Abstract
Context: There is an unmet need to discover new treatments for Alzheimer’s disease. This study determined the anti-acetylcholinesterase (AChE) activity, DPPH free radical scavenging and antioxidant properties of Carpolobia lutea G. Don (Polygalaceae).
Objective: The objective of this study is to quantify C. lutea anti-AChE, DPPH free radical scavenging, and antioxidant activities and cell cytotoxicity.
Materials and methods: Plant stem, leaves and roots were subjected to sequential solvent extractions, and screened for anti-AChE activity across a concentration range of 0.02–200 μg/mL. Plant DPPH radical scavenging activity, reducing power, and total phenolic and flavonoid contents were determined, and cytotoxicity evaluated using human hepatocytes.
Results:Carpolobia lutea exhibited concentration-dependent anti-AChE activity. The most potent inhibitory activity for the stem was the crude ethanol extract and hexane stem fraction oil (IC50 = 140 μg/mL); for the leaves, the chloroform leaf fraction (IC50 = 60 μg/mL); and for roots, the methanol, ethyl acetate and aqueous root fractions (IC50 = 0.3–3 μg/mL). Dose-dependent free radical scavenging activity and reducing power were observed with increasing stem, leaf or root concentration. Total phenolic contents were the highest in the stem: ∼632 mg gallic acid equivalents/g for a hexane stem fraction oil. Total flavonoid content was the highest in the leaves: ∼297 mg quercetin equivalents/g for a chloroform leaf fraction. At 1 μg/mL, only the crude ethanol extract oil was significantly cytotoxic to hepatocytes.
Discussion and conclusions:Carpolobia lutea possesses anti-AChE activity and beneficial antioxidant capacity indicative of its potential development as a treatment of Alzheimer’s and other diseases characterized by a cholinergic deficit.
Keywords: Anti-acetylcholinesterase, Carpolobia lutea, antioxidant, Alzheimer's disease
Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease; with symptomology that typically includes confusion, memory loss, impaired cognitive and emotional function, and dementia. In 2015, the number of people living with dementia was estimated to be 46.8 million, with an associated economic burden of 818 billion US dollars (Alzheimer’s Disease International 2015). The pathological hallmarks of AD are the deposition of extracellular amyloid plaques composed of insoluble amyloid beta (Aβ) peptides and intra-neuronal neurofibrillary tangles (NFTs) of hyperphosphorylated Tau protein. Collectively, these peptide and protein accumulations are thought to be toxic to neuronal tissue and contribute to the neuronal death and cerebral atrophy observed in AD patients (Parihar and Hemnani 2004; Ballard et al. 2011).
The aetiopathology of AD is complex and heterogeneous due to multifaceted disease mechanisms. These include mitochondrial dysfunction, redox and inflammatory stress, and alteration of neurotransmitter activities including cholinergic dysfunction (Parihar and Hemnani 2004; Mufson et al. 2008; Ballard et al. 2011). Acetylcholine signalling is terminated within the synaptic cleft through cleavage by acetylcholinesterase (AChE). Hence, drugs that mimic acetylcholine activity (cholinomimetics), or drugs that limit acetylcholine breakdown (AChE inhibitors) have provided a therapeutic strategy to augment cholinergic signalling in AD patients (Seltzer 2006; Mufson et al. 2008; Ballard et al. 2011; Sun et al. 2012).
Physostigmine (eserine), tacrine, rivastigmine, donepezil, galantamine and huperzine A are drugs that have been employed for symptomatic treatment of AD. Their mode of action is primarily through inhibition of AChE to limit the cholinergic deficit (Sun et al. 2012). However, a search continues for other anti-AChE drugs that temper AD progression, but without induction of unwanted side effects.
Globally, natural products and secondary metabolites are utilized in traditional or alternative medicines. Herbal medicines, particularly Chinese and Indian traditional medicines, have for many centuries been specifically utilized to restore declining cognitive functions (Howes and Houghton 2003; Gurib-Fakim 2006; Adams et al. 2007; Shiksharthi et al. 2011). Medicinal plants may possess an array of secondary metabolites such as polyphenols and flavonoids with broad-spectrum pharmacological activities including cellular antioxidant activities capable of scavenging damaging free radicals. Indeed, the mechanism by which medicinal plants are able to induce therapeutic effects in AD patients may be diverse and include anti-amyloid production, anti-apoptotic, antioxidant and anti-inflammatory activities, in addition to targeting cholinergic deficits (Howes and Houghton 2003; Anekonda and Reddy 2005; Akhondzadeh and Abbasi 2006; Adams et al. 2007; Mukherjee et al. 2007; Hajiaghaee and Akhondzadeh 2012; Orhan 2013; Ansari and Khodagholi 2013; Menon et al. 2013; Shakir et al. 2013; Syad and Devi 2014). Furthermore, herbal drugs may be less toxic, display improved blood–brain barrier penetrance and exhibit beneficial synergistic effects when compared with single moiety synthetic drugs.
Systematic in vitro and in vivo screening of natural plant extracts and fractions will potentially provide new active material able to treat AD and other cognitive dysfunctions. Carpolobia lutea G. Don (Polygalaceae) is a perennial shrub native to West and Central Tropical Africa. It is widely distributed in rainforests and the Guinea savannah of Sierra Leone, Cameroon and Nigeria. Extracts and fractions of C. lutea have hitherto only been reported anecdotally to possess benefits to cognition, with the study of C. lutea that has included an examination of its antidiarrheal (Nwidu, Essien et al. 2011), anti-nociceptive (Nwidu, Nwafor et al. 2011) and gastro-protective effects (Nwidu et al. 2014). Herein extracts and fractions of the stem, leaves and roots of C. lutea were scrutinized for therapeutic promise as an AD treatment via their ability to inhibit AChE. Additionally, extracts and fractions were examined for radical scavenging and reducing (antioxidant) activities, polyphenol and flavonoid contents determined, and C. lutea cytotoxicity was also assessed.
Materials and methods
Materials
All chemicals were purchased from Sigma Aldrich (Irvine, UK) unless specified otherwise.
Preparation of plant material
Plant leaves, stem and roots from Carpolobia lutea grown in the wild were collected in January 2015 from Itak Ikpa village in the Ikono Local Government Area of Akwa Ibom State, Nigeria. Plant parts were supplied by Mr. Okon Etefia, a traditional herbalist, attached to the Pharmacognosy Department, University of Uyo, Nigeria. Plant identification was authenticated by Dr. Margaret Bassey, Department of Botany, University of Uyo, Nigeria. A voucher specimen (UUH 999) was deposited at the University Herbarium, University of Uyo, Nigeria. Plant parts were air-dried under shade for 2 weeks at 30 °C and then powdered with a hammer mill. The pulverized material was stored at room temperature until required.
Extraction of leaf, stem-bark and root of C. lutea
A flow chart depicting the complete preparation of plant extracts and fractions from C. lutea is included in Supplementary Figure S1.
Powdered leaves (750 g) were subjected to sequential extraction by maceration for 72 h in 2.5 L of n-hexane to obtain the n-hexane fraction (n-HLF). The marc was air-dried and the procedure repeated with chloroform, ethyl acetate, and then ethanol solvents to obtain the corresponding chloroform leaf fraction (CHLF), ethyl acetate leaf fraction (EALF) and ethanol leaf fraction (ETLF), respectively, by filtration and concentration under reduced pressure by the use of rotary evaporation at 40 °C.
The crude ethyl acetate leaf extract (cEALE), crude ethanol leaf extract (cETLE), crude ethanol stem-bark extract (cETSE) and crude methanol root extract (cMTRE) were obtained after 72 h of maceration in each respective solvent, and filtered and concentrated under reduced pressure as above.
A hot aqueous leaf extract (HALE) and hot aqueous stem-bark extract (HASE) were obtained by the addition of 1 L of boiling water to 500 g of the powdered leaf or stem-bark material, shaking for 1 min and then the material allowed to stand for 60 min. Solvent extracts were filtered and the process repeated after 24 h. The marc obtained from the leaf extract was dried to a constant weight after 48 h at 50 °C. The residue remaining was immersed in 1 L of butanol for 72 h after which it was processed as above to yield a crude butanol leaf extract (cBULE). The yield obtained for each extract is listed in Table 1.
Table 1.
Percentage yield, AChE and DPPH radical scavenging IC50 concentrations of stem, leaf, and root extracts and fractions of C. lutea.
| Extract or fraction | Yield (%) | IC50 concentration (μg/mL) |
|
|---|---|---|---|
| AChE | DPPH radicalscavenging | ||
| Stem | |||
| cETSE | 21.7 | 140 | 343 |
| EASF | 1.75 | 472 | 675 |
| n-HSF oil | 22.0 | 140 | >1000 |
| EASF oil | 9.0 | 503 | 123 |
| n-HSF | 19.1 | 912 | >1000 |
| MTSF | 52.8 | 142 | 118 |
| MTSF oil | 12.9 | 472 | 825 |
| cSSE | 22.0 | 811 | 351 |
| DCSF | 19.5 | >1000 | 449 |
| cETSE oil | 15.6 | 840 | 334 |
| HASE | 12.5 | 547 | 504 |
| Leaf | |||
| cHALE | 13.4 | >1000 | 196 |
| cETLE | 7.8 | 478 | 849 |
| CHLF | 3.0 | 60 | 500 |
| cETLF | 27.1 | 81 | >1000 |
| cBULE | 10.2 | 738 | >1000 |
| n-HLF | 3.7 | 461 | 141 |
| cEALE | 3.6 | 500 | >1000 |
| Root | |||
| cMTRE | 44.1 | 3 | 509 |
| CHRF | 12.3 | 137 | 849 |
| BURF | 8.0 | 247 | >1000 |
| n-HRF | 16.5 | 173 | 500 |
| EARF | 5.8 | 0.3 | >1000 |
| AQRF | 20.2 | 2 | 141 |
Fractionation of stem-bark and root of C. lutea
The crude ETSE of C. lutea (60 g) was divided into four equal aliquots, and then further fractionated by mixing with 60 g of silica gel. This material was placed in a glass column and eluted sequentially, under pressure, with 500 mL each of n-hexane, dichloromethane, ethyl acetate and then methanol to obtain the corresponding fractions: n-hexane stem fraction (n-HSF), dichloromethane stem fraction (DCSF), ethyl acetate stem fraction (EASF) and methanol stem fraction (MTSF), respectively. The same procedure was followed to fractionate 15 g of crude ethanol stembark extract oil (cETSE oil), n-hexane stem fraction oil (n-HSF oil), ethyl acetate stem fraction oil (EASF oil) and methanol stem fraction oil (MTSF oil). The fractions obtained were reduced in volume using rotary evaporation before drying under nitrogen flow. Thereafter, the extracts were stored in an airtight container at 4 °C until required. HASE was subjected to further sequential extraction with n-hexane, ethyl acetate and butanol, but only the ethyl acetate fraction of HASE yielded significant material for use.
To prepare the crude methanol root extract (cMTRE), 50 g was mixed with 100 g silica gel and placed in a glass column and sequentially eluted with n-hexane (n-hexane root fraction [n-HRF]), chloroform (chloroform root fraction [CHRF]), ethyl acetate (ethyl acetate root fraction, EARF), butanol (butanol root fraction, BURF)) and aqueous fractions (aqueous root fraction, AQRF). The dry weight of each fraction was obtained after complete evaporation of the solvent in a fume cupboard. Extracts and fractions were stored at 4 °C until use. Each extract or fraction was reconstituted in pure (milli Q) water before use in experimental assays.
Preparation of crude saponins
From the hot aqueous stem extract (HASE), a crude saponin stem extract (cSSE) was prepared. A portion of HASE, weighing 5 g, was transferred to a conical flask and soaked with 25 mL of 20% methanol. The mixture was heated for 12 h at constant temperature of 55 °C with stirring. Thereafter, it was filtered and material extracted with 60 mL of 20% methanol. The volume of the liquid extracted was reduced to 30 mL using a water bath and transferred to a separating funnel. Diethyl ether (40 mL) was added, and, after vigorous shaking, the organic layer was removed and retained. n-Butanol (60 mL) was added to the aqueous fraction. The combined aqueous-butanol mixture was washed with 5% NaCl solution, before evaporation of the solvents in a fume cupboard using a water bath to yield crude saponins.
Once prepared all extracts were stored at 4 °C, with experimental assays performed within a 4-month period.
Animals
For rat brain AChE measurements, male F344 strain rats weighing between 200 and 230 g were used for experiments. Rats were maintained in cages under controlled temperature (21 ± 1 °C) and light (16 h light/8 h dark cycle) with ad libitum access to food intake and water. All animal procedures were approved by the University of Nottingham Local Ethical Review Committee (study reference CHE 10) and were carried out in accordance with the Animals Scientific Procedures Act (UK) 1986.
AChE activity assay
AChE activity was measured based on the method of Ellman et al. (1961), adapted for a microtitre plate format. Briefly, for each assay data point, 50 μL of 3 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 50 μL of recombinant AChE (1 mg/mL) (Sigma, C3389, Irvine, UK) or rat brain homogenate (prepared at 10% (w/v) according to Carter et al. (2007) and Tarhoni et al. (2011), 35 μL of 50 mM Tris/HCl pH 8.0 and 40 μL of plant extract or fraction was mixed and incubated at 37 °C. The assay was initiated by the addition of 25 μL of 15 mM acetylthiocholineiodide (ATCI), with production of 5-thio-2-nitrobenzoate anion read at 412 nm every 5 s for 10 min using a Spectramax microplate reader (ThermoFisher, Stafford, UK). Assay reactions with plant extracts or fractions were all performed in triplicate at concentrations of 200, 20, 2, 0.2 and 0.02 μg/mL. A negative control assay performed in the absence of AChE provided a reagent blank. Eserine (Sigma, E8375, Irvine, UK) or the organophosphate pesticide, azamethiphos-oxon (QMX Laboratories Ltd, Thaxted, UK), was used as a positive control to inhibit electric eel or rat brain AChE in a dose-dependent fashion (Supplementary Figure S2). The percentage inhibition of AChE by plant extracts or fractions was calculated relative to inhibition by eserine, with the plant extract or fraction concentration producing 50% inhibition (IC50) of AChE determined.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
Stock solutions of extracts or fractions (5 mg/mL) were diluted to final concentrations of 200, 100, 50, 25, 12.5 and 6.25 μg/mL in ethanol. One hundred and sixty microlitre of 0.1 mM DPPH in ethanol was added to 20 μL of extract or fraction, or vitamin E (as a positive control), and the material mixed with 20 μL of water. A control solution of β-tocopherol was also assayed over a concentration range of 1.56, 0.78, 0.39, 0.195 and 0.0975 mg/mL. The mixture was incubated at 37 °C for 40 min in the dark, before reading the absorbance at 517 nm using a Spectramax microplate reader (ThermoFisher, Stafford, UK). Experimental blanks were performed in the absence of extracts or fractions. The percentage antioxidant activity was estimated as the percent DPPH radical scavenging activity. All tests were performed in triplicate and inhibition percentages reported as means ± SD.
Reducing power assessment
The reducing power of plant extracts or fractions was assessed as the ability to reduce ferric iron (Fe3+) to ferrous iron (Fe2+). Concentrations of plant extracts or fractions were prepared across a concentration range of 6.25–50 μg/mL. Aliquots of 4 μL of 5 mg/mL of each plant sample were added to 400 μL of phosphate buffer pH 7.4 and 250 μL of 1% potassium ferricyanide and incubated at 50 °C for 20 min. Then 250 μL of 10% trichloroacetic acid was added, and after mixing, samples were centrifuged at 3000 rpm for 10 min. One hundred microlitres of the supernatant was mixed with a similar volume of water and added to a microtiter plate well. Freshly prepared ferric chloride solution (20 μL) was added, and the formation of Perls’ Prussian blue followed at 700 nm using a Spectramax plate reader (ThermoFisher, Stafford, UK). A blank was prepared without addition of antioxidant. All assay points were conducted in triplicates. l-Ascorbic acid was used as a positive control antioxidant. The percentage increase of reduction activity to that of control for plant extracts and fractions, and percentage of ascorbic acid reducing capacity were calculated.
Determination of total phenolic content
Total phenolic content was determined using the Folin–Ciocalteau reagent (FCR) method as described in a previous publication (Nwidu et al. 2012). Plant extract (20 μL) or fraction (20 μL) (a concentration range of 1–100 μg/mL) was mixed with 90 μL of water, followed by the addition of 30 μL of FCR and vigorous shaking on a plate reader. Within 8 min, 60 μL of 7.5% Na2CO3 solution was added and the material incubated at 40 °C in a shaking incubator. After 40 min, the mixture was read in a spectrophotometer at 760 nm. Gallic acid (Sigma, CAS14991-7, Irvine, UK) was similarly processed over a concentration range of 0.1–0.5 mg/mL as a positive control, and used to generate a calibration curve for quantification of total phenolic content in extracts and fractions across the concentration range of 0.01–0.05 mg/mL. Total phenolic content was expressed as mg gallic acid equivalents/gram of plant extract or fraction (mg GAE/g).
Determination of total flavonoid content
Total flavonoid content of plant extracts was determined using quercetin as a reference compound. Briefly, 20 μL of plant extract (5 mg/mL) in ethanol was mixed in a microtiter plate well with 200 μL of 10% aluminium chloride solution and 1 M potassium acetate solution. The mixture was incubated for 30 min at room temperature before reading the absorbance at 415 nm using a Spectramax plate reader (ThermoFisher, Stafford, UK). Total flavonoid content of plant extracts or fractions was determined as mg quercetin equivalents/gram of extract or fraction (mg QUER equivalent/g).
Cell culture
Human hepatocellular carcinoma (HepG2) cells from the ATCC collection were cultured in Eagles’s minimum essential media (EMEM) with 2 mM glutamine, 1% non-essential amino acids (NEAA), 10% foetal bovine serum (FBS) and antibiotics (100 μg/mL penicillin and 100 μg/mL streptomycin). Cells were grown in a humidified atmosphere at 37 °C and 5% CO2.
Cytotoxicity assays
Cells were seeded at 3 × 104 cells per well in 96-well plates. At ∼80–90% confluence, extracts or fractions of C. lutea were added to wells at 0.1, 1, 10 and 100 μg/mL. After a 48 h treatment, media were removed and cells were incubated with fresh medium containing 0.5% thiazolyl blue tetrazolium bromide (MTT) reagent (Sigma, M2128, Irvine, UK). After 4 h at 37 °C in the humidified incubator, the media were replaced with 100 μL of isopropanol and DMSO solution [1:1 (v/v)] and the absorbance was read at 540 nm in a Spectramax plate reader (ThermoFisher, Stafford, UK). Each assay point was conducted in triplicate. Control assays performed in the absence of extract/fractions, and with extracts/fractions and in the absence of cells, were subtracted from absorbance values. The percentage of cells surviving was calculated for each concentration of C. lutea extract or fraction.
Statistical analysis
All statistical procedures were performed using PRISM 5 (GraphPad Software Inc., San Diego, CA). IC50 values were calculated using non-linear regression analysis. One-way ANOVA test with Dunn’s multiple comparisons post-test were used for comparisons of different group’s data. Results were expressed as means ± SD. Statistical significance was defined as p < 0.05.
Results
Carpolobia lutea extracts display AChE inhibitory activity
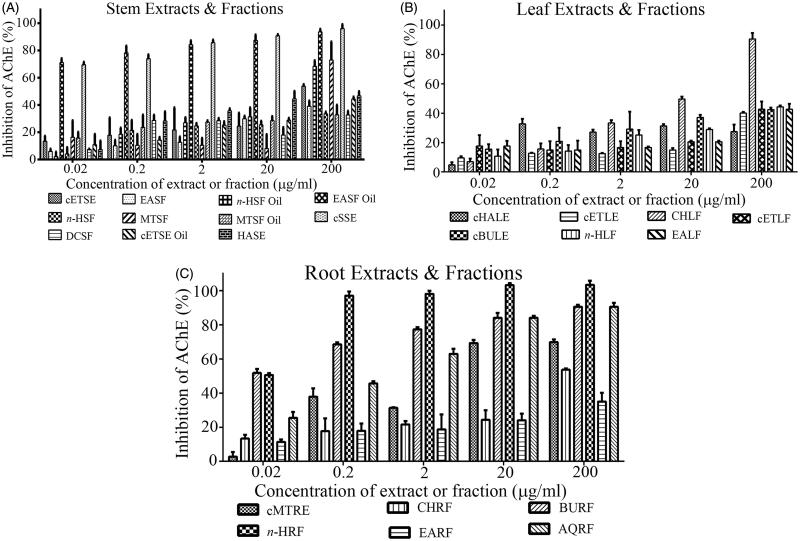
Carpolobia lutea stem-bark, leaf and root extracts inhibited AChE activity in a dose-dependent manner (Figures 1(A–C)). At the highest dose assessed (200 μg/mL), the EASF oil and cSSE (Figure 1(A)); CHLF (Figure 1(B)); and BURF, n-HRF, and AQRF (Figure 1(C)) inhibited AChE to ∼90–100% of that observed using 0.02 μg/mL eserine. From these five assay concentrations (0.02–200 μg/mL), an approximate IC50 concentration was calculated. The roots were observed to retain the highest inhibitory potency (lowest IC50 concentrations) (Table 1).
Figure 1.
AChE inhibitory activity of plant extracts and fractions of C. lutea. Plant inhibition of AChE was measured using a modified Ellman assay, with percentage inhibition of AChE calculated relative to eserine. (A) Stem, (B) leaf and (C) root. Results are expressed as means ± SEM for three separate experiments at each concentration.
The descending order of AChE inhibitory activity for the stem extracts and fractions was cETSE and n-HSF oil > MTSF > EASF, MTSF oil > EASF oil > HASE > cSSE > cETSE oil > n-HSF > DCSF. For the leaves, the descending order of AChE inhibitory potency was CHLF > cETLF > n-HLF > cETLE > EALF > cBULE > cHALE; and for the roots EARF > AQRF > cMTRE > CHRF > n-HRF > BURF.
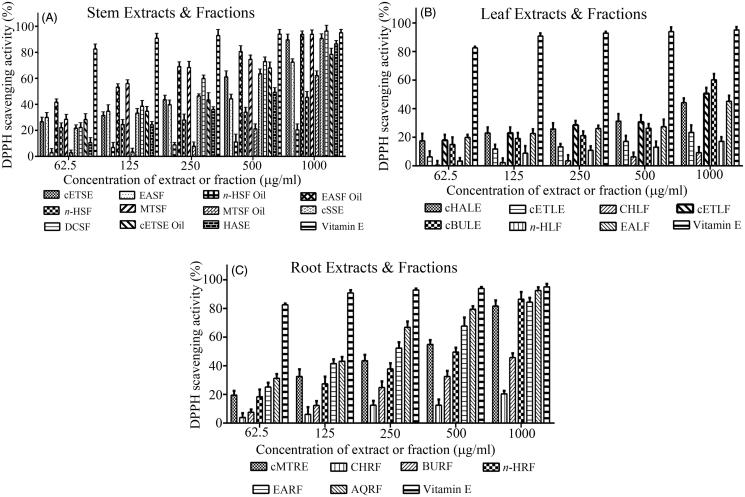
Carpolobia lutea extracts display DPPH scavenging activity
Carpolobia lutea stem, leaf and root extracts and fractions exhibited DPPH radical scavenging activity in a concentration-dependent manner (Figures 2(A–C)). The quantitation of DPPH radical scavenging ability across the concentration range of 62.5–1000 μg/mL was used to calculate IC50 concentrations (Table 1). The MTSF, n-HLF and AQRF displayed the most potent DPPH radical scavenging activity (lowest IC50s) for the stem, leaves and roots, respectively (Table 1). Collectively, for the stem, the descending order of DPPH radical scavenging activity was the following: MTSF > EASF oil > cETSE oil > cETSE > cSSE > DCSF > HASE > EASF > MTSF oil > n-HSF, n-HSF oil (Table 1). For the leaves, DPPH radical scavenging activity was in the descending order: n-HLF > cHALE > CHLF > cETLE > cBULE, EALF, cETLF; and for the roots: AQRF > n-HRF > cMTRF > CHRF > EARF, BURF.
Figure 2.
DPPH radical scavenging activity of plant extracts and fractions of C. lutea. Plant antioxidant activity was assessed via the percent inhibition (radical scavenging) of DPPH. Vitamin E was used as a positive control. (A) Stem, (B) leaf, and (C) root. Results are expressed as means ± SEM for three separate experiments at each concentration.
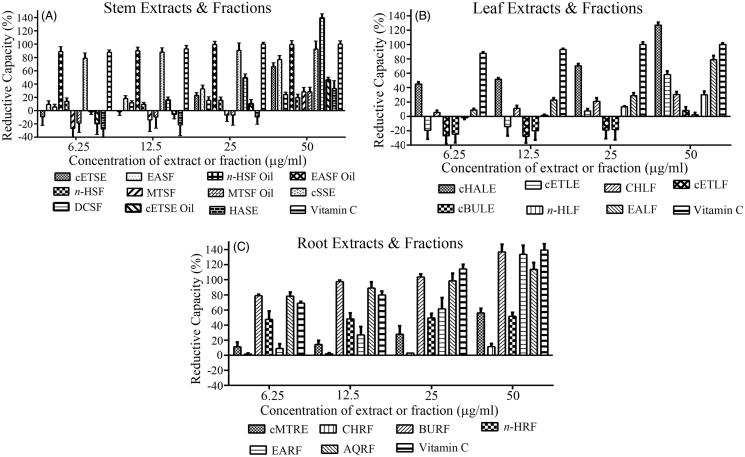
Carpolobia lutea extracts display reducing (antioxidant) activity
Carpolobia lutea stem, leaf and root extracts and fractions displayed reducing (antioxidant) capacity in a concentration-dependent fashion (Figures 3(A–C)). Some extracts and fractions of stem and leaves displayed no reductive capacity, or indeed were oxidative in nature (values below zero) for concentrations of 6.25–25 μg/mL. At the highest concentration examined (50 μg/mL), the reducing capacity of the stem extracts and fractions was DCSF > EASF oil > cSSE > EASF > cETSE > ETSE oil > HASE > MTSF > MTSF oil > n-HSF oil > n-HSF (Figure 3(A)). Notably the DCSF had a higher percentage reducing capacity than the assayed standard, ascorbic acid (vitamin C). At 50 μg/mL, the reducing capacity of the leaf extracts and fractions was cHALE > EALF > cETLE > CHLF > n-HLF > ETLF > cBULE (Figure 3(B)). For the root extracts and fractions, the order was BURF > EARF > AQRF > MTRE > n-HRF > CHRF. Collectively, the roots displayed the greatest reducing capacity, with both BURF and EARF possessing comparable reductive capacity to vitamin C.
Figure 3.
Reductive capacity of plant extracts or fractions of C. lutea. Plant reducing power was assessed via the ability to reduce ferric (Fe3+) to ferrous (Fe2+) iron. The percentage increase of reductive capacity with increasing plant extract concentration was determined. Ascorbic acid (vitamin C) was used as a positive control. (A) Stem, (B) leaf, and (C) root. Results are expressed as means ± SEM for three separate experiments at each concentration.
Total phenolic and total flavonoid content of C. lutea
The total phenolic content measured as mg gallic acid equivalents/gram and total flavonoid content measured as mg quercetin equivalents/gram were determined for each extract and fraction (Table 2). For the stem, the n-HSF oil and the DCSF possessed the highest total phenolic content. The total phenolic content decreased in the following order: n-HSF oil > DCSF > cETSE > MTSF > n-HSF > cETSE oil > HASE > EASF > MTSF oil > cSSE > EASF oil. Similarly, the n-HSF oil possessed the highest total flavonoid content,; with a descending order of n-HSF oil > n-HSF > MTSF oil > EASF > cSSE > cETSE oil > cETSE > EASF oil > DCSF > HASE > MTSF.
Table 2.
Total phenolic and total flavonoid content of stem, leaf and root extracts and fractions of C. lutea.
| Extract or fraction | Total phenoliccontent (mg GAE/g) | Total flavonoid content(mg QUER E/g) |
|---|---|---|
| Stem | ||
| cETSE | 235.33 ± 0.01 | 30.59 ± 0.01 |
| EASF | 177.23 ± 0.01 | 100.37 ± 0.20 |
| n-HSF oil | 632.28 ± 0.00 | 196.21 ± 0.10 |
| EASF oil | 3.00 ± 0.00 | 29.54 ± 0.20 |
| n-HSF | 226.46 ± 0.00 | 176.87 ± 0.20 |
| MTSF | 234.99 ± 0.02 | 1.75 ± 0.01 |
| MTSF oil | 128.42 ± 0.01 | 116.49 ± 0.04 |
| cSSE | 26.0 ± 0.00 | 78.72 ± 0.30 |
| DCSF | 581.49 ± 0.00 | 19.59 ± 0.01 |
| cETSE oil | 198.50 ± 0.00 | 40.85 ± 0.01 |
| HASE | 178.37 ± 0.00 | 11.94 ± 0.02 |
| Leaf | ||
| cHALE | 256.04 ± 0.01 | 17.04 ± 0.00 |
| cETLE | 257.02 ± 0.02 | 24.69 ± 0.02 |
| CHLF | 144.67 ± 0.01 | 296.68 ± 0.03 |
| cETLF | 120.32 ± 0.01 | 63.28 ± 0.02 |
| cBULE | 292.29 ± 0.01 | 19.21 ± 0.00 |
| n-HLF | 285.63 ± 0.00 | 246.22 ± 0.20 |
| cEALE | 100.85 ± 0.00 | 118.23 ± 0.00 |
| Root | ||
| cMTRE | 246.28 ± 0.00 | 9.36 ± 0.01 |
| CHRF | 33.40 ± 0.00 | 104.52 ± 0.08 |
| BURF | 462.00 ± 0.00 | 193.02 ± 0.00 |
| n-HRF | 357.00 ± 0.00 | 123.06 ± 0.00 |
| EARF | 296.50 ± 0.00 | 53.55 ± 0.01 |
| AQRF | 28.00 ± 0.00 | 44.39 ± 0.00 |
For the leaves, the total phenolic content was cBULE > n-HLF > cETLE > cHALE > CHLF > cETLF > EALF; and, for total flavonoid content, CHLF > n-HLF > EALF > cETLF > cETLE > cBULE > cHALE.
For the roots, the total phenolic content was BURF > n-HRF > EARF > cMTRE > CHRF > AQRF; and, for total flavonoid content, BURF > n-HRF > CHRF > EARF > AQRF > cMTRE.
Assessment of C. lutea cytotoxicity
Carpolobia lutea stem, leaf or root extracts and fractions were evaluated for cytotoxicity to human liver HepG2 cells over a broad concentration range of 0.1–100 μg/mL. Evaluation of cytotoxicity included extracts/fractions of relatively potent AChE inhibitory activity (cETSE, n-HSF Oil, MTSF, cMTRE, CHRF, EARF), DPPH radical scavenging activity (MTSF, cHALE) and high phenolic content (n-HSF Oil, DCSF). At the highest dose employed, HepG2 cell viability was still ∼100% with some of the stem (cETSE, n-HSF Oil, MTSF, MTSF Oil) and root (cMTRE, EARF) extracts/fractions, with IC50s of >2000 μg/mL (Table 3). However, certain stem (EASF, n-HSF, DCSF, cETSE Oil, HASE), leaf (cHALE) or root (CHRF) extracts/fractions displayed dose-dependent inhibition of cell viability albeit with relatively high IC50 values (∼691 to >2000 μg/mL). Interestingly, incubation of cells with several plant extracts/fractions (cETSE, MTSF, cBULE, cMTRE, EARF) at concentrations of 0.1–10 μg/mL triggered a significant increase of cell metabolic activity with MTT assay results above 100% (Table 3).
Table 3.
Influence of plant extracts or fractions of C. lutea upon HepG2 cell viability.
| Extracts or fractions | 0.1 μg/mL | 1 μg/mL | 10 μg/mL | 100 μg/mL | IC50 (μg/mL) |
|---|---|---|---|---|---|
| cETSE | 101.20 ± 7.34 | 118.23 ± 9.23* | 121.34 ± 4.35*** | 97.23 ± 2.34 | > 2000 |
| EASF | 105.60 ± 10.24 | 102.40 ± 9.26 | 95.60 ± 7.25 | 35.5 ± 12.24*** | ∼754 |
| n-HSF oil | 99.80 ± 11.25 | 103.20 ± 9.35 | 115.43 ± 7.36 | 93.23 ± 8.35 | >2000 |
| n-HSF | 101.30 ± 8.35 | 113.54 ± 13.24 | 74.30 ± 8.35** | 46.2 ± 5.36*** | ∼895 |
| MTSF | 105.60 ± 7.24 | 130.30 ± 12.13* | 112.20 ± 12.34 | 97.56 ± 10.23 | >2000 |
| MTSF oil | 105.40 ± 6.35 | 113.50 ± 6.35 | 110.60 ± 9.39 | 95.65 ± 9.34 | >2000 |
| DCSF | 107.50 ± 6.45 | 103.40 ± 7.35 | 98.50 ± 10.24 | 42.34 ± 8.34*** | ∼843 |
| cETSE oil | 87.13 ± 8.34* | 79.23 ± 5.54*** | 64.82 ± 4.35*** | 56.20 ± 3.34*** | ∼1010 |
| HASE | 101.23 ± 11.32 | 99.65 ± 6.34 | 91.26 ± 9.45 | 85.45 ± 8.36** | >2000 |
| cBULE | 108.15 ± 0.57** | 110.72 ± 3.64* | 112.49 ± 3.14** | 73.39 ± 7.49** | >2000 |
| cEALE | 102.87 ± 5.06 | 94.85 ± 1.19 | 104.89 ± 4.36 | 89.13 ± 5.29 | >2000 |
| cHALE | 99.34 ± 12.23 | 98.12 ± 10.32 | 78.34 ± 8.34** | 61.23 ± 10.2*** | >2000 |
| cMTRE | 101.23 ± 9.24 | 123.20 ± 8.65* | 112.48 ± 5.34 | 99.50 ± 12.34 | >2000 |
| CHRF | 99.30 ± 9.35 | 92.35 ± 10.23 | 82.50 ± 12.23 | 32.14 ± 10.24*** | ∼691 |
| EARF | 101.70 ± 8.24 | 112.64 ± 6.45 | 122.40 ± 7.35* | 95.23 ± 13.23 | >2000 |
Hep G2 cells were incubated with plant extracts or fractions at the concentrations specified for 48 h and the percentage of viable cells determined using a MTT assay. Extract or fraction was assessed at least in triplicate across a 0.1–100 μg/mL concentration range, and an approximate IC50 calculated. For marked significance from controls,
p < 0.05,
p < 0.01,
p < 0.001.
Discussion and conclusions
The prevalence of neurodegenerative disease is increasing due to an ever ageing population, with the number of individuals living with dementia expected to double every 20 years, reaching 74.7 million by 2030 (Alzheimer’s Disease International 2015). The World Health Organization has recognized this as a global healthcare problem of potentially epidemic proportion (Global Health and Aging report 2011). Treatment of neurodegenerative diseases such as AD is currently limited, and new medicines that alleviate symptomology and restrict disease progression are required.
An ethnopharmacological approach of systematic screening of natural products is a cost effective strategy of developing novel drug treatments. Indeed, rivastigmine and galantamine are FDA-approved AChE inhibitors that were derived from medicinal plants, and are utilized for the treatment of mild to moderate AD (Muñoz-Torrero 2008; Ballard et al. 2011; Syad and Devi 2014). Our screening study demonstrated that extracts and fractions of the stems, leaves and roots of C. lutea inhibit AChE in a dose-dependent fashion. The cETSE, MTSF and n-HSF oil from the stem-bark were approximately equipotent at inhibiting AChE with IC50 values of ∼140 μg/mL. Two leaf fractions, CHLF and ETLF, exhibited even lower IC50 values of 60 and 81 μg/mL, respectively. For roots, the AQRF, EARF and MTRE were the most potent inhibitors of AChE, with IC50 values of 0.3–3.0 μg/mL. Pure eserine (physostigmine) at 0.02 μg/mL (∼72 nM) was employed as a positive control in our assays. At this concentration, eserine was able to inhibit electric eel AChE to approaching 100% (results not included), and likewise inhibit rat brain AChE (Supplementary Data Figure S2). Human brain AChE should also be inhibited to approaching 100% at this eserine concentration (IC50 of ∼14 nM) (Thomsen et al. 1991; Triggle et al. 1998). Hence, root extracts of C. lutea show particular promise for provision of a useful AChE inhibitor since in a partially purified state their IC50 was only an order of magnitude less potent than eserine. By comparison, the anti-AChE drug tacrine has an IC50 value of ∼1 μM against human AChE (Thomsen et al. 1991), approximately 72 times less potent than eserine.
In addition to notable AChE inhibitory activity, our study also highlights the existence and activity of C. lutea secondary metabolites. Carpolobia lutea contains polyphenols and flavonoids (Table 2), compounds that exhibit scavenging (antioxidant) activities. These antioxidants may counter free radical damage produced during metabolism and for which radical levels may be exacerbated in AD (Parihar and Hemnani 2004; Zhao and Zhao 2013). For comparison to standards, the majority of stem and root extracts/fractions at a 1 mg/mL concentration displayed DPPH free radical scavenging ability comparable with vitamin E (Figures 2(A,C)). Likewise, reductive (antioxidant capacity) of certain stem (EASF oil, cSSE, DCSF) and root (BURF, EARF) extracts/fractions at 50 μg/mL was comparable with vitamin C (ascorbic acid) (Figures 3(A,C)). The majority of leaf extracts/fractions were relatively weak DPPH radical scavengers, in keeping with a previous preliminary screening of C. lutea leaves (Nwidu et al. 2012). At concentrations of 6.25–25 μg/mL, some of the stem and leaf extracts of C. lutea were oxidative in nature (Figure 3(A,B)), indicative of the presence of pro- as well as anti-oxidant chemicals.
There is a growing list of plants that have similarly been screened for anti-AChE activity and that also possess beneficial natural antioxidants (secondary metabolites) that can complement endogenous antioxidant systems (Mukherjee et al. 2007; Calderón et al. 2010; Şenol et al. 2010; Hlila et al. 2013; Natarajan et al. 2013; Syad and Devi 2014). By comparison with these, C. lutea roots exhibit sub-micromolar IC50 values and are, therefore, among the more potent phytochemical inhibitors of AChE (Natarajan et al. 2013; Pinho et al. 2013; Tundis et al. 2016).
Some plant constituents are also able to inhibit butyrylcholinesterase (BChE) in vitro (Pinho et al. 2013; Tundis et al. 2016). The AD drug rivastigmine is a weak inhibitor of both AChE and BChE in vitro (Pinho et al. 2013), but in vivo, the AD drugs huperzine A and donepezil specifically inhibit AChE rather than BChE (Duysen et al. 2007), and similarly, in vitro, AChE displays a 1000-fold higher affinity than BChE to inhibition by huperzine A (Ashani et al. 1992). It has been suggested that BChE activity may not normally be needed for acetylcholine hydrolysis since BChE-deficient mice are healthy, and similarly, humans with no BChE activity do not display signs of ill-health, or a loss of fertility or longevity (Manoharan et al. 2007; Lockridge 2015). BChE may certainly substitute for AChE upon requirement since AChE knockout mice are also viable (Mesulam et al. 2002), but the importance of anti-BChE activity in AD drug development will need further substantiation; and so the ability of C. lutea to inhibit BChE has not yet been investigated further.
A recent in vivo study by Ajiwhen and Bisong (2013) administered a low-dose (1500 mg/kg p.o.) of C. lutea root extract to mice and reported cognitive memory enhancing activity, and this may reflect the potent anti-AChE activity described herein. At this dose of C. lutea roots, no toxicity to mice was reported (Ajiwhen and Bisong 2013), and our in vitro study suggests that at levels producing AChE inhibition (0.1–1 μg/mL), the majority of C. lutea extracts/fractions are not toxic to human liver cells (Table 3).
Carpolobia lutea possesses flavonoids (Table 2): polyphenols that include flavones and isoflavones that are notable anti-AChE inhibitors (Uriarte-Pueyo and Calvo 2011; Pinho et al. 2013). However, there was not a direct correlation between total flavonoid levels in either stem, leaf or roots with corresponding anti-AChE inhibitory potency (Table 1). Thus, we cannot yet comment on the active agent that produces AChE inhibition, but similar to other studies (Mukherjee et al. 2007; Calderón et al. 2010; Şenol et al. 2010; Hlila et al. 2013; Natarajan et al. 2013; Syad and Devi 2014), initial screening provides a means to identify fractions suitable for further purification and active agent identification. Thus, C. lutea remains a potentially beneficial pharmacotherapy that could be further developed for the treatment of AD and/or other diseases such as Parkinson’s and myasthenia gravis that also require agents able to address cholinergic deficits.
Supplementary Material
Acknowledgements
The authors are thankful to the following funding sources: International Visiting Fellowship grants to W. G. C. to support L. L. N. and E. E.; The Physiological Society summer scholarship grant to W. G. C. to support J. T.; A. W. is a Commonwealth Scholar, funded by the UK government.
Disclosure statement
The authors can confirm that there are no conflicts of interest associated with the production or publication of this manuscript.
References
- Adams M, Gmünder F, Hamburger M.. 2007. Plants traditionally used in age related brain disorders— a survey of ethnobotanical literature. J Ethnopharmacol. 113:363–381. [DOI] [PubMed] [Google Scholar]
- Ajiwhen IO, Bisong SA.. 2013. Effect of ethanolic extract of Carpolobia lutea G. Don (Polygalaceae) root on learning and memory in CD1 mice. Niger J Physiol Sci. 28:141–145. [PubMed] [Google Scholar]
- Akhondzadeh S, Abbasi SH.. 2006. Herbal medicine in the treatment of Alzheimer's disease. Am J Alzheimers Dis Other Demen. 21:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Disease International. 2015. World Alzheimer Report 2015: The Global Impact of Dementia. [accessed 2016 Oct 4]. http://www.alz.co.uk/research/world-report-2015. [Google Scholar]
- Anekonda TS, Reddy PH.. 2005. Can herbs provide a new generation of drugs for treating Alzheimer's disease? Brain Res Brain Res Rev. 50:361–376. [DOI] [PubMed] [Google Scholar]
- Ansari N, Khodagholi F.. 2013. Natural products as promising drug candidates for the treatment of Alzheimer's disease: molecular mechanism aspect. Curr Neuropharmacol. 11:414–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashani Y, Peggins JO, Doctor BP.. 1992. Mechanism of inhibition of cholinesterases by huperzine A. Biochem Biophys Res Commun. 184:719–726. [DOI] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. 2011. Alzheimer's disease. Lancet. 377:1019–1031. [DOI] [PubMed] [Google Scholar]
- Calderón AI, Cubilla M, Espinosa A, Gupta MP.. 2010. Screening of plants of Amaryllidaceae and related families from Panama as sources of acetylcholinesterase inhibitors. Pharm Biol. 48:988–993. [DOI] [PubMed] [Google Scholar]
- Carter WG, Tarhoni M, Rathbone AJ, Ray DE.. 2007. Differential protein adduction by seven organophosphorus pesticides in both brain and thymus. Hum Exp Toxicol. 26:347–353. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Darvesh S, Lockridge O.. 2007. Sensitivity of butyrylcholinesterase knockout mice to (−)-huperzine A and donepezil suggests humans with butyrylcholinesterase deficiency may not tolerate these Alzheimer's disease drugs and indicates butyrylcholinesterase function in neurotransmission. Toxicology. 233:60–69. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM.. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. [DOI] [PubMed] [Google Scholar]
- Global Health and Aging report. 2011. World Health Organization. [accessed 2016 Oct 4]. www.who.int/ageing/publications/global_health.pdf.
- Gurib-Fakim A.2006. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 27:1–93. [DOI] [PubMed] [Google Scholar]
- Hajiaghaee R, Akhondzadeh S.. 2012. Herbal medicine in the treatment of Alzheimer’s disease. J Med Plants. 11:41. [Google Scholar]
- Hlila MB, Omri A, Jannet HB, Lamari A, Aouni M, Selmi B.. 2013. Phenolic composition, antioxidant and anti-acetylcholinesterase activities of the Tunisian Scabiosa arenaria. Pharm Biol. 51:525–532. [DOI] [PubMed] [Google Scholar]
- Howes M-JR, Houghton PJ.. 2003. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav. 75:513–527. [DOI] [PubMed] [Google Scholar]
- Lockridge O.2015. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther. 148:34–46. [DOI] [PubMed] [Google Scholar]
- Manoharan I, Boopathy R, Darvesh S, Lockridge O.. 2007. A medical health report on individuals with silent butyrylcholinesterase in the Vysya community of India. Clin Chim Acta. 378:128–135. [DOI] [PubMed] [Google Scholar]
- Menon JS, Krishnakumar K, Dineshkumar B, John A, Paul D, Cherian J.. 2013. Herbs for Alzheimer disease – a review. Int J Res Plant Sci. 3:54–56. [Google Scholar]
- Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O.. 2002. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 110:627–639. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Perez SE, Ginsberg SD.. 2008. Cholinergic system during the progression of Alzheimer's disease: therapeutic implications. Expert Rev Neurother. 8:1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Kumar V, Mal M, Houghton PJ.. 2007. Acetylcholinesterase inhibitors from plants. Pytomedicine. 14:289–300. [DOI] [PubMed] [Google Scholar]
- Muñoz-Torrero D.2008. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer's disease. Curr Med Chem. 15:2433–2455. [DOI] [PubMed] [Google Scholar]
- Natarajan S, Shunmugiah KP, Kasi PD.. 2013. Plants traditionally used in age-related brain disorders (dementia): an ethanopharmacological survey. Pharm Biol. 51:492–523. [DOI] [PubMed] [Google Scholar]
- Nwidu LL, Aprioku JS, Guluye FH.. 2014. Study on radical scavenging and gastroprotective effects of ethanolic stem-bark extract of Carbolobia lutea in rodents. W J Pharm Res. 3:73–88. [Google Scholar]
- Nwidu LL, Cilli EM, Vilegas W.. 2012. Amino acid, antioxidant and ion profiles of Carpolobia lutea leaf (Polygalaceae). Trop J Pharm Res. 11:807–813. [Google Scholar]
- Nwidu LL, Essien GE, Nwafor PA, Vilegas W.. 2011. Antidiarrheal mechanism of Carpolobia lutea leaf fractions in rats. Pharm Biol. 49:1249–1256. [DOI] [PubMed] [Google Scholar]
- Nwidu LL, Nwafor PA, da Silva VC, Rodrigues CM, dos Santos LC, Vilegas W, Nunes-de-Souza RL.. 2011. Anti-nociceptive effects of Carpolobia lutea G. Don (Polygalaceae) leaf fractions in animal models. Inflammopharmacology. 19:215–225. [DOI] [PubMed] [Google Scholar]
- Orhan IE.2013. Nature: a substantial source of auspicious substances with acetylcholinesterase inhibitory action. Curr Neuropharmacol. 11:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MS, Hemnani T.. 2004. Alzheimer's disease pathogenesis and therapeutic interventions. J Clin Neurosci. 11:456–467. [DOI] [PubMed] [Google Scholar]
- Pinho BR, Ferreres F, Valentão P, Andrade PB.. 2013. Nature as a source of metabolites with cholinesterase-inhibitory activity: an approach to Alzheimer's disease treatment. J Pharm Pharmacol. 65:1681–1700. [DOI] [PubMed] [Google Scholar]
- Seltzer B.2006. Cholinesterase inhibitors in the clinical management of Alzheimer's disease: importance of early and persistent treatment. J Int Med Res. 34:339–347. [DOI] [PubMed] [Google Scholar]
- Şenol FS, Yilmaz G, Şener B, Koyuncu M, Orhan I.. 2010. Preliminary screening of acetylcholinesterase inhibitory and antioxidant activities of Anatolian Heptaptera species. Pharm Biol. 48:337–341. [DOI] [PubMed] [Google Scholar]
- Shakir T, Coulibaly AY, Kehoe PG.. 2013. An exploration of the potential mechanisms and translational potential of five medicinal plants for applications in Alzheimer's disease. Am J Neurodegener Dis. 2:70–88. [PMC free article] [PubMed] [Google Scholar]
- Shiksharthi AN, Mittal S, Ramana J.. 2011. Systematic review of herbals as potential memory enhancers. Int J Res Pharm Biomed Sci. 2:918–925. [Google Scholar]
- Sun X, Jin L, Ling P.. 2012. Review of drugs for Alzheimer's disease. Drug Discov Ther. 6:285–290. [PubMed] [Google Scholar]
- Syad AN, Devi KP.. 2014. Botanics: a potential source of new therapies for Alzheimer’s disease. Botanics: Targets Therapy. 4:11–26. [Google Scholar]
- Tarhoni MH, Vigneswara V, Smith M, Anderson S, Wigmore P, Lees J, Ray DE, Carter WG.. 2011. Detection, quantification, and microlocalisation of targets of pesticides using microchannel plate autoradiographic imagers. Molecules. 16:8535–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen T, Kaden B, Fischer JP, Bickel U, Barz H, Gusztony G, Cervos-Navarro J, Kewitz H.. 1991. Inhibition of acetylcholinesterase activity in human brain tissue and erythrocytes by galanthamine, physostigmine and tacrine. Eur J Clin Chem Clin Biochem. 29:487–492. [DOI] [PubMed] [Google Scholar]
- Triggle DJ, Mitchell JM, Filler R.. 1998. The pharmacology of physostigmine. CNS Drug Rev. 4:87–136. [Google Scholar]
- Tundis R, Bonesi M, Menichini F, Loizzo MR.. 2016. Recent knowledge on medicinal plants as source of cholinesterase inhibitors for the treatment of dementia. Mini Rev Med Chem. 16:605–618. [DOI] [PubMed] [Google Scholar]
- Uriarte-Pueyo I, Calvo MI.. 2011. Flavonoids as acetylcholinesterase inhibitors. Curr Med Chem. 18:5289–5302. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhao B.. 2013. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev. 2013:316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.