Abstract
Context:Punica granatum L (Punicaceae) flower is an important diabetes treatment in oriental herbal medicine.
Objective: This study investigates the inflammation effects of pomegranate flower (PFE) ethanol extract in LPS-induced RAW264.7 cells.
Materials and methods: PFE (10, 25, 50, 100 μg/mL) was applied to 1 μg/mL LPS-induced RAW 264.7 macrophages in vitro. Levels of nitric oxide (NO), prostaglandin E2 (PGE2) and pro-inflammatory cytokines interleukin (IL)-1β (IL-1β), interleukin (IL)-6 (IL-6) and tumor necrosis factor (TNF-α) in the supernatant fraction were determined using enzyme-linked immunosorbent assay (ELISA). Expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), phosphorylation of mitogen-activated protein kinase (MAPK) subgroups extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and P38, as well as nuclear factor-κB (NF-κB) activation in extracts were detected via Western blot.
Results: 10–100 μg/mL PFE decreased the production of NO (IC50 value = 31.8 μg/mL), PGE2 (IC50 value = 54.5 μg/mL), IL-6 (IC50 value = 48.7 μg/mL), IL-1β (IC50 value = 71.3 μg/mL) and TNF-α (IC50 value = 62.5 μg/mL) in LPS-stimulated RAW 264.7 cells significantly. A mechanism-based study showed that phosphorylation of ERK1/2, p38, JNK and translocation of the NF-B p65 subunit into nuclei were inhibited by the PFE treatment.
Discussion and conclusion: These results show that PFE produced potential anti-inflammatory effect through modulating the synthesis of several mediators and cytokines involved in the inflammatory process.
Keywords: Punica granatum, anti-inflammation, NF-κB, MAPK
Introduction
Punica granatum L. (Punicaceae), commonly known as pomegranate, is commercially cultivated for its edible fruit in the drier regions of Southeast Asia, the Mediterranean region, and the United States (Yuan et al. 2012). According to recent reports, pomegranate is a polyphenol-rich fruit, and showed potential as an anti-inflammatory and antioxidative medicine in several experimental models (Shukla et al. 2008). The various extracts/constituents of different parts of this plant possess a number of biological activities such as antitumor, antibacterial, antifungal, and antiulcer (Celik et al. 2009). The phytochemical and pharmacological actions of all Punica granatum components suggest a wide range of clinical applications where inflammation is believed to play an essential etiologic role (Lansky and Newman 2007). The pomegranate flower extract also contains a large amount of polyphenols and possesses potent antioxidant and hepatoprotective property (Kaur et al. 2006). The Unani and Ayurvedic medicinal systems use the flowers of this plant as a remedy for diabetes (Huang et al. 2005). The effect and action mechanism of a methanol extract from pomegranate flowers on hyperglycemia in vivo and in vitro were investigated, and the studies indicated that pomegranate flowers improve postprandial hyperglycemia in type 2 diabetes by inhibiting glucosidase activity (Li et al. 2005). In a previous study, preliminary studies reported the anti-inflammatory effect of pomegranate flower extract (Rahima et al. 2009); however, the functional components and underlying mechanism remained uncertain. Inflammation is a common pathophysiology of many different diseases and the most primitive protective response to a variety of stimuli (Shi et al. 2009). Macrophages play critical roles in immune reactions, allergy, inflammation and they protect the body from external intruders through phagocytosis (Shi et al. 2009). During this process, macrophages produce many kinds of inflammatory mediators such as IL-1β, TNF-α, NO, and prostaglandins (Shao et al. 2013). LPS, a component of the Gram-negative bacteria cell wall, has been often used in inflammatory response because it can activate macrophages (Poltorak et al. 1998). Activated macrophages transcriptionally express iNOS, which catalyzes the oxidative deamination of l-arginine to produce NO. Excessive generation of NO by iNOS can trigger deleterious consequences such as septic shock and inflammatory diseases (Jung et al. 2014). Prostaglandins (PGs) also functions as mediators of the inflammatory response to induce pain, fever and other symptoms (Jin et al. 2010). LPS-stimulated macrophages activate several intracellular signaling pathways, including NF-κB and MAPK pathways (Guha & Mackman 2001). The present study examined the potential for PFE to reduce inflammation effects in LPS-stimulated RAW 264.7 murine macrophages in vitro. In order to correlate the phenolic composition with the bioactivity, high performance liquid chromatography coupled to quadruple time-of-flight with tandem mass spectrometry (HPLC/QTOF–MS/MS) was used to analyze the active components in PFE.
Materials and methods
LPS, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide], phenylmethylsulfonyl fluoride (PMSF) and the components of the whole cell lysis buffer for Western blot analysis and dimethylsufoxide (DMSO) were purchased from the Sigma Chemical Co. (St. Louis, MO). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY). The Griess reagent kit was purchased from Beyotime Chemical Co. (Jiang Su, China). Antibodies against COX-2, iNOS, β-actin, NF-κB p65, IκBα, Lamin B, phospho-ERK1/2, ERK1/2, phospho-p38, p38, phospho-JNK/SAPK and JNK/SAPK were purchased from Cell Signaling Technology (Danvers, MA). RAW 264.7 mouse macrophage cells were purchased from Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, China). Methanol (HPLC grade) was purchased from Fisher Scientific (Fair Lawn, NJ). All other chemicals were commercial products of reagent grade.
Preparation of PFE
The pomegranate flower was commercial cultivar from Hotan County, Xinjiang Uygur Autonomous Region, China, and identified by Prof. Guanmian Shen (Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences). The herbarium number of PEF was 00022757 and deposited in Xinjiang Institute of Ecology and Geography. The dried pomegranate flower was extracted with ethanol under reflux three times. After removal of ethanol with rotary evaporator and under vacuum conditions at 45 °C, the residual extracts were subjected to a D-101 macroporous resin glass chromatography column with a 1:4 diameter height ratio. 70% (v/v) ethanol was used as elution solvent, and the desired fractions were concentrated in vacuo to produce PFE.
Conditions of HPLC/QTOF–MS/MS
The HPLC analyses were performed using an Agilent 1200 series HPLC system (Agilent Technologies, Waldbronn, Germany), equipped with a quaternary solvent delivery system. The sample was eluted at a flow rate of 0.1 mL/min in a gradient mode of A (0.1% formic acid:water) and B (methanol): 0–25 min 10–70% B, 25–40 min 70–90% B, 40–45 min, 90–10% B. The column temperature was set at 35 °C and the injection volume was 10 μL. Mass spectrometry was performed using a QSTAR Elite LC–MS/MS system from Applied Biosystems/MDS Sciex (Concord, ON, Canada) equipped with an electrospray ionization (ESI) source. The TOF mass range was set from m/z 80 to 1000 and the mass range for product ion scan was m/z 50-1500. The collision energy (CE) was set from 10 eV to 70 eV to optimize signals to obtain maximal structure information from the ions of interest.
Cell culture and MTT assay
The cells were cultured in DMEM medium supplemented with 10% heat-inactivated FBS, 1% streptomycin/penicillin at 37 °C in a humidified atmosphere of 5% CO2. The cells were treated with PFE at different concentrations and then stimulated with 1 μg/mL LPS for 18 h.
Cell viability assay
The cells were seeded in a 96-well plate and treated with various concentrations of PFE for 24 h. The cell viability was measured by an MTT assay according to our previously described method (Tursun et al. 2016).
Measurement of NO production
RAW264.7 cells (1 × 105 cells/mL) were pre-incubated for 1 h with various concentrations of PFE and stimulated with LPS (1 μg/mL) at 37 °C for 18 h in medium. NO levels were determined by measuring nitrite levels in the culture media using Griess reagent assay according to our previously described method (Tursun et al. 2016).
Measurement of PGE2, IL-6, TNF-α and IL-1β
RAW264.7 cells (1 × 106 cells/well) were pretreated with different concentrations of PFE for 1 h and then stimulated with LPS (1 μg/mL) for 18 h. The concentration of PGE2, IL-6, TNF-α and IL-1β were assayed using the ELISA kits according to the manufacturer's instructions.
Western blot analysis
Protein extracts were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Protein concentrations were determined using the BCA assay. Whole cell extracts, cytosolic and nuclear proteins were extracted respectively and the western blot analysis were as described previously (Tursun et al. 2016). The membranes were blocked at room temperature for 1 h with 5% nonfat dry milk, and then incubated with each primary antibody at 4 °C overnight. Blots were visualized and quantified using a ChemiDoc system with ImageLab software (Bio Rad, CA).
Statistical analysis
All the experiments were repeated three times. Data are expressed as the means ± standard error of the mean (SEM) for the number of experiments. Statistical significance was calculated to compare treated and control groups and determined by Student's t-tests. A value of p < 0.05 and p < 0.01 was considered significant.
Results and discussion
Phenolic compound identification
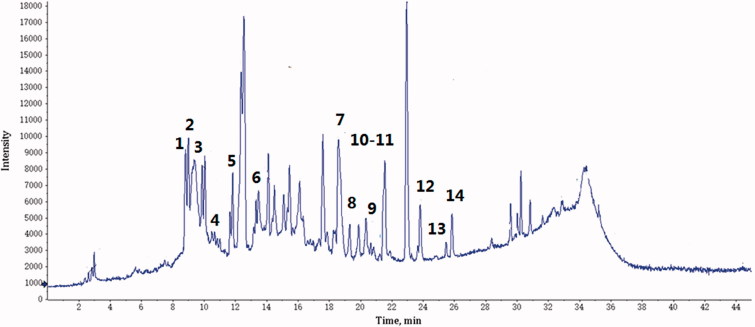
Chemical compositions were separated and investigated by LC/QTOF–MS/MS in negative ESI mode at different CE values (Figure 1). The maximal structural information was obtained for the identification of components. A total of 14 compounds were identified according to accurate mass and the characteristic fragments at low and high CE. Table 1 shows the deprotonated molecular ions, retention time and characteristic fragment ions of identified compounds.
Figure 1.
Total ion chromatograms of PFE in negative ESI mode.
Table 1.
Characterisation of compounds in PFE by HPLC-QTOF-MS/MS.
| Peak | tR (min) | [M-H]− | Major and important MS2 ions | Identification |
|---|---|---|---|---|
| 1 | 8.66 | 633.1440 | 481,301, 275, 247, 203, 175, 169 | Galloyl-HHDP-glucoside |
| 2 | 9.01 | 799.1565 | 781, 479, 301, 273, 257 | Granatin A |
| 3 | 9.57 | 483.1295 | 301,275,169,125 | Digalloyl-glucoside |
| 5 | 11.35 | 635.1635 | 465,313,271, 169 , 125 | Tri-O-galloyll-glucoside |
| 6 | 12.14 | 633.1528 | 481, 301, 275, 247, 203, 175, 169 | Galloyl-HHDP-glucoside |
| 7 | 13.45 | 951.1904 | 933,463,301,275,229,167 | Granatin B |
| 8 | 18.44 | 301.0272 | 284, 229 ,185 145, 129 | Ellagic acid |
| 9 | 19.22 | 491.1155 | 453,301,275,209,177,169 | Ellagic acid derivative |
| 10 | 19.81 | 477.1152 | 301,275,187,151,125 | Ellagic acid- rhamnoside |
| 11 | 21.35 | 301.0651 | 255,179,151,121,107 | quercetin |
| 12 | 21.43 | 463.1409 | 301, 271 ,255, 229, 179 ,151 | Quercetin-O-glucoside |
| 13 | 23.74 | 285.0703 | 71,255,227,151 | Kaempferol |
| 14 | 25.41 | 285.6710 | 285,241,175,151,133 | Luteolin |
tR: retention time; HHDP: hexahydroxydiphenoyl.
Effect of PFE on RAW 264.7 cell viability
RAW264.7 cells were initially seeded in microplates followed by different concentrations of PFE. Treating RAW 264.7 cells with PFE (0–100 μg/mL) did not affect the viability of the RAW 264.7 cells (Figure 2).
Figure 2.
Cytotoxicity of PFE in RAW 264.7 cells. Cells were treated with different concentrations of PFE for 24 h, and viability was assayed by the MTT assay. Data represent mean values of triple determinations ± SEM. PFE at 100 μg/mL was not cytotoxic.
Effect of PFE on NO, PGE2 production and iNOS, COX-2 expression
The amounts of nitrite, a stable metabolite of NO, were determined via the Griess reaction. As presented in Figure 2(A,B), in unstimulated RAW 264.7 cells, NO and PGE2 production were almost undetectable. Upon LPS treatment, nitrite concentrations and PGE2 production in the medium increased markedly. The different concentrations of PFE inhibited NO and PGE2 production in LPS-stimulated RAW264.7 cells. We then tested the effect of PFE on iNOS and COX-2 expressions. Western blot analysis demonstrated that unstimulated RAW264.7 cells did not express iNOS and COX-2 proteins, but LPS treatment induced iNOS and COX-2 expressions. Western blotting with PFE of anti-iNOS and COX-2 antibodies showed lower iNOS and COX-2 protein levels, indicating that PFE could regulate inflammatory effects through inhibiting the iNOS and COX-2 pathway (Figure 3).
Figure 3.
Effects of PFE on LPS-induced NO, PGE2 production and iNOS, COX-2 protein expression levels in LPS-induced RAW264.7 cells. Cells were incubated in the presence of PFE or in combination with 1 μg/mL LPS for 18 h. The culture supernatant was analyzed for NO (A), PGE2 (B) production. The iNOS and COX-2 (C) expression levels were determined by Western blotting. Data show mean ± SEM values of three independent experiments. *p < 0.05 and **p < 0.01 indicate significant differences from LPS-stimulation value.
Effects on pro-inflammatory cytokines TNF-α, IL-6 and IL-1β
In response to LPS stimulation, macrophages could release pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6. The RAW264.7 cells were treated with LPS in the presence or absence of PFE, and the levels of TNF-α, IL-1β and IL-6 were measured by ELISA. As shown in Figure 4(A–C), PFE suppressed the productions of TNF-α, IL-1β and IL-6 in LPS-induced RAW264.7 cells in a concentration-dependent manner.
Figure 4.
Effects of PFE on TNF-α (A), IL-6 (B) and IL-1β (C) in LPS-induced RAW264.7 cells. The cells were pretreated with the different concentrations of PFE for 1 h and then exposed to 1 μg/mL LPS for 18 h. The levels of TNF-α, IL-1β and IL-6 in the supernatant were determined by ELISA. Data show mean ± SEM values of three independent experiments. *p < 0.05 and **p < 0.01 indicate significant differences from LPS stimulation value.
Inhibitory effect of PFE on LPS-induced signal transduction pathways
The data above showed that PFE downregulated proinflammatory mediators as well as cytokines production. Under unstimulated conditions, NF-κB remains in the cytoplasm inactively bound to its inhibitor IκB and becomes active through phosphorylation and degradation of IκB and the subsequent nuclear translocation of NF-κB p65 induced by LPS (Zandi et al. 1997). As shown in Figure 5, PFE significantly inhibited LPS-induced degradation of IκB-α and nuclear translocation of NF-κB p65.
Figure 5.
Effects of PFE on NF-κB p65 and IκBα activity in LPS-stimulated RAW 264.7 cells. The cells were pretreated with the different concentrations of PFE for 1 h and then exposed to 1 μg/mL LPS for additional 30 min. Cytoplasm and nuclear extracts proteins of cells were harvested for measurements of NF-κB p65 and IκB-α protein by Western blotting. β-Actin and Lamin B were used as the internal control. Data show mean ± SEM values of three independent experiments. *p < 0.05 and **p < 0.01 indicate significant differences from LPS-stimulation value.
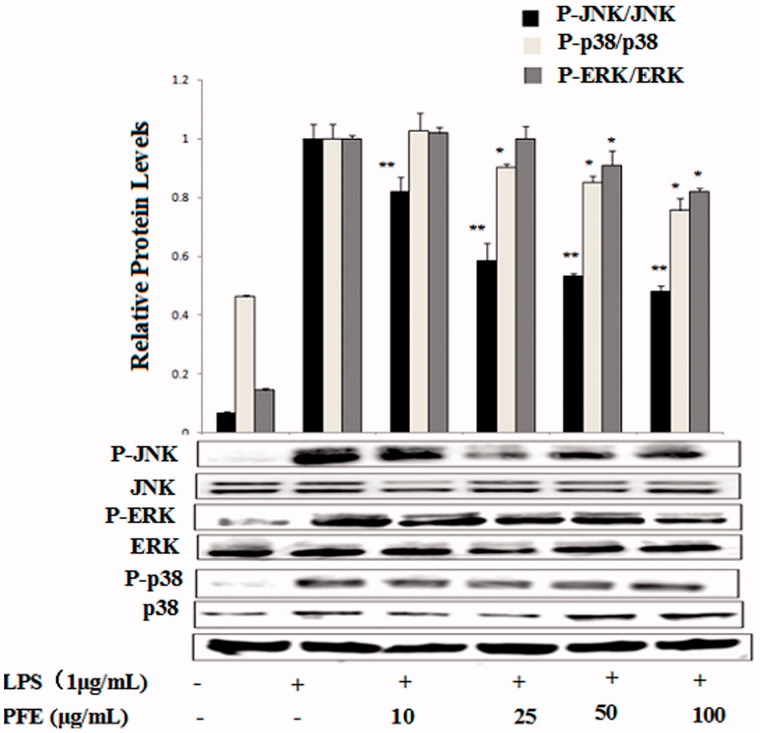
Effect of PFE on LPS-induced protein expression of p-ERK1/2, p-JNK, and p-p38 in RAW 264.7macrophages
NF-κB is activated by phosphorylation of IκB via activation of MAPKs, which plays a key role in the signalling pathways of cell proliferation, differentiation, survival, apoptosis, and transduces various extracellular signals to the nucleus (Kim et al. 2010). In this study, we examined the effects of PFE on the phosphorylation of ERK1/2, JNK and p38. As shown in Figure 6, PFE pretreatment significantly suppressed the phosphorylation of ERK, p38 and JNK.
Figure 6.
Effects of PFE on phosphorylation of MAPKs activity in LPS-stimulated RAW 264.7 cells. The cells were pretreated with the different concentrations of PFE for 1 h and then exposed to LPS for 30 min. Total cellular proteins of cells were harvested for measurements of total or phosphorylated ERK1/2, JNK, and p38 by Western blotting. Data show mean ± SEM values of three independent experiments. *p < 0.05 and **p < 0.01 indicate significant differences from LPS-stimulation value.
Pomegranate flower is an important oriental herbal medicine. The present study examined the anti-inflammatory effects of pomegranate flower using the accepted LPS-induced RAW 264.7 model. LPS stimulated macrophages trigger the secretion of cytokines and mediators, and upon treatment with LPS, RAW 264.7 cells produce induced NO and PGE2 as well as other inflammatory cytokines (Le et al. 2017). NO is an important inflammatory product produced by iNOS and is primarily involved in promoting inflammatory responses. Its excess production, especially in macrophages, can lead to cytotoxicity, inflammation, carcinogenicity, and autoimmune disorders (Lee et al. 2009).
PFE inhibited the pro-inflammatory mediators PGE2 and NO, we thereafter examined its effects on the LPS-induced production of TNF-α, IL-1β and IL-6 by ELISA. NF-κB is a transcription factor that regulates a number of genes, including iNOS, COX-2, TNF-α, IL-1β and IL-6, which are important for immunity in LPS-induced inflammation (Lee et al. 2012). In this study, we found that PFE significantly inhibited LPS-induced degradation of IkBα and the NF-κB p65 translocation from cytosol to nucleus. Activation of MAPKs is involved in LPS-induced expression of inflammatory mediators and NF-κB activation (Lee et al. 2012). It has reported that blockage of RAW 264.7 cells p38, ERK and JNK pathways causes down regulation of COX-2 expression, TNF-α and IL-1β production during inflammation (Ruberlei et al. 2017). To further investigate the mechanisms underlying the anti-inflammatory effects of PFE, phosphorylation of ERK1/2, JNK and p38 were also examined.
Phenolic compounds are plant secondary metabolites known for their antioxidative and anti-inflammatory properties (Dussossoy et al. 2011). In previous studies, quercetin has been reported to exhibit anti-inflammatory effects through regulation of nitric oxide and TNF-α production by NF-κB pathway in LPS-stimulated macrophages (Nakamura and Omura 2008). The effect of ellagic acid on inflammation also has been studied using in vitro and in vivo models (Angeles Rosillo et al. 2012; Anitha et al. 2013). Ellagic acid exerted a renal protective effect in high fat diet/low-dose streptozotocin (HFD/STZ)-induced type 2 diabetic rats by multifactorial approach (Ahad et al. 2014). The anti-inflammatory properties and underlying molecular mechanisms of luteolin in LPS RAW264.7 macrophages have been investigated. Luteolin reduced the expression of pro-inflammatory cytokines and it has potential applications as a functional food component in regulating inflammatory responses (Chen et al. 2007). In the present study, 14 chemical compositions were identified or partially characterized by LC/QTOF–MS/MS. The phenolic compounds mentioned above were identified in a previous study; therefore, it can be assumed that the anti-inflammatory effect by the PFE extracts was due to these compounds. Based on these results, pomegranate flower extracts represent potential natural sources that will be useful for the treatment of inflammatory-related diseases.
Conclusions
The current main task of our study is to investigate the mechanisms underlying the anti-inflammatory activity of PFE in LPS-induced RAW 264.7 cells. Our findings suggest that PFE is able to inhibit the production of NO, PGE2, and pro-inflammatory cytokines (TNF-α, IL-6, IL-1β), as well as the protein expression of iNOS and COX2 in LPS-stimulated RAW264.7 macrophages. Moreover, PFE treatment significantly inhibited LPS-induced NF-κB activation through blocking nuclear translocation of NF-κB and IκBα degradation and PFE treatment also inhibited the phosphorylation of MAPKs. Therefore, we suggest that PFE should be considered as candidate potential anti-inflammatory agents for the treatment of inflammation-related diseases.
Funding Statement
This work was funded by the Joint Funds of the National Natural Science Foundation of China [Grant No. U1203203].
Disclosure statement
The authors declare no conflict of interest.
References
- Ahad A, Ganai A, Mujeeb M, Siddiqui A.. 2014. Ellagic acid, an NF-kappa B inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem-Bilol. Interact. 219:64–75. [DOI] [PubMed] [Google Scholar]
- Angeles Rosillo M, Sanchez-Hidalgo A, Aparicio-Soto M, Sanchez-Fidalgo S, Villegas I, Alarcon C.. 2012. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol Res. 66:235–242. [DOI] [PubMed] [Google Scholar]
- Anitha P, Priyadarsini RV, Kavitha K, Thiyagarajan P, Nagini S.. 2013. Ellagic acid coordinately attenuates Wnt/beta-catenin and NF-kappa B signaling pathways to induce intrinsic apoptosis in an animal model of oraloncogenesis. Eur J Nutr. 52:75–84. [DOI] [PubMed] [Google Scholar]
- Celik I, Temur A, ISIK I.. 2009. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol. 47:145–149. [DOI] [PubMed] [Google Scholar]
- Chen CY, Peng WH, Tsaik D, Hsu SL.. 2007. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappa B and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 81:1602–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussossoy E, Brat P, Bony E, Boundard F, Poucheret P, Mertz C, Giaimis J, Michel A.. 2011. Characterization, antioxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.). J Ethnopharmacol. 133:108–115. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N.. 2001. LPS induction of gene expression in human monocytes. Cell Signal. 13:85–94. [DOI] [PubMed] [Google Scholar]
- Huang THW, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalls BD, Li YH.. 2005. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharm. 207:160–169. [DOI] [PubMed] [Google Scholar]
- Jin M, Suh SJ, Yang JH, Lu Y, Kim SJ, Kwon S, Jo TH, Kim JW, Park YI, Ahn GW, et al. 2010. Anti-inflammatory activity of bark of Dioscorea batatas DECNE through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-kappa B and ERK1/2 inactivation. Food Chem Toxicol. 48:3073–3079. [DOI] [PubMed] [Google Scholar]
- Jung YS, Kim DH, Hwang JY, Yun NY, Lee YH, Han SB, Hwang BY, Lee MS, Jeong HS, Hong JT.. 2014. Anti-inflammatory effect of tricin 4′-O-(threo-beta-guaiacylglyceryl) ether, a novel flavonolignan compound isolated from Njavara on in RAW264.7 cells and in ear mice edema. Toxicol Appl Pharm. 277:67–76. [DOI] [PubMed] [Google Scholar]
- Kaur G, Jabbar Z, Athar M, Alam MS.. 2006. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 44:984–993. [DOI] [PubMed] [Google Scholar]
- Kim KN, Heo SJ, Yoon WJ, Kang SM, Ahn G, Yi TH, Jeon YJ.. 2010. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol. 649:369–375. [DOI] [PubMed] [Google Scholar]
- Lansky EP, Newman RA.. 2007. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 109:177–206. [DOI] [PubMed] [Google Scholar]
- Lee HS, Ryu DS, Lee GS, Lee DS.. 2012. Anti-inflammatory effects of dichloromethane fraction from Orostachys japonicus in RAW 264.7 cells: suppression of NF-κB activation and MAPK signaling. J Ethnopharmacol. 140:271–276. [DOI] [PubMed] [Google Scholar]
- Lee MY, Park BY, Kwon OK, Yuk JE, Oh SR, Kim HS, Lee HK, Ahn KS.. 2009. Anti-inflammatory activity of (-)-aptosimon isolated from Daphne genkwa in RAW264.7 cells. Int Immunopharmacol. 9:878–885. [DOI] [PubMed] [Google Scholar]
- Li YH, Wen SP, Kota BP, Peng G, Li GQ, Yamahara J, Roufogalis BD.. 2005. Punica granatum flower extract, a potent alpha-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J Ethnopharmacol. 99:239–244. [DOI] [PubMed] [Google Scholar]
- Le D, Bing T, Nguyen D, Jeong H, Byung S, Mi H.. 2017. Lignan derivatives from Selaginella tamariscina and their nitric oxide inhibitory effects in LPS-stimulated RAW 264.7 cells. Bioorg Med Chem Lett. 27:524–529. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Omura S.. 2008. Quercetin regulates the inhibitory effect of monoclonal non-specific suppressor factor beta on tumor necrosis factor-alpha production in LPS-stimulated macrophages. Biosci Biotech Bioch. 72:1915–1920. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He XL, Smirnova I, Liu MY, Van HC, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10Sc Cr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- Rahima A, Li L, Jie Z, Haji A.. 2009. Study on the polyphenols purification technology of pomegranate flowers. China J Chin Mater Med. 17:2265–2267. [Google Scholar]
- Ruberlei O, Geovane R, André C, Fábio M, Domingos T.. 2017. Dilodendron bipinnatum Radlk. inhibits pro-inflammatory mediators through the induction of MKP-1 and the down-regulation of MAPKp38/JNK/NF-κB pathways and COX-2 in LPS-activated RAW 264.7 cells. J Ethnopharmacol. 202:127–137. [DOI] [PubMed] [Google Scholar]
- Shao J, Li Y, Wang Z, Xiao M, Yin P, Lu Y, Qian X, Xu Y, Liu J.. 2013. 7b, A novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2- and p38 MAPK-mediated activation of NF-κB in LPS-stimulated RAW264.7 macrophages. Int Immunopharmacol. 17:216–228. [DOI] [PubMed] [Google Scholar]
- Shi C, Yuan S, Zhang H, Zhang T, Wang L, Xu Z.. 2009. Cell-mediated immune responses and protective efficacy against infection with mycobacterium tuberculosis induced by Hsp65 and hIL-2 fusion protein in mice. Scand J Immunol. 69:140–149. [DOI] [PubMed] [Google Scholar]
- Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM.. 2008. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 24:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursun X, Zhao YX, Talat Z, Xin XL, Adila T, Abdulla R, AkberAisa H.. 2016. Anti-inflammatory effect of Rosa rugosa flower extract in lipopolysaccharide-stimulated RAW264.7 macrophages. Biomol Ther. 24:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Ding YQ, Wan CP, Li LY, Xu JL, Liu K, Slitt A, Ferreira D, Khan IA, Seeram NP.. 2012. Antidiabetic ellagitannins from pomegranate flowers: inhibition of alpha-glucosidase and lipogenic gene expression. Org Lett. 14:5358–5361. [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M.. 1997. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 91:243–252. [DOI] [PubMed] [Google Scholar]