Analysis of the noncrystallographic symmetry of crystals of the C-terminal domain of Burkholderia cenocepacia TssA indicates a quaternary structure of 32 subunits in D 16 symmetry.
Keywords: type VI secretion system, TssA, Burkholderia cenocepacia
Abstract
TssA is a core component of the type VI secretion system, and phylogenetic analysis of TssA subunits from different species has suggested that these proteins fall into three distinct clades. Whilst representatives of two clades, TssA1 and TssA2, have been the subjects of investigation, no members of the third clade (TssA3) have been studied. Constructs of TssA from Burkholderia cenocepacia, a representative of clade 3, were expressed, purified and subjected to crystallization trials. Data were collected from crystals of constructs of the N-terminal and C-terminal domains. Analysis of the data from the crystals of these constructs and preliminary structure determination indicates that the C-terminal domain forms an assembly of 32 subunits in D 16 symmetry, whereas the N-terminal domain is not involved in subunit assocation.
1. Introduction
The type VI secretion system (T6SS) is composed of 14 core components, TssA–TssM and PAAR, which together form a contractile machine that injects protein effectors into target cells (Pukatzki et al., 2007 ▸; Shalom et al., 2007 ▸; Zheng & Leung, 2007 ▸; Boyer et al., 2009 ▸; Leiman et al., 2009 ▸; Cianfanelli et al., 2016 ▸). The core components form a number of subcomplexes, including the sheath/tube complex, membrane complex and baseplate (Leiman et al., 2009 ▸; Basler et al., 2012 ▸; Shneider et al., 2013 ▸; Brunet et al., 2015 ▸; Durand et al., 2015 ▸). TssB and TssC form the contractile sheath, which surrounds the TssD inner tube (Bönemann et al., 2009 ▸; Leiman et al., 2009 ▸; Basler et al., 2012 ▸; Brunet et al., 2014 ▸). TssI and PAAR sit on top of the tube and may be decorated with covalently or noncovalently attached effector proteins (Pukatzki et al., 2007 ▸; Leiman et al., 2009 ▸; Shneider et al., 2013 ▸). Upon contraction of the sheath against the baseplate, the tube, which can also carry effector proteins, passes through the baseplate and is ejected from the cell (Pukatzki et al., 2006 ▸; Basler et al., 2012 ▸; Silverman et al., 2013 ▸). TssH then acts to recycle the sheath components post-contraction for reassembly in the extended state (Bönemann et al., 2009 ▸; Basler et al., 2012 ▸; Kapitein et al., 2013 ▸). TssJ, TssL and TssM form a complex that anchors the contractile machinery to the cell envelope through interactions with the baseplate (Aschtgen et al., 2010 ▸; Zoued et al., 2013 ▸; Brunet et al., 2015 ▸; Durand et al., 2015 ▸). TssE, TssF, TssG and TssK are components of the baseplate, which serves as a platform for priming of the sheath and tube assembly (Basler et al., 2012 ▸; English et al., 2014 ▸; Brunet et al., 2015 ▸).
Following phylogenetic analysis, TssA proteins have been classified into three clades (TssA1–TssA3; Planamente et al., 2016 ▸), with representative TssAs belonging to clades TssA1 (PA0082 from Pseudomonas aeruginosa; P.a TssA1) and TssA2 (Ec042_4540 from enteroaggregative Escherichia coli; E.c TssA2) having previously been the subject of biochemical and structural studies (Planamente et al., 2016 ▸; Zoued et al., 2016 ▸). The P.a and E.c TssAs have been shown to interact with the T6SS sheath, inner tube and components of the baseplate, with E.c TssA2 also interacting with the membrane complex (Planamente et al., 2016 ▸; Zoued et al., 2016 ▸). P.a TssA1 is proposed to contain two domains which form a ring structure comprising the C-terminal domain with a flexible peripheral N-terminal domain (Planamente et al., 2016 ▸). Similarly, in E.c TssA2 the C-terminal domain oligomerizes to produce a dodecameric structure of two stacked rings with D 6 symmetry (Zoued et al., 2016 ▸). In E.c TssA2 the N-terminal region is predicted to form arms which extend out from the C-terminal dodecamer core (Zoued et al., 2016 ▸). The structure of part of the E.c TssA2 N-terminal region, Nt2, forms a dimer (Zoued et al., 2016 ▸). Bioinformatic analysis identified a conserved region within both the P.a TssA1 and E.c TssA2 N-terminal domains, referred to as ImpA_N (Planamente et al., 2016 ▸; Zoued et al., 2017 ▸). P.a TssA1 was observed to localize at one end of the sheath, whilst E.c TssA2 was seen to move away from an initial site at the membrane concomitant with sheath polymerization (Planamente et al., 2016 ▸; Zoued et al., 2016 ▸). Based on these observations of P.a TssA1 and some low-level predicted similarities in secondary structure, it was suggested that the TssA1 C-terminal region corresponds to the C-terminal region of the phage T4 baseplate component gp6, thereby identifying P.a TssA1 as a possible baseplate component (Planamente et al., 2016 ▸). On the other hand, E.c TssA2 was predicted to be involved in recruitment of the baseplate assembly to the membrane complex and subsequent polymerization of the TssD tube and TssB/TssC sheath (Zoued et al., 2016 ▸).
Burkholderia cenocepacia TssA (B.c TssA), locus tag I35_RS01755, is a member of TssA3, the TssA clade that has yet to be investigated (Planamente et al., 2016 ▸). In this paper, we describe the production of constructs of B.c TssA representing the predicted N-terminal and C-terminal domains. Overexpression of the domains, their purification and their crystallization resulted in the production of diffraction-quality crystals, analysis of which provides preliminary information on their stoichiometry, symmetry and structural organization.
2. Materials and methods
2.1. Macromolecule production
Coding sequences for the predicted N-terminal domain (Nt1) and C-terminal domain (CTD) of B.c TssA (UniProt A0A1V2WLD6) were amplified from B. cenocepacia strain H111 (Römling et al., 1994 ▸) by PCR using the appropriate combinations of primers as shown in Table 1 ▸. The DNA products were ligated to either plasmid pET-14b (Novagen; encoding a His6 purification tag and a thrombin cleavage site) or pMAL-c5X [NEB; encoding a maltose-binding protein (MBP) solubility tag and a Factor Xa cleavage site], followed by transformation of E. coli strain JM83 (Yanisch-Perron et al., 1985 ▸). Construct details are shown in Table 1 ▸.
Table 1. Construct information.
| Construct | His6-TssA Nt1 | MBP-TssA CTD |
|---|---|---|
| B.c TssA amino acids | 1–255 | 303–373 |
| Tag details | MGSSHHHHHHSSGLVPRGSH | (MBP)NSSSNNNNNNNNNNLGIEGR/ISHM† |
| Construct sequence | MGSSHHHHHHSSGLVPRGSHMPINLPELLTPISEASPSGDDLLFSNEFDAIQDARRYDDPTLDQGEWVTEIKEADWGFVVDHAGELLRTRTKDLRLAVWLTEALALEDGITGLTEGYALLEGLCREFWDTFHPLPEDDDIEHRLGNVAWLSGRTAELLRAVPLTDGASNAFSTLDWEVAQHVAQSIKRDPEHADDIARGKPSIEQIDASRRVTSIAFYTALLANLKAFEFALDAFEERLVERAGDSAPSFRQARDAFETVYRLAERFAREQGYTG | (MBP)NSSSNNNNNNNNNNLGIEGR/ISHMIQNRAQAVDQLRAVARYFRQTEPHSPVAYLADKAAEWADMPLHKWLESVVKDDGSLSHIRELLGVRPDEQS |
| Forward primer‡ | pET-14b-iotAfor, GCGCCATATGCCGATCAATCTCCCCGA | pET-14b-ACTDfor3, GCGCCATATGATCCAGAACCGTGCGCAGGC |
| Reverse primer‡ | pET-14b-TssA-NTDrev, GCGCGGATCC TTAGCCGGTATAGCCCTGTTCGC | pET-14b-iotArev, GCGCGGATCCTGCGTTTACGACTGCTCGTC |
| Restriction enzymes | NdeI and BamHI | NdeI and BamHI |
| Expression vector | pET-14b | pMAL-c5X |
| Antibiotic | 100 µg ml−1 ampicillin | 100 µg ml−1 ampicillin |
| Expression host | E. coli BL21 (DE3) | E. coli ER2523 (NEB Express) |
MBP is maltose-binding protein; IEGR is the Factor Xa recognition site; / indicates the Factor X cleavage site. Tags are highlighted as underlined regions of the construct sequences.
Bold sequences indicate restriction sites. Translation initiation and termination codons are underlined.
A B.c His6-TssA Nt1 construct was overexpressed in E. coli BL21 (DE3) cells (Studier & Moffatt, 1986 ▸) grown in brain heart infusion broth (Becton Dickinson) at 37°C to an OD600 of 0.5–0.7, whereupon expression was induced by the addition of 1 mM IPTG followed by incubation for a further 2–3 h. B.c His6-TssA Nt1 was purified from clarified cell lysate in 50 mM Tris pH 8.0, 500 mM NaCl, applied onto a HisTrap HP column (GE Healthcare Life Sciences) and eluted with a linear gradient of imidazole (0–350 mM) in 50 mM Tris pH 8.0, 500 mM NaCl. Fractions containing the B.c His6-TssA Nt1 protein were concentrated using a 30 kDa molecular-weight cutoff concentrator and washed with 10 mM Tris pH 8.0 for crystallization.
The B.c MBP-TssA CTD fusion protein was overproduced in E. coli ER2523 cells (NEB Express) grown in Lennox broth containing 0.2% glucose (Lennox, 1955 ▸) at 37°C to an OD600 of 0.6–0.7, whereupon protein overexpression was induced with 0.3 mM IPTG and incubation of the culture continued at the same temperature for a further 2–3 h. To prepare selenomethionine-incorporated B.c MBP-TssA CTD protein (B.c SeMet-MBP-TssA CTD), 2 × 500 ml of cells were grown as described above and harvested prior to induction. The cell pellets were then resuspended in selenomethionine minimal medium, harvested and the resuspension was added to 2 × 500 ml selenomethionine minimal medium [10.5 g l−1 K2HPO4, 1.0 g l−1 (NH4)2SO4, 4.5 g l−1 KH2PO4, 0.5 g l−1 trisodium citrate·2H2O, 5.0 g l−1 glycerol and 0.5 g l−1 each of adenine, guanosine, thymine and uracil] supplemented with 1.0 g l−1 MgSO4·7H2O, 4.0 mg l−1 thiamine, 100 mg l−1 each of l-lysine, l-phenylalanine and l-threonine, 50 mg l−1 each of l-isoleucine, l-leucine and l-valine, 40 mg l−1 seleno-l-methionine and 2.0 g l−1 glucose. Growth was continued for 1 h at 37°C prior to the induction of B.c MBP-TssA CTD expression by the addition of 0.3 mM IPTG and subsequent growth at 37°C overnight.
Harvested B.c MBP-TssA CTD- and B.c SeMet-MBP-TssA CTD-overexpressing cells were each resuspended in column-binding buffer (50 mM Tris–HCl pH 8.0, 200 mM NaCl) and the cells were lysed by sonication. The cell lysate was cleared by centrifugation before being applied onto amylose resin (NEB) equilibrated with column-binding buffer. After washing the resin with column-binding buffer, the bound material was then eluted in the same buffer with the addition of 10 mM maltose. Fractions containing the fusion protein were concentrated and then digested with Factor Xa (5 µg per milligram of protein; NEB) in the presence of 2 mM CaCl2 at room temperature for ∼14 h to release the target protein from MBP. Following cleavage of the MBP, the products were separated by gel filtration on a Superdex 200 column (GE Healthcare Life Sciences) in 50 mM Tris–HCl pH 8.0, 500 mM NaCl. The B.c TssA CTD protein obtained from gel filtration was applied to a final amylose column to remove any uncleaved B.c MBP-TssA CTD fusion molecules. This step was added to avoid heterogeneity of the sample. The protein was then concentrated and buffer-exchanged into 5 mM Tris–HCl pH 8.0, 50 mM NaCl for crystallization. The purified B.c TssA CTD protein has an additional four non-native residues (ISHM) at the N-terminus following proteolytic release from the N-terminal MBP solubility tag (Table 1 ▸).
2.2. Crystallization
B.c TssA protein constructs were set down into sitting-drop crystallization trials using a Matrix Hydra II Plus One robot, with 200 nl:200 nl drops and a 50 µl reservoir, or hanging-drop crystallization trials, and stored at 17°C. A selection of crystallization condition suites were used for screening, including JCSG+, ProPlex, PACT, Classics, MPD, AmSO4, PEGs and pHClear (Qiagen, Molecular Dimensions). B.c His6-TssA Nt1 crystals grew in sitting-drop trial conditions consisting of 0.16 M calcium acetate, 0.08 M sodium cacodylate buffer pH 6.5, 14.4%(w/v) PEG 8000, 20%(v/v) glycerol. B.c TssA CTD produced crystals in sitting-drop trial conditions consisting of 0.1 M sodium chloride, 0.1 M Tris pH 8.0, 15%(v/v) ethanol, 5%(v/v) MPD. B.c SeMet-TssA CTD produced crystals in hanging-drop trial conditions consisting of 1 M ammonium sulfate, 2%(w/v) PEG 3350, 0.1 M bis-tris pH 6.0. Crystallization information is shown in Table 2 ▸.
Table 2. Crystallization.
| Construct | His6-TssA Nt1 | TssA CTD | SeMet-TssA CTD |
|---|---|---|---|
| Method | Sitting-drop vapour diffusion | Sitting-drop vapour diffusion | Hanging-drop vapour diffusion |
| Composition of reservoir solution | 0.16 M calcium acetate, 0.08 M sodium cacodylate buffer pH 6.5, 14.4%(w/v) PEG 8000, 20%(v/v) glycerol | 0.1 M sodium chloride, 0.1 M Tris pH 8.0, 15%(v/v) ethanol, 5%(v/v) MPD | 1 M ammonium sulfate, 2%(w/v) PEG 3350, 0.1 M bis-tris pH 6.0 |
| Volume and ratio of drop | 200 nl:200 nl | 200 nl:200 nl | 2 µl:1 µl (protein:reservoir) |
| Volume of reservoir (µl) | 50 | 50 | 1000 |
2.3. Data collection, processing and analysis
Data were collected from B.c His6-TssA Nt1 crystals [cryoprotected in 0.16 M calcium acetate, 0.08 M sodium cacodylate buffer pH 6.5, 16.4%(w/v) PEG 8000, 30%(v/v) glycerol] on beamline I04-1 at Diamond Light Source. The data were processed to 1.87 Å resolution using the -3daii option in xia2 (Collaborative Computational Project, Number 4, 1994 ▸; Evans, 2006 ▸; Kabsch, 2010 ▸; Winter, 2010 ▸) and indicated that the crystals belonged to space group P21212 (unit-cell parameters a = 49.7, b = 125.4, c = 45.5 Å). Data-collection and processing statistics are shown in Table 3 ▸.
Table 3. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Construct | His6-TssA Nt1 | His6-TssA Nt1 + iodine | TssA CTD | SeMet-TssA CTD |
|---|---|---|---|---|
| Wavelength (Å) | 0.92000 | 1.70000 | 0.97949 | 0.97922 |
| Beamline | I04-1 | I03 | I04 | I03 |
| Detector | PILATUS 2M | PILATUS 6M | PILATUS 6M-F | PILATUS3 6M |
| Rotation range per image (°) | 0.2 | 0.2 | 0.2 | 0.1 |
| Total rotation range (°) | 90 | 720 | 180 | 360 |
| Exposure time per image (s) | 0.2 | 0.2 | 0.2 | 0.05 |
| Data-processing package | xia2 -3daii | fast_dp | xia2 -3daii | xia2 -3d |
| Space group | P21212 | P21212 | I222 | I222 |
| a, b, c (Å) | 49.7, 125.4, 45.5 | 45.6, 49.8, 126.1 | 46.9, 203.0, 267.9 | 46.3, 201.3, 263.9 |
| α, β, γ (°) | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Resolution range (Å) | 62.69–1.87 (1.92–1.87) | 29.68–2.01 (2.06–2.01) | 55.90–3.79 (3.89–3.79) | 80.61–2.86 (2.93–2.86) |
| R merge | 0.067 (0.495) | 0.131 (0.761) | 0.221 (0.871) | 0.207 (2.520) |
| R p.i.m. | 0.051 (0.372) | 0.031 (0.174) | 0.112 (0.487) | 0.064 (0.747) |
| 〈I/σ(I)〉 | 10.8 (2.0) | 20.6 (3.4) | 7.6 (2.0) | 11.1 (1.1) |
| Completeness (%) | 98.4 (99.3) | 98.4 (79.0) | 99.4 (99.7) | 100.0 (100.0) |
| Multiplicity | 3.3 (3.3) | 23.0 (18.7) | 5.7 (5.3) | 13.0 (13.2) |
| Total reflections | 77962 | 448717 | 75744 | 382400 |
| Unique reflections | 23839 | 19523 | 13207 | 29304 |
Data were collected from B.c TssA CTD crystals [cryoprotected in 0.1 M sodium chloride, 0.1 M Tris pH 8.0, 15%(v/v) ethanol, 25%(v/v) glycerol] on beamline I04 at Diamond Light Source. Data were processed to 3.79 Å resolution using the -3daii option in xia2 (Collaborative Computational Project, Number 4, 1994 ▸; Sauter et al., 2004 ▸; Evans, 2006 ▸; Zhang et al., 2006 ▸; Kabsch, 2010 ▸; Winter, 2010 ▸) and indicated that the crystals belonged to space group I222. Data-collection and processing statistics are shown in Table 3 ▸. A self-rotation function was calculated on all of the data from crystals of B.c TssA CTD using an integration radius of 44 Å in POLARRFN (Winn et al., 2011 ▸). Self Patterson functions on the native data were calculated using FFT for Patterson (Winn et al., 2011 ▸).
B.c His6-TssA Nt1 crystals were subjected to the sublimination of elemental iodine for ∼3 h prior to cryoprotection in 0.16 M calcium acetate, 0.08 M sodium cacodylate buffer pH 6.5, 16.4%(w/v) PEG 8000, 30%(v/v) glycerol and data collection on beamline I03 at Diamond Light Source. The data were processed to 2.01 Å resolution with fast_dp (Winter & McAuley, 2011 ▸) and indicated that the crystals belonged to space group P21212 (unit-cell parameters a = 45.6, b = 49.8, c = 126.1 Å). Data-collection statistics are shown in Table 3 ▸.
Data were collected from B.c SeMet-TssA CTD crystals [cryoprotected in 1–1.2 M ammonium sulfate, 3–4%(w/v) PEG 3350, 0.1 M bis-tris pH 6.0, 30%(v/v) glycerol] on beamline I03 at Diamond Light Source. The data were processed to 2.86 Å resolution using the -3d option in xia2 (Collaborative Computational Project, Number 4, 1994 ▸; Evans, 2006 ▸; Kabsch, 2010 ▸; Winter, 2010 ▸) and indicated that the crystals belonged to space group I222 (unit-cell parameters a = 46.3, b = 201.3, c = 263.9 Å). Data-collection statistics are shown in Table 3 ▸.
3. Results and discussion
3.1. Construct design
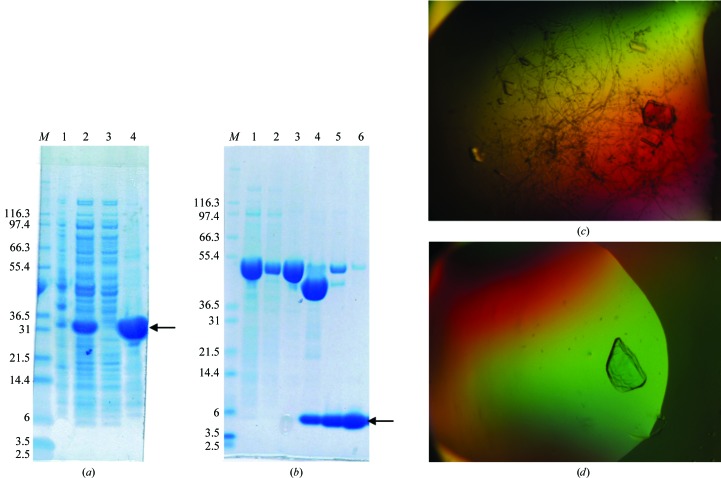
The design of the constructs for the N- and C-terminal domains of B.c TssA was based on an analysis of the pattern of sequence conservation across the different clades of TssA and resulted in stable, soluble samples of the proteins encoded by these regions of the gene. Constructs of the predicted N-terminal domain (Nt1) and C-terminal domain (CTD) of B.c TssA produced crystals (Fig. 1 ▸) which diffracted to high resolution.
Figure 1.
(a) SDS–PAGE analysis of protein purification of the B.c His6-TssA Nt1 construct. Lane M, Mark12 marker (labelled in kDa). Lane 1, cell debris. Lane 2, cell-free extract. Lane 3, flowthrough (Ni column). Lane 4, final preparation. (b) SDS–PAGE analysis of protein purification of the B.c SeMet MBP-TssA CTD construct. The protein, indicated by the arrow, is shown to be ∼90% pure in the final preparation samples and thus was used for crystallization. Lane M, Mark12 marker (labelled in kDa). Lane 1, cell-free extract. Lane 2, unbound material (amylose column). Lane 3, B.c SeMet MBP-TssA CTD. Lane 4, after MBP cleavage. Lane 5, after gel filtration. Lane 6, final preparation. (c) B.c His6-TssA Nt1 crystals grown in 0.16 M calcium acetate, 0.08 M sodium cacodylate buffer pH 6.5, 14.4%(w/v) PEG 8000, 20%(v/v) glycerol. (d) B.c TssA CTD crystals grown in 0.1 M sodium chloride, 0.1 M Tris pH 8.0, 15%(v/v) ethanol, 5%(v/v) MPD.
3.2. TssA data analysis
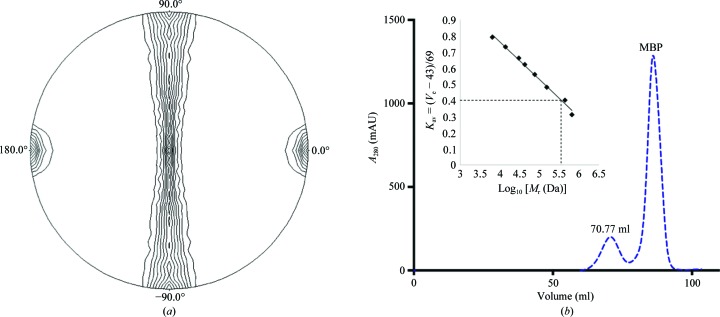
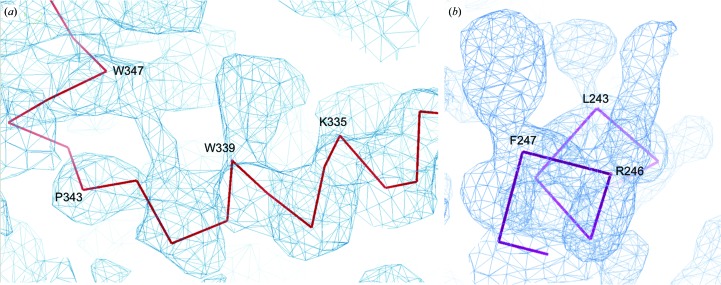
Analysis of the Matthews coefficient of the B.c His6-TssA Nt1 crystals suggests that the crystals contain one molecule of ∼30.7 kDa in the asymmetric unit, with a V M of 2.31 Å3 Da−1 (Matthews, 1968 ▸; Kantardjieff & Rupp, 2003 ▸; Winn et al., 2011 ▸). In contrast, the contents of the asymmetric unit of the B.c TssA CTD crystals could not be confidently assigned from analysis of the Matthews coefficient. Consideration of possible values of V M suggests that the asymmetric unit contains between nine and 21 subunits (4.13–1.77 Å3 Da−1; Matthews, 1968 ▸; Kantardjieff & Rupp, 2003 ▸; Winn et al., 2011 ▸). It has previously been reported that the TssA CTD acts as an oligomerization domain which produces ring-like or star-like structures, as has been shown for P.a TssA1 and E.c TssA2, respectively (Planamente et al., 2016 ▸; Zoued et al., 2016 ▸). Self Pattersons calculated using the native data to 6 or 3.79 Å resolution showed no significant peaks above 15% of the origin, consistent with the absence of translational symmetry in the crystal. A self-rotation function was calculated on the B.c TssA CTD data using an integration radius of 44 Å to detect the presence of noncrystallographic symmetry. This identified peaks on the κ = 180° section with a height of approximately 70% of the origin corresponding to twofold axes located in the bc plane every 11.25° (Fig. 2 ▸). Consideration of the rotational symmetry and apparent molecular weight, determined by gel-filtration analysis (Fig. 2 ▸), suggests that the quaternary structure of B.c TssA CTD is composed of 32 subunits in D 16 symmetry. This suggests that the asymmetric unit contains eight subunits with an unusually high V M (4.64 Å3 Da−1; Matthews, 1968 ▸; Kantardjieff & Rupp, 2003 ▸; Winn et al., 2011 ▸). A preliminary electron-density map was calculated using SAD data to 2.86 Å resolution from the selenomethionine derivative of the B.c TssA CTD crystals, with an initial chain trace confirming the presence of B.c TssA CTD in the crystals and the nature of the symmetry indicated by the self-rotation function (Fig. 2 ▸). Similarly, a preliminary electron-density map has been calculated using SAD data to 2.01 Å resolution from iodine-exposed B.c His6-TssA Nt1 crystals (Fig. 3 ▸). Refinement of the structures and attempts to extend the resolution of the data are ongoing.
Figure 2.
(a) The self-rotation function for B.c TssA CTD crystals calculated on all data from 55.90 to 3.79 Å resolution using a 44 Å radius of integration. The section shown corresponds to κ = 180°, with the orthogonal x, y and z axes along the crystallographic a, b and c axes, respectively. Noncrystallographic twofold axes can be seen every 11.25° in the bc plane at approximately 70% of the origin. The image was generated in POLARRFN (Winn et al., 2011 ▸). (b) Gel-filtration chromatogram of B.c SeMet-MBP-TssA CTD protein, with UV absorption (280 nm) shown as a dotted blue line and the elution volume (70.77 ml) corresponding to B.c TssA CTD shown above the curve. Inset: calibration curve for the gel-filtration column, with K av = 0.4 (V e = 70.77 ml) highlighted corresponding to a molecular mass of ∼3.55 × 105 Da for B.c TssA CTD.
Figure 3.
Preliminary electron-density maps, contoured at ∼1.0σ, representing (a) a helical section of B.c TssA CTD. The large side chain visible towards the C-terminal end of the helix corresponds to Trp339, and density can also be seen for the nearby Trp347. Phases were obtained from SHELX (Sheldrick, 2008 ▸). (b) A helical section of the B.c His6-TssA Nt1 domain. The large side chain visible at the C-terminal end of the helix corresponds to Phe247, and density can also be seen for the side chain of the adjacent Arg246. Phases were obtained from fast_ep and SHELX (Sheldrick, 2008 ▸). Images were generated in Coot (Emsley et al., 2010 ▸).
Acknowledgments
We would like to thank Diamond Light Source and the beamline scientists for access to beamlines I03, I04 and I04-1 and support during data collection.
Funding Statement
This work was funded by BBSRC grant BB/J014443/1 to Hayley J. Owen. Ministry of Higher Education and Scientific Research grant .
References
- Aschtgen, M.-S., Gavioli, M., Dessen, A., Lloubès, R. & Cascales, E. (2010). Mol. Microbiol. 75, 886–899. [DOI] [PubMed]
- Basler, M., Pilhofer, M., Henderson, G. P., Jensen, G. J. & Mekalanos, J. J. (2012). Nature (London), 483, 182–186. [DOI] [PMC free article] [PubMed]
- Bönemann, G., Pietrosiuk, A., Diemand, A., Zentgraf, H. & Mogk, A. (2009). EMBO J. 28, 315–325. [DOI] [PMC free article] [PubMed]
- Boyer, F., Fichant, G., Berthod, J., Vandenbrouck, Y. & Attree, I. (2009). BMC Genomics, 10, 104. [DOI] [PMC free article] [PubMed]
- Brunet, Y. R., Hénin, J., Celia, H. & Cascales, E. (2014). EMBO Rep. 15, 315–321. [DOI] [PMC free article] [PubMed]
- Brunet, Y. R., Zoued, A., Boyer, F., Douzi, B. & Cascales, E. (2015). PLOS Genet. 11, e1005545. [DOI] [PMC free article] [PubMed]
- Cianfanelli, F. R., Alcoforado Diniz, J., Guo, M., De Cesare, V., Trost, M. & Coulthurst, S. J. (2016). PLoS Pathog. 12, e1005735. [DOI] [PMC free article] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Durand, E., Nguyen, V. S., Zoued, A., Logger, L., Péhau-Arnaudet, G., Aschtgen, M. S., Spinelli, S., Desmyter, A., Bardiaux, B., Dujeancourt, A., Roussel, A., Cambillau, C., Cascales, E. & Fronzes, R. (2015). Nature (London), 523, 555–560. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- English, G., Byron, O., Cianfanelli, F. R., Prescott, A. R. & Coulthurst, S. J. (2014). Biochem. J. 461, 291–304. [DOI] [PMC free article] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kantardjieff, K. A. & Rupp, B. (2003). Protein Sci. 12, 1865–1871. [DOI] [PMC free article] [PubMed]
- Kapitein, N., Bönemann, G., Pietrosiuk, A., Seyffer, F., Hausser, I., Locker, J. K. & Mogk, A. (2013). Mol. Microbiol. 87, 1013–1028. [DOI] [PubMed]
- Leiman, P. G., Basler, M., Ramagopal, U. A., Bonanno, J. B., Sauder, J. M., Pukatzki, S., Burley, S. K., Almo, S. C. & Mekalanos, J. J. (2009). Proc. Natl Acad. Sci. USA, 106, 4154–4159. [DOI] [PMC free article] [PubMed]
- Lennox, E. S. (1955). Virology, 1, 190–206. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Planamente, S., Salih, O., Manoli, E., Albesa-Jové, D., Freemont, P. S. & Filloux, A. (2016). EMBO J. 35, 1613–1627. [DOI] [PMC free article] [PubMed]
- Pukatzki, S., Ma, A. T., Revel, A. T., Sturtevant, D. & Mekalanos, J. J. (2007). Proc. Natl Acad. Sci. USA, 104, 15508–15513. [DOI] [PMC free article] [PubMed]
- Pukatzki, S., Ma, A. T., Sturtevant, D., Krastins, B., Sarracino, D., Nelson, W. C., Heidelberg, J. F. & Mekalanos, J. J. (2006). Proc. Natl Acad. Sci. USA, 103, 1528–1533. [DOI] [PMC free article] [PubMed]
- Römling, U., Fiedler, B., Bosshammer, J., Grothues, D., Greipel, J., von der Hardt, H. & Tümmler, B. (1994). J. Infect. Dis. 170, 1616–1621. [DOI] [PubMed]
- Sauter, N. K., Grosse-Kunstleve, R. W. & Adams, P. D. (2004). J. Appl. Cryst. 37, 399–409. [DOI] [PMC free article] [PubMed]
- Shalom, G., Shaw, J. G. & Thomas, M. S. (2007). Microbiology, 153, 2689–2699. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shneider, M. M., Buth, S. A., Ho, B. T., Basler, M., Mekalanos, J. J. & Leiman, P. G. (2013). Nature (London), 500, 350–353. [DOI] [PMC free article] [PubMed]
- Silverman, J. M., Agnello, D. M., Zheng, H., Andrews, B. T., Li, M., Catalano, C. E., Gonen, T. & Mougous, J. D. (2013). Mol. Cell, 51, 584–593. [DOI] [PMC free article] [PubMed]
- Studier, F. W. & Moffatt, B. A. (1986). J. Mol. Biol. 189, 113–130. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Winter, G. (2010). J. Appl. Cryst. 43, 186–190.
- Winter, G. & McAuley, K. E. (2011). Methods, 55, 81–93. [DOI] [PubMed]
- Yanisch-Perron, C., Vieira, J. & Messing, J. (1985). Gene, 33, 103–119. [DOI] [PubMed]
- Zhang, Z., Sauter, N. K., van den Bedem, H., Snell, G. & Deacon, A. M. (2006). J. Appl. Cryst. 39, 112–119.
- Zheng, J. & Leung, K. Y. (2007). Mol. Microbiol. 66, 1192–1206. [DOI] [PubMed]
- Zoued, A., Durand, E., Bebeacua, C., Brunet, Y. R., Douzi, B., Cambillau, C., Cascales, E. & Journet, L. (2013). J. Biol. Chem. 288, 27031–27041. [DOI] [PMC free article] [PubMed]
- Zoued, A., Durand, E., Brunet, Y. R., Spinelli, S., Douzi, B., Guzzo, M., Flaugnatti, N., Legrand, P., Journet, L., Fronzes, R., Mignot, T., Cambillau, C. & Cascales, E. (2016). Nature (London), 531, 59–63. [DOI] [PubMed]
- Zoued, A., Durand, E., Santin, Y. G., Journet, L., Roussel, A., Cambillau, C. & Cascales, E. (2017). Bioessays, 39, 1600262. [DOI] [PubMed]