Significance
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by severe cognitive decline. The connection between neuroinflammation and the progressive loss of neurons is well known; however, the fact that most patients with AD suffer from concomitant seizure disorders is underappreciated. We have previously shown that the NLRP3 inflammasome, a major source of the proinflammatory cytokines IL1β and IL18, drives the pathology of AD in APP/PS1 mice. Here we report that surprisingly, IL18-deficient APP/PS1 mice develop a lethal seizure disorder due to an increase in neuronal network transmission. While targeting the NLRP3 inflammasome might be one potential method of halting AD progression, it should be recognized that specific cytokine inhibition in the brain may result in unintended deleterious consequences.
Keywords: Alzheimer’s disease, neuroinflammation, inflammasome, IL18, seizures
Abstract
Alzheimer’s disease (AD) is characterized by the progressive destruction and dysfunction of central neurons. AD patients commonly have unprovoked seizures compared with age-matched controls. Amyloid peptide-related inflammation is thought to be an important aspect of AD pathogenesis. We previously reported that NLRP3 inflammasome KO mice, when bred into APPswe/PS1ΔE9 (APP/PS1) mice, are completely protected from amyloid-induced AD-like disease, presumably because they cannot produce mature IL1β or IL18. To test the role of IL18, we bred IL18KO mice with APP/PS1 mice. Surprisingly, IL18KO/APP/PS1 mice developed a lethal seizure disorder that was completely reversed by the anticonvulsant levetiracetam. IL18-deficient AD mice showed a lower threshold in chemically induced seizures and a selective increase in gene expression related to increased neuronal activity. IL18-deficient AD mice exhibited increased excitatory synaptic proteins, spine density, and basal excitatory synaptic transmission that contributed to seizure activity. This study identifies a role for IL18 in suppressing aberrant neuronal transmission in AD.
Alzheimer’s disease (AD) is a global health problem that affects ∼5.4 million people in the United States (1). Mechanism-based cures do not exist. Modern theories of AD are based on the hypothesis that amyloid deposits are important in initiating AD pathogenesis (2). Nevertheless, the role of beta-amyloid (Aβ) in AD is controversial (3). Downstream processes are also important, in particular, the formation of aggregated phosphorylated tau proteins (4). Although many AD patients have autosomal dominant mutations that result in the aberrant processing of amyloid protein, the majority of individuals with AD do not (5). Nevertheless, in 2007, the Alzheimer’s Association estimated that ∼200,000 Americans have the familial form of AD, resulting from a mutation in amyloid precursor protein or presenilin, and the essential role of amyloid in these cases seems incontrovertible. The lack of consensus on the pathophysiological mechanism has made achieving a cure difficult, although it is generally accepted that inflammation plays an important role in AD pathogenesis.
We reported a key role for NLRP3 inflammasome activation in AD pathogenesis (6, 7). AD mice [APPswe/PS1ΔE9 (APP/PS1)] lacking the expression of inflammasome components such as NLRP3, ASC, or caspase-1 were protected from behavioral abnormalities and associated biochemical and physiological pathologies. In addition, autopsy specimens of human AD patients had evidence of robust inflammasome activation (7). Inflammasome complexes seem to play a critical role in the spreading of Aβ pathology within and between brain areas (8). These results suggested that the NLRP3 inflammasome plays a central role in AD pathology.
Mature IL18 is one of two proinflammatory inflammasome-derived cytokines, the other being IL1β. It is synthesized as an inactive 24-kDa precursor protein (pro-IL18) and cleaved by activated caspase-1 to generate an 18-kDa biologically active form (9). Many cell types in the central nervous system (CNS) produce IL18 mRNA, although only microglia have the complete repertoire of NLRP3 inflammasome components (10). IL18 increases the production of amyloid peptide in human neurons and enhances tau phosphorylation (11). IL18 is elevated in the cerebrospinal fluid (CSF) of AD patients with mild cognitive impairment (12). Furthermore, IL18 polymorphisms are associated with increased susceptibility to AD (13). We hypothesized that APP/PS1 mice that were IL18 deficient would be protected from AD. Unexpectedly, we noticed an increased mortality of APP/PS1 mice lacking IL18 due to grand mal seizure activity.
Epilepsy is a common complication of AD; studies indicate that up to two-thirds of AD patients have seizures, both nonmotor and motor (14). In a recent study, AD patients with epilepsy were more likely to develop accelerated memory loss, other cognitive symptoms, and a more widespread loss of brain cells than AD patients without epilepsy (15). While epilepsy does not cause AD, the two disorders may share common biological pathways. Indeed, antiseizure treatment suppressed neuronal network dysfunction and reduced cognitive deficits in a common AD mouse model (16).
Impaired glutamatergic synaptic function is one of the most profound pathophysiological hallmarks of AD (17); however, it has not been addressed as to whether the vulnerability of epilepsy in AD patients can be attributed to the dysregulated glutamatergic synaptic function or the exact role, if any, of inflammatory mediators in synaptic dysfunction. Aβ oligomers cause the loss of dendritic spines, increase internalization of AMPA- and NMDA-type glutamate receptors, and impair synaptic plasticity in the hippocampus (18–20). Here, we examined the electrophysiology of seizures that complicate AD at the level of the synapse. APP/PS1 mice lacking IL18 exhibited an increase in neuronal network transmission. These results suggest that IL18 suppresses aberrant neuronal activity due to Aβ-induced inflammation.
Results
IL18 Deficiency Causes the Death of Young APP/PS1 Mice.
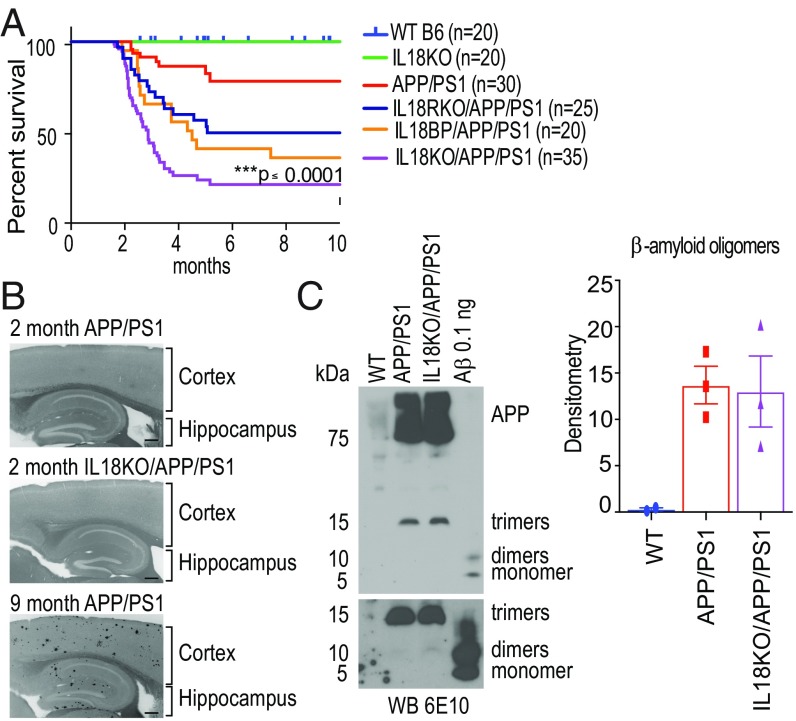
We previously demonstrated that APP/PS1 mice were protected from AD-like learning and memory deficits when bred into NLRP3, caspase-1, or ASC knockouts (7). These data suggested that inflammasome activation enhances disease progression. IL1β and IL18 are NLRP3-derived proinflammatory cytokines and both play major roles in a variety of inflammatory diseases. To understand the role of IL18 in AD, we bred APP/PS1 with IL18-deficient mice (IL18KO), predicting the mice would be protected from AD symptoms. Surprisingly, IL18KO/APP/PS1 mice had a sharply increased mortality rate beginning at ∼2 mo of age (Fig. 1A). We also bred APP/PS1 mice with IL18 binding protein (BP) transgenic (Tg) mice, where overexpression of an IL18 antagonist prevents signaling (21). We found that the survival of APP/PS1 mice on the IL18BP Tg background was similarly compromised (Fig. 1A). Finally, we bred APP/PS1 mice with IL18R-deficient mice (Fig. 1A). These mice also had increased mortality at 2 mo of age.
Fig. 1.
IL18 deficiency causes the death of young APP/PS1 mice before amyloid plaque formation in IL18KO/APP/PS1 mice. (A) Six groups of mice (n = 20–35) were monitored for survival over 10 mo. Note the increased mortality in all groups of mice lacking IL18 activity on the APP/PS1 background. Asterisks indicate a statistical difference in outcome of IL18KO/APP/PS1 mice compared with WT (blue) or APP/PS1 (red) mice [log-rank (Mantel–Cox) test, ***P < 0.0001]. (B) Brain sections of cortices and hippocampi from 2-mo-old APP/PS1, IL18KO/APP/PS1, and 9-mo-old APP/PS1 mice were stained with methoxy-X04 to detect the presence of amyloid plaques. In contrast to 9-mo-old APP/PS1 mice, plaques were not detected in younger mice. (Scale bar: 200 μm.) (C) Lysates from 2-mo-old WT C57BL/6, APP/PS1, and IL18KO/APP/PS1 mice were probed with 6E10 mAb to Aβ peptide. Synthetic oligomeric Aβ peptides were probed as a control. Monomers, dimers, and trimers of Aβ migrate at 4 kDa, 8 kDa, and 13 kDa, respectively. Bottom represents a longer exposure of the lower portion of the full panel. C, Right shows the densitometry analysis of the Western blot results.
Amyloid deposition is thought to be one of the major causes of AD through its activation of inflammatory responses. Oligomeric and fibrillar forms of Aβ impair long-term potentiation (LTP) and synaptic dysfunction, and are thought to accelerate the formation of neurofibrillary tangles (22). We evaluated amyloid plaque formation in the brains of IL18KO/APP/PS1 mice using methoxy-X04 staining. Amyloid deposits were seen in 9-mo-old APP/PS1 mice but not in 2-mo-old APP/PS1 or IL18KO/APP/PS1 mice (Fig. 1B), suggesting that the death of IL18KO/APP/PS1 mice was not due to the acceleration of Aβ plaque formation. Western blots of brain extracts clearly demonstrated the presence of equal quantities of Aβ oligomers in 2-mo-old APP/PS1 and IL18KO/APP/PS1 mice (Fig. 1C).
Because IL18 is an inflammatory mediator, lack of IL18 may induce a compensatory increase of inflammation responsible for the premature death we observed. However, serum levels of inflammatory cytokines, including IL1β, IL6, TNFα, IFNγ, and IL12 were undetectable over the period observed. Other cytokines, such as IL1α, MIP2, RANTES, and KC were unchanged (SI Appendix, Fig. S1A). We next addressed the possibility that IL18 might modulate the expression of IL1β in the brain. We analyzed cytokines by ELISA on hippocampus and cortex samples from 2.5-mo-old IL18KO/APP/PS1 mice and controls and found increased IL1β levels in both APP/PS1 and IL18KO/APP/PS1 mice (SI Appendix, Fig. S1B).
Several AD mouse models display modest susceptibility to seizure-induced death. A recent study showed a selective increase in hyperactive neurons in hAPPJ20 mice (a strain of mice with pathologically elevated levels of human Aβ in the brain) long before the formation of plaques, suggesting that soluble Aβ may cause this dysfunction (16). We observed that APP/PS1 mice had a ∼5% mortality rate at 2 mo of age (∼20% at 10 mo) similar to another report (23). We found no evidence of infection or an obvious anatomic abnormality in these mouse lines. Thus, we wondered whether the sharply increased mortality rate in IL18KO/APP/PS1 mice could be due to increased seizure activity. Accordingly, we used a video system to observe a cohort of mice 24 h a day. We recorded grand mal seizures in six IL18KO/APP/PS1 mice, five of whom died acutely (an example of a fatal seizure is shown in Movie S1). We concluded that IL18 signaling is important for suppressing epileptogenic stimuli.
Although IL18 has multiple roles, it was initially described as an IFNγ-inducing factor (24). To test whether the increased mortality rate of IL18KO/APP/PS1 mice was due to the lack of induction of IFNγ, we bred IFNγKO mice onto the APP/PS1 background. However, we did not observe an enhanced death phenotype (SI Appendix, Fig. S2).
IL18KO/APP/PS1 Mice Are More Sensitive to Chemically Induced Seizures.
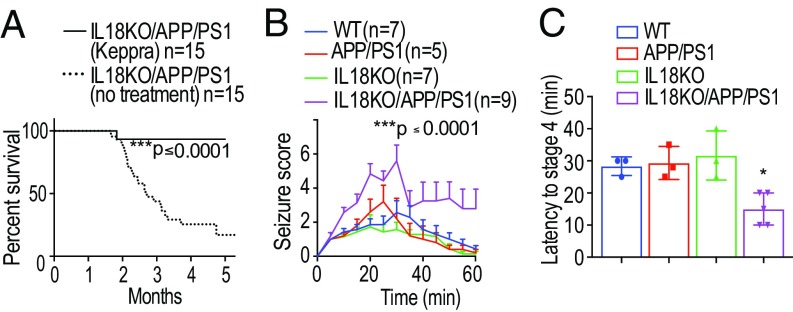
To confirm that seizure activity was the cause of death, we treated IL18KO/APP/PS1 mice with the anticonvulsant drug levetiracetam (Keppra). Levetiracetam is thought to be a ligand of synaptic vesicle glycoprotein 2A and to reduce neurotransmitter release by inhibiting presynaptic calcium channels (25). Mice treated with levetiracetam for 8 wk were protected from death (Fig. 2A). Only 1 of 15 died, and this mouse received levetiracetam for only 6 d, suggesting that the intervention was too late.
Fig. 2.
Levetiracetam treatment restores the survival rate while picrotoxin treatment lowers the seizure threshold of IL18KO/APP/PS1 mice. (A) IL18KO/APP/PS1 mice received the anticonvulsant drug levetiracetam orally at 1.8 mg/mL starting at 6 wk of age for 8 wk (n = 15 per group). Note that levetiracetam almost completely prevented death [log-rank (Mantel–Cox) test, ***P < 0.0001]. (B) Severity of seizure behavior over time following i.p. injection of 2 mg/kg PTX in 10-wk-old IL18KO/APP/PS1 mice and control groups. Higher scores correspond to more severe seizure status; a score of seven indicates death. Seizure score was monitored manually as described (26). In brief, there are a total of seven stages: immobility (stage 1), forelimb or tail extension (stage 2), repetitive scratching or circling (stage 3), rearing and falling (stage 4), repeated observations of stages 2–4 (stage 5), rigidity with uncontrolled spasms (stage 6), and death (stage 7). Data are represented as mean ± SEM (n = 5–9 per group). IL18KO/APP/PS1 mice had significantly increased seizure activities compared with other groups (ANOVA, Tukey’s test, ***P < 0.0001). (C) Summary of the time in minutes to reach seizure stage 4 in IL18KO/APP/PS1 and control mice. *P < 0.05, significant difference from controls.
Increased seizure activity in IL18KO/APP/PS1 mice suggested a hyperexcitable state of the neuronal network due to altered synaptic transmission. To test this, we treated mice with picrotoxin (PTX), a noncompetitive inhibitor of GABAA receptors, and followed their seizure activity. Seizure severity was determined using a previously established rating system (26) with higher values corresponding to more severe seizures up to a score of seven, which indicates death. Over the 1-h test period, IL18KO/APP/PS1 mice showed a dramatically enhanced severity of their seizure score (Fig. 2B). Half of the IL18KO/APP/PS1 mice died during the observation period, in contrast to zero mortality among the control groups. Moreover, IL18KO/APP/PS1 mice exhibited a significantly decreased latency to reach seizure stage four (forepaw clonus with rearing) (Fig. 2C). This reduced threshold to chemically induced seizures suggested that the neuronal network in IL18KO/APP/PS1 mice reflects a hyperexcitable condition. APP/PS1 mice did not exhibit increased susceptibility to seizures, presumably due to the age of APP/PS1 mice we tested, as aged APP/PS1 mice (6 mo old) displayed a significant increase in PTX-induced seizure activity compared with WT mice (SI Appendix, Fig. S3).
IL18KO/APP/PS1 Mice Have a Gene Signature Associated with Neuropeptide Signaling and Cell Migration.
We hypothesized that IL18 deficiency causes abnormal gene expression related to the regulation of neuronal excitability. Thus, we performed RNA sequencing (RNA-seq) analysis of hippocampal samples from 2.5-mo-old IL18KO/APP/PS1 mice and three groups of control mice (WT, IL18KO, and APP/PS1 mice). We identified 1,070 genes with significant differential expression between APP/PS1 mice and IL18KO/APP/PS1 mice (SI Appendix, Table S1; posterior probability of ≥0.95, false discovery rate controlled at 0.05). Among the 75 genes with the largest fold change between IL18KO and IL18KO/APP/PS1 mice (1.6-fold change or greater and posterior probability of ≥0.95), there was a statistical enrichment of genes involved in various neurological processes including sleep, neuropeptide signaling pathways, and cell migration (SI Appendix, Fig. S4A).
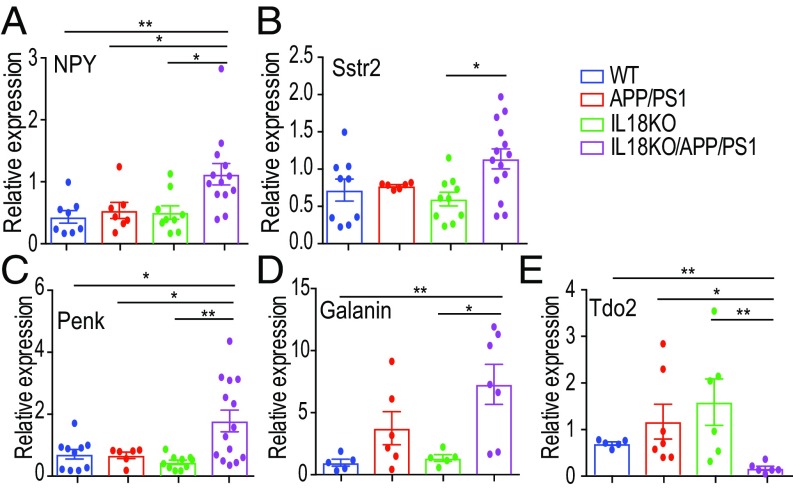
We next used quantitative PCR (qPCR) to confirm the relative gene expression of select genes identified by RNA-seq. One of the genes that was significantly increased in IL18KO/APP/PS1 mice was neuropeptide Y (Npy). Alterations in the expression of NPY are a sensitive indicator of network overexcitation in the hippocampus (27). Indeed, increased NPY expression was reported in other AD mouse models (28, 29). qPCR (Fig. 3A) and immunohistochemistry (SI Appendix, Fig. S5) confirmed the increased expression of NPY in the hippocampus of 2.5-mo-old IL18KO/APP/PS1 mice. The increased NPY expression was mainly in the dentate gyrus and the mossy fibers of IL18KO/APP/PS1 (SI Appendix, Fig. S5), suggesting that chronic elevation of neuronal excitability in dentate gyrus causes the lethal epilepsy in these mice.
Fig. 3.
Differentially expressed signaling genes in IL18KO/APP/PS1 mice. (A) Results of qPCR to detect increased NPY mRNA in hippocampi of IL18KO/APP/PS1 mice (n = 8–12 per group, ANOVA test, *P < 0.05, **P < 0.01). (B–E) qPCR detecting somatostatin receptor 2 (Sstr2), proenkephalins (Penk), galanin, and tryptophan 2,3-dioxygenase (Tdo2) mRNAs in hippocampi from IL18KO/APP/PS1 mice (n = 8–12 per group, ANOVA test, *P < 0.05, **P < 0.01).
In addition to NPY, other neuropeptides believed to be involved in seizure regulation were altered. For example, somatostatin receptor 2, proenkephalin, and galanin were increased (Fig. 3 B–D). In contrast, the expression of the metabolism-related gene Tdo2 was decreased dramatically in IL18KO/APP/PS1 mice (Fig. 3E). In general, genes related to neuronal activities were altered in IL18KO/APP/PS1.
The proepileptogenic dopamine receptor gene Drd1 (30) was increased 1.7-fold in IL18KO/APP/PS1 mice compared with controls (SI Appendix, Fig. S4B). However, Western blots showed that the DRD1 protein level did not change in IL18KO/APP/PS1 mice compared with APP/PS1 mice (SI Appendix, Fig. S6). Furthermore, SCH-23390, a specific antagonist of the type-I dopamine receptor, failed to improve survival in IL18KO/APP/PS1 mice (SI Appendix, Fig. S6D).
IL18KO/APP/PS1 Mice Exhibit Increased Expression of Excitatory but Not Inhibitory Synaptic Proteins.
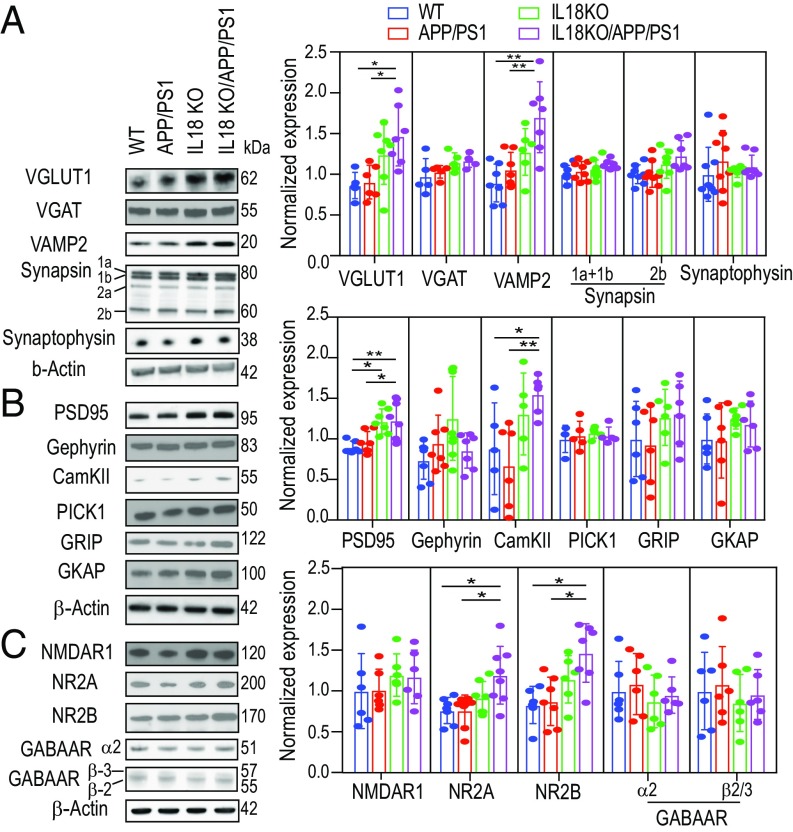
We next addressed whether the lack of IL18 in APP/PS1 mice changes the expression of proteins that regulate neuronal excitability (Fig. 4). In the CNS, glutamate and GABA are the primary excitatory and inhibitory neurotransmitters, respectively. Runaway neuronal network excitation by enhanced glutamatergic excitatory neuronal activity and/or suppression of GABAergic inhibitory neuronal activity cause epileptic seizures. We prepared hippocampal synaptosomal fractions from the mice and performed immunoblots against proteins expressed in excitatory and inhibitory synapses. In the CNS, fast glutamatergic synaptic transmission is mediated by N-methyl-d-aspartate (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR). We detected an increase of NMDAR subunits 2A and 2B (Fig. 4C), suggesting enhanced glutamate signaling. Postsynaptic density protein 95, a scaffold protein enriched in the postsynaptic density, regulates the number of AMPAR and NMDAR (31) and was increased in IL18KO/APP/PS1 (Fig. 4B). In addition, calcium/calmodulin-dependent protein kinase II (CamKII), the highly abundant Ca2+-activated enzyme in postsynaptic density regulating synaptic transmission and LTP (32), was elevated in IL18KO/APP/PS1 mice (Fig. 4B). We also observed increased expression of presynaptic proteins that regulate neurotransmitter release, such as vesicular glutamate transporter 1 (VGluT1) and vesicle-associated membrane protein 2 (VAMP2) in IL18KO/APP/PS1 mice (Fig. 4A). In contrast, vesicular GABA transporter (VGAT), GABA receptors, and gephyrin (Fig. 4 B and C), which are expressed in inhibitory GABAergic synapses, were unchanged. These data indicate that IL18 deficiency increased the expression of excitatory synaptic proteins in APP/PS1 mice, and suggest that the increased mortality due to seizures may be caused by increased excitatory gene and protein expression. Thus, our results strongly suggest that IL18KO/APP/PS1 mice have enhanced excitatory synaptic function.
Fig. 4.
Immunoblot analysis of synaptosomal proteins shows increased expression of excitatory but not inhibitory synaptic proteins in IL18KO/APP/PS1 mice. (A) Presynaptic proteins including VGluT1, VGAT, VAMP2, synapsin, and synaptophysin. (B) Postsynaptic proteins including postsynaptic density protein (PSD-95), glutamate receptor interacting protein (GRIP), CamKII, and scaffold proteins. (C) Neurotransmitter receptors including NMDA receptors and GABA receptors. Right of A–C shows the quantification of synaptosomal protein expression. Results were normalized to the expression of β-actin and then normalized to WT expression levels. One-way ANOVA test was used for statistical analysis. Asterisks indicate a significant difference between IL18KO/APP/PS1 and various groups (n = 6 per group, ANOVA test, *P < 0.05, **P < 0.01).
IL18KO/APP/PS1 Mice Exhibit Increased Spine Density and Hence Enhanced Basal Excitatory Synaptic Transmission.
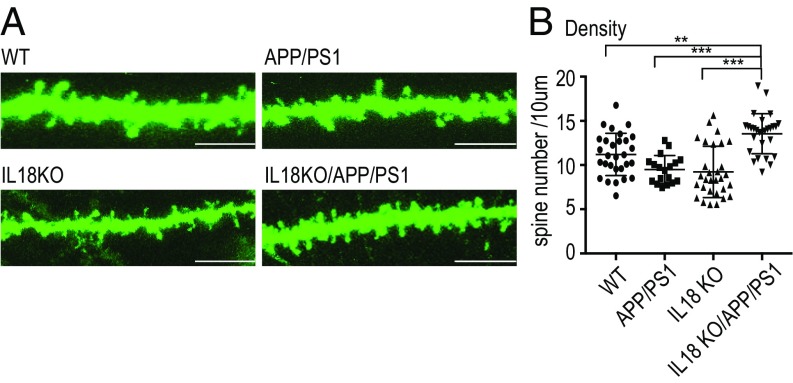
Dendritic spines are the primary postsynaptic sites for excitatory synaptic inputs in most excitatory neurons of the CNS. The density and morphology of the spines correlate with the number of active synapses and glutamate receptors, respectively (33). To address whether the increased expression of excitatory synaptic proteins correlates with a structural change in the spines, we analyzed dendritic morphology in hippocampal CA1 pyramidal neurons. Dendritic segments were visualized by lipophilic tracer (DiO) to assess spine morphology (Fig. 5A). We found a significant increase in spine density, but not in height or width in IL18KO/APP/PS1 mice. Therefore, increased expression of excitatory synaptic protein can be explained by the increase in spine numbers in the hippocampus (Fig. 5B).
Fig. 5.
IL18KO/APP/PS1 mice exhibited increased dendritic spine density in hippocampal CA1 pyramidal neurons. (A) DiO-labeled dendritic segments in CA1 pyramidal neurons from 2.5-mo-old WT, APP/PS1, IL18KO, and IL18KO/APP/PS1 mice. (Scale bar: 5 μm.) (B) Quantification of dendritic spine density from WT, APP/PS1, IL18KO, and IL18KO/APP/PS1 mice. Asterisks indicate a significant difference between IL18KO/APP/PS1 and various groups (ANOVA test, **P < 0.01, ***P < 0.001).
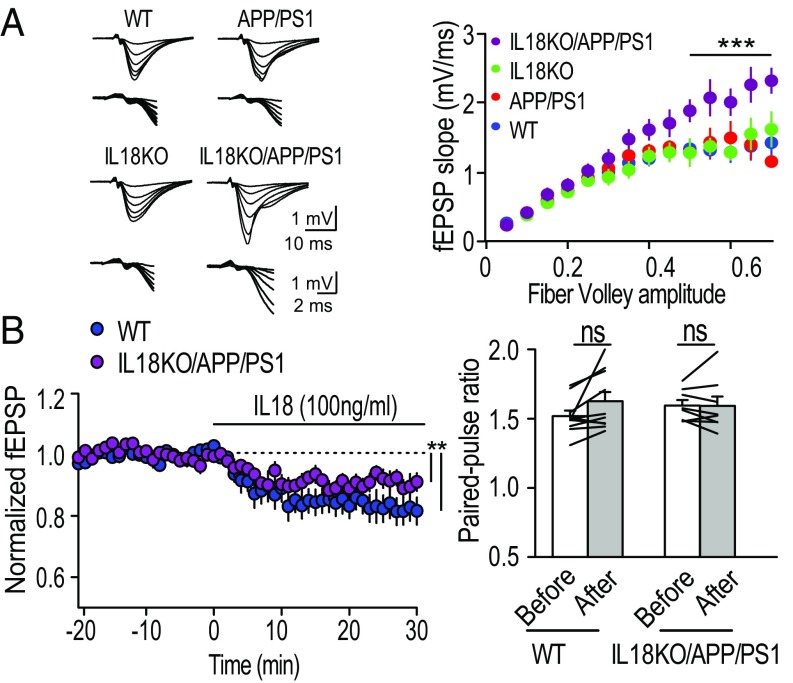
The increased spine density raised the question of whether excitatory neuronal functions were elevated in the hippocampus. We measured field excitatory postsynaptic potentials (fEPSP) in hippocampal CA1 synapses (Fig. 6A). The input–output relationships in IL18KO/APP/PS1 were dramatically increased compared with other genotypes, suggesting that basal excitatory synaptic transmission was elevated in IL18KO/APP/PS1. This is consistent with the increased expression of excitatory synaptic proteins in the synaptosomal fractions (Fig. 4). Paired-pulse facilitation was comparable among genotypes, including IL18KO/APP/PS1 mice, indicating that the presynaptic release probability was not affected (SI Appendix, Fig. S7A).
Fig. 6.
Increased basal excitatory synaptic transmission in IL18KO/APP/PS1 mice. (A, Left) Averaged sample traces (10 events) represent the response evoked with six different stimulus strengths from WT, APP/PS1, IL18KO, or IL18KO/APP/PS1 mice. The same sample traces are shown at two different scales. Stimulus artifacts were truncated. (A, Right) Summary of input–output relationships of field EPSPs at the Schaffer collateral-CA1 synapses. Number of data: WT [n = 15 slices per seven mice (15/7)], APP/PS1 (13/6), IL18KO (12/6), and IL18KO/APP/PS1 (11/6). ***P < 0.0001, two-way ANOVA with Tukey’s multiple comparison test. (B) Decreased excitatory synaptic transmission by IL18. (Left) Sample traces of field EPSPs of WT and IL18KO/APP/PS1 mice recorded at the times indicated in the summary graph. (Right) Summary graph of the averaged time course of recombinant IL18 protein (100 ng/mL). Initial EPSP slopes were measured, and the values were normalized to the averaged slope value measured during the baseline period (−15–0 min). Recombinant mouse IL18 was applied at 0 min. Number of data: WT [n = 10 slices per five mice (10/5)] and IL18KO/APP/PS1 (9/4). **P < 0.01, paired t test. Data shown are means ± SEM. ns, not significant.
Dysfunction of excitatory and inhibitory balance (E–I balance) is one of the hallmarks of epileptogenesis. To address whether excitatory and inhibitory synaptic transmissions are dysregulated in IL18KO/APP/PS1 mice, we performed whole-cell voltage clamp recordings and measured the amplitudes and frequencies of the AMPAR- and GABAAR- mediated miniature excitatory and inhibitory postsynaptic currents (mEPSC and mIPSC) from hippocampal CA1 pyramidal neurons (SI Appendix, Fig. S7 B and C). There was no significant difference in the average mEPSC amplitude between the four genotypes. However, the frequency of the mEPSC from IL18KO/APP/PS1 mutants was significantly increased, suggesting that elevated basal transmission is primarily caused by the increased number of functional excitatory synapses. We found no difference in mIPSC amplitude and frequency, implying that the shift of E–I balance observed in IL18KO/APP/PS1 mice does not alter inhibitory synaptic function. Therefore, elevated excitatory synaptic transmission in IL18KO/APP/PS1 mice led to the development of seizures due to an increased number of active synapses.
Finally, we tested the effect of IL18 in excitatory synaptic transmission to address the mechanism of the protective role of IL18 in neuronal excitability (Fig. 6B). Acute application of recombinant IL18 protein on the hippocampal CA1 area significantly reduced excitatory synaptic transmission in both WT and IL18KO/APP/PS1 mice without changing the presynaptic release probability (Fig. 6B), suggesting that IL18 has a protective role in the brain by reducing excitatory synaptic transmission.
Discussion
Our study resulted from the unpredictable finding that IL18KO/APP/PS1 mice, rather than being protected from the deleterious effects of amyloid in the brain, were instead rendered hypersusceptible to fatal grand mal seizures. This is surprising, given IL18’s numerous proinflammatory effects. Furthermore, IL18 has been reported to suppress LTP in the hippocampus, presumably through its proinflammatory activity (34).
Immune-mediated alteration of neuronal activity leading to fatal seizures is, as far as we know, unprecedented. While neuronal cross-talk with the immune system has been documented, the effects of IL18 deletion were so dramatic that we were unable to identify previous evidence to help understand the phenomena. RNA-seq provided unbiased guidance to sort out the mechanism of the seizure disorder. Among the 75 genes with the greatest fold change between IL18KO and IL18KO/APP/PS1 mice, there was a statistical enrichment of genes involved in various processes, including sleep, neuropeptide signaling pathways, and cell migration. Thus, the broad effects of IL18 deletion in the context of amyloid toxicity (i.e., high levels of circulating Aβ oligomers) were confirmed at a transcriptional level.
Dendritic spine structure and density are positively correlated with synaptic strength and expression of synaptic proteins. Here, we found that deletion of IL18 in APP/PS1 mice increases basal excitatory synaptic transmission in the hippocampus, likely through the increased number of dendritic spines. Our finding highlights the well-known phenotypes in AD mouse models, in which basal synaptic transmission and synaptic plasticity are down-regulated. Epilepsy is one of the phenotypes in several AD models, including APP/PS1 and hAPPJ20 mice (23, 28). More importantly, the risk of epilepsy in AD patients is far higher than age-matched individuals, and reduction of hyperactivity can ameliorate cognitive impairment in both AD patients and mouse models (16, 35). These results indicate that Aβ increases basal excitatory synaptic transmission or LTP (36), suggesting that dysregulation of the neuroinflammasome synergistically potentiates neuronal excitability by Aβ.
IL1β levels are increased during seizures and blocking IL1β signaling ameliorates seizures in rat hippocampal neurons (37). Conversely, applying exogenous IL1β can induce seizure activity (38). Oligomeric Aβ, which we found to be increased in APP/PS1 mice (Fig. 1C), increases IL1β processing in cultured microglia (39). In turn, IL1β enhances neuronal function and circuit activity through activation of NMDAR function via Src kinase (40) and the inhibition of GABA response (41). On the other hand, IL18 has been reported to have an antiinflammatory effect in experimental colitis (42, 43). In fact, the countereffect of IL18 and IL1β has been documented in a mouse model of cerebellar ataxia (44). Importantly, we found that acute application of IL18 protein reduced excitatory synaptic transmission in hippocampus, providing evidence that IL18 has a protective function in neuronal excitability. Thus, we speculate that IL18 directly suppresses these proepileptogenic effects of IL1β in APP/PS1 mice. Importantly, in IL18KO mice, kainate-induced seizures reduced the number of activated microglia and increased the rate of neuronal cell death compared with WT mice (45).
Seizures are an increasingly recognized cause of clinical deterioration in AD. The last few decades have seen the rapid emergence of anticytokine therapies in numerous inflammatory diseases, including diseases that affect the CNS. As a result, there is increasing enthusiasm for the empiric treatment of individuals with inflammatory diseases as new monoclonal Abs and inhibitors are added to the pharmacopeia. Our observations in an AD mouse model suggest that IL18 modulation might negatively affect the course of human AD. This underscores the fact that caution needs to be exercised when engaging in novel therapies against IL18 in AD.
Methods
Mice.
C57BL/6, APP/PS1, IL18, IFNγ, and the IL18R KO mice were from The Jackson Laboratory. IL18BP Tg mice were kindly given by Charles A. Dinarello, University of Colorado Denver, Denver). All KO mice were backcrossed at least 10 generations into C57BL/6 before breeding into APP/PS1. Mice were maintained in specific pathogen-free conditions; mouse protocols were approved by the Institutional Animal Care and Use Committee at University of Massachusetts Medical School.
Drug Treatments.
Levetiracetam (Sequoia Research Products) was diluted in sterile drinking water to achieve a final concentration of 1.8 mg/mL. Water intake was recorded daily. Based on the average weight of mice and the average ingestion of medicated water, mice received about 150 mg/kg of levetiracetam per day. Mice were given levetiracetam orally for 8 wk starting at 6 wk of age. Similarly, mice received oral treatment with 1 mg/kg of SCH23390 for 6 wk beginning at 10 wk of age.
Behavioral Analysis.
Mice were housed individually in polycarbonate cages with minimal bedding and monitored continuously using a digital video camera mounted on one side of the wall. The video camera recorded all events that occurred whenever there was movement of one or more mice. Each mouse was recorded in the cage with 12-h daylight and 12-h dim red light cycle until death occurred or for 2 wk.
Picrotoxin-Induced Seizures.
Ten- to 12-wk-old mice were given 2.0 mg/kg PTX by i.p. injection. Mice were monitored and recorded for 60 min. Seizure behavior and severity were scored using an established rating system (26) with a score of 0 indicating no clinical signs of seizure activity up to a score of 7 indicating death. Time elapsed from injection to the various stages was also recorded.
Reagents and Antibodies.
PTX was obtained from Sigma. Detailed antibody information is in SI Appendix.
Immunoblot Analysis of Synaptosomal Proteins.
Brains from 10-wk-old mice were isolated. Synaptosomal fraction was purified by sucrose-gradient centrifugation from mouse cortex and hippocampus (46). Synaptosomes were lysed in RIPA buffer (Sigma) containing protease and phosphatase inhibitor “mixtures” (Roche) for further analysis. Protein concentrations were determined with the BCA protein assay kit (Thermo Scientific). Equal amounts of protein were loaded onto 10% gels (Invitrogen Bis-Tris), separated by electrophoresis, and then transferred to a PVDF membrane. The signal was visualized with the SuperSignal West Dura Chemiluminescent Substrate (GE).
DiOlistic Morphological Analysis.
Hippocampal neurons from mice were labeled using DiOlistic staining on fixed slices (47). Briefly, mouse brains were fixed with 4% paraformaldehyde (PFA) and cut into 200-μm slices with a vibratome (VT 1200S; Leica). Tungsten particles (1.7 μm in diameter; Bio-Rad) were coated with the lipophilic dye, DiI (Invitrogen). Dye-coated particles were delivered to the acute slices using a hand-held gene gun (Bio-Rad). The secondary dendrite segments were imaged using a Leica SP8X laser-scanning confocal microscope (Zeiss). The spine density, length, and head width of pyramidal cells in the CA1 area were measured.
Immunohistochemistry and Confocal Microscopy.
Mice (2 mo or 9 mo old) were perfused with cold PBS and 4% PFA, followed by incubation in 4% PFA overnight. Sections were cut with a vibratome to a thickness of 40 μm. For staining plaques with methoxy-X04, sections were washed with PBS, incubated with 10 mM methoxy-X04 in 50% DMSO/50% NaCl (0.9%), pH 12 for 10 min, and washed twice with PBS. Sections were imaged with a Leica SP8X laser-scanning confocal microscope.
Electrophysiology.
Mice (2–3 mo old) were anesthetized by isoflurane and then transcardially perfused with 50 mL of ice-cold dissection media containing (in millimoles): 92 N-methyl-d-glucamine (NMDG), 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 Hepes, 11 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2, and 10 MgCl2 gassed with 5% CO2/95% O2, pH 7.4. Transverse acute hippocampal slices (400-μm thickness) were prepared by the vibratome slicer (Leica VT1200S) and incubated in an interface incubation chamber containing extracellular artificial CSF (aCSF) (in millimoles): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 26 NaHCO3, 1 NaH2PO4, 11 glucose, gassed with 5% CO2/95% O2, pH 7.4 for 1 h at 28 °C and maintained at room temperature (26–27 °C) for at least 30 min. The glass pipettes (1.5–2 MΩ) filled with 3 M NaCl and the tungsten bipolar electrode were placed in the stratum radiatum, and the Schaffer collateral/commissural fibers were stimulated at 0.1 Hz (47). Recombinant mouse IL18 protein (100 ng/mL, 9139-IL; R&D) was dissolved in aCSF and bath applied to hippocampal slices. All experiments and the analysis of data were performed in a blind manner. Recordings were performed using a MultiClamp 700B amplifier and Digidata 1322A. The signal was filtered at 10 kHz and digitized at 20 kHz, and data were acquired and analyzed using Clampex 9.2 and Clampfit 10.7 (Molecular Devices).
Statistics.
Values are expressed as mean ± SEM. Statistical significance was set at P < 0.05. Statistics were calculated with Prism software (GraphPad). For two group comparisons, a two-tailed unpaired or paired t test was used. Comparisons of multiple groups were analyzed by one-way analysis of variance ANOVA with Bonferroni’s multiple-comparison test or Dunnett’s test for comparison of all groups with the control group.
Supplementary Material
Acknowledgments
We thank Chunxing Yang and Zuoshang Xu for providing the home cage system and Melanie Trombly for editing the manuscript. Research reported in this publication was supported in part by National Center for Advancing Translational Sciences and National Institute of Neurological Disorders and Stroke of NIH Grant UL1-TR001453 (to D.T.G.), Grant R01NS085215 (to K.F.), and the Riccio Fund for Neuroscience (D.T.G. and K.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. In addition, the work was partially funded by the University of Massachusetts Research Trust Fund. The authors have no competing financial interests.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the ArrayExpress database (accession no. E-MTAB-5668).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801802115/-/DCSupplemental.
References
- 1.Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat Neurosci. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 4.Noble W, Hanger DP, Miller CC, Lovestone S. The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol. 2013;4:83. doi: 10.3389/fneur.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dosunmu R, Wu J, Basha MR, Zawia NH. Environmental and dietary risk factors in Alzheimer’s disease. Expert Rev Neurother. 2007;7:887–900. doi: 10.1586/14737175.7.7.887. [DOI] [PubMed] [Google Scholar]
- 6.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heneka MT, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venegas C, et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature. 2017;552:355–361. doi: 10.1038/nature25158. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutinen EM, Pirttilä T, Anderson G, Salminen A, Ojala JO. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J Neuroinflammation. 2012;9:199. doi: 10.1186/1742-2094-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojala J, et al. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Yu JT, et al. Interleukin-18 promoter polymorphisms and risk of late onset Alzheimer’s disease. Brain Res. 2009;1253:169–175. doi: 10.1016/j.brainres.2008.11.083. [DOI] [PubMed] [Google Scholar]
- 14.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer’s disease: Causes and clinical relevance. Lancet Neurol. 2017;16:311–322. doi: 10.1016/S1474-4422(17)30044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vossel KA, et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol. 2016;80:858–870. doi: 10.1002/ana.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez PE, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci USA. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng M, Sabatini BL, Südhof TC. Synapses and Alzheimer’s disease. Cold Spring Harb Perspect Biol. 2012;4:a005777. doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantuzzi G, et al. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. J Leukoc Biol. 2003;74:889–896. doi: 10.1189/jlb.0503230. [DOI] [PubMed] [Google Scholar]
- 22.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 23.Minkeviciene R, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogl C, Mochida S, Wolff C, Whalley BJ, Stephens GJ. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol Pharmacol. 2012;82:199–208. doi: 10.1124/mol.111.076687. [DOI] [PubMed] [Google Scholar]
- 26.Bateup HS, et al. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 2013;78:510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: Emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 28.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan J, Jones NC, Bush AI, O’Brien TJ, Kwan P. A mouse model of Alzheimer’s disease displays increased susceptibility to kindling and seizure-associated death. Epilepsia. 2015;56:e73–e77. doi: 10.1111/epi.12993. [DOI] [PubMed] [Google Scholar]
- 30.Costa C, et al. Epilepsy, amyloid-β, and D1 dopamine receptors: A possible pathogenetic link? Neurobiol Aging. 2016;48:161–171. doi: 10.1016/j.neurobiolaging.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Futai K, et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: A more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 34.Cumiskey D, Curran BP, Herron CE, O’Connor JJ. A role for inflammatory mediators in the IL-18 mediated attenuation of LTP in the rat dentate gyrus. Neuropharmacology. 2007;52:1616–1623. doi: 10.1016/j.neuropharm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Bakker A, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abramov E, et al. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 37.Vezzani A, et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43:30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 38.Chiavegato A, Zurolo E, Losi G, Aronica E, Carmignoto G. The inflammatory molecules IL-1β and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front Cell Neurosci. 2014;8:155. doi: 10.3389/fncel.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parajuli B, et al. Oligomeric amyloid β induces IL-1β processing via production of ROS: Implication in Alzheimer’s disease. Cell Death Dis. 2013;4:e975. doi: 10.1038/cddis.2013.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viviani B, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Cheng Q, Malik S, Yang J. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther. 2000;292:497–504. [PubMed] [Google Scholar]
- 42.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andoh T, et al. Protective effect of IL-18 on kainate- and IL-1 beta-induced cerebellar ataxia in mice. J Immunol. 2008;180:2322–2328. doi: 10.4049/jimmunol.180.4.2322. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XM, et al. Kainic acid-induced microglial activation is attenuated in aged interleukin-18 deficient mice. J Neuroinflammation. 2010;7:26–34. doi: 10.1186/1742-2094-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamat PK, Kalani A, Tyagi N. Method and validation of synaptosomal preparation for isolation of synaptic membrane proteins from rat brain. MethodsX. 2014;1:102–107. doi: 10.1016/j.mex.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hung AY, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.