Significance
The acid adaptation systems of the pathogen Helicobacter pylori are complex and critical to the survival of this bacterium in the harsh environment of the human stomach. One of the relatively few transcription factors in H. pylori is HpNikR, a metal-responsive protein that controls homeostasis of nickel, an essential nutrient for this organism. In this study, we show that HpNikR also regulates gene expression in direct response to acidic pH. Exposure of H. pylori to acid shock conditions depresses its cytosolic pH, which activates the regulation of multiple promoters by HpNikR. These results demonstrate that HpNikR has a multifaceted response that links nickel homeostasis and acid acclimation, two critical functions in this infectious organism.
Keywords: Helicobacter pylori, acid shock, NikR, nickel
Abstract
Helicobacter pylori is a human pathogen that infects the stomach, where it experiences variable pH. To survive the acidic gastric conditions, H. pylori produces large quantities of urease, a nickel enzyme that hydrolyzes urea to ammonia, which neutralizes the local environment. One of the regulators of urease expression in H. pylori is HpNikR, a nickel-responsive transcription factor. Here we show that HpNikR also regulates urease expression in response to changes in pH, linking acid adaptation and nickel homeostasis. Upon measuring the cytosolic pH of H. pylori exposed to an external pH of 2, similar to the acidic shock conditions that occur in the human stomach, a significant drop in internal pH was observed. This decrease in internal pH resulted in HpNikR-dependent activation of ureA transcription. Furthermore, analysis of a slate of H. pylori genes encoding other acid adaptation or nickel homeostasis components revealed HpNikR-dependent regulation in response to acid shock. This regulation was consistent with pH-dependent DNA binding to the corresponding promoter sequences observed in vitro with purified HpNikR. These results demonstrate that HpNikR can directly respond to changes in cytosolic pH during acid acclimation and illustrate the exquisitely coordinated regulatory networks that support H. pylori infections in the harsh environment of the human stomach.
The human pathogen Helicobacter pylori is a gram-negative bacterium that causes a variety of conditions including gastritis, ulcers, gastric cancer, and mucosa-associated lymphoid tissue lymphoma (1), and is listed as a class I carcinogen by the National Institutes of Health and categorized as a “high priority” on the World Health Organization’s global priority list of antibiotic-resistant bacteria (2, 3). H. pylori is unique because it primarily inhabits the human stomach (4), where it must survive a fluctuating pH that drops as low as 1.8 during digestion (5). However, H. pylori is a neutralophile (6, 7), so to thrive in its ecological niche the bacteria must have robust mechanisms of acid adaptation (8). The focal component of pH homeostasis in H. pylori is urease, a dodecameric nickel enzyme that hydrolyzes host urea into ammonia to neutralize the internal pH as well as the local microenvironment (8).
In addition to mounting a resilient response to variable acid conditions, H. pylori must control the availability and distribution of the nickel ions needed to supply the urease enzyme as well as the [NiFe]-hydrogenase enzyme, which allows it to utilize molecular H2 supplied by other gut bacteria as an energy source (9). The substantial requirement for nickel in the production of these enzymes is a challenge because of the intrinsic cytotoxicity of the metal ions (10). To balance these opposing factors, H. pylori possesses a robust network of metalloregulation and nickel homeostasis systems, with the nickel-responsive transcription factor NikR playing a central role (11, 12). H. pylori NikR (HpNikR) is an activator or repressor of a variety of genes encoding the urease precursor proteins as well as nickel transporters, nickel storage proteins, and others (13–18). HpNikR enacts this activity via nickel-activated DNA binding to the corresponding promoters (18–20). The critical role of HpNikR is further highlighted by the observation that a ∆nikR strain of H. pylori has attenuated colonization in mice (21).
How H. pylori generate a coordinated response to the severe environmental demands is not clear. Transcription in H. pylori is governed by a limited number of factors that appear to control a network of complex, intersecting regulatory circuits (22). As a case in point, HpNikR was implicated in the cascade response to acid in H. pylori (21, 23, 24). In particular, in vitro analysis of HpNikR demonstrated that the protein could selectively bind several DNA promoter sequences in a pH-responsive manner in the absence of nickel ions (25). However, it was unclear if the cytoplasmic pH of H. pylori would undergo a sufficient change to impact the activity of HpNikR, and if this activity was a functional component of H. pylori acid acclimation.
In the present study, whether HpNikR can actively respond when H. pylori are exposed to acidic shock conditions was investigated. Our experiments reveal that the cytosolic pH of H. pylori drops by several units upon acid shock. Quantitative PCR was used to examine the transcription of multiple genes associated with nickel homeostasis and acid adaptation. Transcription of ureA was up-regulated in an HpNikR-dependent fashion in response to acid shock, and six genes exhibited HpNikR-dependent regulation during acid exposure and nickel supplementation: ureA, nikR, amiE, amiF, arsR, and ureG. Furthermore, analysis of HpNikR regulation of the transporter-encoding genes nixA and frpB2 revealed HpNikR-dependent activation at acidic pH, on top of the nickel-responsive repression, suggestive of a complex mechanism of gene regulation by HpNikR during acid acclimation. HpNikR binding to the corresponding DNA promoters at acidic pH in vitro was consistent with the changes in the bacteria, indicating the basis for the pH-responsive transcriptional activity by HpNikR. Altogether, this work demonstrates that an H. pylori cytoplasmic regulator can respond directly to a drop in pH, and affords in HpNikR a link between acid acclimation and nickel homeostasis, two key components of H. pylori survival in the stomach environment.
Results
Determination of Cytosolic pH.
Although there is evidence that the pH of H. pylori may be modulated by acidic growth conditions (26–28), it was not clear how much of that change reaches the cytoplasm, or how quickly acid acclimation can occur. To address this issue, the cytosolic pH was measured in H. pylori exposed to an external pH of 2, to mimic the conditions of the stomach, using a pH-sensing variant of green fluorescent protein called ratiometric pHluorin (RpH) (29). Previous studies have probed the cytoplasmic pH of bacteria by using exogenous indicators, but these methods require additional loading steps to internalize the small molecules, which would compromise the internal state of the cell (26–28). Furthermore, there is evidence that membrane-permeable dyes partition into both the periplasm and cytoplasm of H. pylori, suggesting that these experiments represent measurements of average intracellular pH, rather than cytoplasmic pH specifically (30). In contrast, the RpH sensor has been used to quantify cytosolic pH in other bacterial species, and can allow specific and real-time measurements without additional manipulation (31–33).
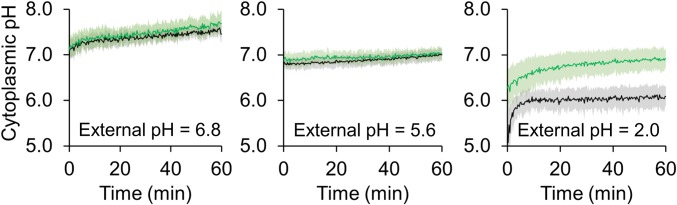
The gene encoding RpH was cloned into an H. pylori expression vector under the control of the constitutive recA promoter (34, 35), and the system was calibrated in a liquid suspension of H. pylori G27 with a collapsed transmembrane pH gradient, as described previously (SI Appendix, Fig. S1) (31). The cytosolic pH of suspensions of wild-type H. pylori G27 was measured in bacteria grown with or without a 10 µM NiSO4 supplement and then exposed to media buffered to decreasing pH values (Fig. 1). Urea concentrations of 50 mM, higher than the physiological concentration of 1 to 15 mM (36), were included to maintain cell viability by ensuring that the supply of urea was not exhausted over the course of the experiment (28). Minor changes to cytosolic pH were observed when the bacteria were exposed to mild acidic conditions of pH 6.8 (Fig. 1, Left), and at an external pH of 5.6 the cell cytoplasm experienced an initial drop to pH 6.8, increasing to pH 7.0 after 1 h (Fig. 1, Center). In both cases, negligible differences were observed between the cytoplasmic pH values of cells grown with or without the nickel supplement.
Fig. 1.
Real-time cytosolic pH changes in H. pylori upon acidic shock. The cytoplasmic pH of H. pylori expressing RpH was determined by measuring the ratio of fluorescence at 508 nm upon excitation at 410 and 470 nm. Fluorescence was measured from a liquid suspension of cells in modified M63 medium at the indicated pH, supplemented with 50 mM urea in the absence (black line) or presence (green line) of 10 µM NiSO4. The data represent the average of six independent biological replicates, with error shown as 1 SD.
In contrast, exposure of H. pylori to an external pH of 2.0 had a dramatic impact on cytosolic pH (Fig. 1, Right). The internal pH initially dropped to about 5.1 and rapidly recovered to a pH of ∼6.0. However, if the growth media were supplemented with nickel, a smaller drop in cytosolic pH was observed. This apparent increase in buffering capacity upon nickel supplementation of H. pylori cultures is likely due to increased nickel uptake (vide infra) and subsequent urease activation (37), suggesting that under these conditions nickel is a limiting intracellular nutrient. These values demonstrate that the cytoplasmic pH of H. pylori decreases during an acid shock similar to that encountered in the human stomach, and indicate that HpNikR would experience an altered pH within the cell.
The cytosolic pH of an isogenic ΔnikR strain expressing RpH was also determined (SI Appendix, Fig. S2). At an external pH of 6.8, the cytosolic pH of the ΔnikR strain was similar to that of the wild-type bacteria. Upon exposure to pH 2.0 conditions, the cytosolic pH of the ΔnikR strain dropped to 6.4 and slowly increased to 6.8; future work will examine why the initial drop in internal pH is not as large as in the wild-type bacteria. Furthermore, in contrast to the wild-type strain, nickel supplementation did not significantly affect the cytosolic pH.
Gene Regulation by HpNikR.
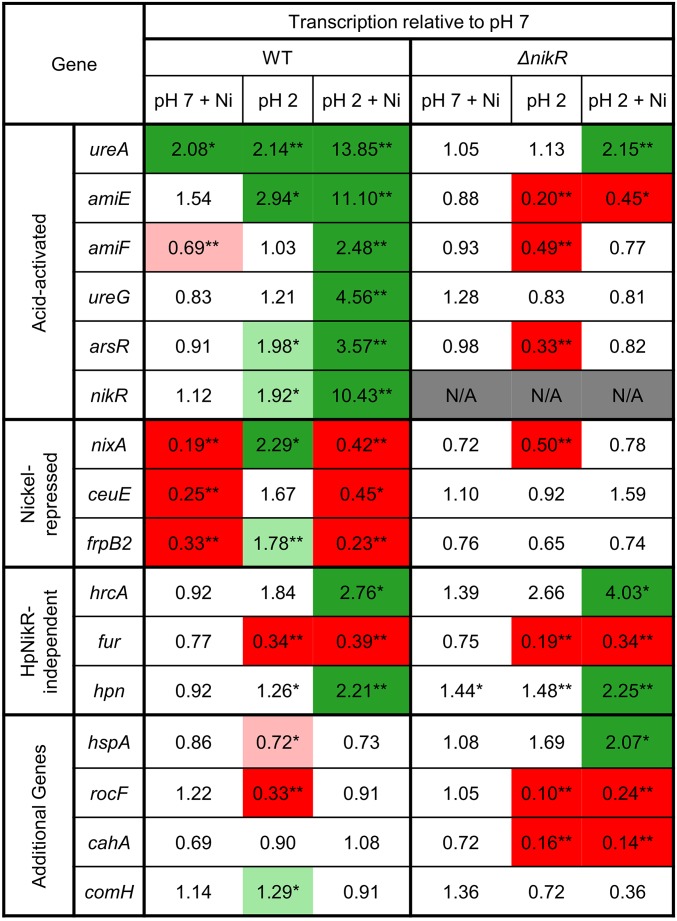
To test if HpNikR modulates gene expression in response to acid shock, H. pylori G27 and the ΔnikR knockout strain were exposed to acidic medium at pH 2 with or without nickel supplementation, and the mRNA levels of multiple known HpNikR targets as well as other genes linked to acid adaptation were monitored by quantitative PCR (Fig. 2 and SI Appendix, Figs. S3 and S4). In total, 16 genes were investigated, and the pattern of changes could be grouped into four broad categories: acid-activated genes, nickel-repressed genes, HpNikR-independent genes, and additional genes ambiguously influenced by HpNikR.
Fig. 2.
Acid- and nickel-induced changes in gene expression. Changes in gene transcription for wild-type and ΔnikR H. pylori grown with or without nickel supplementation following exposure to pH 7 or 2 for 30 min were measured by qPCR. The numbers represent fold changes relative to the levels of mRNA measured at pH 7 in the absence of nickel supplementation for each respective strain. The asterisks indicate changes with statistical significance versus pH 7 (*P < 0.05, **P < 0.01). Boxes shaded green indicate statistically significant up-regulation and red indicates significant down-regulation, and greater than twofold changes are noted by darker shading. Each data point represents the average from at least three independent biological replicates.
Acid-activated genes.
The first group of genes includes ureA, encoding a urease enzyme subunit. HpNikR-dependent up-regulation of ureA was observed upon the addition of nickel (Fig. 2 and SI Appendix, Fig. S3), as expected (15–17, 23, 38). Notably, transcription of ureA was also up-regulated at low pH in the absence of added nickel, and this activation was not observed in the ∆nikR strain, indicating that HpNikR is responding to acidic pH. The strongest activation was induced by the combination of acid shock and nickel supplementation. A significant but much lower level of up-regulation was observed in the ∆nikR strain exposed to pH 2 in the presence of extra nickel, and it is likely that this response partially includes coregulation of ureA by the HpArsR transcription factor (23). In addition to ureA, transcription of amiE, amiF, ureG, arsR, and nikR, encoding an aliphatic amidase, a formamidase, a nickel-binding protein of the urease maturation pathway, the HpArsR acid-responsive transcription factor, and HpNikR itself, respectively, were all significantly up-regulated during acid shock in the presence of nickel. No activation of these genes was detected in the ΔnikR strain undergoing acid shock, and in several cases repression was observed.
Nickel-repressed and acid-activated genes.
The second category includes the nixA, ceuE, and frpB2 genes, representing two inner-membrane nickel importers and one outer-membrane transporter implicated in the uptake of nickel, respectively. Supplementing the medium with nickel caused HpNikR-dependent repression regardless of pH. However, the nickel-induced repression of nixA at pH 2 was significantly less than at pH 7, representing a 2.4-fold decrease versus a 5.3-fold decrease, respectively (SI Appendix, Fig. S4). Similarly, the repression observed for ceuE in the presence of nickel supplementation at pH 2 was smaller than at pH 7 (2.2-fold versus 4-fold). Furthermore, in the absence of added nickel both nixA and frpB2 were up-regulated in wild-type bacteria exposed to acid shock, but not in the ΔnikR strain. To our knowledge, none of these genes have been previously observed to respond to acid (39–41).
HpNikR-independent genes.
The hrcA gene encodes a transcription factor involved in heat shock, stress response, and motility in H. pylori and was previously implicated as a target of HpNikR regulation through a ChIP-seq study (14). However, the patterns of transcriptional changes for hrcA are similar for the wild-type and ΔnikR strains, suggesting that hrcA is not a target of HpNikR under these conditions. Similarly, the impact of acid shock on fur and hpn transcription was independent of HpNikR, which was unexpected given that both of these genes have been previously identified as targets of HpNikR (16, 19), although not in all studies (18), which may be due to the different experimental conditions used.
Additional genes.
The final four genes investigated were hspA, rocF, comH, and cahA, encoding a nickel-binding protein-folding chaperone, an arginase, a periplasmic protein involved in transformation competence, and alpha-carbonic anhydrase, respectively. The hspA gene has been designated a target of HpNikR, previously observed to be up-regulated in the presence of nickel (16). In the present study, mild repression was observed in the wild-type strain at pH 2 and twofold activation was observed in the ∆nikR cells exposed to pH 2 with a nickel supplement. This result suggests that HpNikR is modulating the expression of hspA by blocking activation by another coregulator, but the specific mechanism behind this effect remains unclear.
The rocF gene was examined because it is a known target of HpArsRS (39) and, as expected, strong repression was observed in both the wild-type and ΔnikR bacteria at pH 2. Notably, nickel supplementation abrogated this repression in an HpNikR-dependent fashion. This lack of repression could be a consequence of the impact of nickel on the cytosolic pH (Fig. 1, Right) or the influence of HpNikR on HpArsR activity, and highlights the integrated nature of the regulatory networks in this organism (13, 42). Finally, transcription of the cahA gene and levels of the ComH protein have been reported to be acid-responsive (39, 43), but under these conditions no impact of acid shock was detected in the wild-type cells and acid-dependent repression of cahA was observed in the ΔnikR knockout strain.
Nickel accumulation.
In addition to gene expression, total intracellular nickel was quantified following acidic shock of H. pylori by using inductively coupled plasma mass spectrometry (ICP-MS; SI Appendix, Fig. S5). Both wild-type and ΔnikR bacteria accumulated significantly more nickel when grown in media supplemented with extra nickel, as observed previously in H. pylori and another Helicobacter species (14, 44, 45). However, no significant difference was observed in the nickel content of cells exposed to pH 2 conditions versus pH 7, suggesting that the differences in HpNikR activity upon acidic shock were not the result of altered nickel accumulation.
pH-Responsive DNA Binding by HpNikR.
The transcriptional analysis suggests that HpNikR regulates multiple genes in response to changes in cytoplasmic pH in H. pylori. Previous in vitro studies demonstrated that the promoters of ureA and nikR are bound by HpNikR in a pH-sensitive manner (25), in a pattern consistent with the changes in mRNA levels observed here (Fig. 2, Table 1, and SI Appendix, Fig. S3). To examine if the acid-responsive changes in transcription observed in this study could be explained by direct DNA binding by HpNikR to the other promoters, DNase I footprinting was performed at pH 7.6 and 6.0, which was chosen to reflect the average cytoplasmic pH in H. pylori undergoing acid shock without a nickel supplement (Fig. 1, Right).
Table 1.
HpNikR–DNA interactions determined by DNase I footprinting
| Apo | 1:1 NiSO4 | |||
| Gene | pH 7.6 | pH 6.0 | pH 7.6 | pH 6.0 |
| ureA* | N/D | Binding† | Binding | Binding |
| nikR* | N/D | N/D | N/D | Binding |
| amiE | N/D | Weak‡ | N/D | Binding |
| amiF | N/D | Weak | N/D | Binding |
| nixA | N/D | Binding | Binding | Binding |
| ceuE | N/D | Binding | Binding§ | Binding |
| frpB2 | N/D | Binding | Binding§ | Binding |
See SI Appendix, Table S2 for the values measured. N/D, none detected.
Previously reported (25).
Nanomolar protein required for half-maximal DNA binding.
More than 1 µM protein is required for half-maximal DNA binding.
Previously reported (14).
Although there is some evidence that transcription of amiE and amiF is influenced by HpNikR (21, 24, 46), whether they are directly controlled by HpNikR was not clear, so DNA-binding analysis was performed on both promoters. For the promoter of amiE (PamiE), apo-HpNikR binding was not observed at pH 7.6, and was only weakly detected at pH 6.0 (SI Appendix, Fig. S6). Furthermore, binding by Ni(II)-loaded HpNikR was not detected at pH 7.6 but clear protection was observed at pH 6.0, with a midnanomolar half-maximal DNA affinity (Table 1 and SI Appendix, Fig. S6 and Table S2). The protection region, −37 to −69 relative to the transcription start site (TSS) (46), contains an HpNikR consensus DNA-binding sequence (19, 47). A second protected region was also observed, bound with the same affinity, downstream of the TSS at +15 and +35. A comparable result was observed for the amiF promoter (PamiF); Ni(II)-HpNikR binding was only detected at acidic pH (SI Appendix, Fig. S7). The protected region extended from −18 to +13, overlapping the TSS. Altogether, these results demonstrate that both PamiE and PamiF are direct targets of HpNikR, and the tight DNA affinity only observed for Ni(II)-HpNikR at pH 6.0 is consistent with the HpNikR-dependent activation of these genes in bacteria exposed to acidic shock in the presence of a nickel supplement (Fig. 2).
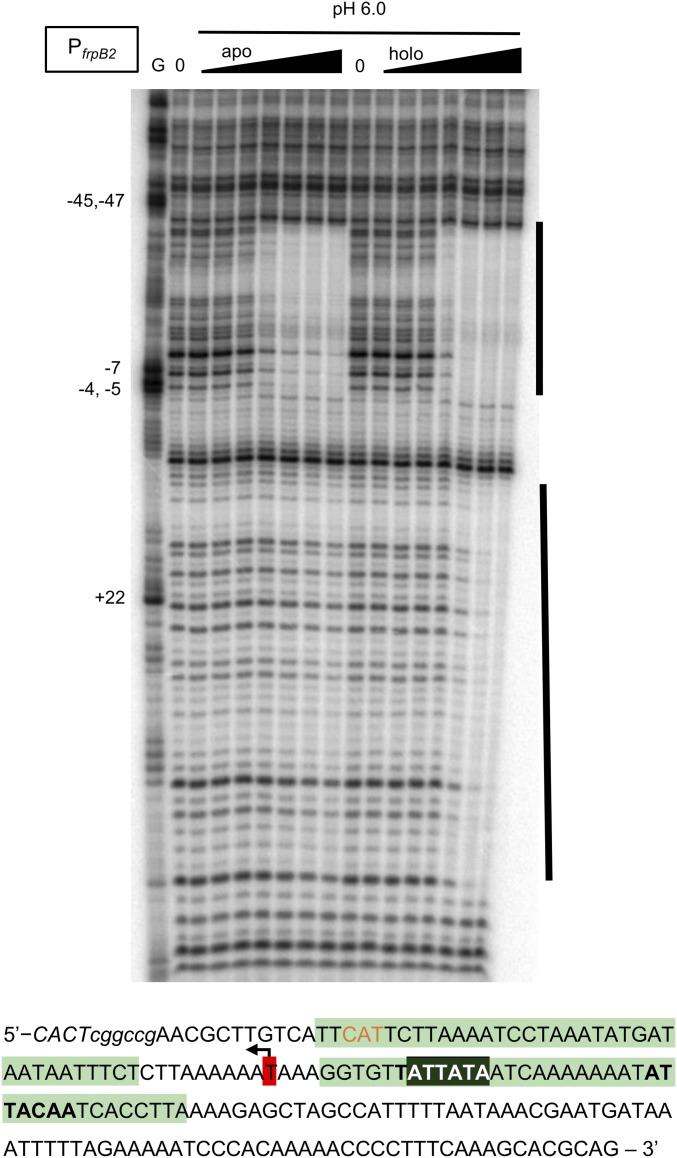
Of the three nickel-repressed genes examined in this study, nixA, ceuE, and frpB2, all three were influenced by acid shock conditions in the presence of HpNikR. In previous DNA-binding experiments performed at pH 7.6, HpNikR was observed to bind all three promoters at a site near or overlapping the transcription start site only if HpNikR was nickel-loaded (Table 1 and SI Appendix, Fig. S8) (14, 48). In contrast, experiments at the lower pH revealed DNA binding by both apo- and nickel-loaded protein (Table 1, Fig. 3, and SI Appendix, Figs. S8 and S9). In the case of the frpB2 promoter, a second HpNikR-binding site was also observed with the nickel-loaded protein at pH 6.0, located +9 downstream of the (putative) transcription start site and 13 bases downstream of the first protection region, overlapping the start codon of the gene. This second protection region was not observed for the apo-protein, but was present for Ni(II)-HpNikR at pH 7.6 (14) and includes a consensus recognition sequence for HpNikR DNA binding (19, 47).
Fig. 3.
HpNikR binding to the frpB2 promoter at pH 6.0. HpNikR binding to a 185-bp DNA probe containing the frpB2 promoter 32P-labeled on the noncoding strand was measured by DNase I footprinting, using apo- or Ni(II)-loaded protein at pH 6.0. Increasing amounts of apo-protein (25 nM to 5 µM) or Ni(II)-loaded protein (12.5 nM to 1 µM) were incubated with the DNA probe for 30 min before digestion by DNase I and analysis on an 8% denaturing urea gel. G indicates a Maxam–Gilbert G reaction, and 0 indicates probe processed without added HpNikR. The black bars indicate regions of protection by HpNikR. The sequence of the frpB2 probe is shown below, with the two regions protected by HpNikR highlighted in green. The Delany consensus sequence (19) within the upstream protection region is shown in bold. Note that the protected regions observed here are identical to the protected regions previously observed for holo-HpNikR at pH 7.6 (14). The putative Pribnow box and transcription start site are shaded in black and highlighted in red, respectively. The orange bases represent the start codon of the gene, and italicized bases represent DNA that does not match the genomic DNA sequence.
Discussion
H. pylori is an unusual component of the human gut microbiome, because it colonizes the acidic environment of the stomach (6, 7). As a pathogen with a specific ecological niche, H. pylori has a compact genome and relatively few regulatory proteins (49). However, to survive under these harsh and highly variable conditions, H. pylori mobilizes a complex and robust acid acclimation response centered around the nickel enzyme urease (50), underscoring the critical link between the need to maintain nickel ion availability and acid adaptation (9, 51). In this study, the results reveal that the activity of the HpNikR transcription factor extends beyond the response to nickel and exerts a direct effect on the regulatory response to acid shock. In this manner, HpNikR coordinates both nickel mobilization and acid acclimation, providing the bacterium with two vital functionalities in one regulator. These results afford insight into the intricate mechanisms of H. pylori acid survival.
The resting pH of the human stomach can reach as low as 1.8 (5), and H. pylori colonies can experience this significantly lower pH during infections (5). H. pylori initiates an acid response when the depression of the periplasmic pH is detected by the HpArsRS two-component system (13, 52), so it was possible that the cytoplasmic pH changed very little during exposure to acidic conditions. The use of a heterologously expressed fluorescent pH sensor protein revealed that the cytoplasmic pH of H. pylori drops to 5.1 when the bacteria are exposed to a medium of pH 2.0. The addition of 10 μM nickel to the growth medium before acidic shock significantly suppressed the initial decrease in pH and enhanced the speed of recovery. While the average concentration of nickel in stomach chyme is currently unknown, supplementation of the cells with additional nickel illustrates the direct impact that nickel has on the ability of H. pylori to restore its cytoplasmic pH following acidic shock. This change may be mediated, at least in part, by enhanced loading of apo-urease by the augmented nickel supply (15, 53), as well as by the activities of HpNikR. Overall, these results demonstrate that, despite the robust acid acclimation systems in H. pylori, the bacteria experience a significant initial drop in cytosolic pH upon acid shock and that nickel is a limiting nutrient during recovery.
Acid shock has a direct impact on transcriptional regulation by HpNikR in the bacteria, and the in vitro experiments suggest that this response is due to pH-responsive DNA binding by the protein. This model is consistent with the whole-cell metal analysis, which indicated that acid shock does not have a dramatic impact on nickel uptake under this time frame. The possibility that the decrease in cytoplasmic pH affects nickel availability cannot be ruled out, but the in vitro studies demonstrate that DNA binding by HpNikR at the lower pH is activated in the absence of nickel. At acidic pH, HpNikR binds to the ureA promoter as an apo-protein as well as a holo-protein (25), and ureA expression was increased under analogous conditions in the cell. The abrogation of these transcriptional changes in the ∆nikR strain suggests that the pH-dependent DNA binding by HpNikR is exerting a quantifiable effect on transcription. In the case of the nikR, amiE, and amiF promoters, strong HpNikR-dependent activation of these genes was observed in H. pylori following acid shock in nickel-supplemented media, consistent with the observation that DNA binding was detected with holo-protein only at acidic pH (Table 1). To date, the only known regulator of nikR is HpNikR itself, so the patterns of DNA binding and transcriptional regulation for PnikR in particular support the relevance of pH-dependent DNA binding by HpNikR in H. pylori.
In addition to acid-sensitive regulation by HpNikR, it is clear that in the context of the living bacteria the activities of HpNikR overlap with those of other regulators (13, 22). Examples include the activation of ureA at pH 2 with nickel and the repression of amiE, amiF, and arsR at pH 2, all in the ΔnikR strain. These results may be due to coregulation by other transcription factors. For instance, arsR and amiE are repressed by HpFur in an iron-dependent manner (42, 54), and ureA, ureG, amiE, and amiF are coactivated by HpArsR (23, 46). Furthermore, arsR is regulated through a complex mechanism involving HpNikR, HpFur, and HpArsR itself, suggesting that the expression of arsR is sensitive to the various stimuli that influence the activities of all three transcription factors (42). These overlapping regulons complicate the interpretation of the global expression data. Nevertheless, the absent or abrogated activation of these genes in the ΔnikR strain indicates that HpNikR is a source of pH-responsive regulation during acid shock. Future work will focus on elucidating the complex interplay between the three major regulatory proteins.
Apo-HpNikR binds the promoters of nixA, ceuE, and frpB2 at acidic pH in vitro, which corresponds to a mild HpNikR-dependent activation for nixA and frpB2 at pH 2. This observation provides a potential mechanism for HpNikR to link nickel uptake to the acidic conditions when urease is needed. A biological effort for increased nickel acquisition and mobilization was previously suggested by apparent changes in Hpn-dependent nickel storage (53) and frpB4-dependent increases in nickel uptake during mildly acidic conditions (55). In addition, these observations add another layer to the simple mechanism of nickel-induced repression observed for these genes under neutral bacterial growth conditions (14, 56), and suggest that the internal pH of the cell can influence the ability of HpNikR to repress or activate a gene, even though the location of the binding site is the same. Acid-dependent changes to HpNikR regulatory activity are also illustrated by the smaller magnitude of nickel-induced repression observed for nixA and ceuE during acidic shock versus neutral conditions. The milder impact on ceuE expression during acid shock without nickel supplementation, despite apo-HpNikR binding PceuE under analogous conditions in vitro, implies that the degree of regulatory activity exerted by HpNikR during acid adaptation is also promoter-specific. Furthermore, there is no direct relationship between the location of HpNikR binding relative to the TSS and whether HpNikR activates or represses transcription, which in H. pylori is not a phenomenon unique to HpNikR (42, 48, 57).
How changes in pH modulate the DNA-binding activity by HpNikR and the subsequent impact on transcription is not yet clear. Previous in vitro analysis demonstrated that the lower pH does not impact the secondary or quaternary structures of the protein, and that nanomolar nickel binding is maintained (25). The N-terminal regions of NikR homologs are variable in length, and in HpNikR there is an extended sequence containing several acidic residues that play a significant role in the specific recognition of DNA promoters and the pH sensitivity of HpNikR (25, 58). An additional level of regulation may be applied by having more than one HpNikR-binding site on the promoters, as observed for PfrpB2, PamiE, and ParsR (vide supra) (42). Furthermore, given the change in cytoplasmic pH it is likely that there are other biochemical impacts on transcription, such as changes in the protein–protein interactions at the various promoters, pH-dependent activation of the cytosolic FlgS sensor kinase, or more global effects on RNA polymerase or DNA architecture (41, 42, 59, 60).
Methods
For details on plasmid preparation, standard culturing protocols for H. pylori, ICP-MS, and DNase I footprinting, please see SI Appendix.
Determination of H. pylori Cytosolic pH.
H. pylori wild-type or ∆nikR G27 was transformed with the pTMRpH plasmid (31) and cultured with 50 μg/mL kanamycin. To construct the calibration curve of pH versus observed fluorescence, liquid cultures were spun down at 8,000 × g and the cells were resuspended in buffered M63AC medium (29), which is a modified M63 minimal medium supplemented with 10 g/L tryptone, 40 mM each sodium benzoate and methylamine hydrochloride and buffered using a 50 mM citric acid/sodium phosphate dibasic buffer system (26). The medium was filtered through a 0.2-μm syringe filter, degassed by vacuum, and backfilled with microaerophilic gas mixture (85% N2, 10% CO2, 5% O2). Filtration and gas exchange did not significantly affect the final pH. Pelleted cells were resuspended to an OD600 of 0.4 and plated in duplicate into sterile black NUNC 96-well plates (Bio-Rad). Fluorescence at 508 nm was measured with excitation wavelengths of 410 and 470 nm at room temperature under ambient atmosphere using a CLARIOstar Fluorescence Plate Reader (BMG Labtech), with M63AC at the corresponding pH measured as background. Fluorescence was monitored over 30 min to ensure that the signals reached equilibrium. The 410/470 ratio was calculated as the average ratio for the final 5 min of the measurement from at least three biological replicates. The plot of the average 410/470 ratios versus external pH was fitted against the Boltzmann formula to construct the calibration curve.
Cytosolic pH was measured in a similar fashion as above but with M63AU medium, which was M63AC containing 50 mM urea and no benzoate or methylamine. Cell pellets were resuspended to OD600 0.4 and immediately plated in technical duplicates, and the fluorescence was monitored upon excitation at 410 and 470 nm over time at room temperature and ambient atmosphere. The dead time in-between cell resuspension and the beginning of the fluorescence measurements was typically 2 to 3 min.
H. pylori Acid Shock.
Acid exposures of H. pylori were performed by diluting cell cultures grown to midlog phase (OD600 0.5 to 0.9) to an OD600 of 0.1 in 10 mL of acid-precipitated medium (APM) at pH 7 or 2 for 30 min at room temperature. To prepare APM, Brucella broth buffered with 100 mM sodium citrate was pH-corrected to 7 or 2 with HCl and then autoclaved. The sterile buffered media were then supplemented with 10% FBS, 50 mM urea, 10 μg/mL vancomycin, 5 μg/mL cefsulodin B, and optionally 10 μM NiSO4 before being centrifuged at 3,600 × g for 15 min to pellet any precipitated protein. The supernatant was removed, degassed under vacuum, backfilled with microaerophilic gas mixture, and then used fresh for each acid exposure.
Following 30 min of acid exposure, the bacteria were pelleted at 3,600 × g for 10 min and then frozen in liquid nitrogen following removal of the supernatant and stored at −80 °C. Viability tests were performed following acid exposure as previously described (14), and no differences were detected between the strains following acid shock. RNA isolation by TRIzol reagent and reverse-transcription and quantitative PCR were performed as previously described (14) with the sequences of the primers used in the qPCR experiments listed in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
We thank Prof. Nicola Jones and Dr. Lisa Willis for their advice on culturing H. pylori, Prof. Peter Chivers for the ∆nikR H. pylori, and Dr. Beth Carpenter for the pTM117 expression vector. This research was funded by operating grants from the Canadian Institutes of Health Research and the Natural Science and Engineering Research Council of Canada, as well as an Ontario Graduate Scholarship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808393115/-/DCSupplemental.
References
- 1.Sibony M, Jones NL. Recent advances in Helicobacter pylori pathogenesis. Curr Opin Gastroenterol. 2012;28:30–35. doi: 10.1097/MOG.0b013e32834dda51. [DOI] [PubMed] [Google Scholar]
- 2.Tacconelli E, Magrini N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. WHO; Geneva: 2017. [Google Scholar]
- 3.Yamada T, et al. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 4.Cho I, Blaser MJ. The human microbiome: At the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley EMM, Turnberg LA. pH of the microclimate lining human gastric and duodenal mucosa in vivo. Studies in control subjects and in duodenal ulcer patients. Gastroenterology. 1987;92:1876–1884. doi: 10.1016/0016-5085(87)90619-6. [DOI] [PubMed] [Google Scholar]
- 6.Morgan DR, Freedman R, Depew CE, Kraft WG. Growth of Campylobacter pylori in liquid media. J Clin Microbiol. 1987;25:2123–2125. doi: 10.1128/jcm.25.11.2123-2125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjöström JE, Larsson H. Factors affecting growth and antibiotic susceptibility of Helicobacter pylori: Effect of pH and urea on the survival of a wild-type strain and a urease-deficient mutant. J Med Microbiol. 1996;44:425–433. doi: 10.1099/00222615-44-6-425. [DOI] [PubMed] [Google Scholar]
- 8.Ansari S, Yamaoka Y. Survival of Helicobacter pylori in gastric acidic territory. Helicobacter. 2017;22:e12386. doi: 10.1111/hel.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benoit SL, Maier RJ. Hydrogen and nickel metabolism in Helicobacter species. Ann N Y Acad Sci. 2008;1125:242–251. doi: 10.1196/annals.1419.014. [DOI] [PubMed] [Google Scholar]
- 10.Macomber L, Hausinger RP. Mechanisms of nickel toxicity in microorganisms. Metallomics. 2011;3:1153–1162. doi: 10.1039/c1mt00063b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones MD, Sydor AM, Zamble DB. Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons; Hoboken, NJ: 2011. Nickel homeostasis: Mechanism and function of NikR; pp. 1–11. [Google Scholar]
- 12.de Reuse H, Vinella D, Cavazza C. Common themes and unique proteins for the uptake and trafficking of nickel, a metal essential for the virulence of Helicobacter pylori. Front Cell Infect Microbiol. 2013;3:94. doi: 10.3389/fcimb.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danielli A, Scarlato V. Regulatory circuits in Helicobacter pylori: Network motifs and regulators involved in metal-dependent responses. FEMS Microbiol Rev. 2010;34:738–752. doi: 10.1111/j.1574-6976.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones MD, Ademi I, Yin X, Gong Y, Zamble DB. Nickel-responsive regulation of two novel Helicobacter pylori NikR-targeted genes. Metallomics. 2015;7:662–673. doi: 10.1039/c4mt00210e. [DOI] [PubMed] [Google Scholar]
- 15.van Vliet AH, et al. NikR mediates nickel-responsive transcriptional induction of urease expression in Helicobacter pylori. Infect Immun. 2002;70:2846–2852. doi: 10.1128/IAI.70.6.2846-2852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller C, et al. Hierarchical regulation of the NikR-mediated nickel response in Helicobacter pylori. Nucleic Acids Res. 2011;39:7564–7575. doi: 10.1093/nar/gkr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contreras M, Thiberge JM, Mandrand-Berthelot MA, Labigne A. Characterization of the roles of NikR, a nickel-responsive pleiotropic autoregulator of Helicobacter pylori. Mol Microbiol. 2003;49:947–963. doi: 10.1046/j.1365-2958.2003.03621.x. [DOI] [PubMed] [Google Scholar]
- 18.Vannini A, et al. Comprehensive mapping of the Helicobacter pylori NikR regulon provides new insights in bacterial nickel responses. Sci Rep. 2017;7:45458. doi: 10.1038/srep45458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delany I, et al. In vitro analysis of protein-operator interactions of the NikR and Fur metal-responsive regulators of coregulated genes in Helicobacter pylori. J Bacteriol. 2005;187:7703–7715. doi: 10.1128/JB.187.22.7703-7715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dosanjh NS, West AL, Michel SL. Helicobacter pylori NikR’s interaction with DNA: A two-tiered mode of recognition. Biochemistry. 2009;48:527–536. doi: 10.1021/bi801481j. [DOI] [PubMed] [Google Scholar]
- 21.Bury-Moné S, et al. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol Microbiol. 2004;53:623–638. doi: 10.1111/j.1365-2958.2004.04137.x. [DOI] [PubMed] [Google Scholar]
- 22.Haley KP, Gaddy JA. Metalloregulation of Helicobacter pylori physiology and pathogenesis. Front Microbiol. 2015;6:911. doi: 10.3389/fmicb.2015.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter BM, et al. Crosstalk between the HpArsRS two-component system and HpNikR is necessary for maximal activation of urease transcription. Front Microbiol. 2015;6:558. doi: 10.3389/fmicb.2015.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Vliet AHM, Kuipers EJ, Stoof J, Poppelaars SW, Kusters JG. Acid-responsive gene induction of ammonia-producing enzymes in Helicobacter pylori is mediated via a metal-responsive repressor cascade. Infect Immun. 2004;72:766–773. doi: 10.1128/IAI.72.2.766-773.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Zamble DB. pH-responsive DNA-binding activity of Helicobacter pylori NikR. Biochemistry. 2009;48:2486–2496. doi: 10.1021/bi801742r. [DOI] [PubMed] [Google Scholar]
- 26.Stingl K, Uhlemann EM, Schmid R, Altendorf K, Bakker EP. Energetics of Helicobacter pylori and its implications for the mechanism of urease-dependent acid tolerance at pH 1. J Bacteriol. 2002;184:3053–3060. doi: 10.1128/JB.184.11.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus EA, Moshfegh AP, Sachs G, Scott DR. The periplasmic α-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J Bacteriol. 2005;187:729–738. doi: 10.1128/JB.187.2.729-738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stingl K, et al. Prolonged survival and cytoplasmic pH homeostasis of Helicobacter pylori at pH 1. Infect Immun. 2001;69:1178–1180. doi: 10.1128/IAI.69.2.1178-1180.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 30.Athmann C, et al. Local pH elevation mediated by the intrabacterial urease of Helicobacter pylori cocultured with gastric cells. J Clin Invest. 2000;106:339–347. doi: 10.1172/JCI9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez KA, et al. Cytoplasmic pH response to acid stress in individual cells of Escherichia coli and Bacillus subtilis observed by fluorescence ratio imaging microscopy. Appl Environ Microbiol. 2012;78:3706–3714. doi: 10.1128/AEM.00354-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol. 2009;55:1–79. doi: 10.1016/S0065-2911(09)05501-5. [DOI] [PubMed] [Google Scholar]
- 33.Yaginuma H, et al. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci Rep. 2014;4:6522. doi: 10.1038/srep06522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter BM, et al. Expanding the Helicobacter pylori genetic toolbox: Modification of an endogenous plasmid for use as a transcriptional reporter and complementation vector. Appl Environ Microbiol. 2007;73:7506–7514. doi: 10.1128/AEM.01084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orillard E, Radicella JP, Marsin S. Biochemical and cellular characterization of Helicobacter pylori RecA, a protein with high-level constitutive expression. J Bacteriol. 2011;193:6490–6497. doi: 10.1128/JB.05646-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blusiewicz K, Rydzewska G, Rydzewski A. Gastric juice ammonia and urea concentrations and their relation to gastric mucosa injury in patients maintained on chronic hemodialysis. Rocz Akad Med Bialymst. 2005;50:188–192. [PubMed] [Google Scholar]
- 37.Wolfram L, Bauerfeind P. Activities of urease and nickel uptake of Helicobacter pylori proteins are media- and host-dependent. Helicobacter. 2009;14:264–270. doi: 10.1111/j.1523-5378.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- 38.Ernst FD, et al. The nickel-responsive regulator NikR controls activation and repression of gene transcription in Helicobacter pylori. Infect Immun. 2005;73:7252–7258. doi: 10.1128/IAI.73.11.7252-7258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loh JT, Gupta SS, Friedman DB, Krezel AM, Cover TL. Analysis of protein expression regulated by the Helicobacter pylori ArsRS two-component signal transduction system. J Bacteriol. 2010;192:2034–2043. doi: 10.1128/JB.01703-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merrell DS, Goodrich ML, Otto G, Tompkins LS, Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect Immun. 2003;71:3529–3539. doi: 10.1128/IAI.71.6.3529-3539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen Y, et al. Acid-adaptive genes of Helicobacter pylori. Infect Immun. 2003;71:5921–5939. doi: 10.1128/IAI.71.10.5921-5939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roncarati D, et al. Metal-responsive promoter DNA compaction by the ferric uptake regulator. Nat Commun. 2016;7:12593. doi: 10.1038/ncomms12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen Y, Feng J, Scott DR, Marcus EA, Sachs G. The HP0165-HP0166 two-component system (ArsRS) regulates acid-induced expression of HP1186 α-carbonic anhydrase in Helicobacter pylori by activating the pH-dependent promoter. J Bacteriol. 2007;189:2426–2434. doi: 10.1128/JB.01492-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benoit SL, Seshadri S, Lamichhane-Khadka R, Maier RJ. Helicobacter hepaticus NikR controls urease and hydrogenase activities via the NikABDE and HH0418 putative nickel import proteins. Microbiology. 2013;159:136–146. doi: 10.1099/mic.0.062976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schauer K, et al. The Helicobacter pylori GroES cochaperonin HspA functions as a specialized nickel chaperone and sequestration protein through its unique C-terminal extension. J Bacteriol. 2010;192:1231–1237. doi: 10.1128/JB.01216-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pflock M, et al. Characterization of the ArsRS regulon of Helicobacter pylori, involved in acid adaptation. J Bacteriol. 2006;188:3449–3462. doi: 10.1128/JB.188.10.3449-3462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoof J, Kuipers EJ, van Vliet AH. Characterization of NikR-responsive promoters of urease and metal transport genes of Helicobacter mustelae. Biometals. 2010;23:145–159. doi: 10.1007/s10534-009-9275-7. [DOI] [PubMed] [Google Scholar]
- 48.Delany I, Pacheco ABF, Spohn G, Rappuoli R, Scarlato V. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. J Bacteriol. 2001;183:4932–4937. doi: 10.1128/JB.183.16.4932-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Reuse H, Bereswill S. Ten years after the first Helicobacter pylori genome: Comparative and functional genomics provide new insights in the variability and adaptability of a persistent pathogen. FEMS Immunol Med Microbiol. 2007;50:165–176. doi: 10.1111/j.1574-695X.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 50.Fischer W, Prassl S, Haas R. 2009. Virulence mechanisms and persistence strategies of the human gastric pathogen Helicobacter pylori. Molecular Mechanisms of Bacterial Infection via the Gut, Current Topics in Microbiology and Immunology, ed Sasakawa C (Springer, Heidelberg), Vol 337, pp 129–171. [DOI] [PubMed]
- 51.de Reuse H. 2017. Nickel and virulence in bacterial pathogens. The Biological Chemistry of Nickel, Metallobiology, eds Zamble DB, Rowińska-Żyrek M, Kozlowski H (The Royal Society of Chemistry, London), pp 339–356.
- 52.Pflock M, Kennard S, Finsterer N, Beier D. Acid-responsive gene regulation in the human pathogen Helicobacter pylori. J Biotechnol. 2006;126:52–60. doi: 10.1016/j.jbiotec.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 53.Seshadri S, Benoit SL, Maier RJ. Roles of His-rich Hpn and Hpn-like proteins in Helicobacter pylori nickel physiology. J Bacteriol. 2007;189:4120–4126. doi: 10.1128/JB.01245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Vliet AH, et al. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori Fur repressor. J Biol Chem. 2003;278:9052–9057. doi: 10.1074/jbc.M207542200. [DOI] [PubMed] [Google Scholar]
- 55.Schauer K, Gouget B, Carrière M, Labigne A, de Reuse H. Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery. Mol Microbiol. 2007;63:1054–1068. doi: 10.1111/j.1365-2958.2006.05578.x. [DOI] [PubMed] [Google Scholar]
- 56.Wolfram L, Haas E, Bauerfeind P. Nickel represses the synthesis of the nickel permease NixA of Helicobacter pylori. J Bacteriol. 2006;188:1245–1250. doi: 10.1128/JB.188.4.1245-1250.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pflock M, Kennard S, Delany I, Scarlato V, Beier D. Acid-induced activation of the urease promoters is mediated directly by the ArsRS two-component system of Helicobacter pylori. Infect Immun. 2005;73:6437–6445. doi: 10.1128/IAI.73.10.6437-6445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benanti EL, Chivers PT. The N-terminal arm of the Helicobacter pylori Ni2+-dependent transcription factor NikR is required for specific DNA binding. J Biol Chem. 2007;282:20365–20375. doi: 10.1074/jbc.M702982200. [DOI] [PubMed] [Google Scholar]
- 59.Nedialkov YA, Burton ZF. Translocation and fidelity of Escherichia coli RNA polymerase. Transcription. 2013;4:136–143. doi: 10.4161/trns.25527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen Y, Feng J, Scott DR, Marcus EA, Sachs G. The pH-responsive regulon of HP0244 (FlgS), the cytoplasmic histidine kinase of Helicobacter pylori. J Bacteriol. 2009;191:449–460. doi: 10.1128/JB.01219-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.