Significance
Guard cells have photosynthetically active chloroplasts in most plant species. However, the significance of their existence in guard cells or their developmental mechanisms is unknown. Here, through a forward-genetic approach, we have identified a key feature and a function of guard cell chloroplasts. We observed that a mutation that impaired chloroplast biogenesis in guard cells also disrupted the regulation of stomatal movements by CO2 and light. We demonstrated that guard cell chloroplasts, compared with those in mesophyll cells, display a unique lipid metabolism, in which the prokaryotic pathway is diminished and the eukaryotic pathway gains control. Our findings highlight the importance of the eukaryotic pathway for developing functional chloroplasts in guard cells.
Keywords: stomata, chloroplast, lipid metabolism, CO2, Arabidopsis
Abstract
Stomatal guard cells develop unique chloroplasts in land plant species. However, the developmental mechanisms and function of chloroplasts in guard cells remain unclear. In seed plants, chloroplast membrane lipids are synthesized via two pathways: the prokaryotic and eukaryotic pathways. Here we report the central contribution of endoplasmic reticulum (ER)-derived chloroplast lipids, which are synthesized through the eukaryotic lipid metabolic pathway, in the development of functional guard cell chloroplasts. We gained insight into this pathway by isolating and examining an Arabidopsis mutant, gles1 (green less stomata 1), which had achlorophyllous stomatal guard cells and impaired stomatal responses to CO2 and light. The GLES1 gene encodes a small glycine-rich protein, which is a putative regulatory component of the trigalactosyldiacylglycerol (TGD) protein complex that mediates ER-to-chloroplast lipid transport via the eukaryotic pathway. Lipidomic analysis revealed that in the wild type, the prokaryotic pathway is dysfunctional, specifically in guard cells, whereas in gles1 guard cells, the eukaryotic pathway is also abrogated. CO2-induced stomatal closing and activation of guard cell S-type anion channels that drive stomatal closure were disrupted in gles1 guard cells. In conclusion, the eukaryotic lipid pathway plays an essential role in the development of a sensing/signaling machinery for CO2 and light in guard cell chloroplasts.
Stomatal pores allow an influx of CO2 in exchange for transpirational water loss. The stomatal aperture is regulated by environmental and physiological factors, especially CO2, the plant hormone abscisic acid (ABA), humidity, light, and ozone (1–4). Chloroplasts in the guard cells of stomata have been proposed to play an important role in osmoregulatory mechanisms mediating stomatal movements (5, 6), although their functions have been a subject of debate. To date, studies on guard cell chloroplasts have largely focused on their photosynthetic activities (7–9), whereas the relevance of lipid synthesis remains poorly investigated.
Chloroplast development accompanies the biogenesis of thylakoid membranes, which requires the coordinated synthesis of membrane proteins and glycerolipids. The thylakoid membranes consist of the glycolipids monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), and sulfoquinovosyldiacylglycerol and the phospholipid phosphatidylglycerol (PG). Fatty acids are exclusively synthesized de novo within plastids, but the assembly of fatty acids into the glycerolipids of thylakoid membranes occurs via two distinct pathways: the prokaryotic pathway and the eukaryotic pathway (10–12). In the prokaryotic pathway, all reaction steps take place within the chloroplast (hence called the plastidial pathway), whereas in the eukaryotic pathway or the cooperative pathway, fatty acids are exported from the chloroplast to the cytosol to be assembled into glycerolipids in the endoplasmic reticulum (ER). Some of the ER-localized glycerolipids return to the chloroplast to serve as a substrate for glycolipid synthesis (10–12) (SI Appendix, Fig. S1). The prokaryotic pathway contributes to chloroplast PG synthesis in all plants, and to glycolipid synthesis only in the so-called 16:3 plants, which contain 16:3 (acyl carbons: double bonds) in the sn-2 position of MGDG. In contrast, the eukaryotic pathway contributes to the glycolipid synthesis in all plants. MGDG synthesized via the eukaryotic pathway contains C18 fatty acids, most abundantly 18:3, in the sn-2 position, and those plants, including pea (Pisum sativum) and rice (Oryza sativa), that exclusively use the eukaryotic pathway in plastid glycolipid synthesis have been referred to as 18:3 plants. In 16:3 plants, the contribution of the prokaryotic and the eukaryotic pathways varies among different plant species (13). The contribution of the lipid flux through the prokaryotic pathway is the highest in nonseed plants, such as mosses and ferns, whereas it has been estimated as 38% in Arabidopsis (14). Moreover, even in the same16:3 plant species, the prokaryotic and the eukaryotic pathways do not necessarily work at a fixed proportion in all tissues. For example, in Arabidopsis, the prokaryotic pathway appears to be strongly diminished during embryo development (15–18). Although each of the prokaryotic and the eukaryotic pathways produces lipid molecular species with distinct fatty acid distribution, the physiological significance of such metabolic divergence remains unclear.
The eukaryotic pathway requires lipid transfer from the ER to chloroplasts. The trigalactosyldiacylglycerol (TGD) protein complex has been identified as playing a major role in the lipid transfer from the ER to chloroplasts (19). The TGD protein complex itself is an ABC-type transporter consisting of three subunits: TGD1, TGD2, and TGD3. TGD1 is an ABC transporter permease located in the inner envelope membranes of chloroplast, TGD3 is an ATPase or a nucleotide-binding protein facing to stroma, and TGD2 is a substrate-binding protein, which is suggested to bind a lipid substrate in outer envelope membranes to deliver it to TGD1. An additional subunit, TGD4, is located in outer envelope membranes to play a tethering role between the ER and the chloroplast envelope (20, 21). Furthermore, TGD5, a small glycine-rich protein, has been hypothesized to play a regulatory role in the ER-to-chloroplast lipid transfer (22).
To date, the studies of lipid metabolism in guard cells have been confined to an 18:3 plant species, Vicia faba (23, 24). Using [14C] acetate labeling, guard cell protoplasts from V. faba have been shown to produce eukaryotic lipid molecular species (23). Guard cells are known to contain a large amount of the triacylglycerols produced by the eukaryotic lipid metabolic pathway (24). Recently, it has been reported that triacylglycerols stored in guard cells are used to produce ATP required for light-induced stomatal opening (25). However, the distinct roles of prokaryotic and eukaryotic lipid metabolic pathways in guard cells have not been understood.
In this study, we have found, through a forward-genetic approach, that lipid synthesis in guard cells is distinct from that in mesophyll cells, and that the prokaryotic pathway is extensively retarded in Arabidopsis guard cells. As a consequence, lipid transfer from ER to chloroplast through the eukaryotic pathway gains more significance and seems essential for guard cell chloroplast development and for stomatal CO2 and light responses in Arabidopsis guard cells.
Results and Discussion
Isolation of Arabidopsis gles1 Mutant That Develops Abnormal Chloroplasts in Guard Cells.
Previously, we isolated a CO2-insensitive mutant line (cdi6) from an M2 population of ∼30,000 ethyl methanesulfonate-mutagenized Arabidopsis plants, using leaf infrared imaging thermography (3). This technology enabled us to isolate a number of mutants that showed abnormal leaf temperature resulting from malfunction in stomatal movement (3). The cdi6 mutant line showed two phenotypes [irregularly shaped stomata (26) and achlorophyllous stomata], but these phenotypes were segregated by backcrossing with WT. In this study, we separated a recessive mutation responsible for achlorophyllous stomata from the cdi6 line and designated it as gles1. Although WT plants displayed normal chlorophyll fluorescence in guard cells and mesophyll cells (Fig. 1A), gles1 exhibited reduced chlorophyll fluorescence specifically in some guard cells (Fig. 1A): gles1 mutants developed different types of stomata with differentially reduced chlorophyll fluorescence, which were categorized as achlorophyllous (SI Appendix, Fig. S2A, type I; 12.2 ± 1.2%), faintly chlorophyllus (SI Appendix, Fig. S2A, type II; 70.0 ± 1.5%), and chlorophyllous stomata (SI Appendix, Fig. S2A, type III; 17.8 ± 1.5%). The fluorescence values differed substantially from WT controls (SI Appendix, Fig. S2A). Similar trends were also observed in guard cell protoplasts (GCPs) isolated from WT and gles1 using flow cytometry. Chlorophyll fluorescence decreased in more than 70% gles1 GCPs (SI Appendix, Fig. S2B).
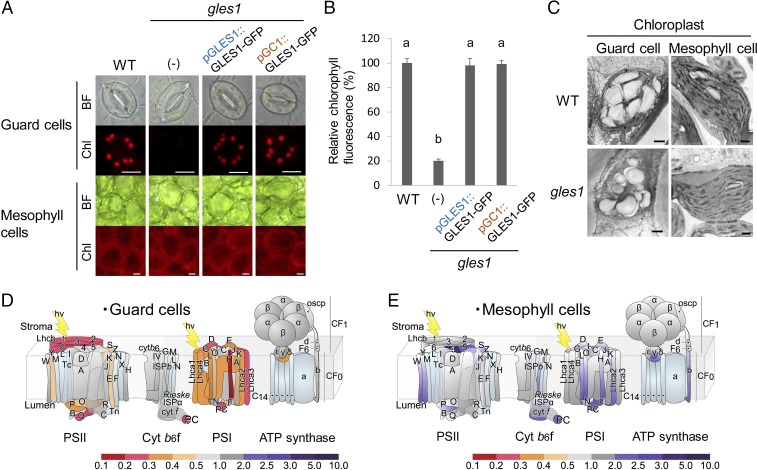
Fig. 1.
Mutation in GLES1 impairs chloroplast development in guard cells. (A) Guard cells in WT contain well-developed chloroplasts, but the imaged gles1 stomata have a negligible amount of chloroplasts and chlorophyll autofluorescence in their guard cells. In contrast, the gles1 mutant has normal chlorophyllous mesophyll cells. (Scale bars, 10 μm.) These gles1 phenotypes were restored in transformants expressing GLES1-GFP driven by GLES1’s own promoter or guard cell-specific GC1 promoter. BF, Bright Field; Chl, Chlorophyll fluorescence. Percentage relative chlorophyll fluorescence values ± SE (n > 100) are shown in B. The statistical significance was determined by a one-way ANOVA with Tukey–Kramer multiple comparison tests. Same letters (a and b) indicate no significant difference (P > 0.05). (C) Ultrastructure of plastids in WT and gles1 mutant plants. Mutant chloroplasts are as developed as WT chloroplasts in mesophyll cells. However, they fail to develop thylakoid membranes in guard cell. (Scale bars, 10 μm.) (D and E) Schematic view of gene expression of thylakoid-associated photosynthetic components integrated with microarray data. Fold-changes were calculated as gles1/WT control and are shown as a heat map. In achlorophyllous gles1 guard cell protoplasts that were sorted by FACS (SI Appendix, Fig. S2B: type I), the expression of photosynthesis genes is significantly down-regulated (D). In contrast, the expression of these genes is not down-regulated in the gles1 mesophyll cell protoplasts (E). Note that only transcripts for nuclear-encoded proteins could be detected on the microarrays used. Further information is available in SI Appendix, Table S1.
We then observed the ultrastructure of WT and mutant chloroplasts from guard cells and mesophyll cells by electron microscopy. In mesophyll cells, WT and gles1 chloroplasts showed comparable thylakoid membrane structure and development with similar numbers of granal stacks and some starch granules (Fig. 1C). In guard cells, however, gles1 chloroplasts showed fewer thylakoid membranes with smaller granal stacks compared with WT (Fig. 1C). These results indicate that the thylakoid-less phenotype of gles1 is specific to guard cells.
Using GCPs and mesophyll cell protoplasts (MCPs) isolated from WT and gles1, we also measured transcription levels of thylakoid membrane system components in guard cells and mesophyll cells by microarray experiments. Compared with WT control cells, the expression of thylakoid-associated photosynthetic components was significantly reduced in the type I population of gles1 guard cells isolated by cell sorting (Fig. 1D and SI Appendix, Table S1). In contrast, the expression was not reduced but was slightly up-regulated in gles1 mesophyll cells (Fig. 1E and SI Appendix, Table S1). The gles1 mutant showed no differences in the maximum efficiency of photosystem II (Fv/Fm; SI Appendix, Fig. S3A) or CO2 assimilation rates at 360 ppm compared with WT in whole-plant measurements (SI Appendix, Fig. S3B). These results suggested that gles1 mutation did not affect photosynthetic activity in whole leaves.
GLES1 Was Identical to TGD5, a Putative Regulatory Component of the TGD Protein Complex.
By map-based cloning, we originally identified that gles1 mutation had a single 160G-to-A substitution in At1g27695, which caused a 54Gly-to-Arg exchange in a small glycine-rich protein (Fig. 2A and SI Appendix, Fig. S4). The 54Gly residue was conserved among four GLES1 orthologs from a variety of higher plant species (Fig. 2A). Introduction of a genomic GLES1 sequence fused in-frame with green fluorescent protein (GFP) marker gene (GLES1-GFP) into gles1 plants fully restored the WT phenotype, verifying that At1g27695 is GLES1 (Fig. 1 A and B). Furthermore, the distribution of GFP fluorescence in the guard cells of GLES1-GFP plants was similar to that of AtOUTER ENVELOPE MEMBRANE PROTEIN 7 (AtOEP7)-GFP (27), which is a marker protein localized to the envelope of plastids (Fig. 2B). These results demonstrated that GLES1 is a chloroplast envelope-localized protein in guard cells. To investigate the expression patterns of GLES1, we examined the expression of the β-glucuronidase reporter driven by the GLES1 promoter in transgenic plants. GLES1 expression was detected in the whole plant, including guard cells (SI Appendix, Fig. S5). However, the molecular function of GLES1 had remained unidentified until Fan et al. (22) reported that At1g27695 encodes TGD5, a putative regulatory component for the TGD protein complex. We therefore investigated whether mutation in components of TGD protein complex and its related protein TDG4 could exhibit gles1-like phenotypes. We found that the null mutant tgd4-3 (20) exhibited similar gles1 phenotypes in guard cells; that is, reduced chlorophyll fluorescence (Fig. 2 C and D). A knockdown mutant tgd3-1 (28) also exhibited reduced chlorophyll fluorescence in guard cells (Fig. 2 C and D), but less severely than tgd4-3. These results suggested that the TGD proteins are important for the development of mature chloroplasts in guard cells.
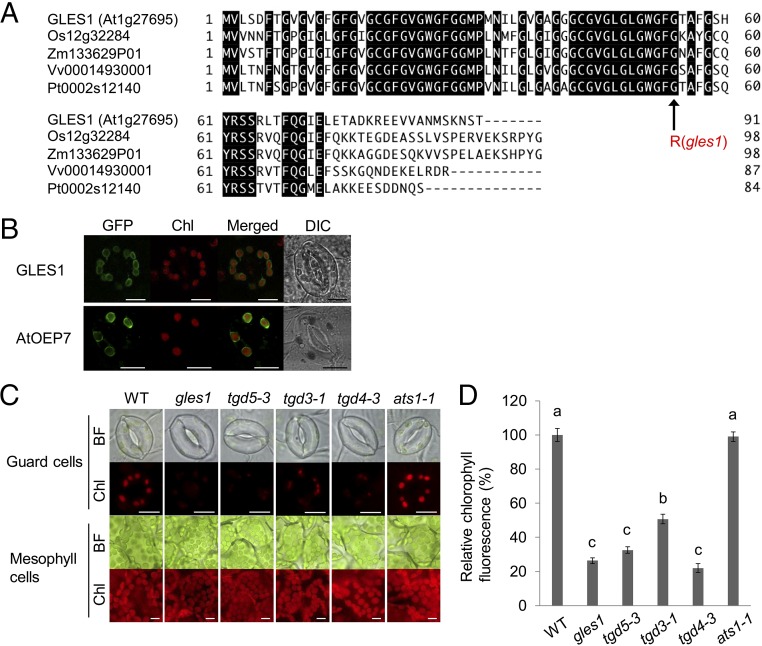
Fig. 2.
TGD proteins involved in lipid transport from ER to chloroplasts are essential for guard cell chloroplast development. (A) Alignment of amino acid sequence of the GLES1(At1g27695) protein and its orthologs assessed using Clustal W. GLES1-like proteins are found in various plants, including Oryza sativa (Os12g32284), Zea mays (Zm133629P01), Vitis vinifera (Vv00014930001) and Populus trichocarpa (Pt0002s12140). Amino acid identity is indicated by black boxes. The gles1 mutation site Glycine 53 is conserved among the orthologs, as indicated by the arrowhead. (B) Chloroplast envelope localization of GLES1-GFP. Expression of GLES1–GFP fusion protein resulted in functional complementation of the gles1 mutant phenotypes (Fig. 1 A and B). GFP fluorescence, autofluorescence of chlorophyll, the overlay of all fluorescence signals, and the differential interference contrast images (DIC) are shown for a representative example. AtOEP7-GFP was used as the chloroplast envelope marker. Chl, Chlorophyll fluorescence. (Scale bars, 10 μm.) (C and D) GLES1 is identical to TGD5, a subunit of TGD protein involved in the ER-to-plastid transport. The T-DNA insertion allele tgd5-3 and loss of function mutants of other TGD complex subunits exhibited achlorophyllous phenotypes in guard cells. (Scale bars, 10 μm.) Total chlorophyll autofluorescence of individual guard cells was analyzed (D). The average chlorophyll autofluorescence measured for WT plants was designated as 100%, and relative fluorescence has been plotted (%). Values shown are means ± SE (n > 100) of five independent experiments. The statistical significance was determined by a one-way ANOVA with Tukey-Kramer multiple comparison tests. Same letters (a–c) indicate no significant difference (P > 0.05).
The Prokaryotic Lipid Metabolic Pathway Is Severely Down-Regulated in Guard Cells.
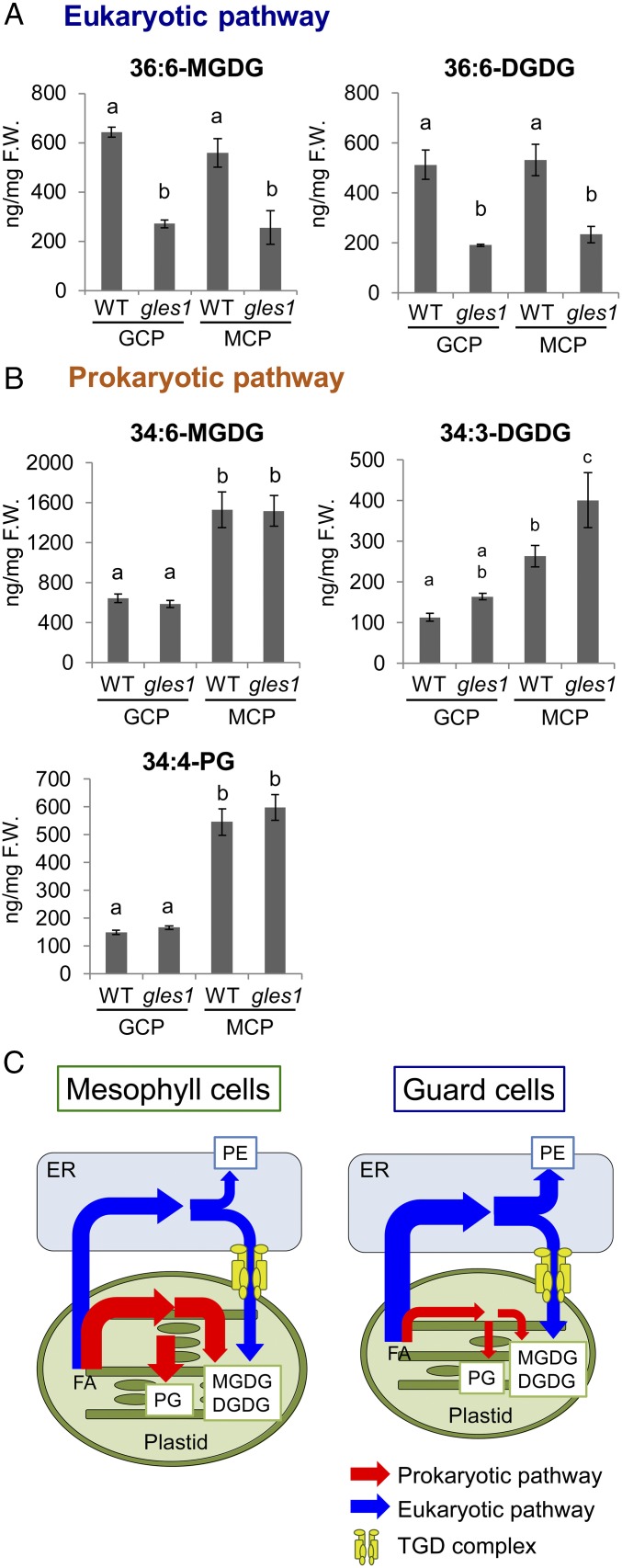
In Arabidopsis, ATS1 encoding plastid-targeted glycerol-3-phosphate acyltransferase catalyzes the biosynthesis of lysophosphatidic acid, the first step of the prokaryotic pathway or the glycerolipid biosynthesis within plastids (SI Appendix, Fig. S1). We found that the ats1-1 mutant that is blocked in the prokaryotic pathway (29) had no effect on chlorophyll fluorescence in guard cells (Fig. 2 C and D). Because the TGD proteins have been shown to be involved in lipid transfer from the ER to plastids in the eukaryotic pathway (30), and the loss-of-function mutants of TGD complex subunits had a reduced chlorophyll fluorescence in guard cells (Fig. 2 C and D), we hypothesized that ER-derived lipid precursors are essential for the synthesis of chloroplast glycolipids, and hence, the development of chloroplasts in guard cells. To address this hypothesis, we determined the content of chloroplast lipids in GCPs and MCPs isolated from leaves of WT and gles1 plants. In both GCPs and MCPs, the gles1 mutation reduced the contents of 36:6-MGDG and 36:6-DGDG, both of which are synthesized from diacylglycerol derived from the eukaryotic pathway (Fig. 3A and SI Appendix, Fig. S6). In WT plants, it is noteworthy that the contents of the prokaryotic glycerolipid molecular species such as 34:6-MGDG, 34:3-DGDG, and 34:4-PG were reduced in guard cells compared with those in mesophyll cells (Fig. 3B and SI Appendix, Fig. S6). In contrast, phosphatidylethanolamine, which is an ER-produced phospholipid, increased in guard cells compared with that in mesophyll cells (SI Appendix, Fig. S6). The Arabidopsis Δ7-desaturase FAD5, which is specific to palmitate esterified at the sn-2 position of MGDG, is responsible for the synthesis of 34:6-MGDG in the prokaryotic pathway, and this enzyme is not present in 18:3 plants (31). To exclude the possibility that the down-regulation of FAD5 decreased the content of 34:6-MGDG in guard cells, we conducted microarray analysis between the transcripts of guard cells and mesophyll cells. The results showed that the expression level of FAD5 in guard cells was comparable to that in mesophyll cells (GCP/MCP ratio = 1.35 ± 0.23; P = 0.4). Thus, the lower content of 34:6-MGDG in guard cells compared with mesophyll cells should be ascribed to a decreased flux of the prokaryotic pathway, and not to the changes in FAD5 expression levels. These results suggest that guard cells have a limited contribution from the prokaryotic pathway and rely on the eukaryotic pathway for chloroplast development (Fig. 3C). Therefore, when the gles1 mutation disrupted the eukaryotic pathway, it must have caused drastic defects in the development of guard cell chloroplasts.
Fig. 3.
The prokaryotic pathway of lipid synthesis is suppressed in guard cells. (A and B) Chloroplast polar lipid species were determined in guard cell protoplasts GCPs and MCPs, which were isolated from WT and gles1 mutants. As an overview, we have depicted representatives of the most abundant species derived from the eukaryotic pathway (A) or prokaryotic pathway (B). MGDG, DGDG, and PG content are shown. Values shown are means ± SE (n = 4). The statistical significance was determined by a one-way ANOVA with Tukey-Kramer multiple comparison tests. Same letters (a–c) indicate no significant difference (P > 0.05). A complete dataset with details on analysis is given in SI Appendix, Fig. S6. (C) Schematic diagram of deduced lipid flux model in Arabidopsis mesophyll cells and guard cells. We propose that the contribution of the prokaryotic and eukaryotic lipid pathways is different between mesophyll cells and guard cells, and that the guard cells rely on the eukaryotic pathway exclusively. Therefore, the ER-to-plastid lipid trafficking mediated by the TGD complex is essential for the formation of guard cell chloroplasts. FA, fatty acid; PE, phosphatidylethanolamine.
Guard Cell Chloroplasts Are Essential for Light-Induced Stomatal Opening and CO2-Induced Stomatal Closure.
To evaluate the roles of guard cell chloroplasts in stomatal movement in WT and gles1 leaves, we measured stomatal responses to CO2, light, and ABA. The type I (achlorophyllous) stomata of gles1 mutants showed significantly reduced responses to CO2 (Fig. 4A and SI Appendix, Fig. S7A) and light (Fig. 4B and SI Appendix, Fig. S7B). Compared with the WT, gles1 stomata opened more slowly and less extensively in response to a CO2 shift from 360 to 0 ppm (Fig. 4D), and closed more slowly in response to a CO2 shift from 0 to 700 ppm (Fig. 4D). Similarly, compared with the WT, gles1 stomata opened more slowly and less extensively in response to illumination at 150 µmol m−2⋅s−1 (Fig. 4E). In contrast, the stomatal aperture showed a similar decrease in size in both WT and achlorophyllous gles1 stomata in response to applied ABA, indicating that the gles1 mutation did not affect stomatal responses to applied ABA (Fig. 4C and SI Appendix, Fig. S7C). These results indicate that the function of guard cell chloroplasts is important not only for the light-induced stomatal opening but also for the CO2-induced stomatal closing. The guard cell S-type anion channel, SLOW ANION CHANNEL 1 (SLAC1), has a crucial role for CO2- and ABA-induced stomatal closure (32, 33). We therefore tested whether the S-type channel activity is impaired in gles1 mutants, using whole-cell patch clamp techniques. Our results showed that elevated CO2 concentrations activated S-type anion channel currents in WT guard cells (Fig. 4F), but the CO2 response was much diminished in gles1 guard cells (Fig. 4G). Interestingly, ABA activation of the S-type anion channel current in the gles1 guard cells was not impaired (Fig. 4H), indicating that the gles1 mutation did not cause structural defects in the S-type anion channel. These results suggest that the gles1 mutation affected the signaling pathway from CO2 sensing to the S-type anion channel activation, and that guard cell chloroplasts could have an important role in CO2-dependent activation of S-type anion channels.
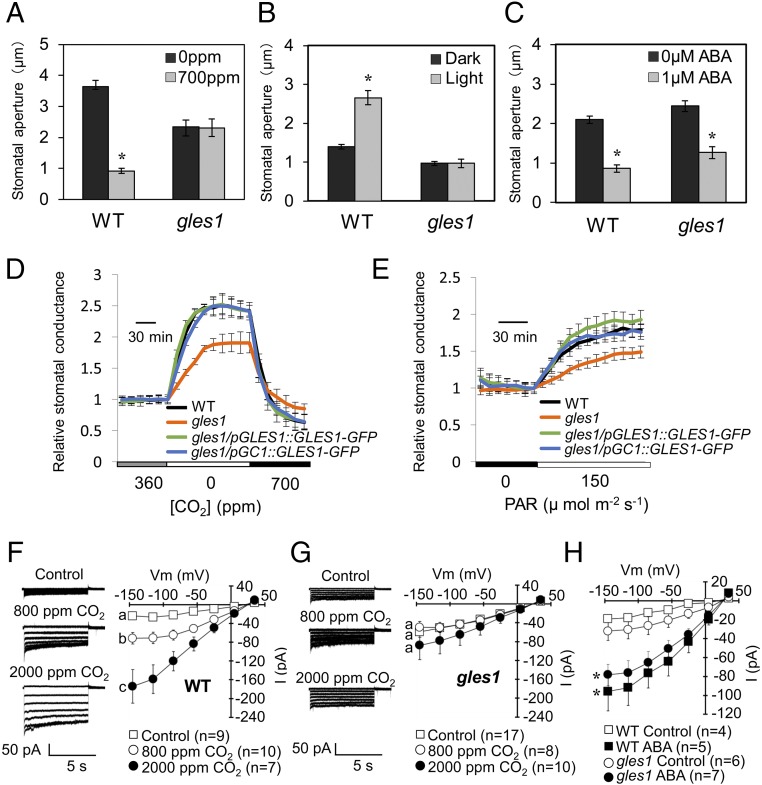
Fig. 4.
Chloroplasts in guard cells have a central role in the regulation of CO2- induced stomatal closure via S-type anion channel activation. (A–C) Stomatal aperture in gles1 mutant and WT. The achlorophyllous stomata (SI Appendix, Fig. S2A: type I) in gles1 mutant fail to respond to high [CO2] (A) and light (B), but show a normal response to ABA (C). Values shown are means ± SE (n = 4 independent experiments with >50 stomata per experiment). Asterisks indicate significant differences (P < 0.05, Student’s t test). (D and E) CO2 and light responses are impaired in the gles1 mutant. Time course of stomatal conductance in gles1 mutant, gles1/pGLES1:GLES1-GFP, gles1/pGC1:GLES1-GFP, and WT in response to changes in CO2 concentrations (D) or in light intensity (E). Stomatal conductance was normalized to the average conductance at the last 360 ppm CO2 data point (D) and the last 0 µmol m−2⋅s−1 PAR data point (E). Values shown are means ± SE (n = 5). (F and G) CO2 activation of S-type anion channels is impaired in gles1 GCPs. Representative current traces (Left) and steady state current–voltage relationships (Right) are shown. CO2 activates S-type anion channel currents in WT GCPs (F), but not in gles1 GCPs (G). Values shown are means ± SE. Different lowercase letters indicate significant differences at −145 mV (P < 0.05, Tukey-Kramer test). (H) ABA activation of S-type anion channels remains intact in gles1 guard cell protoplasts. Steady state current-voltage relationships of the whole-cell currents recorded in the WT (squares) and gles1 mutant (circles) with (black) or without (white) ABA are shown. Error bars indicate ± SE. Asterisks indicate significant differences (without ABA vs with ABA at −145 mV; P < 0.02, Student’s t test).
We found that tgd4-3 (20) stomata also showed reduced CO2 sensitivity in intact Arabidopsis leaves (SI Appendix, Fig. S8). Interestingly, the knockdown mutant tgd3-1 (28) also exhibited reduced chlorophyll fluorescence in guard cells, but its stomatal CO2 responsiveness was not affected (SI Appendix, Fig. S8), suggesting that the residual activity of lipid transfer from the ER to chloroplasts in tgd3-1 mutants was not sufficient to maintain the WT levels of photosynthetic activity, but was sufficient to maintain the perception and signaling events involved in CO2-induced stomata closure. In contrast, the prokaryotic pathway mutant ats1-1 (29) had no effect on the stomatal CO2 response (SI Appendix, Fig. S8). These results suggested that ER-derived lipids have an important role within the chloroplast, possibly in an osmoregulatory mechanism mediating stomatal movements. Alternatively, down-regulation of the lipid flux from the ER to chloroplasts may have a secondary effect on the lipid metabolism in the extrachloroplastic compartments, which could eventually affect the perception and signaling events involved in CO2-induced stomatal closure. However, our study did not exclude the possibility that, in addition to the lipid transfer, GLES1/TGD5 could play an unknown regulatory role in CO2-induced signaling processes.
It has been proposed that stomatal conductance is affected by photosynthetic activity in the mesophyll (34, 35). However, gles1 mutants showed normal leaf photosynthetic activity in our experimental conditions (SI Appendix, Fig. S3). Therefore, it seems unlikely that photosynthetic activity of the mesophyll cells could have affected stomatal CO2 and light responses in gles1 mutants, despite the fact that GLES1 expression was recognized in whole-plant tissues (SI Appendix, Fig. S5). However, to exclude this possibility, we created a transgenic gles1 plant that expressed GLES1 under the control of the guard-cell-specific promoter pGC1 (SI Appendix, Fig. S9). The resultant transformant developed normal chloroplasts in guard cells (Fig. 1 A and B) and showed normal stomatal responses (Fig. 4 D and E). These results demonstrated that GLES1 plays a crucial role in the regulation of stomatal movements by CO2 and light when expressed in guard cells, and that gles1 mutation in mesophyll cells had little influence on the stomatal responses in gles1 guard cells.
Conclusions
We conclude that the lipid supply pathway from the ER to chloroplasts has a significant contribution in the development of guard cell chloroplasts and the regulation of stomatal movements in response to CO2 and light. Chloroplasts of nonseed plants are essentially autonomous in membrane lipid synthesis, but the relative contributions of prokaryotic pathways to plastid glycolipid synthesis have diminished during the course of evolution, and have even become extinct in 18:3 plants (13). To date, the physiological relevance of the prokaryotic pathways in the extant 16:3-plants has not been well described, except that it is dispensable for the development both of male and female gametophytes and of embryos before the heart stage (15–18). Thus, our present finding that the prokaryotic pathway to chloroplast glycolipids is significantly down-regulated in guard cells compared with mesophyll cells in Arabidopsis thaliana, a 16:3 plant (Fig. 3), provides additional evidence for the advantage of the eukaryotic pathway in plant lipid metabolism and would help unravel the physiological significance of the evolution of lipid metabolic pathways in plants. The present study also points to key functions of the eukaryotic lipid pathway in the physiological regulation of stomatal movements.
Materials and Methods
The details and procedures of plant materials and growth conditions, fluorescence microscopy, electron microscopy, isolation of guard cell protoplasts and mesophyll cell protoplasts, cell sorting of guard cell protoplasts, Arabidopsis gene expression microarray, construction of binary vectors for plant transformation, transgene expression analysis, measurement of lipid content, whole-plant stomatal conductance and photosynthesis measurements, microscopic analysis of stomatal responses, and patch clamp analyses are provided in SI Appendix, Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. F. Beisson for critical reading of the manuscript. We also thank N. Kawahara and Y. Johno for the technical assistance. We appreciate the technical assistance from The Research Support Center, Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Areas (JP25891020 and JP15K18556 to J.N., and JP26221103 to K.I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Core Research for Evolution Science and Technology, Japan Science and Technology Agency (JPMJCR1505 to K.I.) and grants from the National Science Foundation (MCB-1616236 to J.I.S.), and in part the National Institutes of Health (GM060396 to J.I.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810458115/-/DCSupplemental.
References
- 1.Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 2.Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negi J, Hashimoto-Sugimoto M, Kusumi K, Iba K. New approaches to the biology of stomatal guard cells. Plant Cell Physiol. 2014;55:241–250. doi: 10.1093/pcp/pct145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engineer CB, et al. CO2 sensing and CO2 regulation of stomatal conductance: Advances and open questions. Trends Plant Sci. 2016;21:16–30. doi: 10.1016/j.tplants.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeiger E, Talbott LD, Frechilla S, Srivastava A, Zhu J. The guard cell chloroplast: A perspective for the twenty-first century. New Phytol. 2002;153:415–424. doi: 10.1046/j.0028-646X.2001.NPH328.doc.x. [DOI] [PubMed] [Google Scholar]
- 6.Lawson T. Guard cell photosynthesis and stomatal function. New Phytol. 2009;181:13–34. doi: 10.1111/j.1469-8137.2008.02685.x. [DOI] [PubMed] [Google Scholar]
- 7.Roelfsema MRG, et al. Guard cells in albino leaf patches do not respond to photosynthetically active radiation, but are sensitive to blue light, CO2 and abscisic acid. Plant Cell Environ. 2006;29:1595–1605. doi: 10.1111/j.1365-3040.2006.01536.x. [DOI] [PubMed] [Google Scholar]
- 8.Suetsugu N, et al. Guard cell chloroplasts are essential for blue light-dependent stomatal opening in Arabidopsis. PLoS One. 2014;9:e108374. doi: 10.1371/journal.pone.0108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azoulay-Shemer T, et al. Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2- and ABA-induced stomatal closing. Plant J. 2015;83:567–581. doi: 10.1111/tpj.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roughan PG, Slack CR. Cellular organization of glycerolipid metabolism. Annu Rev Plant Physiol. 1982;33:97–132. [Google Scholar]
- 11.Somerville C, Browse J. Dissecting desaturation: Plants prove advantageous. Trends Cell Biol. 1996;6:148–153. doi: 10.1016/0962-8924(96)10002-7. [DOI] [PubMed] [Google Scholar]
- 12.Benning C, Xu C, Awai K. Non-vesicular and vesicular lipid trafficking involving plastids. Curr Opin Plant Biol. 2006;9:241–247. doi: 10.1016/j.pbi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Mongrand S, Bessoule J-J, Cabantous F, Cassagne C. The C16:3 C18:3 fatty acid balance in photosynthetic tissues from 468 plant species. Phytochemistry. 1998;49:1049–1064. [Google Scholar]
- 14.Browse J, Warwick N, Somerville CR, Slack CR. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3’ plant Arabidopsis thaliana. Biochem J. 1986;235:25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B, Wakao S, Fan J, Benning C. Loss of plastidic lysophosphatidic acid acyltransferase causes embryo-lethality in Arabidopsis. Plant Cell Physiol. 2004;45:503–510. doi: 10.1093/pcp/pch064. [DOI] [PubMed] [Google Scholar]
- 16.Kim HU, Huang AH. Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol. 2004;134:1206–1216. doi: 10.1104/pp.103.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, Fan J, Froehlich JE, Awai K, Benning C. Mutation of the TGD1 chloroplast envelope protein affects phosphatidate metabolism in Arabidopsis. Plant Cell. 2005;17:3094–3110. doi: 10.1105/tpc.105.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Zienkiewicz A, Lavell A, Benning C. Co-evolution of domain interactions in the chloroplast TGD1, 2, 3 lipid transfer complex specific to Brassicaceae and Poaceae plants. Plant Cell. 2017;29:1500–1515. doi: 10.1105/tpc.17.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roston RL, Gao J, Murcha MW, Whelan J, Benning C. TGD1, -2, and -3 proteins involved in lipid trafficking form ATP-binding cassette (ABC) transporter with multiple substrate-binding proteins. J Biol Chem. 2012;287:21406–21415. doi: 10.1074/jbc.M112.370213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Fan J, Cornish AJ, Benning C. Lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis requires the extraplastidic TGD4 protein. Plant Cell. 2008;20:2190–2204. doi: 10.1105/tpc.108.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Xu C, Benning C. TGD4 involved in endoplasmic reticulum-to-chloroplast lipid trafficking is a phosphatidic acid binding protein. Plant J. 2012;70:614–623. doi: 10.1111/j.1365-313X.2012.04900.x. [DOI] [PubMed] [Google Scholar]
- 22.Fan J, Zhai Z, Yan C, Xu C. Arabidopsis TRIGALACTOSYLDIACYLGLYCEROL5 interacts with TGD1, TGD2, and TGD4 to facilitate lipid transfer from the endoplasmic reticulum to plastids. Plant Cell. 2015;27:2941–2955. doi: 10.1105/tpc.15.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato N. Lipid biosynthesis in epidermal, guard and mesophyll cell protoplast from leaves of Vicia faba L. Plant Cell Physiol. 1985;26:805–811. [Google Scholar]
- 24.Sakaki T, Satoh A, Tanaka K, Omasa K, Shimazaki K-I. Lipids and fatty acids in guard-cell protoplasts from Vicia faba leaves. Phytochemistry. 1995;40:1065–1070. [Google Scholar]
- 25.McLachlan DH, et al. The breakdown of stored triacylglycerols is required during light-induced stomatal opening. Curr Biol. 2016;26:707–712. doi: 10.1016/j.cub.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negi J, et al. A Dof transcription factor, SCAP1, is essential for the development of functional stomata in Arabidopsis. Curr Biol. 2013;23:479–484. doi: 10.1016/j.cub.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YJ, Kim DH, Kim YW, Hwang I. Identification of a signal that distinguishes between the chloroplast outer envelope membrane and the endomembrane system in vivo. Plant Cell. 2001;13:2175–2190. doi: 10.1105/tpc.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu B, Xu C, Awai K, Jones AD, Benning C. A small ATPase protein of Arabidopsis, TGD3, involved in chloroplast lipid import. J Biol Chem. 2007;282:35945–35953. doi: 10.1074/jbc.M704063200. [DOI] [PubMed] [Google Scholar]
- 29.Kunst L, Browse J, Somerville C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA. 1988;85:4143–4147. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurlock AK, Roston RL, Wang K, Benning C. Lipid trafficking in plant cells. Traffic. 2014;15:915–932. doi: 10.1111/tra.12187. [DOI] [PubMed] [Google Scholar]
- 31.Heilmann I, Mekhedov S, King B, Browse J, Shanklin J. Identification of the Arabidopsis palmitoyl-monogalactosyldiacylglycerol delta7-desaturase gene FAD5, and effects of plastidial retargeting of Arabidopsis desaturases on the fad5 mutant phenotype. Plant Physiol. 2004;136:4237–4245. doi: 10.1104/pp.104.052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vahisalu T, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negi J, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 34.Mott KA, Sibbernsen ED, Shope JC. The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ. 2008;31:1299–1306. doi: 10.1111/j.1365-3040.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 35.Fujita T, Noguchi K, Terashima I. Apoplastic mesophyll signals induce rapid stomatal responses to CO2 in Commelina communis. New Phytol. 2013;199:395–406. doi: 10.1111/nph.12261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.