Abstract
Context: The available treatments for the abnormal proliferation of vascular smooth muscle cells (VSMCs) are still dismal. Berberine has been demonstrated to possess extensive medicine activity, yet relatively little is known about its effect on VSMCs proliferation. Many studies showed that PPARα and NO participated in the process of VSMCs proliferation.
Objective: To evaluate the effect of berberine and its possible influence on PPARα-NO pathway in angiotensin IV-stimulated VSMCs.
Materials and methods: The primary VSMCs were cultured with the tissue explants method, and the proliferation was characterized by MTT and protein content. Protein and mRNA expression were measured by Western blot and real-time RT-PCR, respectively. NO synthase (NOS) activity was measured using a spectrophotometric assay, and NO concentration was measured using the Griess assay.
Results: Angiotensin IV (0.1 nmol/L)-induced VSMCs proliferation was evidenced by increasing the optical density at A490 and total protein content (p < 0.01), which was inhibited by berberine (10, 30 and 100 μmol/L) in a concentration-dependent manner (p < 0.05). Angiotensin IV decreased the expression of PPARα at mRNA and protein level (p < 0.05), which occurred in parallel with declining eNOS mRNA expression, NOS activity and NO concentration (p < 0.01). Berberine at 30 μmol/L reversed the effects of angiotensin IV in VSMCs (p < 0.05), which were abolished by MK 886 (0.3 μmol/L) (p < 0.05).
Discussion and conclusion: The results support the therapeutic effects of berberine on angiotensin IV-induced proliferation in cultured VSMCs at least partially through targeting the PPARα-NO signalling pathway.
Keywords: Peroxisome proliferator-activated receptor-α, eNOSl, NOS
Introduction
Cardiovascular disease, with high morbidity and mortality, is not only one of the most prevalent physical disorders but also an enormous economic burden on society. According to the estimate of the World Health Organization, there will be over 23 million people losing their lives because of cardiovascular disease by 2030 (Lim 2013). Studies on the pathogenesis underlying cardiovascular diseases, including hypertension, coronary heart disease, and atherosclerosis and restenosis after angioplasty, suggest that the abnormal proliferation of vascular smooth muscle cells (VSMCs) is associated with the pathophysiology of these diseases (Shi & Chen 2015). VSMCs, as an important type of cells in vessel walls, play a key role in maintaining the blood vessel elasticity. To date, treatment options of abnormal VSMCs proliferation are still limited.
Berberine, one of the active components of Coptis chinensis Franch., is an isoquinoline alkaloid endowed with multiple pharmacological activities, including antimicrobial, glucose- and cholesterol-lowering, antitumour and immunomodulatory properties (Pirillo & Catapano 2015), and has been widely used to treat some diseases for many centuries in China. In the context of the present work, some studies have demonstrated that berberine can inhibit the proliferation of VSMCs (Liang et al. 2008; Wu et al. 2010; Liu et al. 2011), yet relatively little is known about the potential mechanism of anti-proliferative effect of berberine. Peroxisome proliferator-activated receptor (PPARs) is one of the nuclear receptor superfamily members and contains three subtypes (α, β/δ and γ). Studies in vitro and in vivo have shown that PPARs, especially PPARα is involved in the abnormal proliferation of VSMCs (Hamblin et al. 2009). PPARα is mainly located in heart, VSMCs, vascular endothelial cell, liver, skeletal muscle, haematopoietic cell and some other organs, the activation of which displays the anti-inflammatory and anti-proliferative effects by regulating some cytokines, e.g., nitric oxide (NO) (Mueller et al. 2010). As well-known, NO is an important factor in regulating blood pressure, dilating blood vessels, inhibiting platelet aggregation and leukocyte adhesion, and is released by catalysis of nitric oxide synthase (NOS). Some studies also showed that NO was involved in the pathophysiology of VSMC abnormal proliferation (Lei et al. 2013). Our previous studies showed that berberine could activate PPARα-NO signalling pathway to inhibit cardiomyocyte hypertrophy induced by high glucose and insulin (Wang et al. 2013). Angiotensin IV (Ang IV), C-terminal hexapeptide fragment of Ang II, has been reported to stimulate the proliferation of VSMCs (Ruiz-Ortega et al. 2007). The goal of the present study was to determine whether berberine could inhibit abnormal proliferation of VSMCs induced by Ang IV through activation of the PPARα-NO signalling pathway.
Materials and methods
Chemicals and reagents
Berberine (C20H18NO4, MW: 384.43, purity: ≥98%) was purchased from Division of Chinese Material Medical and Natural Products, National Institute for the Control of Pharmaceutical and Biological Products, Ministry of Public Health, Beijing, China, and dissolved in 0.1% DMSO before use; all other chemicals and reagents were purchased from Sigma (St. Louis, MO).
VSMC isolation of primary rats and culture
Thoracic aortas were isolated from 8 to 10 weeks-old Sprague-Dawley rats (male or female, 160–180 g, provided by Animal Laboratory Center of Chongqing Medical University, Chongqing, China). Primary VSMCs culture was conducted using the explants method. VSMCs were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% heat-inactivated foetal bovine serum. Cells were serum-starved before stimulation or treatment with reagents for 24 h. Ang IV (0.1 nmol/L) was used to stimulate the proliferation of VSMCs. The anti-proliferative effects of berberine (10, 30 and 100 μmol/L) were studied. In addition, MK886 (0.3 μmol/L), a selective PPARα antagonist, was used for investigating the relationship between the anti-proliferative effects of berberine and the PPARα-NO signalling pathway. The experimental procedures were approved by the Animal Laboratory Administration Center and Ethics Committee of Chongqing Medical University [SYXK (Chongqing) 2007-0001], particularly with respect to the ethical animal care.
MTT assay for VSMC proliferation
VSMCs proliferation was determined by adding MTT solution at 5 g/L, and then incubating at 37 °C for 4 h. After removing the medium, 100 μl DMSO was added followed by 10 min vortex, and the optical density (OD) was read at 490 nm with six times repeating in each group.
Measurement of VSMCs protein content
VSMCs were collected and separated by trypsin, counted and washed three times with ice-cold phosphate-buffered solution (PBS), then homogenized with RIPA lysis buffer, and finally centrifuged at 12,000 g for 15 min at 4 °C. The protein concentration in the supernatant was determined with a BCA protein assay kit (Beyotime, Shanghai, China), and then the protein concentration per 106 cells was calculated for a six times repeating.
Real time RT-PCR analysis of mRNA
Total RNA was isolated from VSMCs with a Trizol reagent kit (Takara Biotech Co., Dalian, China), quantified by ultraviolet spectrometric detection (Eppendorf, Hamburg, Germany) and reverse transcribed into cDNA using a PrimeScript™ RT reagent kit (Takara Biotech Co., Dalian, China), according to the manufacturer’s instructions. Real time RT-PCR was performed according to the standard protocol of SYBR®Premix Ex Taq™ II (Takara Biotech Co., Dalian, China) on the IQ5 real time RT-PCR system (Bio-Rad, Hercules, CA). The standard cycling conditions were 95 °C for 15 min, followed by 40 cycles of 95 °C for 10 s, annealing for 1 min at different temperature (PPARα: 60.9 °C; eNOS: 59.1 °C; β-actin: 59.1 °C), and then 72 °C for 32 s. The quantification of gene expression relative to β-actin was calculated using the ΔCt (Ct = cycle threshold) method as follows: the relative expression =2−ΔΔCt, ΔCt = Ct (target gene) _Ct (β-actin). The sequences of the primer were designed and synthesized as follows: 5′-CCT CGT GGT AGC GTT GCT GA-3′ (forward) and 5′-AGC TGG TGA AGC CGG TGA C-3′ (reverse) for eNOS; 5′-TTG CAG CTT CGA TCA CAC TTG C-3′ (forward) and 5′-GAC AAG GCC TCA GGA TAC CAC TAT G-3′ (reverse) for PPARα; 5′-GGC CAA CCG TGA AAA GAT GA-3′ (forward) and 5′-CAG CCT GGA TGG CTA CGT ACA-3′ (reverse) for β-actin. Results of four independent experiments were used for statistical analysis.
Western blot analysis
The isolated protein (50 μg) from VSMCs was separated by 10% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes. The blots were blocked by 5% fat-free milk in PBST for 1 h, and then probed with rabbit anti-rat PPARα (1:1000 dilution) (Santa Cruz, CA) or mouse anti-rat β-actin (1:1500 dilution) (Takara Biotech Co., Dalian, China) primary antibodies for another 1 h followed by horseradish peroxidase-conjugated secondary antibodies (1:2000 dilution) (Beyotime, Shanghai, China) for 1 h at room temperature. The blots were visualized using an ECL reagent (Pierce Chemical, Rockford, IL). The optical densities of the bands were quantified with a quantitative imaging system (Bio-Rad, Hercules, CA). All Western blot experiments were repeated four times.
Measurement of NOS activity and NO level
NOS activity in the conditioned medium of VSMCs was measured using a NOS detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions, and the OD was detected at 530 nm with a spectrophotometer (DU-640 Beckman, Fullerton, CA). In addition, NO level was determined using a nitrite detection kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions, and the OD was detected at 540 nm using a microplate reader (Tecan, Groedig, Austria). Results of six independent experiments were used for statistical analysis.
Statistical analysis
Data were presented as mean ± SEM and analyzed by the one-way ANOVA and SNK-q test using SPSS 17.0 statistical software. Significance was defined as p < 0.05.
Results
Effect of berberine on VSMCs proliferation induced by Ang IV
The stimulation of Ang IV at 0.1 nmol/L caused a significant reaction of VSMCs proliferation, which was exhibited by elevating OD value (12.5%) at A490 and total protein content (23.5%) (p < 0.01) (Figure 1). Treatment with berberine (10, 30 and 100 μmol/L) significantly inhibited the increasing of OD and total protein induced by Ang IV in a concentration-dependent manner (p < 0.05). MK 886 (0.3 μmol/L), a selective PPARα antagonist, could completely abolish the effects of berberine at 30 μmol/L (p < 0.05) (Figure 1).
Figure 1.
Effect of berberine (BBR) on VSMCs proliferation induced by angiotensin IV (Ang IV). Ang IV (0.1 nmol/L) stimulation promoted significant VSMCs proliferation, which was displayed by increasing OD value at A490 and total protein content. Treatment with BBR (10, 30 and 100 μmol/L) significantly inhibited VSMCs proliferation induced by Ang IV in a concentration-dependent manner. MK 886 (0.3 μmol/L) could abolish the effects of BBR at 30 μmol/L. Results are represented by mean ± SEM, n = 6. **p < 0.01 vs control group; #p < 0.05, ##p < 0.01 vs Ang IV group; △p < 0.05 vs Ang IV + BBR at 30 μmol/L. ‘+’ or ‘−’: treatment with or without relevant reagent.
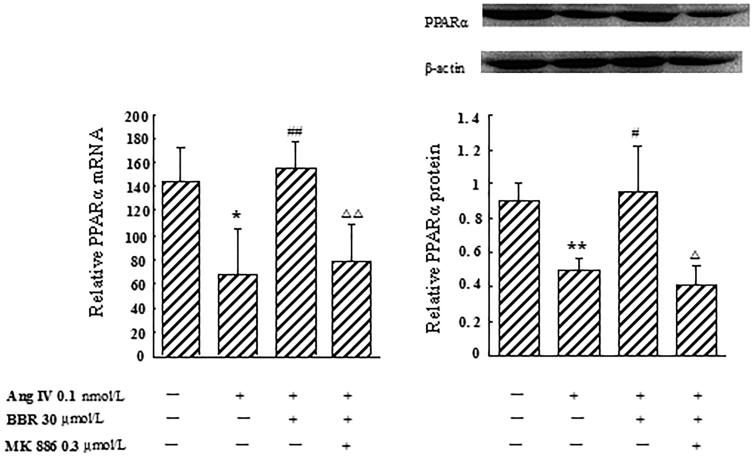
Effect of berberine on PPARα mRNA and protein expression in Ang IV-stimulated VSMCs
In Ang IV-induced VSMCs, the expression of PPARα decreased by 53.5% at the mRNA level, and by 45.4% at the protein level, respectively, compared with control (p < 0.05). Berberine treatment markedly elevated PPARα mRNA and protein expression, by 132.8 and 93.6%, respectively (p < 0.05), which were significantly blocked by MK 886 (p < 0.05) (Figure 2).
Figure 2.
Effect of berberine (BBR) on the expression of PPARα at the mRNA and protein level in angiotensin IV (Ang IV)-stimulated VSMCs. In Ang IV-treated VSMCs, the expression of PPARα decreased at the mRNA and protein levels. BBR (30 μmol/L) treatment markedly elevated PPARα mRNA and protein expression, which were significantly blocked by MK 886 (0.3 μmol/L). Results are represented by mean ± SEM, n = 4. *p < 0.05, **p < 0.01 vs control group; #p < 0.05, ##p < 0.01 vs Ang IV group; Δp < 0.05, ΔΔp < 0.01 vs Ang IV + BBR group. ‘+’ or ‘−’: treatment with or without relevant reagent.
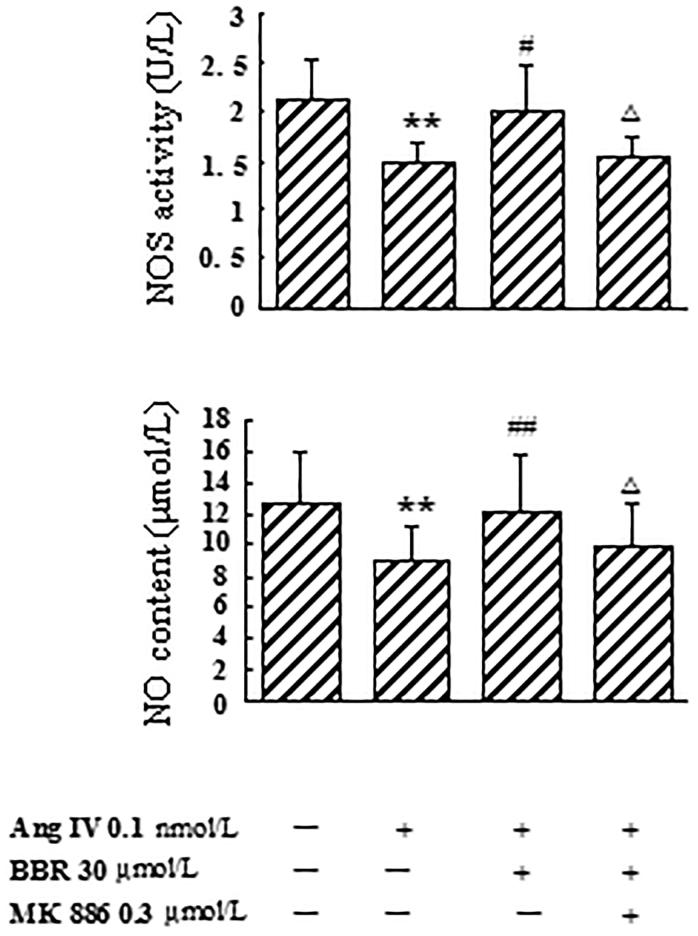
Effects of berberine on eNOS mRNA expression, NOS activity and NO level in Ang IV-stimulated VSMCs
In Ang IV-induced VSMCs, the expression of eNOS mRNA, NOS activity and NO level were significantly decreased by 75.3, 30.4 and 29.4%, respectively, compared with control (p < 0.01). Berberine treatment counteracted the effects caused by Ang IV and made eNOS mRNA, NOS activity and NO level increase by 195.2, 37.8 and 34.2%, respectively (p < 0.05). The rescue effects of berberine on eNOS mRNA expression, NOS activity and NO concentration were abolished by MK 886 at 0.3 μmol/L (p < 0.05) (Figures 3 and 4).
Figure 3.
Effect of berberine (BBR) on eNOS mRNA expression in angiotensin IV (Ang IV)-stimulated VSMCs. The expression of eNOS mRNA was significantly decreased in Ang IV-induced VSMCs, which was counteracted by BBR at 30 μmol/L. MK 886 (0.3 μmol/L) could abolish the effect of BBR. Results are represented by mean ± SEM, n = 4. **p < 0.01 vs control group; ##p < 0.01 vs Ang IV group; △p < 0.05 vs Ang IV + BBR group. ‘+’ or ‘−’: treatment with or without relevant reagent.
Figure 4.
Effects of berberine (BBR) on NOS activity and NO content in angiotensin IV (Ang IV)-stimulated VSMCs. NOS activity and NO level were significantly decreased from the culture medium of the VSMCs treated with Ang IV, which were counteracted by BBR at 30 μmol/L. The rescue effects of BBR were abolished by MK 886 (0.3 μmol/L). Results are represented by mean ± SEM, n = 6. **p < 0.01 vs control group; #p < 0.05, ##p < 0.01 vs Ang IV group; △p < 0.05 vs Ang IV + BBR group. ‘+’ or ‘−’: treatment with or without relevant reagent.
Discussion
The renin-angiotensin system (RAS) has been demonstrated to play a critical role in the initiation and progression of VSMCs proliferation (Sata & Fukuda 2010). Ang II, a major substrate in RAS, maintains the circulating blood volume, regulates blood pressure, and mediates chronic inflammation and other various responses including VSMC proliferation and migration. Many studies reported that Ang II stimulated the abnormal proliferation of VSMCs through multiple signalling pathways such as Akt, ERK 1/2 Smad, Ras/MAPK kinase 1 and NF-κB pathway (Rodríguez-Vita et al. 2005; Ma et al. 2006; Xu et al. 2015). Ang IV is an active shorter peptide fragment of Ang II, but the action of Ang IV remains controversial in different cells or tissues. Dominska et al. (2013) found that Ang IV could modulate tumour cell proliferation in the early stage of androgen-dependent prostate cancer. The study by Yang et al. (2011) also showed that Ang IV could inhibit Ang II-induced proliferation and collagen synthesis of cardiac fibroblasts. In contrast, some researchers reported that Ang IV could stimulate the proliferation of various cells such as lung endothelial cell, anterior pituitary cell and adrenocortical cell (Pawlikowski et al. 2001; Ptasinska-Wnuk et al. 2003; Lu et al. 2005). These results suggested that Ang IV is also involved in a variety of cell proliferation through autocrine and paracrine. In the present study, Ang IV significantly increased the OD value at A490 and protein synthesis of VSMCs, which indicated that Ang IV could stimulate the proliferation in cultured VSMCs in rat.
Berberine has been widely used in the traditional Chinese medicine as an antimicrobial agent in the treatment of dysentery and infectious diarrhoea. Recent studies have suggested that berberine has pharmacological activities that could potentially be used in cardiovascular diseases management such as hyperlipidaemia, hypertension and atherosclerosis (Derosa et al. 2012). The anti-atherosclerosis mechanism of berberine might be related to regulating lipids, anti-inflammation, decompression, reducing blood sugar and inhibiting VSMCs proliferation (Wu et al. 2010). In this regard, berberine significantly inhibited the increase of OD value at the A490 and protein synthesis caused by Ang IV in VSMCs in a concentration-dependent manner, suggesting that berberine could effectively inhibit Ang IV-induced VSMCs proliferation. Some studies showed that the anti-proliferative effect of berberine was through inhibiting MMP-2/9, u-PA, AP-1 and NF-κB expression in human aortic smooth muscle cells (Liu et al. 2014), or by increasing the activity of PI3K/Akt/eNOS signalling pathway in endothelial progenitor cells (Xiao et al. 2014). But the mechanism of berberine effect on Ang IV-stimulated VSMC proliferation is still unclear.
Extensive research has revealed that the activation of PPARs protects the vascular complications from diabetes, hypertension, atherosclerosis, myocardial infarction and stroke, through exerting anti-inflammatory, anti-atherogenic and antioxidant effects (Cheang et al. 2015). PPARα is also involved in diverse processes such as energy metabolism, oxidative stress, inflammation, circadian rhythm, immune response and cell differentiation (Moran & Ma 2015). PPARα activators showed improving effects on the VSMCs proliferation through negatively regulating the NF-κB signalling pathway, decreasing the cyclooxygenase-2 expression and repressing and delaying the cell cycle from G1 phase to S phase (Hu et al. 2002; Gizard et al. 2008; Ji et al. 2009). Similarly, our investigation indicated that PPARα expression, at both the mRNA and protein level, was suppressed by Ang IV stimulation in VSMCs. It was noteworthy that berberine could not only reverse Ang IV-induced VSMCs proliferation, but also markedly up-regulated PPARα expression. Meanwhile, MK 886 abolished these effects of berberine. The observations confirmed the hypothesis that PPARα is a major intermediate in facilitating the beneficial effects of berberine; however, the down-stream molecular mechanisms of PPARα signalling pathway are unclear.
NO is an important signal molecule and participates in multiple functions of body, especially in cardiovascular system. As an indispensable factor, NO shows cardiovascular protection by regulating blood pressure and blood flow, inhibiting platelet aggregation and leukocyte adhesion, and preventing VSMCs proliferation. NO is synthesized from L-arginine by the catalytic reaction of NOS, including neuronal NOS, inducible NOS and eNOS; among which, eNOS is constitutively expressed in VSMCs. Then, NO activates soluble guanylate cyclase by binding its prosthetic haem group, thereby catalyzing cyclic guanosine monophosphate (cGMP) synthesis. cGMP causes vasodilation and may inhibit smooth muscle cell proliferation and platelet aggregation (Dasgupta et al. 2015). Recently, Yousefipour and Newaz (2014) found that clofibrate-mediated reduction in blood pressure and proteinuria was probably associated with the up-regulation of PPARα expression and the increase of NO production in spontaneously hypertensive rats. Similarly, Yakubu et al. (2010) also demonstrated that the activation of PPARα could increase eNOS expression at the transcriptional and translational levels and further enhance NO production in cerebral microvascular endothelial cells. Collectively, these studies indicated that NO may be an important downstream effector of PPARα-related signal pathway. In the present study, with PPARα deactivation in Ang IV-induced proliferative VSMCs, eNOS mRNA expression level, as well as culture medium NOS activity and NO concentration were also decreased, which suggested that the negative regulation of PPARα-NOS-NO signalling pathway was involved in the VSMCs proliferation induced by Ang IV. However, this deterioration could be restored by berberine treatment, which was correlated with decreased measures of VSMCs proliferation and evidence of PPARα activation. Moreover, co-administration of MK 886 abolished the improved effects of berberine. We interpret these results to suggest that NO plays an important role in the anti-proliferative effect of berberine-modulated PPARα activation.
Conclusions
These results demonstrated that the down-regulation of PPARα-NO signalling pathway was involved in Ang IV-induced VSMCs proliferation; and the anti-proliferative effects of berberine in Ang IV-treated VSMCs might be, at least partially, associated with the activation of PPARα, promotion of eNOS and finally led to a beneficial increase of NO production.
Disclosure statement
The authors report no declarations of interest.
References
- Cheang WS, Tian XY, Wong WT, Huang Y.. 2015. The peroxisome proliferator-activated receptors in cardiovascular diseases: experimental benefits and clinical challenges. Br J Pharmacol. 172:5512–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Bowman L, D'Arsigny CL, Archer SL.. 2015. Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin Pharmacol Ther. 97:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa G, Maffioli P, Cicero AF.. 2012. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 12:1113–1124. [DOI] [PubMed] [Google Scholar]
- Dominska K, Piastowska-Ciesielska AW, Pluciennik E, Lachowicz-Ochedalska A, Ochedalski T.. 2013. A comparison of the effects of angiotensin IV on androgen-dependent and androgen-independent prostate cancer cell lines. J Renin Angiotensin Aldosterone Syst. 14:74–81. [DOI] [PubMed] [Google Scholar]
- Gizard F, Nomiyama T, Zhao Y, Findeisen HM, Heywood EB, Jones KL, Staels B, Bruemmer D.. 2008. The PPARalpha/p16 INK4a pathway inhibits vascular smooth muscle cell proliferation by repressing cell cycle-dependent telomerase activation. Circ Res. 103:1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M, Chang L, Fan Y, Zhang J, Chen YE.. 2009. PPARs and the cardiovascular system. Antioxid Redox Signal. 11:1415–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZW, Kerb R, Shi XY, Wei-Lavery T, Hoffman BB.. 2002. Angiotensin II increases expression of cyclooxygenase-2: implications for the function of vascular smooth muscle cells. J Pharmacol Exp Ther. 303:563–573. [DOI] [PubMed] [Google Scholar]
- Ji YY, Liu JT, Liu N, Wang ZD, Liu CH.. 2009. PPARalpha activator fenofibrate modulates angiotensin II-induced inflammatory responses in vascular smooth muscle cells via the TLR4-dependent signaling pathway. Biochem Pharmacol. 78:1186–1197. [DOI] [PubMed] [Google Scholar]
- Lei J, Vodovotz Y, Tzeng E, Billiar TR.. 2013. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide. 35:175–185. [DOI] [PubMed] [Google Scholar]
- Liang KW, Yin SC, Ting CT, Lin SJ, Hsueh CM, Chen CY, Hsu SL.. 2008. Berberine inhibits platelet-derived growth factor-induced growth and migration partly through an AMPK-dependent pathway in vascular smooth muscle cells. Eur J Pharmacol. 590:343–354. [DOI] [PubMed] [Google Scholar]
- Lim GB.2013. Global burden of cardiovascular disease. Nat Rev Cardiol. doi: 10.1038/nrcardio.2012.194. [DOI] [PubMed] [Google Scholar]
- Liu J, Xiu J, Cao J, Gao Q, Ma D, Fu L.. 2011. Berberine cooperates with adrenal androgen dehydroepiandrosterone sulfate to attenuate PDGF-induced proliferation of vascular smooth muscle cell A7r5 through Skp2 signaling pathway. Mol Cell Biochem. 355:127–134. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Yin CX, Ding MC, Xia SY, Shen QM, Wu JD.. 2014. Berberine suppresses in vitro migration of human aortic smooth muscle cells through the inhibitions of MMP-2/9, u-PA, AP-1, and NF-κB. BMB Rep. 47:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang J, Block ER, Patel JM.. 2005. Angiotensin IV enhances phosphorylation of 4EBP1 by multiple signaling events in lung endothelial cells. Mol Cell Biochem. 275:181–188. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang L, Peng T, Cheng J, Taneja S, Zhang J, Delafontaine P, Du J.. 2006. Angiotensin II stimulates transcription of insulin-like growth factor I receptor in vascular smooth muscle cells: role of nuclear factor-kappaB. Endocrinology. 147:1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EP, Ma JX.. 2015. Therapeutic effects of PPAR α on neuronal death and microvascular impairment. PPAR Res. 2015:595426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Hobiger S, Jungbauer A.. 2010. Red clover extract: a source for substances that activate peroxisome proliferator-activated receptor alpha and ameliorate the cytokine secretion profile of lipopolysaccharide-stimulated macrophages. Menopause. 17:379–387. [DOI] [PubMed] [Google Scholar]
- Pawlikowski M, Gruszka A, Mucha S, Melen-Mucha G.. 2001. Angiotensins II and IV stimulate the rat adrenocortical cell proliferation acting via different receptors. Endocr Regul. 35:139–142. [PubMed] [Google Scholar]
- Pirillo A, Catapano AL.. 2015. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studies. Atherosclerosis. 243:449–461. [DOI] [PubMed] [Google Scholar]
- Ptasinska-Wnuk D, Kunert-Radek J, Pawlikowski M.. 2003. Angiotensins II and IV stimulate the rat anterior pituitary cell proliferation independently of the AT1 receptor subtype. Neuro Endocrinol Lett. 24:397–400. [PubMed] [Google Scholar]
- Rodríguez-Vita J, Sánchez-López E, Esteban V, Rupérez M, Egido J, Ruiz-Ortega M.. 2005. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 111:2509–2517. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Esteban V, Egido J.. 2007. The regulation of the inflammatory response through nuclear factor-kappa B pathway by angiotensin IV extends the role of the renin angiotensin system in cardiovascular diseases. Trends Cardiovasc Med. 17:19–25. [DOI] [PubMed] [Google Scholar]
- Sata M, Fukuda D.. 2010. Crucial role of renin-angiotensin system in the pathogenesis of atherosclerosis. J Med Invest. 57:12–25. [DOI] [PubMed] [Google Scholar]
- Shi N, Chen SY.. 2015. Smooth muscle cell differentiation: model systems, regulatory mechanisms, and vascular diseases. J Cell Physiol. 231:777–787. [DOI] [PubMed] [Google Scholar]
- Wang M, Wang J, Tan R, Wu Q, Qiu H, Yang J, Jiang Q.. 2013. Effect of berberine on PPAR α /NO activation in high glucose- and insulin-induced cardiomyocyte hypertrophy. Evid Based Complement Alternat Med. 2013:285489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Wang J, Liu LT.. 2010. Advance of studies on anti-atherosclerosis mechanism of berberine. Chin J Integr Med. 16:188–192. [DOI] [PubMed] [Google Scholar]
- Xiao M, Men LN, Xu MG, Wang GB, Lv HT, Liu C.. 2014. Berberine protects endothelial progenitor cell from damage of TNF-α via the PI3K/AKT/eNOS signaling pathway. Eur J Pharmacol. 743:11–16. [DOI] [PubMed] [Google Scholar]
- Xu T, Zhu H, Li D, Lang Y, Cao L, Liu Y, Wu W, Chen D.. 2015. Luteolin inhibits angiotensin II-stimulated VSMC proliferation and migration through downregulation of Akt phosphorylation. Evid Based Complement Alternat Med. 2015:931782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubu MA, Nsaif RH, Oyekan AO.. 2010. Regulation of cerebrovascular endothelial peroxisome proliferator activator receptor alpha expression and nitric oxide production by clofibrate. Bratisl Lek Listy. 111:258–264. [PubMed] [Google Scholar]
- Yang H, Zeng XJ, Wang HX, Zhang LK, Dong XL, Guo S, Du J, Li HH, Tang CS.. 2011. Angiotensin IV protects against angiotensin II-induced cardiac injury via AT4 receptor. Peptides. 32:2108–2115. [DOI] [PubMed] [Google Scholar]
- Yousefipour Z, Newaz M.. 2014. PPARα ligand clofibrate ameliorates blood pressure and vascular reactivity in spontaneously hypertensive rats. Acta Pharmacol Sin. 35:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]