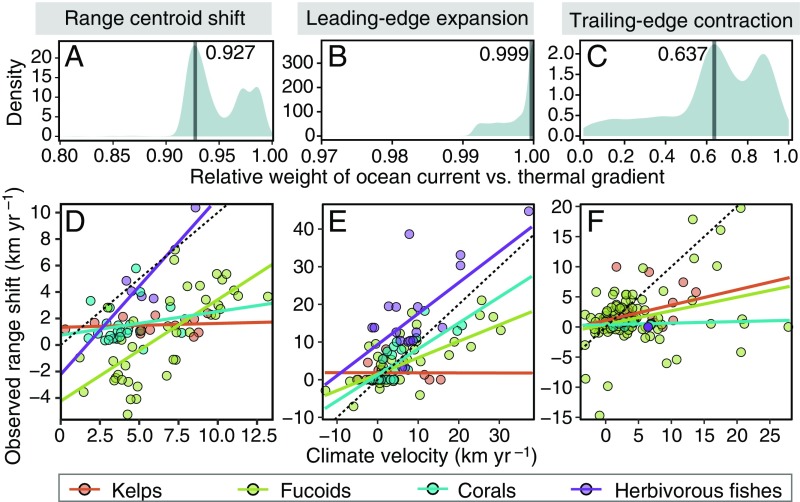
As expected, we found ocean currents to have a much higher relative weight than thermal gradient in explaining observed shift responses for range centroids (0.927) (Fig. 3A) and expansions (0.999) (Fig. 3B), but the relative weight was much more balanced for contractions (0.637) (Fig. 3C). These results were akin to those derived from traditional statistics (SI Appendix, SI Results and Fig. S4). This weight pattern indicated that climate-related range shifts involving dispersal process (i.e., expansion and centroid) would explicitly follow ocean currents rather than thermal gradients. Predicted centroid shifts and leading-edge expansions were fastest in herbivorous fishes and slowest in kelps (Fig. 3 D and E and SI Appendix, Fig. S4 G and H). Importantly, however, all taxa lagged behind climate velocity on average: slope of regression lines were more gradual than the 1:1 line (Fig. 3E). Predicted trailing-edge contractions were slowest in corals and fastest in kelps and fucoids (Fig. 3F and SI Appendix, Fig. S4I). No corresponding analysis was possible for fish as we only have one trailing-edge observation that remained stationary (i.e., no contraction). The proportion of variance in shift response explained by the model predictions was larger for range centroids (52.8%) and expansions (65.0%) than for contractions (26.6%). As initially expected, these results suggest that ocean currents strongly facilitate centroid and expansion shifts, but only weakly contractions.
Fig. 3.
Predicted shift rates based on climate velocities for (A and D) range centroid shifts (n = 82), (B and E) leading-edge expansions (n = 114), and (C and F) trailing-edge contractions (n = 124). (A–C) Bayesian posterior distribution of the relative weight of effects by ocean current against thermal gradient on climate velocity. (D–F) Relationship between the observed and predicted shifts by a climate velocity based on the coupled climate and current effects (Fig. 1C and SI Appendix, Fig. S3), estimated together with the relative weight. Bold vertical line in A–C indicates the maximum probability in the relative weight. Dotted and solid lines in D–F represent, respectively, the 1:1 line and the mean of model fits for kelps, fucoids, corals, and deforestation by herbivorous fishes.
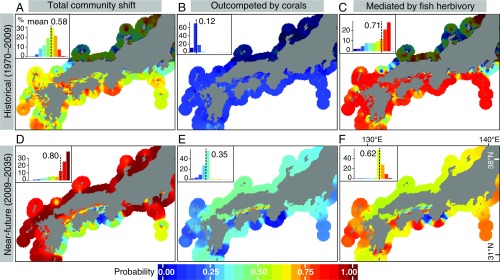
Based on the Bayesian posterior distributions of the species range-shift responses predicted by the optimized coupled climate and current model (SI Appendix, Fig. S5), we estimated probabilities of potential community shifts from macroalgae to corals for historical (1970–2009) and near-future (2009–2035) climates (Materials and Methods and Fig. 4). The mean probability of contemporary community shift across the Japanese coast was 0.58, with the largest values along the southwestern to southeastern coasts (Fig. 4A), where the warm Kuroshio and Tsushima currents flow nearby and vigorously (Fig. 1A). The mean probability of community shift is predicted to increase to 0.8 in the near future (Fig. 4D), with high probabilities becoming widespread apart from the southern-middle coasts around Shikoku, where coral community have already expanded (13). In both periods, community shifts are estimated to be driven more by herbivorous deforestation by fishes (Fig. 4 C and F) than through competition displacement by the colonizing corals (Fig. 4 B and E), although the relative importance of the latter is predicted to increase significantly under future warming (difference between mean probabilities of both processes of 0.59 and 0.24 for historical and future periods).
Fig. 4.
Probability of potential community shift from macroalgae to corals based on predicted shift rates and climate velocity. (A and D) Total probability of macroalgae-to-corals shifts, probability of the community shift being driven by (B and E) competition with corals, and (C and F) deforestation by herbivorous fishes. (A–C) Prediction based on historical (1970–2009) and (D–F) near-future climates (2009–2035). Inset histograms represent frequency distribution of probabilities with their mean value. Probabilities of shifts were obtained from the Bayesian posterior distributions of the relative difference in the predicted shift rates among taxa with the coupled climate–current model (SI Appendix, Fig. S5). The darkened area in the north coast in A–C covers the region where no observed macroalgal–coral shift was detected. Calculations were restricted to coastal grids, since the study species inhabit coastal environments. Figures were spatially interpolated to 50 km from the coast using an inverse distance-weighted method to aid visualization.
Our results reveal a clear mechanistic pattern for the mediation of ocean currents and climate change to shifts from macroalgae to coral dominance over the warm temperate coast of Japan. As expected, the range expansion of herbivorous fishes was fastest and most influenced by current transport among the study species. This is generating increased grazing pressure on macroalgae communities already under direct pressure from warming, as indicated by their high contraction and slow expansion rates. Whereas the rapidly expanding tropical corals can colonize into existing temperate macroalgae communities (12, 22), this mechanism can create a cascading effect, facilitating coral recruitment and accelerating the change in dominance between both taxa. The outcome of the combination of all these factors is the shifts from macroalgae- to coral-dominated communities that are being currently reported across the Japanese warm temperate zone (12–16).
Our results highlight the complexity of managing for climate-driven range shifts and anticipating transient dynamics where species are currently expanding faster than they are contracting, thus increasing their distribution range. For example, tropical macroalgal species have expanded their range into a warm temperate zone at the expense of temperate species (32), a process that is intimately related to their differences in physiology (33). Similarly, despite degradation of coral communities in tropical regions (7), endangered tropical reef-forming coral species are migrating into Japanese temperate waters (as in the case of Acropora muricata in our study) (1), supporting the role of the temperate zone as a potential refuge for coral from the effects of global warming (1, 17). However, coral expansions may still be limited by nonclimatic factors, such as availabilities of carbonate (34), food (35), and light (16, 35). Nevertheless, and although expansion rates are faster than contraction rates overall, we found that not only macroalgae but even corals might be unable to keep pace with climate change. This may suggest that the apparent expansion in overall range might be a transient effect potentially masking risk of metapopulation collapse with decreasing connectivity among local populations (extinction debts) and future range collapses (36). Furthermore, such range expansions often entail the replacement of temperate macroalgae (12–16), and these range shifts are accompanied by those of coral-associated organisms, such as tropical reef fishes (37) and obligate-dwelling crabs (38). On the other hand, expansion of coral-eating species is equally likely. The starfish (Acanthaster planci), for example, is suspected to have expanded its reproduction range into Japanese temperate waters over the last decade (39). Indeed, not only coral communities (1, 12–16, 22) but also deforested barrens are expanding along temperate marine coasts (4, 18–20, 32). Thus, future management for corals and macroalgae might need to consider proactive species conservation strategies, including artificial assistance for thermal adaptation through selection of thermal-tolerant lineages (40) or relocation and translocation into cooler areas (17).
Our study provides evidence of how the combination of climate warming and external directional drivers can elicit differential rates of shift response by habitat-competing and higher trophic species. Consideration of such interacting effects is necessary for anticipating the ecosystem-wide implications of species shifts under future climate warming.
Materials and Methods
Environmental Variables.
We used bias-corrected (41, 42) SST (1950–2035) extracted from the MIROC4h model (43) and surface current (1985–2015; longitudinal and latitudinal velocity) sourced from assimilation data of the MOVE/MRI.COM (44) because the study species mainly inhabit the infra-littoral zone (see SI Appendix, SI Methods for a detailed description of data). The data were downscaled to 0.05° × 0.05° using bilinear interpolation. We chose this resolution to adequately reproduce coastal outlines in the climate-velocity trajectory models. To account for interannual variation and the possibility of lagged response to environmental change, we used 5-y means of SST and current values instead of just the values at the year of the species record.
Species Occurrence Data.
We used a long-term (mainly 1950–2015; 2.4% of the records were made before 1950) database of habitat-forming macroalgae, corals, and herbivorous fishes (22,253 survey records) compiled from 439 literature sources. The dataset thus provides good spatial coverage of most of the Japanese coastline. The occurrence data for macroalgae are publicly available (45). Coral data consisted of the dataset used in ref. 1 updated with 65 records for 3 new species from the literature sources. From the database, we selected 8 kelp, 22 fucoid macroalgae, and 12 coral species that are representative of the macroalgal flora and coral fauna in the Japanese temperate zone (see SI Appendix, Table S1 for a description of the species). The records used for this study are shown in Dataset S1.
Shifts of herbivorous fishes were inferred from records on deforestation of temperate macroalgal communities due to overgrazing by three herbivorous (omnivorous) fish species that have been associated with increases in sea temperature (Dataset S1). We therefore excluded records linked to other factors, such as increases in pollution levels or turbidity. Such herbivorous fishes have long been found in the Japanese temperate zone as individuals or small schools of possibly nonbreeding populations (46). However, rising sea temperature is speculated to have facilitated their overwintering survival and reproduction, resulting in increased school size (19), and subsequent overgrazing of macroalgal beds (47, 48). Although using information on fish density data would have been desirable, such records are quite limited across Japan. Therefore, given the available data, we believe our records on deforestation of macroalgae provide an adequate indicator of the direct effects of herbivorous impacts.
We then identified the reported geographical locations for 85% of all survey sites with an accuracy of 1 km. There were no apparent spatial or temporal gaps in survey data: at least two decades of replications were available in most locations, except for coral data in Okinawa Prefecture (approximately lower than 27°N) before the 1970s (SI Appendix, Fig. S2).
Detection and Prediction of Range Shifts.
We estimated centroid shifts using multiresponse linear models on longitudinal and latitudinal locations as a function of time for each study species and Japanese coast (SI Appendix, SI Methods). Range-edge shifts consisted of either documented shifts or shifts estimated from two or more occurrence records separated by more than 5 y (SI Appendix, SI Methods and Dataset S1). We then calculated the distance shifted as the least-cost distance between the cell locations corresponding to the historical and present records. Distances were restricted to coastal waters and took into account the coastline configuration. Finally, shift rates were calculated by dividing the distance shifted (kilometers) by the number of years elapsed between the historical and present records.
Range-edge shifts were predicted using a coastal adaptation of climate-velocity trajectories (30), which consisted of finding the least-cost path an isotherm will move over a specific time period from a starting fixed location (Fig. 1C). We first located those target coastal cells intersected by the future location of the isotherm corresponding to the temperature existing at the historical location (focal cell) using the “contourLines” function of the “maptools” R package (49). We then identified the target cell providing minimum accumulated movement cost from the focal cell using a least-cost path analysis with conductance matrices, representing the ease of movement across the seascape and created using spatial thermal gradients in combination with ocean current speed and direction using combinations of weights between 0 and 1 at 0.001 intervals (see the next section and SI Appendix, SI Methods). Two leading and 12 trailing edges were lost because no coastline was available along isotherm trajectory (e.g., southern archipelago). Predicted shift rates were then calculated by dividing the resulting least-cost distance by the number of years between periods. For contractions and expansions, this was a single cell-to-cell estimate (i.e., localized shifts), while for the calculation of shift rates for range centroids (i.e., whole-distribution shifts) we used the mean distance from all of the least-cost path estimates for the historical occurrence points for each species grouped by coast (i.e., two shift estimates per species). Accumulated costs were calculated using the “accCost” function of the “gdistance” R package (50).
Statistical Analysis and Prediction.
To account for the observed shift rates, the statistical analysis included: (i) the estimations of the relative weight of ocean current against thermal gradient in the climate-velocity trajectory model, (ii) the selection of the optimum SST index (see SI Appendix, SI Methods for a detailed description of the index) for each species and range edge, and (iii) the relationship between the observed shift rates and the climate velocities, performed each at a time under a common Bayesian inference. Briefly, we assigned a uniform prior distribution from 0 to 1 for the relative weight of ocean currents, and a uniform categorical prior for the SST index. Then, we calculated the estimates (posterior distributions) of these parameters by Markov chain Monte Carlo simulations (MCMC). Analyses of range shifts were conducted by fitting linear models including as covariates climate velocity and differences of taxonomic group (kelps, fucoids, corals, and herbivorous fishes), both in intercept and slope (i.e., analysis of covariance model) (51). For edge shifts, but not centroid shifts, sample size varied among species. This is because multiple shifts are often reported in the literature at different locations within a species’ distribution range, which is usually filled discontinuously by local populations due to the complicated configuration of the coast line along the Japanese archipelago. Therefore, we included species as a random effect on the intercept (i.e., the linear mixed-effects model) (51) for edge shifts, to estimate the response of each taxon equally by reducing bias of uneven sample size among species. The climate velocities were fitted after standardization (i.e., with a mean of 0 and a SD of 1) to obtain standardized coefficients. We also calculated indices of explained variance by the model using ordinal R2 for centroids, but marginal R2 (explained by fixed effects) (52) for edge shifts.
The probability of potential macroalgal–coral shift and its driving processes were estimated for each cell over historical (1970–2009) and near-future (2009–2035) climates. We first assumed that both contraction rates of macroalgae (Mc) and expansion rates of corals (Ce) must be positive for a dominance shift from macroalgae to corals to occur: that is, Mc > 0 and Ce > 0. Next, we assumed that the relative difference between range expansions and contractions is indicative of the temporal order with which the events take place at each cell (local scale): that is, faster expansions rates of corals or herbivorous fishes (Fe) than macroalgae indicate potential causality between the earlier arrival of corals/fishes and the latter extirpation of macroalgae from that cell. We then identified four types of processes underpinning macroalgal–coral shifts: (i) coral expansions after macroalgal contractions (e.g., contractions caused directly by warming), satisfying the condition Mc > 0 and Ce > 0 and Fe – Mc < 0 and Ce – Mc < 0; (ii) coral expansions after deforestation of macroalgae by herbivorous fishes (Mc > 0 and Ce > 0 and Fe – Mc > 0 and Ce – Mc < 0); (iii) macroalgae outcompeted by expanded corals (Mc > 0 and Ce > 0 and Fe – Mc < 0 and Ce – Mc > 0 and Fe – Ce < 0); and (iv) combined displacement by herbivorous deforestation and competition with corals (Mc > 0 and Ce > 0 and Ce – Mc > 0 and Fe – Mc > 0), where the relative importance of each process is specified by a weighting ratio of shift rates between expansion rates of corals and herbivorous fishes.
For the analyses, we obtained Bayesian posterior distributions of parameter estimates for the linear mixed-effects models on expansion and contraction shift rates using the optimized combination of conductance weights for thermal gradients and ocean currents, under nearly noninformative normal prior distributions. Using the estimated intercept and slope, and the climate velocity (monthly minimum SST for expansions and maximum SST for contractions) from the climate–current model, we predicted macroalgae (average of kelps and fucoids), expansion rates of corals, and expansion rates of herbivorous fishes for each cell along the Japanese coast. The predicted shift rate was multiplied by the sign of the climate velocity at the cell to keep shifts in the same direction of the climate velocity positive. We then calculated Bayesian probabilities (the number of posterior estimates over all estimates) meeting each of the four processes, and summarized them into the total probability of the macroalgae–coral shift, and the probability of each process driving the community shift. Note that these probabilities represent the likelihood for each process in terms of time-order rather than causal relationships.
Bayesian inferences were performed in WinBUGS 1.4.3 (53) specifying nearly noninformative normal prior distributions for coefficients. We obtained 500 estimates per parameter from 70,000 MCMC iterations after a 20,000-iteration burn-in by thinning at intervals of 100, using three chains. Convergence was verified by checking that the R-hat values ranged between 1 and 1.01 (54). All of the other analyses were performed in R (55).
Supplementary Material
Acknowledgments
Collection and taxonomical update of coral records were supported by Chuki Hongo, Yumiko Yara, and Kaoru Sugihara. This work was conducted under the “Program for Risk Information on Climate Change (SOUSEI Program)” and the “Integrated Research Program for Advancing Climate Models (TOUGOU Program)” of the Ministry of Education, Culture, Sports, Science, and Technology in Japan (MEXT), and partially by the Environment Research and Technology Development Fund (S9 and S15) of the Ministry of the Environment, Japan. J.G.M. acknowledges additional support received from the International Research Fellow Programme of the Japan Society for the Promotion of Science (JSPS/FF1/434) and the “Tenure-Track System Promotion Program” of MEXT.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716826115/-/DCSupplemental.
References
- 1.Yamano H, Sugihara K, Nomura K. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys Res Lett. 2011;38:L04601. [Google Scholar]
- 2.Poloczanska ES, et al. Global imprint of climate change on marine life. Nat Clim Chang. 2013;3:919–925. [Google Scholar]
- 3.Straub SC, Thomsen MS, Wernberg T. The dynamic biogeography of the Anthropocene: The speed of recent range shifts in seaweeds. In: Hu ZM, Fraser C, editors. Seaweed Phylogeography. Springer; Dordrecht, The Netherlands: 2016. pp. 63–93. [Google Scholar]
- 4.Wernberg T, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016;353:169–172. doi: 10.1126/science.aad8745. [DOI] [PubMed] [Google Scholar]
- 5.Knowlton N. The future of coral reefs. Proc Natl Acad Sci USA. 2001;98:5419–5425. doi: 10.1073/pnas.091092998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 7.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 8.Pecl GT, et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science. 2017;355::eaai9214. doi: 10.1126/science.aai9214. [DOI] [PubMed] [Google Scholar]
- 9.Done TJ. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia. 1992;247:121–132. [Google Scholar]
- 10.Hughes TP, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature. 2015;518:94–97. doi: 10.1038/nature14140. [DOI] [PubMed] [Google Scholar]
- 12.Nojima S, Okamoto M. Enlargement of habitats of scleractinian corals to north and coral bleaching events. Bull Jpn Soc Sci Fish. 2008;74:884–888. [Google Scholar]
- 13.Mezaki T, Kubota S. Changes of hermatypic coral community in coastal sea area of Kochi, high-latitude, Japan. Aquabiology. 2012;201:332–337. [Google Scholar]
- 14.Serrano E, Coma R, Ribes M. A phase shift from macroalgal to coral dominance in the Mediterranean. Coral Reefs. 2012;31:1199. [Google Scholar]
- 15.Denis V, et al. Recruitment of the subtropical coral Alveopora japonica in the temperate waters of Jeju Island, South Korea. Bull Mar Sci. 2015;91:85–96. [Google Scholar]
- 16.Tuckett CA, de Bettignies T, Fromont J, Wernberg T. Expansion of corals on temperate reefs: Direct and indirect effects of marine heatwaves. Coral Reefs. 2017;36:947–956. [Google Scholar]
- 17.Beger M, Sommer B, Harrison PL, Smith SDA, Pandolfi JM. Conserving potential coral reef refuges at high latitudes. Divers Distrib. 2014;20:245–257. [Google Scholar]
- 18.Serisawa Y, Imoto Z, Ishikawa T, Ohno M. Decline of the Ecklonia cava population associated with increased seawater temperatures in Tosa Bay, southern Japan. Fish Sci. 2004;70:189–191. [Google Scholar]
- 19.Bennett S, Wernberg T, Harvey ES, Santana-Garcon J, Saunders BJ. Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol Lett. 2015;18:714–723. doi: 10.1111/ele.12450. [DOI] [PubMed] [Google Scholar]
- 20.Vergés A, et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc Natl Acad Sci USA. 2016;113:13791–13796. doi: 10.1073/pnas.1610725113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floeter SR, Behrens MD, Ferreira CEL, Paddack MJ, Horn MH. Geographical gradients of marine herbivorous fishes: Patterns and processes. Mar Biol. 2005;147:1435–1447. [Google Scholar]
- 22.Thomson D. Range extension of the hard coral Goniopora norfolkensis (Veron & Pichon 1982) to the south-east Indian Ocean. J R Soc West Aust. 2010;93:81–83. [Google Scholar]
- 23.Gaylord B, Gaines SD. Temperature or transport? Range limits in marine species mediated solely by flow. Am Nat. 2000;155:769–789. doi: 10.1086/303357. [DOI] [PubMed] [Google Scholar]
- 24.Sorte CJB. Predicting persistence in a changing climate: Flow direction and limitations to redistribution. Oikos. 2013;122:161–170. [Google Scholar]
- 25.García Molinos J, Burrows MT, Poloczanska ES. Ocean currents modify the coupling between climate change and biogeographical shifts. Sci Rep. 2017;7:1332. doi: 10.1038/s41598-017-01309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothäusler E, Gutow L, Thiel M. Floating seaweeds and their communities. In: Wiencke C, Bischof K, editors. Seaweed Biology. Springer; Heidelberg: 2012. pp. 403–420. [Google Scholar]
- 27.Vergés A, et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc R Soc B. 2014;281:20140846. doi: 10.1098/rspb.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, et al. Enhanced warming over the global subtropical western boundary currents. Nat Clim Chang. 2012;2:161–166. [Google Scholar]
- 29.Sunday JM, et al. Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecol Lett. 2015;18:944–953. doi: 10.1111/ele.12474. [DOI] [PubMed] [Google Scholar]
- 30.Burrows MT, et al. Geographical limits to species-range shifts are suggested by climate velocity. Nature. 2014;507:492–495. doi: 10.1038/nature12976. [DOI] [PubMed] [Google Scholar]
- 31.Bates AE, et al. Defining and observing stages of climate-mediated range shifts in marine systems. Glob Environ Change. 2014;26:27–38. [Google Scholar]
- 32.Tanaka K, Taino S, Haraguchi H, Prendergast G, Hiraoka M. Warming off southwestern Japan linked to distributional shifts of subtidal canopy-forming seaweeds. Ecol Evol. 2012;2:2854–2865. doi: 10.1002/ece3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beardall J, Beer S, Raven JA. Biodiversity of marine plants in an era of climate change: Some predictions based on physiological performance. Bot Mar. 1998;41:113–123. [Google Scholar]
- 34.Yara Y, et al. Ocean acidification limits temperature-induced poleward expansion of coral habitats around Japan. Biogeosciences. 2012;9:4955–4968. [Google Scholar]
- 35.Sommer B, Harrison PL, Beger M, Pandolfi JM. Trait-mediated environmental filtering drives assembly at biogeographic transition zones. Ecology. 2014;95:1000–1009. doi: 10.1890/13-1445.1. [DOI] [PubMed] [Google Scholar]
- 36.Kuussaari M, et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol Evol. 2009;24:564–571. doi: 10.1016/j.tree.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Feary DA, Kanda M, Yamaoka K. Tropical fishes dominate temperate reef fish communities within western Japan. PLoS One. 2013;8:e81107. doi: 10.1371/journal.pone.0081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamano H, et al. Ranges of obligate coral-dwelling crabs extend northward as their hosts move north. Coral Reefs. 2012;31:663. [Google Scholar]
- 39.Nomura K. Recent changes in coral community in Kushimoto, the southernmost part of Honshu, Japan. J Jpn Coral Reef Soc. 2009;11:39–49. [Google Scholar]
- 40.van Oppen MJH, Oliver JK, Putnam HM, Gates RD. Building coral reef resilience through assisted evolution. Proc Natl Acad Sci USA. 2015;112:2307–2313. doi: 10.1073/pnas.1422301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabor K, Williams JW. Globally downscaled climate projections for assessing the conservation impacts of climate change. Ecol Appl. 2010;20:554–565. doi: 10.1890/09-0173.1. [DOI] [PubMed] [Google Scholar]
- 42.Yara Y, et al. Projection and uncertainty of the poleward range expansion of coral habitats in response to sea surface temperature warming: A multiple climate model study. Galaxea. 2011;13:11–20. [Google Scholar]
- 43.Sakamoto TT, et al. MIROC4h—A new high-resolution atmosphere—Ocean coupled general circulation model. J Meteorol Soc Jpn. 2012;90:325–359. [Google Scholar]
- 44.Usui N, et al. Meteorological Research Institute multivariate ocean variational estimation (MOVE) system: Some early results. Adv Space Res. 2006;37:806–822. [Google Scholar]
- 45.Kumagai NH, Yamano H, Fujii M, Yamanaka Y. Habitat-forming seaweeds in Japan (fucoids and temperate kelps) Ecol Res. 2016;31:759. [Google Scholar]
- 46.Yamaguchi A. Biological aspects of herbivorous fishes in the coastal areas of western Japan. Bull Fish Res Agen. 2010;32:89–94. [Google Scholar]
- 47.Noda M. Characteristics in grazing behavior of Siganus fuscescens. In: Fujita D, Noda M, Kuwahara H, editors. Marine Herbivorous Fish—Ecology, Fishery and Utilization. Seizando-Shoten; Tokyo: 2006. pp. 114–125. [Google Scholar]
- 48.Michael PJ, Hyndes GA, Vanderklift MA, Vergés A. Identity and behaviour of herbivorous fish influence large-scale spatial patterns of macroalgal herbivory in a coral reef. Mar Ecol Prog Ser. 2013;482:227–240. [Google Scholar]
- 49.Bivand R, Lewin-Koh N. 2015. maptools: Tools for Reading and Handling Spatial Objects. R package Version 0.8-39. Available at cran.r-project.org/package=maptools. Accessed October 13, 2016.
- 50.van Etten J. 2015. gdistance: Distances and Routes on Geographical Grids. R package Version 1.1-9. Available at cran.r-project.org/package=gdistance. Accessed October 13, 2016.
- 51.Kéry M. Introduction to WinBUGS for Ecologists: Bayesian Approach to Regression, ANOVA, Mixed Models and Related Analyses. 1st Ed Academic; Burlington, VT: 2010. [Google Scholar]
- 52.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- 53.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—A Bayesian modelling framework: Concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 54.Gelman A, et al. Bayesian Data Analysis. 3rd Ed Chapman & Hall; New York: 2013. [Google Scholar]
- 55.R Core Team 2015. R: A Language and Environment for Statistical Computing. Version 3.3.1. Available at cran.r-project.org. Accessed October 13, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.