Abstract
Context: Abnormal angiogenesis and evasion of apoptosis are hallmarks of cancer. Accordingly, anti-angiogenic and pro-apoptotic therapies are effective strategies for cancer treatment. Medicinal plants, namely, Eugenia jambolana Lam. (Myrtaceae), Musa paradisiaca L. (Musaceae), and Coccinia indica Wight & Arn. (Cucurbitaceae), have not been greatly investigated for their anticancer potential.
Objective: We investigated the anti-angiogenic and pro-apoptotic efficacy of ethyl acetate (EA) and n-butanol (NB) extracts of E. jambolana (seeds), EA extracts of M. paradisiaca (roots) and C. indica (leaves) with respect to mammary neoplasia.
Materials and methods: Effect of extracts (2–200 μg/mL) on cytotoxicity and MCF-7, MDA-MB-231 and endothelial cell (EC) proliferation and in vitro angiogenesis were evaluated by MTT, 3[H]thymidine uptake and EC tube formation assays, respectively. In vivo tumour proliferation, VEGF secretion and angiogenesis were assessed using the Ehrlich ascites tumour (EAT) model followed by rat corneal micro-pocket and chicken chorioallantoic membrane (CAM) assays. Apoptosis induction was assessed by morphological and cell cycle analysis.
Results: EA extracts of E. jambolana and M. paradisiaca exhibited the highest cytotoxicity (IC50 25 and 60 μg/mL), inhibited cell proliferation (up to 81%), and tube formation (83% and 76%). In vivo treatment reduced body weight (50%); cell number (16.5- and 14.7-fold), secreted VEGF (∼90%), neoangiogenesis in rat cornea (2.5- and 1.5-fold) and CAM (3- and 1.6-fold) besides EAT cells accumulation in sub-G1 phase (20% and 18.38%), respectively.
Discussion and conclusion: Considering the potent anti-angiogenic and pro-apoptotic properties, lead molecules from EA extracts of E. jambolana and M. paradisiaca can be developed into anticancer drugs.
Keywords: Anti-angiogenic, pro-apoptotic, cytotoxicity
Introduction
Angiogenesis involves the formation of new vasculature from the already existing blood vessels and is a characteristic phenomenon in numerous diseases, such as tumour formation, rheumatoid arthritis, diabetic retinopathy and psoriasis to name a few (Yu et al. 2009; Miao et al. 2011). This physiological process is effected by various factors such as vascular endothelial growth factor (VEGF), angiopoietins (Ang), platelet-derived growth factor (PDGF), matrix metalloproteinase (MMP) which expedite cell proliferation, tube formation and migration of endothelial cells (Carmeliet & Jain 2011; Wang et al. 2013). There is a rapid increase in angiogenesis when a tumour transits into the malignant state as a result of a process called the ‘angiogenic switch’ (Guo et al. 2013). VEGF-A is one of the critical factors responsible for augmentation of the tumour vascular bed, which is characterized by abnormal features such as high turnover of neovessels, poor perfusion and increased leakage (Claesson-Welsh & Welsh 2013). VEGF is overexpressed in hypoxic tumour cells (Carbajo-Pescador et al. 2013); endothelial cells (ECs) and tumour-associated macrophages (TAMs) (Guo et al. 2013). The majority of studies have shown that VEGF signalling in tumour cells is autocrine in nature, characteristic of more aggressive cancers, although paracrine signalling also occurs (Goel & Mercurio 2013).
Breast cancer, being the second most common malignancy worldwide, with 1.7 million new cases in 2012 (Ferlay et al. 2015), is known to have a poor prognosis due to the specific pattern of metastasis (Steeg 2006). High levels of VEGF mRNA or VEGF have been reported in invasive ductal breast carcinoma compared to benign or normal tissue (Shivakumar et al. 2009; Ali et al. 2011). Among all the other types of invasive ductal breast carcinomas, the most aggressive, highly metastatic and challenging to treat is the triple-negative breast cancer (TNBC) (Fan et al. 2006), which cannot be treated with targeted therapy such as herceptin or tamoxifen and is bound to have the worst prognosis (Yin et al. 2009). However, VEGF has become a potential therapeutic target in a number of solid malignancies, including TNBC. Clinical trials are now evaluating the potential therapeutic agents that either inhibit VEGF or block VEGFR-2 (Dent 2009; Lu et al. 2016). Thus, targeting tumour growth by angiotherapy is considered as one of the best strategies for anticancer therapy (Bikfalvi & Bicknell 2002), thereby the problems of cytotoxicity and chemo-resistance associated with the classical chemotherapies can be overcome to a certain extent.
Apart from antagonizing VEGF, small molecules such as vatalanib, tivozanib, cediranib, and lenvatinib have been shown to inhibit receptor tyrosine kinase (RTK) signalling (Hojjat-Farsangi 2014). A wide variety of compounds of plant origin has been reported to exhibit anti-angiogenic activity through various molecular pathways. Development of new small molecules is based on the discovery of bioactive secondary plant metabolites and new phytochemical drugs that target tumour angiogenesis and induce apoptosis in cancer cells. Plant polyphenols, catechins, flavonoids, terpenes, tannins, alkaloids and polyacetylenes comprise the natural anti-angiogenic phytochemicals (Lu et al. 2016). Compounds such as taxol, camptothecin and combretastatin have been reported to have potent anti-angiogenic properties (Fan et al. 2006). Further, anti-angiogenic effects through inhibition of VEGF signalling have been reported from dietary functional foods such as genistein from soybean, epigallocatechin gallate from green tea, and resveratrol from red grapes. With respect to cancer therapy, a number of important new commercialized drugs have been synthesized, by structural modification of natural compounds (Gordaliza 2007).
This study was conducted to validate the effectiveness of complementary and alternative medicine by evaluating the anti-angiogenic properties of four plant extracts; ethyl acetate (EA) and n-butanol (NB) fractions from seeds of Eugenia jambolana Lam. (Myrtaceae), EA fraction from the roots of Musa paradisiaca L. (Musaceae) and EA fraction from leaves of Coccinia indica Wight & Arn. (Cucurbitaceae). Although our previous studies have reported the antihyperglycemic and antioxidative bioactivities of these plant sources (Chatterjee et al. 2009), various other studies have reported a myriad of biological activity that also includes a potent anticancer activity (Baliga 2011; Pekamwar et al. 2013; Nadumane & Timsina 2014). In order to study the role of these extracts on tumour growth and inhibition of VEGF-induced angiogenesis in vitro, two invasive ductal breast carcinoma cell lines, namely, MCF-7, MDA-MB-231 (triple-negative) and human umbilical vein endothelial cells (HUVEC) were used. Ehrlich ascites tumour (EAT), a murine mammary carcinoma model was used for in vivo studies. In the current study, we report the effect of plant extracts on tumour burden, ascites volume, tumour cell number, peritoneal angiogenesis, and VEGF secretion in ascites. Besides, the anti-angiogenic property of the plant extracts has also been evaluated in a non-tumour context using corneal micro-pocket and CAM assays. Experiments to evaluate the mode of EAT cell death was studied by nuclear staining and cell cycle analysis. Comprehensively, out of the four extracts, our data suggests that two extracts, namely, EA fraction of E. jambolana and EA fraction of M. paradisiaca have promising anti-angiogenic and pro-apoptotic activity. Further, the active molecules from these plant extracts are potential candidates for developing alternative and complementary treatments for breast cancer patients.
Materials and methods
Plant material collection and extraction
Fresh seeds, leaves and roots of E. Jambolana, M. paradisiaca and C. indica were collected from rural areas of Paschim Midnapur District, West Bengal, India in May–July 2012. Identification of the plants was made and a voucher specimen (HPCH No. 8, 7 and 6) was deposited in the Botany Department, Vidyasagar University, Midnapur, India. The plant materials were separated washed thoroughly first with tap water then with deionized water and dried in an incubator completely at 37 °C. About 900 g of dried seeds were collected from 1 kg fresh seeds, 200 g of dried leaves were collected from 1 kg of fresh leaves and 650 g of dried roots were collected from 1 kg of fresh roots and pulverized separately in an industrial electrical grinder. The pulverized material was macerated in separate 20 L percolators with hydro-methanol solvent (H2O: MeOH:: 40:60, v/v) (for each 50 g of plant part used at least 250 mL of solvent) at 35 °C with an intermittent stirring for the first 2 h and left for 36 h at 37 °C. This extraction process was repeated four times using freshly prepared hydro-methanol solvent and the final extracts were collected on the 4th day. The extracts were then filtered first through cotton filter followed by No. 1 Whatman filter paper. The hydro-methanol filtrates were vacuum evaporated using Rotavapor (HAHN-SHIN, HS-2000NS, Hahn-Shin Scientific Co., Korea) at 38 °C and lyophilized on bench top K Lyophilizer and finally stored in amber glass containers refrigerated under vacuum for subsequent fractionation.
These dried hydro-methanol extracted powders were subjected to fractionation with laboratory grade solvents (non-polar to polar), dried under partial vacuum at 38 °C to collect the solvent free residues. Each fraction was stored in amber glass containers at 4 °C for experimental use. The extract preparation and fractionation were performed following the method described earlier (Wagner & Bladt 1996) with slight modification.
Preparation of the plant extracts
The plant extracts (5 mg); EA and NB fractions from seeds of E. jambolana; EA fractions from the roots of M. paradisiaca and leaves of C. indica were dissolved in 0.1% dimethyl sulfoxide (DMSO). Then the volume was made up to 5 mL with phosphate buffered saline (PBS) and filter sterilized using a syringe filter (Millipore, 22 microns) to give a final concentration of 1 μg/μL. All extracts were stored at 4 °C.
Animals, cell lines, and chemicals
Female Albino-Wistar rats (4–6 months old) and Swiss albino mice (6–8 weeks old) were obtained from the animal house, department of studies in Zoology, University of Mysore, Mysuru, India. EAT cells are maintained in our laboratory and are routinely used for in vivo transplantation.
All experiments were approved by the Institutional animal care and use committee of the University of Mysore, according to the guidelines from the committee for purpose of control and supervision of experiments on animals (approval number 122/GO/ReBi/S/99/CPCSEA), the government of India. HUVECs and endothelial growth medium (EGM) were obtained from Cambrex Biosciences, Walkersville, MD. MCF-7 and MDA-MB-231 cell lines were from National Centre for Cell Sciences, Pune, India. 3[H]thymidine was from Baba Atomic Research Center, Mumbai, India. DMEM, L15, FBS and penicillin-streptomycin were from Invitrogen, Carlsbad, CA. Poly-2 hydroxyethyl methacrylate (poly 2-HEMA) from Sigma-Aldrich, St. Louis, MO, Fertilized chicken eggs were from the government poultry farm, Bangalore, India. Matrigel was from Becton Dickinson Labware, Bedford, MA. Recombinant vascular endothelial growth factor (rVEGF165) was expressed and purified in-house using the pET-3d-VEGF plasmid. All the other reagents were of the highest analytical grade.
MTT assay
MDA-MB-231 cells, growing in exponential phase were seeded into 96-well plates (Nunc MicroWell™) in triplicates at an initial density of 3 × 104 cells per well in 100 μL of complete medium. Following 16 h incubation, filter sterilized plant extracts were added to the culture medium at 2, 5, 10, 20, 40, 80, 100, and 200 μg/mL concentrations with appropriate controls and blanks. Cells were incubated for 24 h. Later, 20 μL MTT (5 mg/mL) reagent (Chemicon International, Inc., Temecula, CA) was added and incubated for 4 h at 37 °C. For solubilization of the resultant formazan product, 100 μL of DMSO was added. The absorbance was measured using a multimode reader at a test wavelength of 570 nm and a reference wavelength of 630 nm. (Varioskan™ Flash Multimode Reader, Thermo Scientific). Data obtained are expressed as the percentage of control mean ± SEM of triplicate values.
Cell proliferation assay using 3[H]thymidine
3[H]thymidine incorporation assay was performed as described previously (Raj et al. 2016). To study the in vitro effect of plant extracts on the proliferation of HUVEC, MCF-7 and MDA-MB-231 cells, (3 × 104) were seeded into separate 12 well plates in their respective growth medium and were grown for 48 h. On the third day, cells were serum starved for 24 h in media containing 0.1% serum and treated with plant extracts (20 μg/mL) along with rVEGF (10 ng). The negative control wells were vehicle (0.1% DMSO) treated while the positive control wells were rVEGF (10 ng) treated. 3[H]thymidine (1 μCi/mL), was added to each well and incubated for 4 h. The cells were washed with PBS, high molecular weight DNA was precipitated using 10% trichloroacetic acid at 4 °C for 30 min. After two washes with ice-cold PBS, the pellet was solubilized in 0.2 N NaOH and 0.1% SDS and taken into scintillation vials containing 5 mL of scintillation cocktail. The incorporated 3[H] radioactivity was measured using a liquid scintillation counter (Perkin Elmer Tri-Carb 2900 TR model, Shelton, CT). All the data expressed as the percent mean ± SEM of triplicate values in comparison to positive control.
In vitro tube formation assay
Tube formation of HUVEC was performed to assess the effect of plant extracts on in vitro angiogenesis as described previously (Ramachandra et al. 2009). A flat bottomed 96 well plate (Nunc) was coated with 50 μL of Matrigel and allowed to solidify at 37 °C for 1 h. HUVECs (5 × 103) were seeded on the Matrigel in 100 μL complete EGM medium containing vehicle (0.1% DMSO) for the negative control, rVEGF (10 ng) for the positive control and the four plant extracts (20 μg/mL) along with rVEGF (10 ng) for tests were incubated for 24 h. After incubation, the enclosed networks of complete tubes formed were quantified by enumerating the network branch points formed in randomly chosen fields by photographing at 200 × magnification under an Olympus inverted microscope (CKX40; Olympus, New York). The average branch points were counted using image J1.49 u software (National Institutes of Health, Bethesda, MD).
Anti-angiogenic effect of plant extracts treatment in vivo
Ehrlich ascites tumour is derived from a murine mammary carcinoma and is an experimental model for breast cancer. EAT cells (5 × 106) were injected intraperitoneally (i.p.) into mice (5 groups, 5n) and tumour growth was recorded every day from the day of transplantation. To verify whether the plant extracts inhibit tumour growth and angiogenesis mediated by EAT cells in vivo, the four different extracts (100 mg/kg body weight/day) was injected into the peritoneum of the respective groups of EAT bearing mice every day from the 6th day of transplantation. The weights of the mice were monitored from the 1st day till the 12th day. On the 12th day, the animals were sacrificed and saline (2 mL) was injected (i.p.), and a small incision was made in the abdominal wall to harvest the tumour cells along with ascites fluid, centrifuged at 3000 g for 10 min. The volume of ascites formed in both untreated and treated mice was recorded. The pelleted EAT cells were counted by trypan blue dye exclusion method using a hemocytometer. The animals were dissected and the exposed peritoneum was examined for neovascularisation and photographed.
VEGF-enzyme linked immunosorbent assay (VEGF-ELISA)
Recombinant human VEGF165 was used to set up the standard curve. An indirect VEGF-ELISA was performed using ascites fluid harvested from untreated tumour bearing mice as well as plant extracts treated mice as described previously (Lingaraju et al. 2008). In brief, 100 μL of 1:1000 diluted ascites sample from all the treated or untreated mice were coated onto 96-well microplate using carbonate-bicarbonate coating buffer (pH 9.6) at 4 °C overnight. Wells were washed with PBS and blocked with blocking buffer (5% skimmed milk powder in PBS) for 2 h at 37 °C, followed by incubation with the anti-VEGF165 primary antibody (1:1000). After 2 h incubation, wells were washed, 100 μL/well of goat anti-rabbit IgG conjugated to alkaline phosphatase secondary antibody (1:2000) was added. Post 2 h incubation; wells were washed prior to addition of 100 μL of the substrate, p-nitrophenyl phosphate (p-NPP). After 30 min, the reaction was terminated by adding 0.1 N NaOH; the absorbance was read at 405 nm using the Infinite 200 PRO multimode plate reader (Tecan, Männedorf, Switzerland).
Microvessel density scoring
For the histological studies of peritoneal neovasculature, the peritoneal tissue was excised from all the mice of each group and fixed in 10% formalin solution. Microtome sections (5 μm) were made from paraffin embedded peritoneum and stained with hematoxylin and eosin. Microvessel density was assessed using a bright field microscope in 10 fields of the vascularized areas under high power (40 ×) and the average MVD/HPF was noted and photographed at 40 × magnification.
Rat corneal micro-pocket assay
The corneal micro-pocket assay was performed as described by Nagaraj et al. (2015) to assess the anti-angiogenic effect of plant extracts in vivo. Briefly, poly 2-HEMA pellets were formulated in ethanol 12.5% W/V. Aliquots of 10 μL of this solution was taken on a Teflon sheet and vehicle (0.1% DMSO) for the negative control group, rVEGF (10 ng) for positive control group and different plant extracts (20 μg/mL) along with rVEGF (10 ng) for the test group were placed onto the pellet and dried overnight at 4 °C. Wister rats weighing 300–350 g were anesthetized with an i.p. injection of ketamine (87 mg/kg) and xylazine (13 mg/kg). A corneal micro-pocket was made using a sterile scalpel, with the pocket’s base 1 mm from the limbus. A single pellet was advanced into the lamellar pocket to the limbus using corneal forceps. Postoperatively, gentamicin eye drops were applied onto the operated eye. On the 7th day, the rats were anesthetized again and the corneas were photographed using a stereo binocular microscope with CCD camera attachment, Stereo Discovery V20, Carl Zeiss, Germany. The lengths of blood vessels from the limbus were measured using image J1.49 u software (National Institutes of Health, Bethesda, MD).
Shell-less CAM
The shell-less CAM assay was performed according to Nataraj and Salimath (2013) to study the effect of the plant extracts on the neovasculature. Fertilized chicken eggs were surface sterilized with 70% alcohol and incubated at 37 °C (fan-assisted humidified incubator). Eggs were gently rolled periodically. On day 4, eggs were cracked open and placed onto the cling film hammocks aseptically in a laminar hood. Egg preparation was covered with a sterile Petri dish and transferred to humidified incubator at 37 °C for 48 h. On day 6, sterile filter discs (2 mm), saturated with the plant extracts (20 μg) along with rVEGF (10 ng), rVEGF (10 ng) alone or vehicle (0.1% DMSO) were placed directly over a blood vessel and placed back in the incubator. After 72 h of incubation, the membrane was examined for inhibition of neovascularization. The CAM was photographed under a stereo binocular microscope at 10 × magnification and the total neovascular blood vessels per unit square millimeter (mm2) were quantified using Image J1.49 u software (National Institutes of Health, Bethesda, MD).
Acridine orange-ethidium bromide nuclear staining
Analysis of morphological changes to assess the mode of cell death in the in vivo treated EAT cells was performed using acridine orange and ethidium bromide (AO/EtBr) dual staining. Briefly, EAT cells were harvested from all the groups of mice post-treatment as mentioned earlier and first washed with 0.4% ammonium chloride solution to remove RBCs, followed by two rounds of PBS washes. 1 mg/mL AO/EtBr mixture (10 μL) was added to the cell pellet in PBS and 20 μL of cells were placed on a microscopic slide with a cover slip and immediately analyzed under a fluorescence microscope with the fluorescein filter at 40 × magnification (Zeiss AxioVert). Nuclei were visualized; apoptotic cells in ten random fields were counted and photographed.
Flow cytometric analysis of cell cycle and apoptosis
EAT cells (3 × 106) were seeded in a 6 well plate in complete DMEM media and treated with plant extracts (20 μg/mL) for 24 h at 37 °C and 5% CO2 along with vehicle (0.1% DMSO) treated control. Post-treatment, the cells were washed with PBS and fixed overnight in ice-cold 70% ethanol. After fixation, 1 × 106 cells were washed twice with PBS, and the cells were resuspended in the propidium iodide (PI) staining buffer (25 μg/mL PI; 40 μg/mL RNase and 0.03% Igepal in PBS) and analyzed after 30 min in FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). Cells were analyzed for DNA content in triplicates and the fraction of every cell cycle phases including the sub G1 phase was determined.
Determination of total phenol and flavonoids contents in the effective plant extracts
The Folin-Ciocalteu’s assay was performed to measure the total amount of polyphenol content (Andzi-Barhé et al. 2015). Eugenia jambolana seed extract (0.25 mL) and M. paradisiaca root extract (1 mg/mL) were mixed with 1.25 mL Folin–Ciocalteu reagent (0.2 N diluted in methanol). Methanol was used as a reagent blank. After 5 min incubation at room temperature, 1 mL of sodium carbonate solution (75 g/L) was added. The absorbance was measured at 765 nm after 2 h incubation at room temperature. The total phenol content was expressed as mg of Gallic acid equivalents (GAE) per 100 g of extract.
Quantification of flavonoids was performed using aluminium chloride (Andzi-Barhé et al. 2015). Different extracts (1 mg/mL) were mixed in 1 mL of AlCl3 (2%). The absorbance was measured at 415 nm after 10 min of incubation. Results were expressed as mg of Quercetin equivalents (EQ) per 100 g of extract.
Statistics
All the experiments were performed in triplicate with a minimum of three replicates. The means and standard deviations were calculated and values are expressed as mean ± SEM. The significance of the differences among the treatments was determined using one-way analysis of variance (ANOVA). p-Values lower than 0.05 (p < 0.05) were considered statistically significant and those lower than 0.01 (p < 0.01) were considered extremely significant.
Results
Cytotoxicity of extracts
The effect of different plant extracts on the metabolic activity and consequently the cytotoxicity was assessed by the MTT assay in MDA-MB-231 cells. Increasing concentrations of the four plant extracts were used and the effective concentration was calculated from the dose-response curve along with the IC50 values. The percentage cytotoxicity in comparison to the vehicle treated control is shown in Figure 1(a). The EA fraction of E. jambolana and the EA fraction of M. paradisiaca exhibited significant cytotoxicity with an IC50 value of 25 and 60 μg/mL, respectively. On the contrary, NB fraction of E. jambolana and the EA fraction of C. indica did not exhibit any substantial cytotoxicity.
Figure 1.
Effects of E. jambolana, M. paradisiaca, and C. indica extracts on cell proliferation. (a) The metabolic and cytotoxic response of MDA-MB-231 cells assessed by MTT assay. MDA-MB-231 cells (3 × 104) were treated with plant extracts (2, 5, 10, 20, 40, 80, 100, and 200 μg/mL) for 24 h, washed with PBS, incubated for 4 h with MTT and the formazan crystals dissolved in DMSO and read at 570 nm. (b) Cell proliferation assessed by the rate of DNA synthesis using 3[H]thymidine. HUVEC, MCF-7 and MDA-MB-231 cells (3 × 104) were cultured in 12-well plates, serum starved and treated with plant extracts (20 μg/mL) along with rVEGF (10 ng) for 24 h. Post incubation for 4 h in presence of 3[H]thymidine, the DNA was harvested and rate of cell proliferation was measured in a liquid scintillation counter. (c) HUVEC tube formation assay. HUVECs (5 × 103) were cultured in EGM on Matrigel with plant extracts (20 μg/mL) along with rVEGF (10 ng), in a 96 well plate. After incubation for 24 h at 37 °C, capillary networks were photographed and quantified (Magnification: 200 ×). (d) Quantification of angiogenesis by counting the average number of branch points. Data in all results presented as mean ± SEM of three independent experiments; **p < 0.01 and *p < 0.05.
Inhibition of cell proliferation
HUVEC, MCF-7 and MDA-MB-231 treated with or without the four plant extracts (20 μg/mL) along with rVEGF (10 ng) for 24 h resulted in considerable decrease in DNA synthesis as measured by the incorporation of 3[H]thymidine, which was prominent in cells treated with two of the plant extracts namely the EA fraction of E. jambolana and the EA fraction of M. paradisiaca as shown in Figure 1(b). Significant inhibition of up to 71.2% and 61.6% in HUVEC, 81% and 71% in MCF-7 and 77% and 75% in case of MDA-MB-231 cells proliferation was observed, respectively.
Suppression of in vitro angiogenesis
The anti-angiogenic potential of all four plant extracts was evaluated by the in vitro tube formation assay, mediated by HUVECs. In the positive control wells with vehicle (0.1% DMSO) and rVEGF (10 ng), HUVECs differentiated into an extensive and enclosed network of tubes. However, the treatment with plant extracts inhibited the tube formation even in presence of rVEGF as shown in Figure 1(c,d). The degree of HUVEC mediated angiogenesis was assessed based on the number of branch points by the image J analysis, which revealed that among all the four extracts the EA fraction of E. jambolana and EA fraction of M. paradisiaca showed sparse tube networks and acute inhibition of tube formation by 83 and 76%, respectively, compared to rVEGF treated positive control.
Inhibition of tumour growth and neoangiogenesis in vivo
According to the results in Figure 2(a–c), the vehicle treated EAT bearing control mice showed a steady increase in body weight (8–10 g), the volume of ascites fluid secreted as well as the EAT cell number over a span of 12 days. However, the mice in the treatment groups, specifically the treatment with EA fraction of E. jambolana and EA fraction of M. paradisiaca exhibited almost 50% reduction in body weight compared to control indicating the effectiveness of the extracts in the prevention of EAT tumour growth. Concurrently a 2.3- and 2.2-fold reduction in secreted ascites fluid along with 16.5- and 14.7-fold reduction in viable cells in comparison to control was observed post-treatment with the above-mentioned extracts, respectively. Although the NB fraction of E .jambolana and EA fraction of C. indica did show a reduction in body weight, ascites volume, and cell number, it was not on par with that of EA fraction of E. jambolana and EA fraction of M. paradisiaca.
Figure 2.
Effects of plant extracts on tumour growth and angiogenesis in vivo. (a) Body weights of EAT-bearing; untreated and treated with plant extracts were recorded. 6th day onwards, plant extracts (100 mg/kg body weight) was administered (i.p) for six days. The animals were sacrificed on the 12th day. EAT cells were collected along with ascites fluid and centrifuged, (b) secreted ascited volume, (c) EAT cell count assessed by trypan blue dye exclusion method, (d) representative photographs of the peritoneum of the untreated and treated mice and (e) peritoneal MVD marked by arrows in each peritoneum assessed by H and E staining of sections. At least five mice were used in each group and the results obtained are an average of three individual experiments and means of ± S.E.M. n = 5 per group.
Decreased microvessel density (MVD)
An acute reduction in peritoneal angiogenesis was conspicuous in treated groups of mice compared to control EAT bearing mice, shown in Figure 2(d). The smaller capillaries and arterioles were affected by the treatment suggesting inhibition of tumour neoangiogenesis.
Further authentication of the angio-inhibitory effect of the compounds was assessed by histological examination of the peritoneum sections by H and E staining and microscopic examination of the microvasculature. From the photomicrographs (40 × magnification), (Figure 2(e)), it is evident that the MVD is drastically reduced in the peritoneum of mice treated with the EA fraction of E. jambolana and EA fraction of M. paradisiaca compared to control or other treatments.
Angio-inhibitory effect of plant extracts
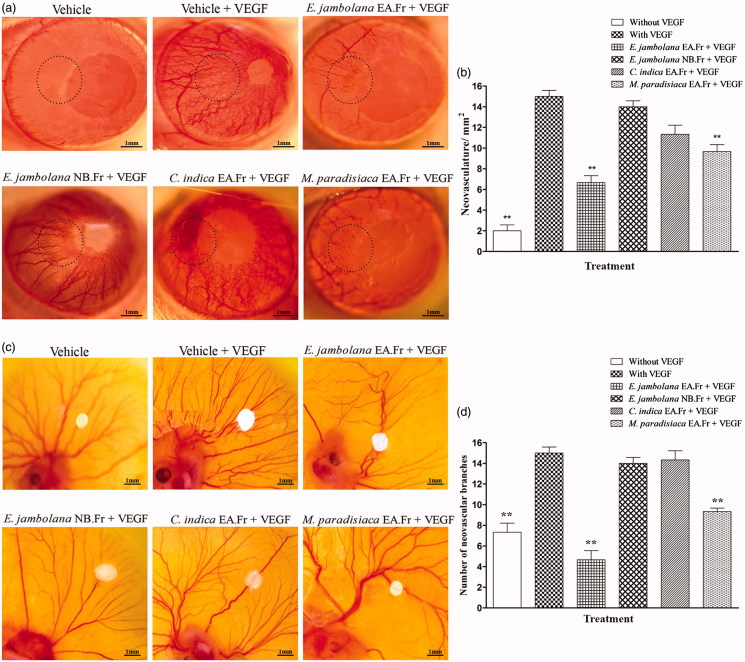
The rat corneal micro-pocket assay and the shell-less chorioallantoic membrane assay are generally used in the in vivo assessment and validation of the angio-inhibitory potential of anti-angiogenic compounds in a non-tumour context. Results (Figure 3(a–d)) clearly indicate that the EA fraction of E. jambolana and EA fraction of M. paradisiaca are efficient inhibitors of neovasculature even in presence of rVEGF (10 ng) in vivo, both in rat cornea and the CAM in comparison to rVEGF (10 ng) treated positive control which showed extensive angiogenesis.
Figure 3.
Effects of plant extracts on neovasculature in the rat cornea and chick embryo CAM assays. (a) Representative photographs of VEGF-induced rat corneal neovascularization, plant extracts 20 μg/hydron polymer pellet were surgically implanted into the micro-pocket in the cornea of one eye. On day 7, the extent of neovascularization or inhibition was visualized and photographed under a dissection microscope. (b) Quantitative comparison of the number of neovascular vessels per mm2 was estimated. (c) Representative photographs of VEGF-induced neovascularization in shell-less CAM of chicken embryos. Plant extracts, 20 μg/filter disc was placed on the CAM of 6-day old chicken embryos. After 72 h of incubation, the area surrounding the filter disc was inspected for changes in neovascularization. (d) Quantitative comparison of the number of neovascular branches surrounding the plant extract containing filter paper discs. The data shown represent the results of experiments that were performed using a maximum of 5 eggs in each group. All quantitative data are presented as mean ± SEM of five independent counts; **p < 0.01.
In the rat cornea, the effect of EA fraction of E. jambolana and EA fraction of M. paradisiaca on the reduction of neovasculature/mm2 was 2.5- and 1.5-fold, respectively. Similarly, in the CAM assay, the reduction in the number of vascular branches was 3- and 1.6-fold, respectively.
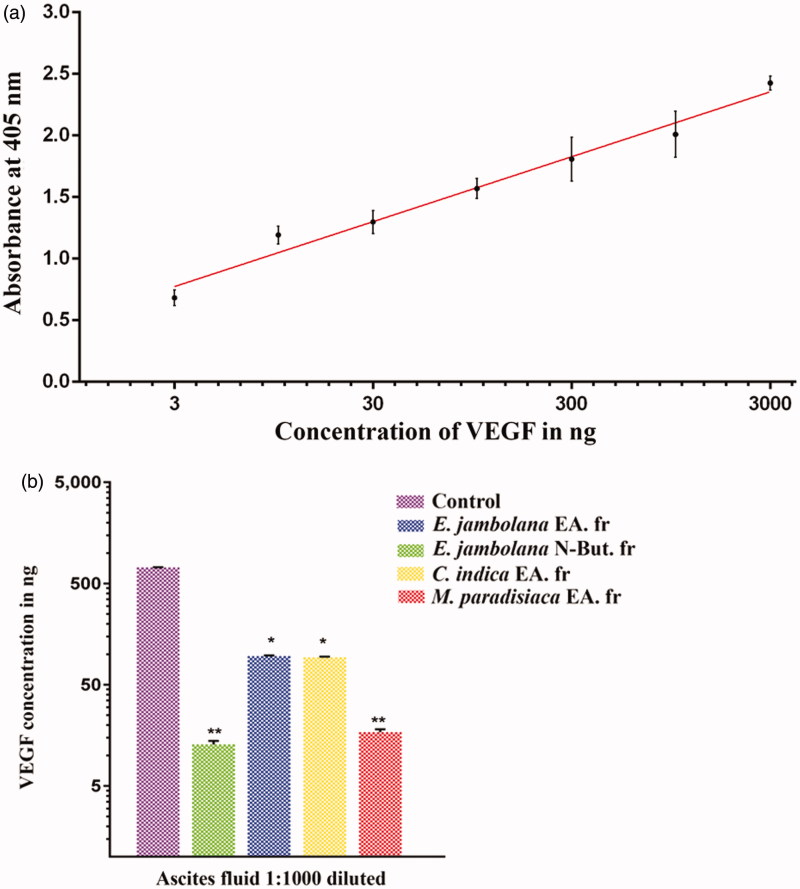
Inhibition of VEGF production in EAT cells
The VEGF standard curve was observed to have good linearity as shown in Figure 4(a). The ELISA results showed elevated levels of VEGF in the ascites fluid taken from the tumour-bearing control mice (721 ng). However, a significant decrease (∼90%) in VEGF levels was observed in the groups treated with EA fraction of E. jambolana (13 ng) and EA fraction of M. paradisiaca (17 ng). But groups treated with NB fraction of E. jambolana and EA fraction of C. indica showed a comparatively lower decrease in VEGF levels of 96 and 93 ng, respectively, (Figure 4(b)), suggesting the inhibition of VEGF secretion in vivo.
Figure 4.
Effects of plant extracts on the secretion of VEGF in ascites fluid of tumour bearing mouse. Indirect ELISA was carried out using the ascites fluid harvested from the control (tumour bearing) and plant extracts treated (100 mg/kg body weight) mice to quantify the VEGF in ascites fluid using anti-VEGF165 antibodies. (a) ELISA standard curve for VEGF. (b) The histogram showing a comparison of VEGF levels in the ascites of untreated control and treated groups. Data are representative of three independent experiments and values are expressed in mean ± SEM, **p < 0.01 and *p < 0.05.
Effects of plant extracts treatment in vivo on EAT cell morphology
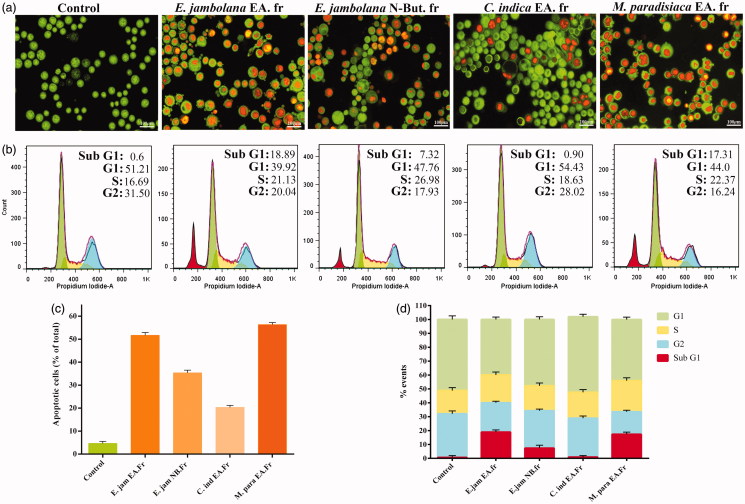
EAT cells, post-harvest from treated and control groups were dual stained with acridine orange and ethidium bromide and examined under a fluorescent microscope. No significant morphological changes were observed in the vehicle-treated control cells, most of them appeared green with intact nuclei. However, the cells from the treated groups showed the early and late stages of apoptosis, manifested by the shrunken and crescent-shaped orange nuclei, membrane blebbing and apoptotic bodies containing fragmented nuclei. The percentage of apoptotic cells was determined by counting the number of apoptotic cells under the microscope in ten random fields in comparison to the vehicle treated control. Results, (Figure 5(a,c)) clearly show increased apoptosis induction by 51% and 56% by the EA fraction of E. jambolana and EA fraction of M. paradisiaca treatments, respectively.
Figure 5.
Apoptosis induction and cell cycle analysis of plant extract treated EAT cells. (a) In vivo treated EAT cells with or without plant extracts were stained with acridine orange and ethidium bromide and verified for apoptotic characteristics such as plasma membrane blebbing, chromatin condensation and apoptotic body formation under a fluorescence microscope. (b) Effect of plant extracts on cell cycle progression. EAT cells, in vitro, were treated with or without different plant extracts (20 μg/mL). After 24 h of treatment, distribution of cell cycle phases and apoptotic cell population was quantitated based on flow cytometric analysis. (c) A quantitative comparison of the number of apoptotic cells in 10 random fields. (d) Bar diagram showing the percentage of cells present in different phases of cell cycle.
Plant extracts induce apoptosis without cell cycle arrest
The inhibitory effect of plant extracts on EAT cell proliferation was ascertained by cell cycle analysis by flow cytometry. EAT cells; post-treatment with plant extracts for 24 h, the cell cycle distribution was analyzed by flow cytometry following staining with propidium iodide (PI). A significant accumulation of cells was observed in sub-G1 phase in cells treated with EA fraction of E. jambolana (20%) and EA fraction of M. paradisiaca (18.38%), characteristic of apoptosis, whereas treatments with NB fraction of E. jambolana and EA fraction of C. indica showed 7.53% and 0.94%, respectively as shown in Figure 5(b,d). However, no cell cycle arrest was observed upon treatment.
Total phenolic and flavonoids content
The results (Table 1) revealed that the bioactive EA fraction of seed extract of E. jambolana had a comparatively lower content of phenolic compounds (18.54 mg GAE/100 g of extract) in comparison to the flavonoids content (122.32 mg EQ/g of extract). Whereas the EA fraction of root extract of M. paradisiaca showed high phenolic compounds content (118 mg GAE/100 g of extract) in comparison to the flavonoids (78 mg EQ/g of extract).
Table 1.
The phytochemical analysis (total phenol and flavonoids content) of the two plant extracts, namely, EA fraction of Eugenia jambolana and EA fraction of Musa paradisiaca were performed and the values obtained are as shown.
| Plant extract | Total phenolic content (mg GAE/g dried extract | Total flavonoids (μg EQ/g dried extract) |
|---|---|---|
| Ethyl acetate fraction of seed of E. jambolana | 18.54 | 122.32 |
| Ethyl acetate fraction of root of M. paradisiaca | 118 | 78 |
GAE: Gallic acid equivalents; EQ: Quercertin equivalents; E. jambolana: Eugenia jambolana; M. paradisiaca: Musa paradisiaca.
Discussion
Sustained angiogenesis, being a hallmark of cancer, has a fundamental role in tumour growth, invasion and metastasis. In pathological conditions like chronic inflammation, diabetic retinopathy, rheumatoid arthritis or atherosclerosis, angiogenesis is typically upregulated (Quesada et al. 2006). Thus, the prime importance and the understanding of new blood vessels formation have led to novel therapies designed to interrupt this process (Miller et al. 2001). As part of our search for a natural product based anti-angiogenic agents, we studied the effects of the four plant extracts; EA and NB fractions from seeds of Eugenia jambolana, EA fraction from the roots of Musa paradisiaca and EA fraction from leaves of Coccinia indica.
Various bioactive chemical compounds of plant origin may influence the angiogenic potential of various cell types and hence upsets the formation of blood vessels (Loboda et al. 2005; Neal et al. 2006). Several alkaloids such as sanguinarine, isolated from the root of Sanguinaria canadensis L. (Papaveraceae) and vinca alkaloids obtained from Catharanthus roseus (L.) G. Don (Apocynaceae) have been exploited to target angiogenesis (Eun & Koh 2004; Ribatti et al. 2005). Halofuginone, an alkaloid isolated from Dichroa febrifuga Lour. (Hydrangeaceae), is a potent inhibitor of crucial steps in angiogenesis cascade (Elkin et al. 2000). The primary strategy for the screening of novel anti-angiogenic compounds is to look for EC growth inhibitors (Quesada et al. 2006). Also, the invasive ability of the EC requires extracellular matrix degradation and involves the activation of many EC signalling pathways which can also be studied. Pharmacologically important bioactive organic compounds such as phenols, flavonoids, tannins, saponins, and terpenes have better solubility in solvents such as ethyl acetate and n-butanol. Hence in this study, we screened the plant extracts obtained from the EA and NB fractions. Furthermore, we chose to study the anti-angiogenic property of these extracts based on our previous work on antioxidant and antidiabetic properties of these extracts (Mallick et al. 2006, 2009; Ghosh 2015). The preliminary toxicity profile of all the four plant extracts exhibited cytotoxicity in a dose-dependent manner as assessed by MTT assay. However, EA fraction of E. jambolana and EA fraction of M. paradisiaca showed high cytotoxicity with lower IC50 values compared to NB fraction of E. jambolana or EA fraction of C. indica. Subsequently, our studies with a subtoxic concentration of all the extracts on the cell proliferation assay using a normal cell line (HUVEC) and two breast cancer cell lines (MCF-7 and MDA-MB-231) revealed a significant reduction in rate of cell proliferation even in the presence of a potent cytokine-VEGF, which may suggest the interference of VEGF signalling by the bioactive constituents in the plant extracts. Numerous plant compounds possess anti-angiogenic property by disrupting angiogenesis signalling cascade (Lee et al. 2015), like the capsaicin, curcumin or quercetin-mediated inhibition of VEGF-induced angiogenesis (Gururaj et al. 2002; Min et al. 2004; Pratheeshkumar et al. 2012).
The inhibition of tumour angiogenesis, growth and metastasis by means of phytochemicals is reportedly due to the regulation of signalling pathways of VEGFR (Fukuda et al. 2006). Thus, to further inspect whether this curtailment in the rate of cell proliferation was directly due to cytotoxicity or an inherent anti-angiogenic property, the in vitro endothelial tube formation assay was performed using HUVECs in presence of VEGF. Our results showed that both the EA fraction of E. jambolana and EA fraction of M. paradisiaca were indeed anti-angiogenic and effectively blocked VEGF-induced angiogenesis in vitro.
To further validate these results, the next step was to evaluate the antitumor, anti-proliferative and anti-angiogenic properties of these extracts in vivo. Hence, we adopted the murine mammary carcinoma, EAT model for the study. The in vivo data showed a drastic reduction in tumour burden, EAT cell number, ascites formation and also secreted VEGF levels which bear significant importance in terms of a clinical correlation with inhibited ascites formation in human tumours. The concomitant reduction in the peritoneal neovasculature and corresponding decrease in the MVD was observed in the peritoneum of the treated mice.
The substantiation of the anti-angiogenic property of the two plant extracts further led us to perform the classical in vivo and ex vivo angiogenic assays, namely, the rat corneal micro-pocket assay and shell-less CAM assay (Wang et al. 2004), respectively. The results of these two experiments were consistent with the earlier results where the number of new blood vessels was significantly decreased upon treatment with EA fraction of E. jambolana or EA fraction of M. paradisiaca, in comparison to VEGF treatment that showed increased neoangiogenesis.
Furthermore, apart from the anti-angiogenic activity of the plant extracts, the EAT cells harvested after in vivo treatment interestingly showed induction of cell death by apoptosis, which was rather pronounced in the EA fraction of E. jambolana and EA fraction of M. paradisiaca, confirmed by the AO/EtBr dual stained morphological study and also by the cell cycle analysis. These results suggest that the extracts may also contain potent pro-apoptotic compounds.
The plant extracts showing significant bioactivity could be attributed to the high flavonoid content in E. jambolana seeds, since flavonoids have been reported to reduce the risk of development of breast cancer and prostate cancer by induction of apoptosis, reducing oxidative stress (Afsar et al. 2016) and also inhibit VEGF-induced cell proliferation and migration in HUVECs, as well as angiogenesis (Wu et al. 2012). Also, the high phenolic content in the M. paradisiaca roots may be contributing to the anti-angiogenic, anti-proliferative and pro-apoptotic properties since phenols have been known to play a critical role in inhibition of VEGF, PDGF receptor phosphorylation, MMP inhibition, cell migration, inhibition of ROS (Sun et al. 2015) and also induction of apoptosis in cancer cells (Roy et al. 2002).
Conclusions
Taken together, representing the sequential events in the angiogenic process, the two plant extracts, namely, EA fractions of E. jambolana seeds and M. paradisiaca roots, showed strong anti-proliferative and anti-angiogenic effects in breast cancer cell lines through the inhibition of VEGF-induced cell proliferation, HUVEC tube formation in vitro as well as suppression of VEGF-induced rat corneal neovascularization in vivo and also CAM neovascularisation ex vivo. In addition, in the in vivo EAT mouse tumour model, there was a profound reduction in EAT tumour growth, ascites volume, ascites VEGF levels, tumour cell number and also peritoneal angiogenesis. The anti-proliferative effect was evident in the EAT cells, which clearly showed cell death by induction of apoptosis. Thus, in conclusion, all the results advocate an authenticated evidence that the two plant extracts have a potent therapeutic potential in the treatment of cancer or angiogenesis-related disorders. The findings of this study contribute to the pharmacological knowledge and the therapeutic efficacy of E. jambolana and M. paradisiaca, and can initiate the development of new anti-angiogenic drugs. However, further studies to identify the individual bioactive compounds and their translational research is essential to elucidate their explicit mechanisms.
Funding Statement
The University Grants Commission Special Assistance Programme Department of Special Assistance (UGC-SPA-DSA) Government of India [No. f 4-1/2013(SAP-II)] is acknowledged.
Disclosure statement
The authors report no declaration of interest.
References
- Afsar T, Trembley JH, Salomon CE, Razak S, Khan MR, Ahmed K.. 2016. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: involvement of multiple signal transduction pathways. Sci Rep. 6:23077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali EM, Sheta M, El Mohsen MA.. 2011. Elevated serum and tissue VEGF associated with poor outcome in breast cancer patients. Alexandria J Med. 47:217–224. [Google Scholar]
- Andzi-Barhé T, Massala KK, Obame LC, Engonga JL.. 2015. Phytochemical studies, total phenolic and flavonoids content and evaluation of antiradical activity of the extracts of the leaves from Dischistocalyx sp. (Acanthaceae). J Pharmacogn Phytochem. 3:174–178. [Google Scholar]
- Baliga MS.2011. Anticancer, chemopreventive and radioprotective potential of black plum (Eugenia jambolana Lam.). Asian Pac J Cancer Prev. 12:3–15. [PubMed] [Google Scholar]
- Bikfalvi A, Bicknell R.. 2002. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol Sci. 23:576–582. [DOI] [PubMed] [Google Scholar]
- Carbajo-Pescador S, Ordonez R, Benet M, Jover R, García-Palomo A, Mauriz JL, González-Gallego J.. 2013. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer. 109:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK.. 2011. Molecular mechanisms and clinical applications of angiogenesis. Nature. 473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee K, Ali KM, Ghosh D.. 2009. Antihyperglycaemic, antioxidative activities of a formulated polyherbal drug MTEC (Modified) in streptozotocin-induced diabetic rat. J Med Plant Res. 3:468–480. [Google Scholar]
- Claesson-Welsh L, Welsh M.. 2013. VEGFA and tumor angiogenesis. J Intern Med. 273:14–127. [DOI] [PubMed] [Google Scholar]
- Dent SF.2009. The role of VEGF in triple-negative breast cancer: where do we go from here? Ann Oncol. 20:1615–1617. [DOI] [PubMed] [Google Scholar]
- Elkin M, Miao HQ, Nagler A, Aingorn E, Reich R, Hemo I, Dou HL, Pines M, Vlodavsky I.. 2000. Halofuginone: a potent inhibitor of critical steps in angiogenesis progression. FASEB J. 14:2477–2485. [DOI] [PubMed] [Google Scholar]
- Eun JP, Koh GY.. 2004. Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem Biophys Res Commun. 317:618–624. [DOI] [PubMed] [Google Scholar]
- Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN.. 2006. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 27:297–309. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F.. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136:359–386. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kaga S, Zhan L, Bagchi D, Das DK, Bertelli A, Maulik N.. 2006. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 44:43–49. [DOI] [PubMed] [Google Scholar]
- Ghosh D.2015. In vitro antioxidant potential of ethyl acetate fraction of seed of Eugenia jambolana. Asian J Pharm Clin Res. 8:167–170. [Google Scholar]
- Goel HL, Mercurio AM.. 2013. VEGF targets the tumour cell. Nat Rev Cancer. 13:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordaliza M.2007. Natural products as leads to anticancer drugs. Clin Transl Oncol. 9:767–776. [DOI] [PubMed] [Google Scholar]
- Guo C, Buranych A, Sarkar D, Fisher PB, Wang XY.. 2013. The role of tumor-associated macrophages in tumor vascularization. Vasc Cell. 5:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururaj AE, Belakavadi M, Venkatesh DA, Marmé D, Salimath BP.. 2002. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 97:934–942. [DOI] [PubMed] [Google Scholar]
- Hojjat-Farsangi M.2014. Small-molecule inhibitors of the receptor tyrosine kinases: promising tools for targeted cancer therapies. Int J Mol Sci. 15:13768–13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Shukla S, Kim JA, Kim M.. 2015. Anti-angiogenic effect of Nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potential. PLoS One. 10:e0118552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingaraju SM, Keshavaiah K, Salimath BP.. 2008. Inhibition of in vivo angiogenesis by Anacardium occidentale L. involves repression of the cytokine VEGF gene expression. Drug Discov Ther. 2:234–244. [PubMed] [Google Scholar]
- Loboda A, Jazwa A, Wegiel B, Jozkowicz A, Dulak J.. 2005. Heme oxygenase-1-dependent and -independent regulation of angiogenic genes expression: effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells. Cell Mol Biol. 51:347. [PMC free article] [PubMed] [Google Scholar]
- Lu K, Bhat M, Basu S.. 2016. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 19:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick C, De D, Ghosh D.. 2009. Correction of protein metabolic disorders by composite extract of Musa paradisiaca and Coccinia indica in streptozotocin-induced diabetic albino rat: an approach through the pancreas. Pancreas. 38:322–329. [DOI] [PubMed] [Google Scholar]
- Mallick C, Maiti R, Ghosh D.. 2006. Antidiabetogenic effects of separate and composite extract of seed of Jamun (Eugenia jambolana) and root of Kadali (Musa paradisiaca) in streptozotocin-induced diabetic male albino rat: a comparative study. Int J Pharmacol. 2:492–503. [Google Scholar]
- Miao S, Shi X, Zhang H, Wang S, Sun J, Hua W, Miao Q, Zhao Y, Zhang C.. 2011. Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia K562 cell line in vitro. Int J Mol Sci. 12:3831–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Rahman ZU, Sledge Jr GW.. 2001. Selection bias in clinical trials. Breast Dis. 14:31–40. [DOI] [PubMed] [Google Scholar]
- Min JK, Han KY, Kim EC, Kim YM, Lee SW, Kim OH, Kim KW, Gho YS, Kwon YG.. 2004. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 64:644–651. [DOI] [PubMed] [Google Scholar]
- Nadumane VK, Timsina B.. 2014. Anti-cancer potential of banana flower extract: an in vitro study. Bangladesh J. Pharmacol. 9:628–635. [Google Scholar]
- Nagaraj SRM, Shilpa P, Rachaiah K, Salimath BP.. 2015. Crosstalk between VEGF and MTA1 signaling pathways contribute to aggressiveness of breast carcinoma. Mol Carcinog. 54:333–350. [DOI] [PubMed] [Google Scholar]
- Nataraj NB, Salimath BP.. 2013. Crosstalk between VEGF and novel angiogenic protein regulates tumor angiogenesis and contributes to aggressiveness of breast carcinoma. Cell Signal. 25:277–294. [DOI] [PubMed] [Google Scholar]
- Neal C, Berry D, Doucas H, Manson M, Steward W, Garcea G.. 2006. Clinical aspects of natural anti-angiogenic drugs. Curr Drug Targets. 7:371–383. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al. . 2012. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PloS One. 7:e47516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekamwar S, Kalyankar T, Kokate S.. 2013. Pharmacological activities of Coccinia grandis. J Appl Pharm Sci. 3:114–119. [Google Scholar]
- Quesada AR, Muñoz-Chápuli R, Medina MA.. 2006. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 26:483–530. [DOI] [PubMed] [Google Scholar]
- Raj MH, Yashaswini B, Rössler J, Salimath BP.. 2016. Combinatorial treatment with anacardic acid followed by TRAIL augments induction of apoptosis in TRAIL resistant cancer cells by the regulation of p53, MAPK and NFκβ pathways. Apoptosis. 21:578–593. [DOI] [PubMed] [Google Scholar]
- Ramachandra S, D'Souza SS, Gururaj AE, Shaila MS, Salimath BP.. 2009. Paracrine action of sFLT-1 secreted by stably-transfected Ehrlich ascites tumor cells and therapy using sFLT-1 inhibits ascites tumor growth in vivo. J Gene Med. 11:422–434. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Finato N, Crivellato E, Marzullo A, Mangieri D, Nico B, Vacca A, Beltrami CA.. 2005. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 193:1961–1965. [DOI] [PubMed] [Google Scholar]
- Roy M, Chakraborty S, Siddiqi M, Bhattacharya RK.. 2002. Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac J Cancer Prev. 3:61–67. [PubMed] [Google Scholar]
- Shivakumar S, Prabhakar BT, Jayashree K, Rajan MGR, Salimath BP.. 2009. Evaluation of serum vascular endothelial growth factor (VEGF) and microvessel density (MVD) as prognostic indicators in carcinoma breast. J Cancer Res Clin Oncol. 135:627–636. [DOI] [PubMed] [Google Scholar]
- Steeg PS.2006. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 12:895–904. [DOI] [PubMed] [Google Scholar]
- Sun Q, Heilmann J, König B.. 2015. Natural phenolic metabolites with anti-angiogenic properties – a review from the chemical point of view. Beilstein J Org Chem. 11:249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Bladt S. 1996. Plant drug analysis: a thin layer chromatography atlas. Peabody, MA: Hendrickson Publishers. [Google Scholar]
- Wang S, Zheng Z, Weng Y, Yu Y, Zhang D, Fan W, Dai R, Hu Z.. 2004. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 74:2467–2478. [DOI] [PubMed] [Google Scholar]
- Wang W, McKinnie SM, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, et al. . 2013. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogs. J Am Heart Assoc. 2:e000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WB, Hung DK, Chang FW, Ong ET, Chen BH.. 2012. Anti-inflammatory and anti-angiogenic effects of flavonoids isolated from Lycium barbarum Linnaeus on human umbilical vein endothelial cells. Food Funct. 3:1068–1081. [DOI] [PubMed] [Google Scholar]
- Yin WJ, Lu JS, Di GH, Lin YP, Zhou LH, Liu GY, Wu J, Shen KW, Han QX, Shen ZZ.. 2009. Clinicopathological features of the triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat 115:325–333. [DOI] [PubMed] [Google Scholar]
- Yu ST, Chen TM, Chern JW, Tseng SY, Chen YH.. 2009. Downregulation of GSTpi expression by tryptanthrin contributing to sensitization of doxorubicin-resistant MCF-7 cells through c-jun NH2-terminal kinase-mediated apoptosis. Anticancer Drugs. 20:382–388. [DOI] [PubMed] [Google Scholar]