Abstract
Context: Soy is the main source of phytoestrogens, which has long been used as traditional food. One major subtype of phytoestrogens includes isoflavones and they are scientifically validated for their beneficial actions on many hormone-dependent conditions.
Objective: The present study examines the effect of soy isoflavones on letrozole-induced polycystic ovary syndrome (PCOS) rat model.
Materials and methods: PCOS was induced in Sprague–Dawley rats with of 1 mg/kg letrozole, p.o. once daily for 21 consecutive days. Soy isoflavones (50 and 100 mg/kg) was administered for 14 days after PCOS induction. Physical parameters (body weight, oestrous cycle determination, ovary and uterus weight) metabolic parameters (oral glucose tolerance test, total cholesterol), steroidal hormone profile (testosterone and 17β-oestradiol), steroidogenic enzymes (3β-hydroxy steroid dehydrogenase (HSD) and 17β-HSD), oxidative stress and histopathology of ovary were studied.
Results: Soy isoflavones (100 mg/kg) treatment significantly altered the letrozole-induced PCOS symptoms as observed by decreased body weight gain (p < 0.05), percentage diestrous phase (p < 0.001), testosterone (p < 0.001), 3β-HSD (p < 0.01) and 17β-HSD (p < 0.001) enzyme activity and oxidative stress. Histological results reveal that soy isoflavones treatment in PCOS rats resulted in well-developed antral follicles and normal granulosa cell layer in rat ovary.
Discussion: Treatment with soy isoflavones exerts beneficial effects in PCOS rats (with decreased aromatase activity) which might be due to their ability to decrease testosterone concentration in the peripheral blood.
Conclusion: Analysis of physical, biochemical and histological evidences shows that soy isoflavones may be beneficial in PCOS.
Keywords: Genistein, daidzein, phytoestrogen, testosterone, oestradiol, oestrogen beta
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in premenopausal women (Kopera et al. 2010). A deficiency in activity of aromatase was one reasonable intraovarian disturbance in steroidogenesis thought to cause PCOS. Because aromatase catalyzes the rate-limiting step in biosynthesis of oestrogens from androgens, decrease in activity of this enzyme could be expected to result in increased ovarian androgen production and development of PCOS (Shi & Vine 2012). In light of these facts, letrozole, a non-steroidal aromatase inhibitor, was administered in female rats to develop PCOS (Kafali et al. 2004).
Apart from high testosterone concentrations, women with PCOS, showed a decrease in the expression of oestrogen receptor beta (ERβ) mRNA and protein in both granulosa and theca cells from follicles derived from subjects with PCOS compared with those from size-matched control follicles (Jakimiuk et al. 2002). Zurvarra et al. (2009) reported that there is a decrease in the expression of ERβ in the granulosa cell layer of cystic follicles in animals treated with letrozole compared with that in normal animals, concomitant with a decrease in total ERβ protein in letrozole-treated animals. A significant increase in androgen receptor expression was also evident in letrozole-induced PCOS animals (Zurvarra et al. 2009). Thus, letrozole-induced PCOS model exhibits many histologic and biochemical findings consistent with human PCOS (Sun et al. 2013).
Isoflavones are naturally occurring plant chemicals belonging to the “phytoestrogen” class; they are currently reported to offer potential alternative therapies for a range of hormone-dependent conditions, including cancer, menopausal symptoms, cardiovascular disease and osteoporosis. Dietary supplements containing isoflavones from soy are widely marketed for menopausal symptoms and are increasingly being used by women as an alternative to oestrogen (Romualdi et al. 2008). The phenolic ring structures of isoflavones enable these compounds to bind ERs and mimic oestrogen. Interestingly, soy isoflavone genistein and daidzein increased the mRNA and protein expression of ERβ in porcine granulosa cells (Nynca et al. 2009, 2013). Soy isoflavones are also known to downregulate androgen receptor (Basak et al. 2008). Hence, the present study was carried out on the hypothesis that the soy isoflavones may exhibit beneficial effects in PCOS associated with deficient aromatase activity. To test the hypothesis, letrozole (aromatase inhibitor) was used to induce PCOS in immature female rats and the effect of soy isoflavones treatment was evaluated.
Materials and methods
Experimental animal and ethics statement
Female Sprague–Dawley rats (42–45 days old), weighing 85 ± 5 g were used in this study. The animals were group-housed (n = 6 per cage) in a room with controlled temperature (21–22 °C), and in a reversed 12 h light-dark cycle. They had free access to oestrogen-free food and water ad libitum. All the experimental protocols employed in this study were approved by our Institutional Animal ethical Committee (Protocol approval number: 224/2014) and experiments were performed according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines on the ethical use of animals.
Study protocol
Twenty-four female Sprague–Dawley rats were randomly assigned into 4 groups of 6 rats each as following: Group I served as control and received 0.3% w/v carboxy methyl cellulose (CMC), p.o.; Group II served as PCOS control group and received vehicle, p.o.; Group III and Group IV PCOS rats were treated with Soy isoflavones 50 and 100 mg/kg, p.o., respectively. PCOS was induced with of 1 mg/kg letrozole, p.o. once daily for 21 consecutive days. Soy isoflavones (a mixture of genistin, genistein, daidzin and daidzein, accounting for 59.91, 12.05, 23.53, and 4.49%, respectively), were obtained from SoyEstro® Gland pharma, Hyderabad and administered for 14 days after PCOS induction.
On day one of the study (i.e., 43 day old), vaginal smears were examined on each group, and their oestrous cycles were observed for 35 consecutive days. Rats were considered as PCOS positive if it exhibited acyclic/irregular ovarian cyclicity. Those rats were chosen for the study.
Peripheral blood, uterus and ovarian tissue samples were collected from the PCOS rats after 14 days of the treatment. During the study period, weekly body weight was measured. The rats were anesthetized with anaesthetic ether, and then blood was collected through retro-orbital sinus puncture on the terminal day of study. The blood samples were centrifuged, and the separated plasma was stored at −20 °C until biochemical analysis. The ovaries and uterus were removed, weighed on terminal day of the study. The ovaries were preserved in Bouin’s fluid and processed for histopathological analysis.
Oestrous cycle determination through vaginal smears
Vaginal smears were performed on all the rats daily at 9:00 am beginning with the 43rd day, and their oestrous cycles were observed till the last day of the study. The cells were stained with methylene blue and evaluated under light microscopy. The smears were classified as 1 of the 4 stages of the oestrous cycle as reported earlier (Sun et al. 2013).
Briefly, three types of cells could be recognized: round and nucleated ones were epithelial cells, irregular ones without nucleus were cornified cells, and the little round ones were leukocytes. The proportion among them was used for determination of the oestrous cycle. When the smear consisted of predominance of nucleated epithelial cells it is considered as proestrous phase. A smear primarily consisted of anucleated cornified cells is considered as oestrous phase. A smear consisted of the same proportion among leukocyte, cornified and nucleated epithelial cells were considered as metaestrous phase. A smear primarily consisted of a predominance of leukocytes is considered as dioestrous phase.
Assessment of biochemical parameters
Oral glucose tolerance test (OGTT)
At terminal day of the study, OGTT was performed after 12 h fasting, in all the experimental group of rats by administering 2 g/kg of glucose (p.o.) (Dong et al. 2014). One hour prior to administration of glucose group I and II rats received 0.3% CMC while group III and IV received soy isoflavones 50 and 100 mg/kg respectively. Blood samples were collected from tail vein at 0, 30, 60, and 120 min to detect the blood glucose levels using Glucometer.
Total cholesterol levels
Total cholesterol levels were measured using Agappe, kit in autoanalyzer according to the manufacturer instructions. The reactions involved in the assay system are as follows: Cholesterol esters are enzymatically hydrolyzed by cholesterol esterase to cholesterol and free fatty acids. Free cholesterol, is then oxidized by cholesterol oxidase to cholest-4-en-3-one and hydrogen peroxide. In presence of peroxidase, the formed hydrogen peroxide formed effects the oxidative coupling of phenol and 4-aminoantipyrine to form a red-coloured quinoneimine dye. The intensity of the colour produced is directly proportional to cholesterol concentration.
Steroidal hormone measurement
The amount of testosterone and oestradiol in plasma samples of experimental rats were estimated using HPLC by previously reported methods with slight modifications (Yamada et al. 2002; Ng & Yuen 2003). Refer Supplementary material for detailed protocols.
Steroidogenic enzymes assay
Preparation of ovarian homogenate
After blood collection, rats were sacrificed by excess anaesthetic ether, the ovaries and uterus were removed. After weighing, 10% ovarian tissue homogenate was prepared in 0.1 M Tris HCl buffer (pH-7.8) and centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was used as a source of steroidogenic enzyme assay, and protein content was estimated according to the method of Lowry et al. (1951).
3β-Hydroxy steroid dehydrogenase enzyme activity assay
3β-Hydroxy steroid dehydrogenase (HSD) was assayed in ovarian homogenate by the method reported earlier (Shivanandappa & Venkatesh 1997). In brief, the enzyme assay was carried out in 0.1 M Tris HCl buffer (pH 7.8) containing NAD (500 μM) and the substrate DHEA (100 μM) for 3β-HSD in a total volume of 3 mL. The reaction was started by adding the enzyme (100 μL ovarian homogenate) together with the colour reagent, iodonitro tetrazolium (INT). The mixture was then incubated at 37 °C for 1 h. The reaction was terminated by the addition of 2.0 mL of phthalate buffer (pH 3.0) and read at 490 nm. The enzyme activity was calculated from the standard curve for NADH and expressed as nano-moles of NADH formed/min/mg protein.
17β- HSD enzyme activity assay
Enzymatic activity of 17β-HSD was determined by Bergmeyer (1974) method. The activity is determined by the optical measurement of the rate of conversion of NADPH to NADP. In brief, the reaction mixture contained 100 μL of ovarian homogenate supernatant, 200 μL of 0.5 μM NADPH, 100 μL of 0.8 μM androsten-3,17-dione in a final volume of 3 mL 100 μM phosphate buffer solution (pH 7.4). The reaction was initiated by the addition of the substrate and the decrease in absorbance of NADPH was followed at 340 nm for 5 min at 20 s interval. The enzyme activity was expressed as nano-moles of NADPH oxidized/min/mg protein.
Estimation of antioxidants
Non-enzymic and enzymic antioxidants were measured in ovarian homogenate as per the following protocols.
Superoxide dismutase estimation
Superoxide dismutase (SOD) activity was determined spectrophotometrically by measuring inhibition of nicotinamide adenine dinucleotide (reduced)-phenazine methosulfate-nitroblue tetrazolium (NBT) reaction system according to the method of Kakkar et al. (1984). The SOD activity was expressed in units/mg protein.
Catalase estimation
The activity of catalase (CAT) was measured according to the method of Beers and Sizer (1952). CAT measurement was done based on the ability of CAT to inhibit oxidation of hydrogen peroxide (H2O2). The change in the absorbance at 240 nm was measured for 3 min. dy/dx for every min for each assay was calculated and the results are expressed as CAT units/mg protein.
Glutathione peroxidase estimation
Glutathione peroxidase (GPx) activity was measured by following the procedure according to Paglia and Valentine (1967). Glutathione peroxidase activity was expressed as Units/mg protein.
Reduced glutathione estimation
Glutathione (GSH) content was estimated according to Jollow et al. (1974) method. The values were expressed in μM/mg protein.
Lipid peroxidation estimation
LPO levels were measured using thiobarbituric acid reacting substance (TBARS) method (Ohkawa et al. 1979). After the reaction with thiobarbituric acid, the reaction product was measured spectrophotometrically at 535 nm. The LPO levels were expressed in μmoles/mg protein.
Nitric oxide estimation
Nitrate/nitrite was assayed in the ovarian homogenate according to the method of Green et al. (1982) method using Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthyl ethylenediamine dihydrochloric acid in water in the ratio of 1:1). The colour intensity of chromogen was read at 540 nm. The results were expressed as μM/mg protein.
Histopathological studies
The collected ovaries at the terminal day of the study were fixed in Bouins fixative. Histological examinations of ovaries from all experimental groups were carried out using standardized histological methods. Sections of 5 μm thickness were cut in paraffin embedded block and stained with hematoxylin eosin.
Statistical analysis
Data were represented as mean ± standard deviation. The collected data were subjected to one way ANOVA (Analysis of Variance) followed by post hoc analysis Tukey’s multiple comparison test. Weekly body weight measurement and OGTT test data alone were subjected to two way ANOVA followed by Bonferroni post hoc test; p value less than 0.05 were considered as significant. The analysis was carried out using Graph pad prism software of version 4.03 (La Jolla, CA).
Results
Effect of soy isoflavones treatment on body weight in PCOS-induced rats
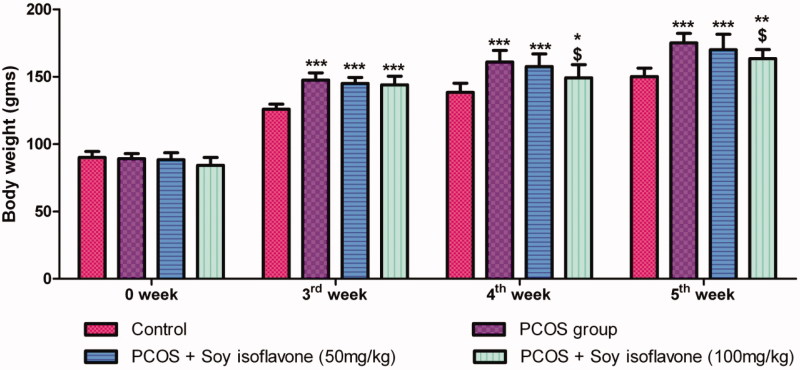
Letrozole administration for 3 weeks resulted in significant increase in body weight in all the groups in comparison to control rats which denotes weight gain (Figure 1). Treatment with soy isoflavones 100 mg/kg in PCOS rats exhibited significant decrease in body weight at 4th week (p < 0.05) and 5th week (p < 0.05), in comparison to vehicle treated PCOS group, at their respective weeks (Figure 1). Treatment with soy isoflavones 50 mg/kg did not induce any significant changes in body weight in comparison to vehicle-treated PCOS group.
Figure 1.
Effect of soy isoflavones treatment in letrozole induced PCOS rats on weekly body weight measurement. All the values are expressed in mean ± S.D. Statistical analysis was carried out by Two way ANOVA followed by Bonferroni post test. *, **, *** denotes statistical significance in comparison to control rats at p < 0.05, p < 0.01 and p < 0.001 respectively. $denotes statistical significance in comparison to vehicle treated PCOS group.
Effect of soy isoflavones treatment in PCOS rats on estrous cycle
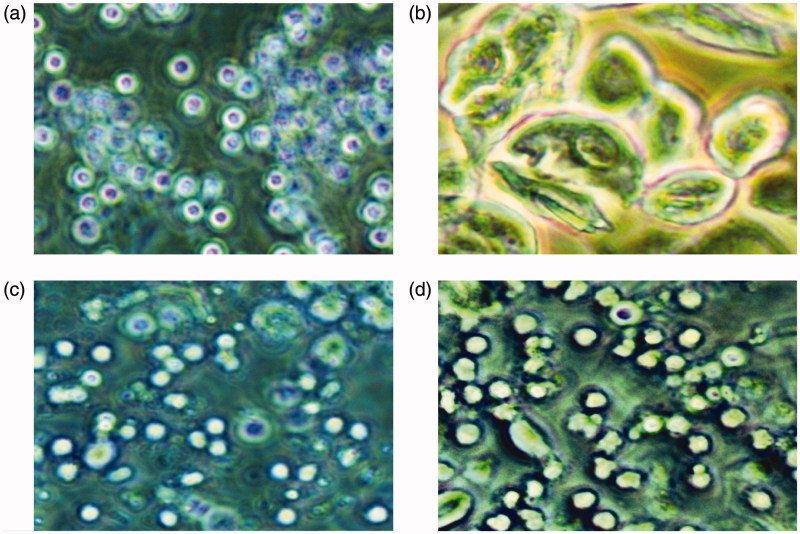
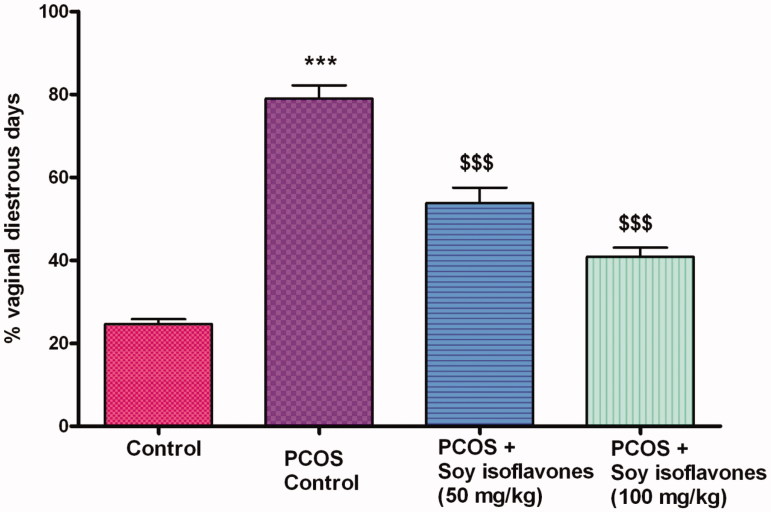
Female rats had 4–5 days of oestrous cycle, comprising proestrous, oestrous, metaestrous and dioestrous phases. The different phases of estrous cycle are represented in Figure 2(a–d). Most of the rats experienced prolonged dioestrous stage beginning on the 7th day after the letrozole administration, with oestrous disappearing on the 15th day. Twenty one days after the gavage of letrozole, the rats were in dioestrous stage were assigned to different treatment groups. After PCOS induction (i.e., after 21 days of the study), the percentage amount of dioestrous days spent by each rat was calculated. PCOS vehicle treated rats exhibited significantly (p < 0.001) higher percentage of dioestrous days (79.00 ± 7.84%) in comparison to control (24.66 ± 2.80%) rats. Soy isoflavones 50 (53.83 ± 9.87%) and 100 mg/kg (40.83 ± 5.56%) exhibited dose-dependent (p < 0.001) decrease in the percentage of dioestrous days in comparison to PCOS group (Figure 3).
Figure 2.
The vaginal smears of rats with different stages of oestrous cycle in the control group. (a) The representative rat's vaginal smears from the control group in proestrous (200×). Oval nucleated epithelial cells, occasionally with a small number of keratinocytes, were detected. (b) The representative rat?s vaginal smears from the control group in estrous (200×). Epithelial keratinocytes with irregular shapes were detected; among which there was a small number of nuclear epithelial cells. (c) The representative rat?s vaginal smears from the control group in metestrous (200×). Irregular epithelial keratinocytes, nucleated epithelial cells, and leukocytes were detected. (d) The representative rat?s vaginal smears from the control group in diestrous (200×). A large number of leukocytes and a small number of nuclear epithelial cells were detected.
Figure 3.
Effect of soy isoflavones treatment in letrozole induced PCOS rats on percentage vaginal dioestrous days. All the values are expressed in mean ± S.D. Statistical analysis was carried out by One way ANOVA followed by Tukey?s multiple comparison test. *** denotes statistical significance as compared to control group rats at p < 0.001. $$$ denotes statistical significance as compared to vehicle treated PCOS group at p < 0.001.
Effect of soy isoflavones treatment in PCOS rats on metabolic profile
Effect of soy isoflavones treatment on OGTT and cholesterol levels in PCOS rats
Administration of letrozole did not show any significant changes in plasma glucose and cholesterol levels in rats as compared to control rats. Similarly, treatment with soy isoflavone (50 and 100 mg/kg) did not exhibit any significant changes in plasma glucose and cholesterol levels as compared to PCOS vehicle-treated rats (Data not shown).
Effect of soy isoflavones treatment in PCOS rats on steroid hormonal profile
Effect of soy isoflavones treatment on testosterone concentrations in PCOS rats
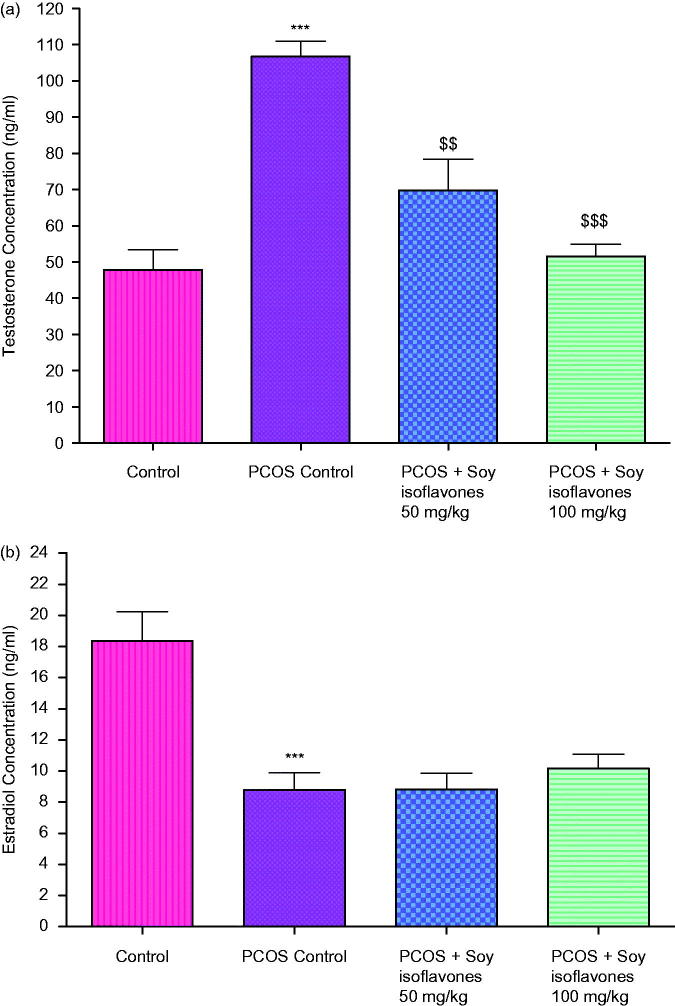
In comparison to control rats, PCOS-induced rats exhibited significant increase (p < 0.001) in testosterone levels at the terminal day of the study. Treatment with soy isoflavones 50 (p < 0.01) and 100 mg/kg (p < 0.001) showed significant decrease in testosterone levels in comparison to vehicle treated PCOS rats (Figure 4(a)).
Figure 4.
(a) Effect of soy isoflavones treatment on plasma concentrations of testosterone in PCOS rats. (b) Effect of soy isoflavones treatment on plasma concentrations of 17β-oestradiol in PCOS rats. All the values are expressed in mean ± S.D. Statistical analysis was carried out by One way ANOVA followed by Tukey?s multiple comparison test. *** denotes statistical significance as compared to control group rats at p < 0.001. $$,$$$ denotes statistical significance as compared to vehicle treated PCOS group at p < 0.01 and p < 0.001 respectively.
Effect of soy isoflavones treatment on 17β-oestradiol concentrations in PCOS rats
Induction of PCOS exhibited significant decrease (p < 0.001) in oestradiol levels at the terminal day of the study. Treatment with soy isoflavones 50 and 100 mg/kg did not show any significant changes in oestradiol levels in comparison to PCOS rats (Figure 4b).
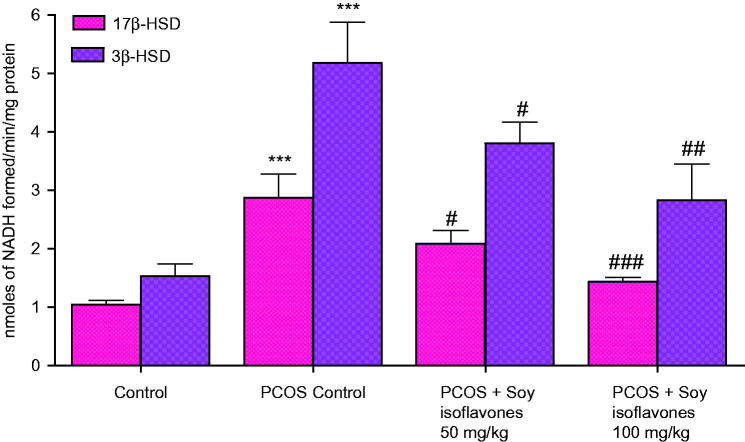
Effect of soy isoflavones treatment on steroidogenic enzymes (3β-HSD and 17β-HSD) in PCOS rats
PCOS rats exhibited significant increase (p < 0.001) in 3β-HSD and 17β-HSD levels in comparison to control rats. Treatment with soy isoflavones at 50 (p < 0.05) and 100 mg/kg (p < 0.01; p < 0.001) exhibited dose-dependent decrease in 3β-HSD and 17β-HSD levels, respectively, in comparison to vehicle-treated PCOS rats (Figure 5).
Figure 5.
Effect of soy isoflavones treatment on steroidogenic enzyme activity in PCOS rat model. All the values are expressed in mean ± S.D. Statistical analysis was carried out by One way ANOVA followed by Tukey?s multiple comparison test. *** denotes statistical significance as compared to control group rats at p < 0.001. #,##,### denotes statistical significance as compared to vehicle treated PCOS group at p < 0.05, p < 0.01 and p < 0.001 respectively.
Effect of soy isoflavones treatment on oxidative stress markers in PCOS rats
Effect of soy isoflavones treatment on SOD activity in PCOS rats
In comparison to control rats, administration of letrozole significantly decreased (p < 0.01) the SOD activity in the ovary. Soy isoflavones (100 mg/kg) treatment significantly increased (p < 0.01) the SOD activity in the ovaries in comparison to vehicle-treated PCOS rats (Table 1).
Table 1.
Effect of soy isoflavones (50 and 100 mg/kg) on ovarian homogenate oxidative markers in letrozole-induced PCOS rat model.
| Treatment and dose | SOD (units/mgprotein) | CAT (units/mgprotein) | GPx (units/mgprotein) | GSH (μM/mgprotein) | LPO (μM/mgprotein) | NO (μM/mgprotein) |
|---|---|---|---|---|---|---|
| Normal control | 59.22 ± 12.49 | 96.54 ± 10.40 | 163.40 ± 34.95 | 47.91 ± 10.97 | 533.51 ± 73.70 | 118.30 ± 27.94 |
| PCOS control | 32.61 ± 08.14** | 50.74 ± 12.17*** | 84.49 ± 17.14** | 18.31 ± 4.22*** | 1166.35 ± 214.30*** | 283.37 ± 54.40*** |
| PCOS + Soy isoflavones 50 mg/kg | 45.29 ± 09.31 | 72.48 ± 14.51 | 134.90 ± 29.99 | 40.81 ± 9.74$$ | 794.50 ± 138.40$$ | 205.30 ± 38.35$ |
| PCOS + Soy isoflavones 100 mg/kg | 61.04 ± 15.49$$ | 90.92 ± 17.56$$ | 165.30 ± 40.38$$ | 48.64 ± 11.99$$$ | 635.70 ± 106.71$$$ | 166.80 ± 25.77$$$ |
Results were expressed as mean ± S.D., n = 6 rats per group. One-way ANOVA followed by post hoc Tukey’s multiple comparison test.
Indicates significance at p < 0.01, p < 0.001 in comparison to control group.
Indicates significance at p < 0.05, p < 0.01 and p < 0.001 in comparison to vehicle treated PCOS group.
Effect of soy isoflavones treatment on CAT activity in PCOS rats
In comparison to control rats, administration of letrozole significantly decreased (p < 0.001) the CAT levels. Treatment with soy isoflavones (100 mg/kg) reversed the letrozole effect as evidenced by significant increase (p < 0.01) in CAT activity, in the ovarian homogenate in comparison to PCOS vehicle-treated rats (Table 1).
Effect of soy isoflavones on GPx activity in PCOS rats
GPx activity was significantly reduced (p < 0.01) in vehicle-treated PCOS rats in comparison to control rats. Treatment with soy isoflavones (100 mg/kg) significantly elevated the GPx activity at p < 0.01 in comparison to PCOS rats (Table 1).
Effect of soy isoflavones on GSH levels in PCOS rats
GSH levels were significantly decreased (p < 0.001) in vehicle-treated PCOS rats in comparison to control rats. Soy isoflavones treatment at 50 (p < 0.01) and 100 mg/kg (p < 0.001) resulted in significant increase in the GSH levels in comparison to vehicle-treated PCOS rats (Table 1).
Effect of soy isoflavones on LPO levels in PCOS rats
In comparison to control rats, PCOS rats exhibited a significant elevation (p < 0.001) in LPO levels. Soy isoflavones 50 (p < 0.01) and 100 mg/kg (p < 0.001) treatment significantly decreased the LPO levels in comparison to vehicle-treated PCOS rats (Table 1).
Effect of soy isoflavones on NO levels in PCOS rats
PCOS rats exhibited a significant increase (p < 0.001) in NO levels in comparison to normal control rats. Treatment with soy isoflavones 50 (p < 0.05) and 100 mg/kg (p < 0.001) significantly decreased the NO levels in comparison to vehicle-treated PCOS rats (Table 1).
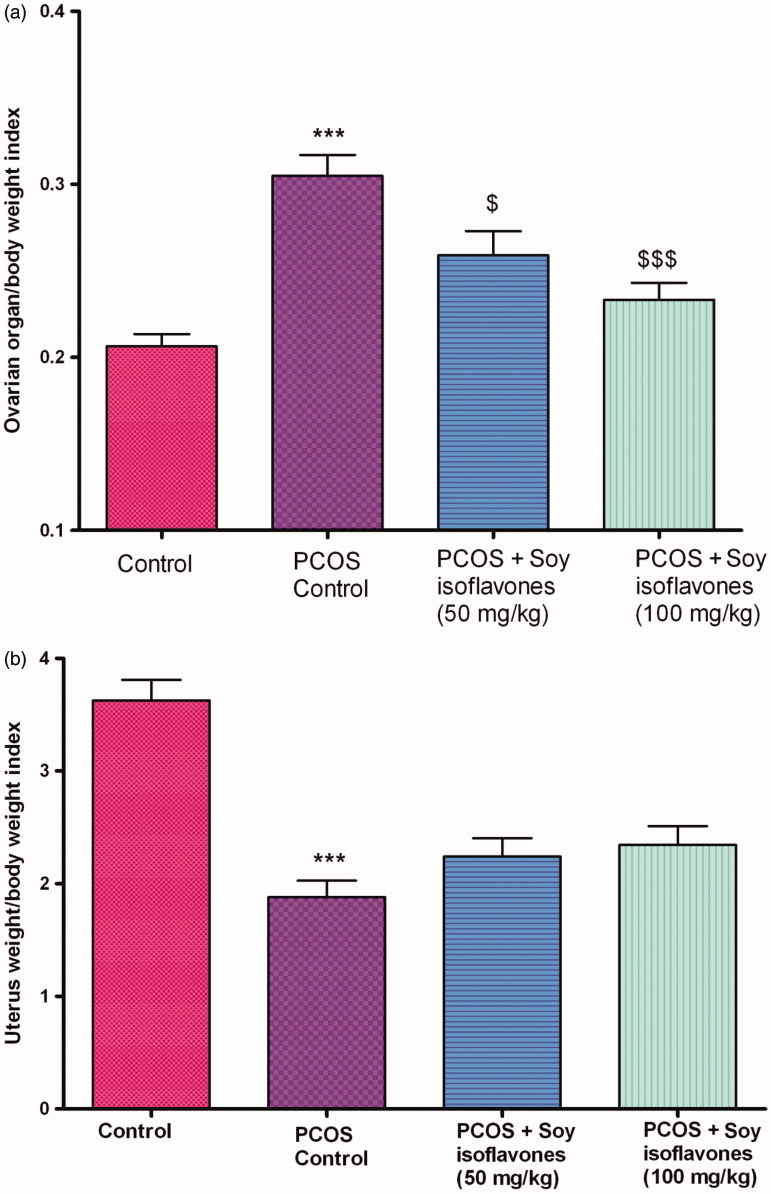
Effect of soy isoflavones on organ weight/body weight index in PCOS rats
Vehicle-treated PCOS rats exhibited a significant increase (p < 0.001) in ovary weight/body weight index in comparison to control rats. Treatment with soy isoflavones at 50 (p < 0.05) and 100 mg/kg (p < 0.001) exhibited a significant decrease in ovary weight/body weight index in comparison to vehicle-treated PCOS rats (Figure 6(a)). Vehicle-treated PCOS rats exhibited a significant decrease (p < 0.001) in uterine weight index in comparison to control rats. Treatment with soy isoflavones (50 and 100 mg/kg) did not exhibit any significant change in uterine weight in comparison to vehicle-treated PCOS rats (Figure 6(b)).
Figure 6.
(a) Effect of soy isoflavones treatment on ovarian weight/body weight index in PCOS rat model. (b) Effect of soy isoflavones treatment on uterine weight/body weight index in PCOS rat model. All the values are expressed in mean ± S.D. Statistical analysis was carried out by One way ANOVA followed by Tukey?s multiple comparison test. *** denotes statistical significance as compared to control group rats at p < 0.001. $,$$$ denotes statistical significance as compared to PCOS control group at p < 0.05, p < 0.001 respectively.
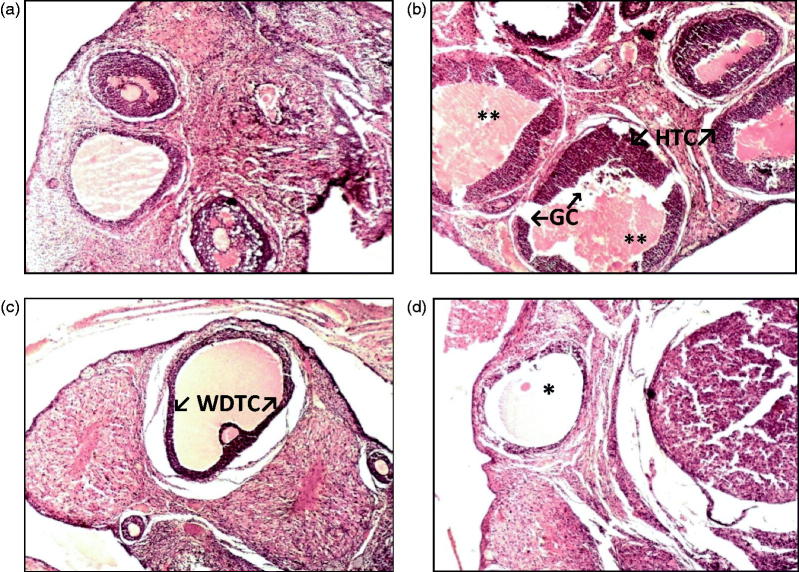
Histopathology results
Control rats exhibited normal ovary histological features (Figure 7(a)). Vehicle-treated PCOS rats displayed more number of follicular cysts, attenuation of granulosa cell layer, and hyperplasia of theca layer. Cell debris in the antrum and occasional corpus lutea are seen (Figure 7(b)). Rats treated with soy isoflavone 50 mg/kg displayed ovarian tissue with some well-developed antral follicles, normal granulosa cell layer, a defined theca layer and few corpus lutea are seen (Figure 7(c)). Soy isoflavone 100 mg/kg treated PCOS rats displayed ovarian tissue with well-developed antral follicles, normal granulosa cell layer, a defined theca layer and containing little cell debris (Figure 7(d)). This indicates that soy isoflavones treatment at 100 mg/kg exhibited protective effect in PCOS rat ovary.
Figure 7.
(a–d) Microscopic study of lateral section of rat ovary. Histological sections of ovary were stained with hematoxylin and eosin. (a) Representative photograph of control rat ovary (H&E, 10×) (b) Representative photograph of letrozole induced PCOS rats (** - indicates large cystic dilated follicles, HTC ? indicates hyperplastic thecal cells, GC ? indicates attenuated granulosa cells) (H&E, 100×) (c) Representative photograph of soy isoflavone 50 mg/kg treated PCOS rats (WDTC ? indicates well-defined thecal cell layer) (H&E, 10×) (d) Representative photograph of soy isoflavone 100 mg/kg treated PCOS rats (* - indicates normal follicle) (H&E, 10×).
Discussion
In the present study, the female rats of control group displayed a regular oestrous cycle. Letrozole induced PCOS rats did not display regular oestrous cycle and exhibited a persistent dioestrous phase. Treatment of soy isoflavones gradually reversed the dioestrous phase to a normal oestrous cycle. The changes in the rat oestrous cycle may be linked to alterations in the circulating concentrations of the sex hormones and gonadotrophins. These hormones control the ovarian function, including follicular maturation and hormonal imbalance which would have led to irregular oestrous cycle thus affecting the ovarian function (Sun et al. 2013). In the present study, there was a significant elevation in testosterone levels in letrozole treated groups in comparison to control group. This reflects the accumulation of androgen because the conversion of androgen substrates into oestrogens was blocked by letrozole (aromatase inhibition). This increased concentration of testosterone in peripheral blood can be the reason for prolonged dioestrous phase and increased body weight of letrozole alone treated rats in the study (Abdulghani et al. 2012). Treatment with soy isoflavones resulted in decreased percentage of vaginal dioestrous days and body weight in PCOS rats which might be due to their ability to decrease testosterone concentration in the peripheral blood. Earlier reports indicate that genistin, daidzin and its aglycone form (genistein and daidzein) have decreased testosterone levels by interfering steroid production in adrenals, in male rats (Weber et al. 2001; Ohno et al. 2003).
In the present study, letrozole-induced PCOS rats displayed reduced levels of plasma oestradiol concentrations which were expected, as letrozole blocks aromatization of testosterone to oestradiol. Although genistein and daidzein are known to exert weak estrogenic effects treatment with soy isoflavones did not cause any significant changes with respect to oestradiol levels. Change in gonadotropin pulse amplitude and frequencies which are typical of PCOS may offer resistance to a possible modulating influence of soy isoflavones. The lack of any effect of isoflavones on the oestradiol levels of letrozole induced PCOS rats adds to the discrepancies already reported in the literature: some studies documented a decrease in oestradiol levels at variable daily doses of isoflavones, others reported a slight increase or no effects on this parameter in humans (Cassidy et al. 1994; Wu et al. 2000; Romualdi et al. 2008). A comparison between the present study results and previous results is difficult to make since the other results were obtained from normoovulatory premenopausal women.
Letrozole-induced PCOS rats in the present study did not exhibit any significant changes in OGTT and total cholesterol levels. This observation is in line with previous findings and the reason for this type of result denotes letrozole does not affect insulin signalling pathways and will not induce insulin resistance or reduction of insulin sensitivity (Walters et al. 2012). However, to analyze the effects of soy isoflavones the metabolic parameters were captured. In the present study, letrozole-induced PCOS rats exhibited significant increase in steroidogenic enzymes (3β-HSD and 17β-HSD) activity which is in agreement with the previous study reports (Zurvarra et al. 2009; Ortega et al. 2012). Treatment with soy isoflavones in PCOS rats significantly decreased the steroidogenic enzymes activity. Previous report indicates that genistein can inhibit 3β-HSD and 17β-HSD enzyme in both human and rat testis microsomes (Hu et al. 2010). It was also reported that isoflavonoids, as well as other molecules that have a phenolic B ring in the 3rd position of the pyran ring can preferentially inhibit 3β-HSD and/or 17β-HSD activities (Le Bail et al. 2000). Apart from inhibition of steroidogenic enzymes, genistein can also interfere with the coupling of transmembrane luteinizing hormone (LH) receptors with G proteins. The uncoupling of LH receptors from G proteins by genistein adversely affects adenylate cyclase function, thus blocking LH induced stimulation of steroidogenesis (Hancock et al. 2009).
Oxidative stress is considered as an important pathological feature of PCOS and women with PCOS have decreased total antioxidant status (Jungbauer & Medjakovic 2014). Treatment with soy isoflavones significantly reduced LPO, NO (markers of oxidative stress) and increased SOD, CAT, GPx, and GSH (markers of antioxidant potential) in letrozole-induced PCOS model of rats. The antioxidant potencies of isoflavones are structurally related and closely associated with the presence of hydroxyl groups at positions 4′ and 5′ and the position of the aromatic ring. Genistein is the most powerful antioxidant among the various soy isoflavones and it is well documented in previous studies (Setchell et al. 2002; Zhang et al. 2003).
In the present study, administration of letrozole decreased the uterus weight. The reduction in uterus weight observed in PCOS rats might be due to the reduction in oestradiol levels. Treatment with soy isoflavones did not significantly increased the uterus weight in PCOS rats and did not elevate the oestradiol levels. Previous findings denote that, genistein and daidzein possess estrogenic activity, have high affinity towards ERβ, can induce specific oestrogen-responsive gene products, interfere with steroid hormone metabolism or action and alter oestrogen receptor structure and transcription (Ososki & Kennelly 2003). Reports also indicate that compounds selectively bind to ERβ will not increase the uterine weight. This type of uterotrophic assay is a classical model to test ERβ agonism (Harris et al. 2003). Hence, the non-uterotrophic feature of soy isoflavones observed in the present study can be justified.
Ovarian morphologic changes in letrozole-induced PCOS rats include development of cysts with hyperplasia of internal theca cells and a thickened ovarian capsule. It is also possible to see the abundance of subcapsular cysts lined with a thin layer of granulosa cells and hyperplasia of theca interna cells in the present study. These histologic findings are indicative of the presence of biologically active levels of FSH, increased LH, and lack of interplay between granulosa and theca cells (Kafali et al. 2004). Treatment with soy isoflavones decreased the ovary weight in letrozole treated rats which may be due to their ability to reduce testosterone levels. Interestingly, soy isoflavones (100 mg/kg) treatment showed protective effects in ovary histological sections with well-developed antral follicles, normal granulosa cell layer and a defined theca layer. Thus, these physical, biochemical and histological results clearly demonstrated that soy isoflavones can exert beneficial effects in PCOS phenotypes for the animals having decreased aromatase activity and elevated plasma testosterone levels.
Supplementary Material
Disclosure statement
The authors report no declarations of interest.
Acknowledgements
We thank Prof. Dr. M. Ramanathan, PSG College of Pharmacy for his critique and valuable comments towards this manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Abdulghani M, Hussin AH, Sulaiman SA, Chan KL.. 2012. The ameliorative effects of Eurycoma longifolia Jack on testosterone-induced reproductive disorders in female rats. Reprod Biol. 12:247–255. [DOI] [PubMed] [Google Scholar]
- Basak S, Pookot D, Noonan EJ, Dahiya R.. 2008. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol Cancer Ther. 7:3195–3202. [DOI] [PubMed] [Google Scholar]
- Beers RF, Sizer IW.. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 195:133–140. [PubMed] [Google Scholar]
- Bergmeyer HU. 1974. beta-Hydroxy steroid dehydrogenase In: Methods of enzymatic analysis. New York: Academic Press; p. 447–489. [Google Scholar]
- Cassidy A, Bingham S, Setchell KD.. 1994. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 60:333–340. [DOI] [PubMed] [Google Scholar]
- Dong Y, Jing T, Meng Q, Liu C, Hu S, Ma Y, Liu Y, Lu J, Cheng Y, Wang D, et al. . 2014. . Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague–Dawley rats. Biomed Res Int. 2014:160980–160991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR.. 1982. . Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. J Anal Biochem. 126:131–138. [DOI] [PubMed] [Google Scholar]
- Hancock KD, Coleman ES, Tao YX, Morrison EE, Braden TD, Kemppainen BW, Akingbemi BT.. 2009. . Genistein decreases androgen biosynthesis in rat Leydig cells by interference with luteinizing hormone-dependent signaling. Toxicol Lett. 184:169–175. [DOI] [PubMed] [Google Scholar]
- Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, et al. . 2003. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 144:4241–4249. [DOI] [PubMed] [Google Scholar]
- Hu GX, Zhao BH, Chu YH, Zhou HY, Akingbemi BT, Zheng ZQ, Ge RS.. 2010. Effects of genistein and equol on human and rat testicular 3beta-hydroxysteroid dehydrogenase and 17beta-hydroxysteroid dehydrogenase 3 activities. Asian J Androl. 12:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakimiuk AJ, Weitsman SR, Yen HW, Bogusiewicz M, Magoffin DA.. 2002. Estrogen receptor alpha and beta expression in theca and granulosa cells from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 87:5532–5538. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR.. 1974. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 11:151–169. [DOI] [PubMed] [Google Scholar]
- Jungbauer A, Medjakovic S.. 2014. Phytoestrogens and the metabolic syndrome. J Steroid Biochem Mol Biol. 139:277–289. [DOI] [PubMed] [Google Scholar]
- Kafali H, Iriadam M, Ozardali I, Demir N.. 2004. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 35:103–108. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN.. 1984. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 21:130–132. [PubMed] [Google Scholar]
- Kopera D, Wehr E, Obermayer-Pietsch B.. 2010. Endocrinology of hirsutism. Int J Trichol. 2:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bail JC, Champavier Y, Chulia AJ, Habrioux G.. 2000. . Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 66:1281–1291. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ.. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275. [PubMed] [Google Scholar]
- Ng BH, Yuen KH.. 2003. Determination of plasma testosterone using a simple liquid chromatographic method. J Chromatogr B Analyt Technol Biomed Life Sci. 793:421–426. [DOI] [PubMed] [Google Scholar]
- Nynca A, Jablonska O, Slomczynska M, Petroff BK, Ciereszko RE.. 2009. Effects of phytoestrogen daidzein and oestradiol on steroidogenesis and expression of estrogen receptors in porcine luteinized granulosa cells from large follicles. J Physiol Pharmacol. 60:95–105. [PubMed] [Google Scholar]
- Nynca A, Nynca J, Wasowska B, Kolesarova A, Kolomycka A, Ciereszko RE.. 2013. Effects of the phytoestrogen, genistein, and protein tyrosine kinase inhibitor-dependent mechanisms on steroidogenesis and estrogen receptor expression in porcine granulosa cells of medium follicles. Domest Anim Endocrinol. 44:10–18. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K.. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358. [DOI] [PubMed] [Google Scholar]
- Ohno S, Nakajima Y, Inoue K, Nakazawa H, Nakajin S.. 2003. Genistein administration decreases serum corticosterone and testosterone levels in rats. Life Sci. 74:733–742. [DOI] [PubMed] [Google Scholar]
- Ortega I, Villanueva JA, Wong DH, Cress AB, Sokalska A, Stanley SD, Duleba AJ.. 2012. Resveratrol reduces steroidogenesis in rat ovarian theca-interstitial cells: the role of inhibition of Akt/PKB signaling pathway. Endocrinology. 153:4019–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ososki AL, Kennelly EJ.. 2003. Phytoestrogens: a review of the present state of research. Phytother Res. 17:845–869. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN.. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 70:158–169. [PubMed] [Google Scholar]
- Romualdi D, Costantini B, Campagna G, Lanzone A, Guido M.. 2008. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil Steril. 90:1826–1833. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown N, Lydeking-Olsen N.. 2002. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 132:3577–3584. [DOI] [PubMed] [Google Scholar]
- Shi D, Vine DF.. 2012. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 98:185–193. [DOI] [PubMed] [Google Scholar]
- Shivanandappa T, Venkatesh S.. 1997. . A colorimetric assay method for 3beta-hydroxy-delta5-steroid dehydrogenase. Anal Biochem. 254:57–61. [DOI] [PubMed] [Google Scholar]
- Sun J, Jin C, Wu H, Zhao J, Cui Y, Liu H, Wu L, Shi Y, Zhu B.. 2013. . Effects of electro-acupuncture on ovarian P450arom, P450c17α and mRNA expression induced by letrozole in PCOS rats. PLoS One. 8:e79382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ.. 2012. Rodent models for human polycystic ovary syndrome. Biol Reprod. 86:12–11. [DOI] [PubMed] [Google Scholar]
- Weber KS, Setchell KD, Stocco DM, Lephart ED.. 2001. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol. 170:591–599. [DOI] [PubMed] [Google Scholar]
- Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, Pike MC.. 2000. Effects of soy foods on ovarian function in premenopausal women. Br J Cancer. 82:1879–1886.10839307 [Google Scholar]
- Yamada H, Yoshizawa K, Hayase T.. 2002. Sensitive determination method of oestradiol in plasma using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 775:209–213. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shu XO, Gao YT, Yang G, Li Q, Li H, Jin F, Zheng W.. 2003. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 133:2874–2878. [DOI] [PubMed] [Google Scholar]
- Zurvarra FM, Salvetti NR, Mason JI, Velazquez MM, Alfaro NS, Ortega HH.. 2009. Disruption in the expression and immunolocalisation of steroid receptors and steroidogenic enzymes in letrozole-induced polycystic ovaries in rat. Reprod Fertil Dev. 21:827–839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.