Abstract
Context: Geraniol, an acyclic monoterpene alcohol is found in medicinal plants, is used traditionally for several medical purposes including diabetes.
Objectives: The present study evaluates the antihyperglycemic potential of geraniol on key enzymes of carbohydrate metabolism in streptozotocin (STZ)-induced diabetic rats.
Materials and methods: Diabetes was induced in experimental rats, by a single intraperitoneal (i.p) injection of STZ [40 mg/kg body weight (b.w.)]. Different doses of geraniol (100, 200 and 400 mg/kg b.w.) and glyclazide (5 mg/kg b.w.) were administrated orally to diabetic rats for 45 days. Body weight, food intake, plasma glucose, insulin, blood haemoglobin (Hb), glycosylated haemoglobin (HbA1c), hepatic glucose metabolic enzymes and glycogen were examined.
Results: The LD50 value of geraniol is 3600 mg/kg b.w. at oral administration in rats. Administration of geraniol in a dose-dependent manner (100, 200, 400 mg/kg b.w.) and glyclazide (5 mg/kg b.w.) for 45 days significantly improved the levels of insulin, Hb and decreased plasma glucose, HbA1C in diabetic-treated rats. Geraniol at its effective dose (200 mg/kg b.w.) ameliorated the altered activities of carbohydrate metabolic enzymes near normal effects compared with two other doses (100 and 400 mg/kg b.w.). Geraniol treatment to diabetic rats improved hepatic glycogen content suggesting its anti-hyperglycemic potential. Geraniol supplement was found to preserve the normal histological appearance of hepatic cells and pancreatic β-cells in diabetic rats.
Discussion and conclusions: The present findings suggest that geraniol can potentially ameliorate key enzymes of glucose metabolism in experimental diabetes even though clinical studies used to evaluate this possibility are warranted.
Keywords: Diabetes mellitus, glucose 6-phosphatase, fructose 1, 6-bisphoshpatase, hexokinase
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycaemia with disturbances in carbohydrate, fat and protein metabolism, secondary to an absolute or relative lack of insulin (Fatima et al. 2010). It is a common public health problem throughout the world, with an estimated prevalence of 383 million people in 2013, expected to increase to 552 million people by 2030 (Colosia et al. 2013). Particularly, in India the number of diabetic people is expected to raise from 62 million to 101.2 million by 2030 (Kaveeshwar & Cornwall 2014). Several studies have demonstrated that alterations in carbohydrate metabolism play a vital role in the pathogenesis of diabetes and accelerate the development of diabetic complications (Soumya & Srilatha 2011).
Liver is an insulin-dependent tissue, which plays a pivotal role in blood glucose homeostasis. The key enzymes of glycolysis and gluconeogenesis are disturbed, leading to the chronic hyperglycaemia (Berg et al. 2001). Hyperglycaemia characterized by an abnormal postprandial blood glucose level has been linked to the onset of type 2 DM, associated with oxidative dysfunction and failure of various organs, especially the eyes, kidneys and nerves (Prathapan et al. 2012). STZ is a selective pancreatic β-cell genotoxicant used to induce experimental diabetes in model organisms. STZ-induced diabetic animals exhibit many of the complications similar to that of human diabetes (Srinivasan & Ramarao 2007). Therefore, the beneficial effects of antidiabetic principles are well-studied in these animal models.
Recently, undesirable side effects have been reported from the pharmacological drugs used for the treatment of diabetes. The search for antidiabetic drugs, focusing on plant-based medicine gains importance due to their potential benefits and provides ways for the treatment of human diabetes to reduce undesirable side effects. Plants provide a predominant resource for a huge number of conventional medicines that have been in existence for hundreds of years in countries like India (Kondeti et al. 2010). Terpenes are a group of secondary plant metabolites that are widespread in nature and have significant hypoglycaemic effect, which is well documented in several experimental studies. Geraniol possesses pharmacological activities such as antimicrobial (Lorenzi et al. 2009), antioxidant (Tiwari & Kakkar 2009), antilipid peroxidative (Chen & Viljoen 2010), anticancer (Pattanayak et al. 2009) and anti-inflammatory properties (Ji et al. 2002). The objective of the present study is to evaluate the antihyperglycemic efficacy of geraniol by assessing the activities of key metabolic enzymes of glucose metabolism in STZ-induced diabetic rats.
Materials and methods
Drugs and chemicals
Geraniol (98%) and STZ were obtained from Sigma Chemical Company (St. Louis, MO). All additional chemicals utilized were obtained either from Hi Media (Mumbai) and SD-Fine Chemicals (Mumbai). All chemicals used were of analytical grade.
Animals and diet
Adult male albino Wistar rats with body weight ranging from 180 to 200 g were procured from Central Animal House, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital, Annamalai University, Annamalainagar, Tamil Nadu, India, and were acclimatized for two weeks prior to start the experiments. They were maintained in a controlled environment under standard conditions of temperature and humidity with light–dark cycle (12 h light/dark cycle). Throughout the experimental period, the animals were fed with a balanced commercial pellet diet (Hindustan Lever Ltd., Mumbai, India) and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethical Committee of Annamalai University (Reg. No.987/2013).
Experimental induction of diabetes
Experimental diabetes was induced in overnight fasted rats by single intraperitoneal injection (i.p.) of STZ (40 mg/kg b.w.) dissolved in 0.1 M of citrate buffer (pH 4.5) Rakieten et al. (1963). As STZ is capable of inducing fatal hypoglycemia due to massive pancreatic insulin release, rats were provided with 10% glucose solution after STZ administration for the next 24 h to overcome drug-induced hypoglycaemia. Rats with blood glucose level ≥250 mg/dL were considered diabetic and were used in the study.
Experimental design
After acclimatization for a period of one week, the rats were randomly divided into seven groups of six animals in each group. Geraniol was dissolved in corn oil (2.5 mL/kg b.w.) and an aqueous solution of glyclazide (5 mg/kg b.w.) was administered orally once in a day for 45 days.
Group 1: Normal (vehicle treated)
Group 2: Normal + geraniol (400 mg/kg b.w.)
Group 3: Diabetic control (vehicle treated)
Group 4: Diabetic + geraniol (100 mg/kg b.w.)
Group 5: Diabetic + geraniol (200 mg/kg b.w.)
Group 6: Diabetic + geraniol (400 mg/kg b.w.)
Group 7: Diabetic + glyclazide (5 mg/kg b.w.)
Sample collection
The initial and final body weights of the various groups were recorded. At the end of the experimental period, the animals were fasted overnight, sacrificed by cervical decapitation. Blood samples were collected in tubes containing potassium oxalate and sodium fluoride (3:1) mixture for the estimation of plasma glucose, insulin, haemoglobin (Hb) and glycosylated haemoglobin (HbA1C) levels. Liver and kidney tissues were excised immediately and washed in ice-cold saline, homogenized in Tris-HCl buffer (0.1 M, pH 7.5), centrifuged (3000 rpm) and the supernatant was collected for various biochemical estimations. A portion of the tissues was used for histopathology studies. The pancreas was dissected out, used for histological and immunohistochemical studies.
Assessment of oral glucose tolerance
On the day prior to sacrifice, oral glucose tolerance test (OGTT) was performed in all the groups. Blood samples were obtained from the lateral tail vein of overnight fasted experimental animals. Successive blood samples were taken at 0, 30, 60, 90 and 120 min after glucose administration of 2 mg/kg b.w. of glucose solution (Young et al. 1995).
Biochemical assays
Plasma glucose levels were estimated using a commercial kit (Sigma Diagnostics Private Limited, Baroda, India) by the method of Trinder (1969). Hb and HbA1c were estimated using diagnostic kit (Agappe Diagnostic Private Limited, Kochi, India) (Bisse & Abraham 1985).
Estimation of carbohydrate metabolic enzymes
Hexokinase was assayed by the method of Brandstrup et al. (1957). Glucose 6-phosphate dehydrogenase was assayed by the method of Ellis and Kirkman (1961). Glucose 6-phosphatase was assayed by the method of Koide and Oda (1959). Fructose 1,6-bisphosphatase activity was measured by the method of Gancedo and Gancedo (1971).
Histopathological study
For the histological study, the pancreas was excised immediately perfused with cold physiological saline and fixed in 10% formalin. Then, dehydrated on treatment with a series of different concentration of ethanol and embedded in paraffin wax. Sections of 3–5 μm thickness were cut using a microtome and stained with haematoxylin and eosin stain. The specimens were evaluated with a light microscope. All histopathological changes were examined by eminent pathologist.
Immunohistochemistry
Immunohistochemical staining was done to confirm the presence of insulin positive cells in the islets of pancreas in all rats, as described by Ozougwu et al. (2013). Paraffin sections were serially cut into 5 μm thickness and placed on microscopic slides sealed with poly-l-lysine (Sigma, USA). The tissue was fixed on the microscopic slide in a 37 °C oven for 1 h. Then, the tissues were deparaffinized by rinsing in xylene for three times and dehydrated with two rinses in absolute alcohol and two rinses in 95% ethanol for 3 min each before being washed with phosphate buffer saline (PBS) and distilled water for 5 min each. The tissue sections were then incubated for 15 min in 3% H2O2 in methanol to quench the endogenous peroxidase. The sections were then washed in PBS for 5 min and excess PBS was wiped around the tissues. The sections were blocked by incubating with diluted normal serum for 20 min and excess serum was blotted from the sections. For the detection of insulin, sections were incubated with primary antibody (Rabbit polyclonal anti-insulin antibody from Cell Signaling, Danvers, MA) diluted to 1:100 in PBS for 60 min, and sections were washed with PBS for 5 min. Excess PBS was wiped from the slides. Then, the sections were processed by indirect immunoperoxidase technique using a One-Step Polymer HRP Detection kit (Biogenex, The Hague, The Netherlands) with secondary antibodies using haematoxylin as the counterstain. Evaluation of immunohistochemical staining was made by the examination of 10 islets for all groups of rats using a Confocal Scanning Microscope (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
The experimental results were expressed as means ± SD and subjected to one-way analysis of variance (ANOVA), using a computer software package (SPSS version 11.5; SPSS Inc., Chicago, IL) and the comparisons of significant differences among the groups were performed using Duncan’s post has multiple range test (DMRT), p < 0.05 was considered as significantly different between means.
Results
Effect of geraniol on changes in body weight, food and fluid intake
Table 1 shows the changes in the levels of body weight of control and experimental groups. At the end of the experimental period, the body weight was significantly decreased in diabetic rats when compared with normal control rats. Food and fluid intake were significantly elevated in diabetic control rats throughout the experimental period. On the other hand, diabetic rats treated with geraniol (100, 200 and 400 mg/kg b.w.) showed significantly decreased food and fluid intake with improved body weight to near normal which is comparable with glyclazide group.
Table 1.
Effect of geraniol on body weight, fluid and food intake of normal control and experimental rats.
| Body weight (g) |
Fluid intake (mL/rat/day) |
Food intake (g/rat/day) |
||||
|---|---|---|---|---|---|---|
| Groups | Initial | Final | Before | After | Before | After |
| Normal control | 206.34 ± 15.71 | 214.30 ± 16.32a | 67.52 ± 5.14 | 67.23 ± 5.12a | 15.68 ± 1.19 | 18.09 ± 1.38a |
| Normal + geraniol (400 mg/kg b.w.) | 207.47 ± 15.89 | 215.84 ± 16.53a | 69.09 ± 5.29 | 71.54 ± 5.48a | 16.15 ± 1.24 | 19.28 ± 1.48a |
| Diabetic control | 211.13 ± 16.08 | 136.60 ± 10.40b | 76.44 ± 5.82 | 187.91 ± 14.31b | 56.88 ± 4.33 | 72.28 ± 5.50b |
| Diabetic + geraniol (100 mg/kg b.w.) | 210.75 ± 16.14 | 188.89 ± 14.4c | 73.27 ± 5.61 | 126.53 ± 9.69c | 53.12 ± 4.07 | 38.90 ± 2.98c |
| Diabetic + geraniol (200 mg/kg b.w.) | 208.28 ± 15.95 | 197.14 ± 15.5d | 74.99 ± 5.74 | 97.63 ± 7.48d | 55.76 ± 4.27 | 30.26 ± 2.09d |
| Diabetic + geraniol (400 mg/kg b.w.) | 207.99 ± 15.93 | 198.25 ± 15.72d | 70.88 ± 5.43 | 94.42 ± 7.23d | 54.97 ± 4.21 | 29.12 ± 2.00d |
| Diabetic + glyclazide (5 mg/kg b.w.) | 206.33 ± 15.80 | 200.43 ± 15.89d | 74.0 ± 5.67 | 90.96 ± 6.97d | 57.14 ± 4.38 | 25.23 ± 1.93d |
Values are given as mean ± SD from six rats in each group.
Values not sharing a common superscript letter(a–d) differ significantly at p < 0.05 (DMRT).
Effect of geraniol on OGTT, plasma glucose and insulin
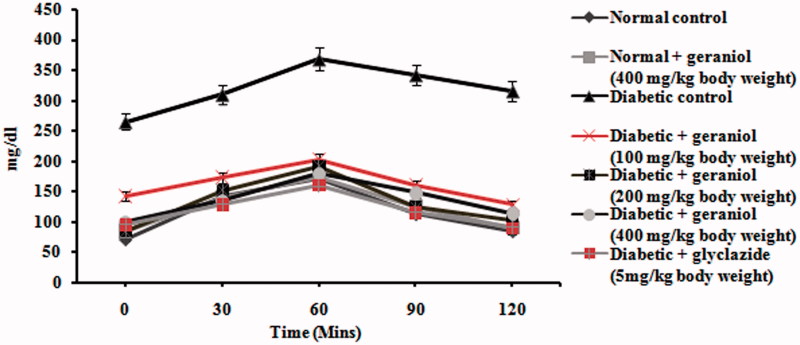
OGTT conducted on control and experimental rats are shown in Figure 1. OGTT of diabetic rats showed a highly pronounced elevation of glucose at fasting state and at 30, 60, 90 and 120 min after oral glucose intake as compared with control rats. Administration of geraniol showed a significant decrease in blood glucose levels, which signifies insulin secretion.
Figure 1.
Effect of geraniol on the levels of oral glucose tolerance test in normal control and experimental rats.
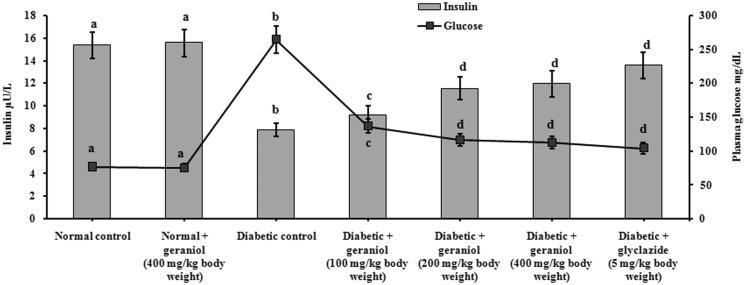
The effect of geraniol on the levels of plasma glucose and insulin in experimental rats are showed in Figure 2. Glucose levels were elevated and plasma insulin was significantly decreased in diabetic rats when compared with normal control rats. Oral administration of geraniol (100, 200 and 400 mg/kg b.w.) for 45 days significantly decreased blood glucose and increased plasma insulin to near normal, as compared with glyclazide treated rats. Geraniol at a dose of 200 and 400 mg/kg b.w. showed a highly significant effect than the lower dose (100 mg/kg b.w.). Based on these data, the effective dose was fixed as 200 mg/kg b.w. and further studies were carried out at this dose.
Figure 2.
Effect of geraniol on the levels of plasma insulin and glucose in normal control and experimental rats.
Effect of geraniol on levels of Hb and HbA1C
Table 2 shows the levels of Hb and HbA1C in normal control and diabetic rats. Decrease in Hb level and an increase in HbA1C level were observed in diabetic rats. These values were reverted back towards near normal on treatment with geraniol and glyclazide.
Table 2.
Effects of geraniol on the levels of Hb and HbA1C in normal control and experimental rats.
| Parameters/Groups | Hb (g/dL) | HbA1c (%Hb) |
|---|---|---|
| Normal control | 15.32 ± 1.32a | 6.46 ± 0.45a |
| Normal + geraniol (200 mg/kg b.w.) | 15.00 ± 1.32a | 6.15 ± 0.44a |
| Diabetic control | 7.9 ± 0.55b | 15.42 ± 0.88b |
| Diabetic + geraniol (200 mg/kg b.w.) | 12.01 ± 0.88c | 11.22 ± 0.88c |
| Diabetic + glyclazide (5 mg/kg b.w.) | 12.95 ± 0.88c | 12.03 ± 0.88c |
Values are given as mean ± SD from six rats in each group.
Values not sharing a common superscript letter(a–c) differ significantly at p < 0.05 (DMRT).
Effect of geraniol on the activities of carbohydrate metabolizing enzymes
Table 3 shows the effects of geraniol on the activities of glycolytic and gluconeogenic enzymes in the liver and kidney tissues of normal control and experimental rats. Significantly elevated activities of gluconeogenic enzymes such as glucose 6-phosphatase and fructose 1, 6-bisphosphatase as well as decreased activities of hexokinase and glucose 6-phosphate dehydrogenase were found in liver tissue of diabetic rats when compared with normal control rats. Oral administration of geraniol (200 mg/kg b.w.) as well as glyclazide (5 mg/kg b.w.) to diabetic rats reversed the activities of these enzymes to near normal.
Table 3.
Effect of geraniol on the activities of carbohydrate metabolic enzymes in the tissues of normal control and experimental rats.
| Parameters/groups | Normal control | Normal + geraniol (200 mg/kg b.w.) | Diabetic control | Diabetic + geraniol (200 mg/kg b.w.) | Diabetic + Glycazide (5 mg/kg b.w.) |
|---|---|---|---|---|---|
| Glycolytic enzyme | |||||
| Hexokinase | |||||
| Liver (units/g protein) | 155.61 ± 11.92a | 154.23 ± 11.75a | 112.16 ± 8.59b | 138.60 ± 10.56c | 140.31 ± 10.75c |
| Glucose 6-phosphate dehydrogenase | |||||
| Liver (×10−4 ml U/mg protein) | 5.71 ± 0.43a | 5.30 ± 0.41a | 3.15 ± 0.24b | 4.75 ± 0.36c | 4.62 ± 0.35c |
| Kidney (μg/mg protein) | 4.55 ± 0.35a | 4.60 ± 0.35a | 2.95 ± 0.22b | 3.82 ± 0.29c | 3.90 ± 0.30c |
| Gluconeogenic enzyme | |||||
| Glucose 6-phosphatase | |||||
| Liver (μg/mg protein) | 0.20 ± 0.02a | 0.16 ± 0.01a | 0.45 ± 0.03b | 0.29 ± 0.02c | 0.26 ± 0.02c |
| Kidney (μg/mg protein) | 0.19 ± 0.01a | 0.17 ± 0.01a | 0.49 ± 0.04b | 0.25 ± 0.02c | 0.27 ± 0.02c |
| Fructose 1,6-bisphosphatase | |||||
| Liver (μg/mg protein) | 0.31 ± 0.02a | 0.35 ± 0.03a | 0.67 ± 0.05b | 0.43 ± 0.03c | 0.47 ± 0.04c |
| Kidney (μg/mg protein) | 0.28 ± 0.02a | 0.31 ± 0.02a | 0.55 ± 0.04b | 0.47 ± 0.04c | 0.43 ± 0.03c |
Values are given as mean ± S.D from six rats in each group.
Values not sharing a common superscript letter(a–c) differ significantly at p < 0.05 (DMRT).
Effect of geraniol on histology of pancreas
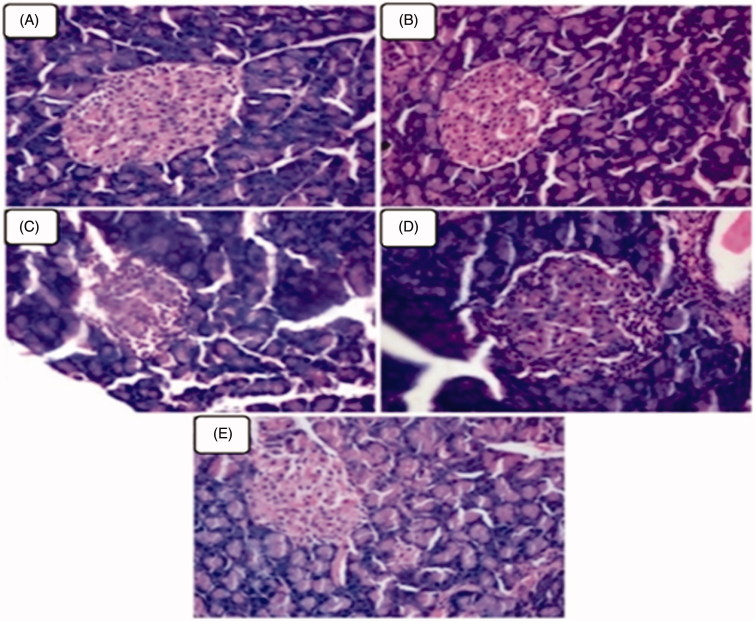
Histopathological studies on the pancreatic islets of experimental rats are shown in Figure 3(A–E). Diabetic rats (Figure 3(C)) showed fatty infiltration with shrinkage of islet cells. Oral administration of geraniol (Figure 3(D)) and glyclazide (Figure 3(E)) restored pancreatic acini with the absence of dilatation.
Figure 3.
(A–E) Represents the microphotographs of pancreatic tissues of normal control and experimental rats in each group (haematoxylin and eosin staining, 40×). (A and B) Normal control and normal control + geraniol (200 mg/kg body weight) treated rats showed normal appearing pancreatic exocrine glands and ducts with Islet of Langerhans. (C) Diabetic rats showing fatty infiltration and shrinkage of islet cells. (D and E) Pancreatic tissues from diabetic + geraniol (200 mg/kg b.w.) and diabetic + glyclazide (5 mg/kg b.w.) treated rats shows normal appearing pancreatic acini with absence of dilation and prominent hyperplastic of islets.
Effect of geraniol on immunohistology of pancreas
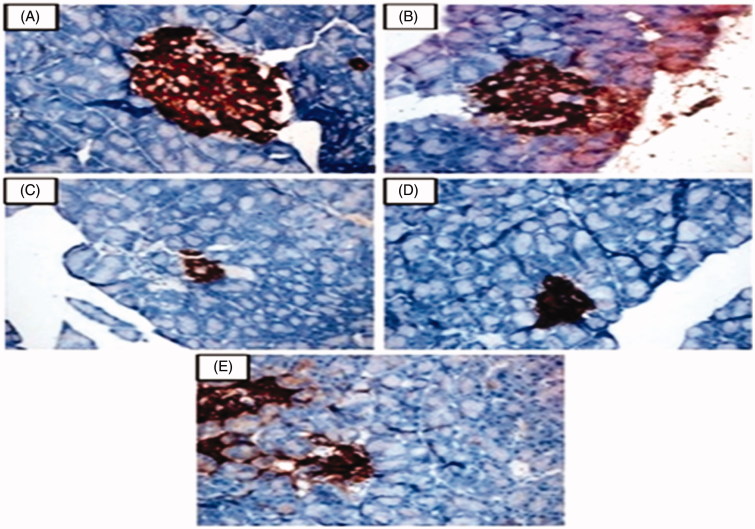
A decrease in the number of insulin-immunoreactive β-cells was observed in the diabetic control group. Immunohistochemical section of the pancreatic islets of diabetic rats treated with geraniol (200 mg/kg b.w.) and glyclazide (5 mg/kg b.w.) showed increased insulin-immunoreactive β-cells (Figure 4(D,E)). This clearly suggests that after treatment with geraniol, positive immunoreactions of β-cells for anti-insulin antibodies were obviously increased in numbers with deep brown granules as interpreted in Figure 4(D).
Figure 4.
This figure shows the immunoexpression pattern of insulin secreting cells in pancreatic islets observed in the normal control and experimental rats in each group (40×). (A and B) Normal control and normal control + geraniol (200 mg/kg b.w.) treated rats showed normal immunohistochemical expression of insulin secreting cells in pancreatic islets. (C) Diabetic control showed decreased staining of insulin secreting cells in pancreatic islets. (D and E) Diabetic + geraniol (200 mg/kg b.w.) and diabetic + glyclazide (5 mg/kg b.w.) treated rats showed increased insulin secreting cells in pancreatic islets.
Table 4 depicts the % of insulin positive staining areas in pancreatic β-cells of normal, control and experimental rats in each group. Normal control and geraniol control rats revealed no significant difference in the % area of positive stain while a significant decline was observed in diabetic rats. The % area of immunoreactivity of insulin was substantially increased in geraniol- (200 mg/kg b.w.) and glyclazide-treated (5 mg/kg b.w.) diabetic rats. This indicates the potential insulinotropic effect of geraniol.
Table 4.
Effects of geraniol on the levels of immunoreactive cells of insulin in pancreatic islets of normal and experimental rats.
| Parameters/groups | % of Insulin positive cells |
|---|---|
| Normal control | 66.14 ± 4.29a |
| Normal + geraniol (200 mg/kg b.w.) | 69.23 ± 4.84a |
| Diabetic control | 39.97 ± 3.74b |
| Diabetic + geraniol (200 mg/kg b.w.) | 67.20 ± 5.04c |
| Diabetic + glyclazide (5 mg/kg b.w.) | 70.06 ± 5.60c |
Values are given as mean ± S.D from six rats in each group.
Values not sharing a common superscript letter(a–c) differ significantly at p < 0.05 (DMRT).
Discussion
Type 2 DM is one of the most common chronic and progressive metabolic disorders with impaired insulin secretion, insulin resistance with augmented hepatic glucose production (Jainandunsing et al. 2015). This study evaluates the effect of geraniol by measuring the activities of key enzymes involved in carbohydrate metabolism in the liver and kidney of control and experimental rats. Recently several studies reported the biological activities and important applications of geraniol (Choi et al. 2000; Ji et al. 2002; Duncan et al. 2004; Vinothkumar & Manoharan 2011). Pancreas is the primary organ involved in sensing the organism’s diet and energetic states via glucose concentration in the blood and in response to elevated blood glucose, releases insulin to control glycaemic status (Sundaram et al. 2013). STZ is a nitrosurea derivative, preferentially taken up by pancreatic β-cells via low affinity GLUT-2 transporter and causes DNA alkylation followed by the activation of poly ADP ribosylation leading to depletion of cytosol concentrations of NAD+ and ATP resulting in destruction of β-cells of pancreas. An insufficient release of insulin leads to hyperglycemia and results in diabetic complications (Grover et al. 2002).
STZ-induced diabetic rats showed increase in water and food intake with a significant loss of body weight. In this study, diabetic rats showed marked reduction in their body weight when compared to normal rats, which could be due to the unavailability of carbohydrate as energy source (Sundaram et al. 2014). We also observed an increase in food and water intake in diabetic rats that result from a chronic reduction in glucose utilization by the cells and considerable loss of glucose through urine. Oral administration of geraniol significantly improved glycaemic control which prevented the loss of body weight and improved food and fluid intake in diabetic control rats in a dose-dependent manner.
An OGTT is a sensitive measure of early abnormality in glucose regulation than HbA1c or plasma glucose. An increase in the plasma glucose level after glucose load during the OGTT was observed in diabetic rats. The data obtained from the OGTT clearly show that the blood glucose level remains high even after 120 min in diabetic control rats (Ceriello 2005). However, in geraniol-treated diabetic rats the blood glucose levels reached a peak and returned again to fasting levels after 120 min. This revealed that the increased glucose tolerance in geraniol-treated STZ rats is due to insulin secretion from existing β-cells and augmented glucose transport and utilization.
Blood glucose control is an important component in delaying or preventing acute or long-term diabetic complications (Gandhi & Sasikumar 2012). In the present study, we observed a low level of plasma insulin in STZ-induced rats denoting perturbations in β-cells function, whereas geraniol-treated rats showed increased plasma insulin levels, indicating that geraniol treatment improved β-cell function in STZ rats. The increased insulin levels after the treatment of geraniol in diabetic rat may be related to the closure of K+-ATP channels and stimulation of Ca2+ influx, an initial key step in insulin secretion from remnant β-cells. These results are in harmony with Lone and Yun (2016), who reported that, a monoterpene, limonene reduced glucose levels and increased insulin secretion in β-cells of diabetic rats.
HbA1C is considered as a gold standard marker for accurate and reliable measurement of fasting glucose, which is strongly associated with the prognosis of the diabetes (Lakshmi et al. 2009). The decreased level of Hb in diabetic rats is mainly due to the increased formation of HbA1C. In DM, the excess blood glucose reacts with Hb to form HbA1C. The increase in HbA1C is directly proportional to the blood glucose level (Nain et al. 2012). Profound studies well documented that during diabetes, increased glucose and dicarbonyl compounds react with haemoglobin to form advanced glycation end products, which leads to the development of diabetic complications (Bunn et al. 1978; Turk 2010). Treatment with geraniol and glyclazide to diabetic rats significantly improved the levels of Hb and decreased HbA1C when compared to normal control rats. This is due to the restoration of blood glucose with a reduction in the glycosylation of Hb.
Glucose homeostasis is related to endogenous production and utilization of glucose by target tissues (Wilding 2014). Insulin regulates the activities of various enzymes such as hexokinase, glucose-6-phosphate dehydrogenase, glucose-6-phosphatase and fructose 1,6-bisphosphatase which are involved in carbohydrate metabolism (Prasath & Subramanian 2014). Hexokinase is one of the key enzymes, in glycolysis, which catalyzes the conversion of glucose to glucose 6‐phosphate in liver and plays a pivotal role in the maintenance of blood glucose homeostasis (Matschinsky 2009). Being an insulin‐dependent enzyme, the hepatic hexokinase activity of diabetic rats is almost entirely inhibited or inactivated due to the lack of insulin (Selvaraja & William Ndyeabura 2011). This impairment results in a marked reduction in the rate of glucose oxidation via glycolysis in tissues with decreased glucose removal from the blood, thus ultimately leads to hyperglycaemia. Several studies documented that hexokinase activity is significantly decreased in STZ-induced diabetic rats (Malini et al. 2011; Ramachandran & Saravanan 2013; Vanitha et al. 2014). Oral administration of geraniol to STZ‐induced diabetic rats resulted in a significant reversal in the activity of hexokinase, thereby increases the oxidation of glucose. Profound studies reported that hexokinase is the potential target for new treatment strategies for the management of type 2 diabetes (Thiebaud et al. 1982; Murali & Saravanan 2012).
Glucose 6-phosphate dehydrogenase is the primary rate limiting enzyme of hexose mono-phosphate shunt and produces NADPH required for the maintenance of glutathione (Kondeti et al. 2010). It has an important role in pancreatic β-cell function and its survival (Cernea & Dobreanu 2013). Previous studies demonstrated that the lack of insulin results in the reduction of glucose 6-phosphate dehydrogenase activity in STZ-induced diabetic models (Baltrusch et al. 2012). Our results corroborate with these findings. In DM, decreased activity of glucose 6-phosphate dehydrogenase affects the concentration of NADPH and induces oxidative stress in various tissues (Stanton 2012). In our study, administration of geraniol and glyclazide significantly improved the activity of glucose 6-phosphate dehydrogenase in liver and kidney tissues of diabetic rats. It provides hydrogen, which binds NADP+ and produces NADPH and enhances the synthesis of fats from carbohydrate, which finally decreases the plasma glucose level.
During starvation, the activities of chief regulatory enzymes of gluconeogenesis, glucose-6-phosphatase and fructose 1, 6-bisphosphatase in liver and kidney were increased. These enzymes play a vital role in the regulation of blood glucose levels. In diabetic state, enhanced activities of gluconeogenic enzymes increases hepatic endogenous glucose production, which leads to chronic hyperglycemia (Nordlie et al. 1999). In the current study, diabetic rats showed a significant increase in the activities of gluconeogenic enzymes. Oral administration of geraniol to diabetic rats significantly reversed the activities of gluconeogenic enzymes. Our results are concomitant with Deepa and Anuradha (2011) who reported that limonene a monoterpene reduced the activities of gluconeogenic enzymes in STZ-induced diabetic rats.
The morphology and number of insulin-positive β-cells in the islets were studied using the immunohistochemical method. The results of the present study showed marked reduction in insulin-positive β-cells in the pancreatic tissue of diabetic rats compared to normal rats. The percentage of insulin positive cells per islet was significantly increased in geraniol- and glyclazide-treated diabetic rats as compared with diabetic control rats. This observation indicates that geraniol and glyclazide increases the number of insulin positive cells in the pancreas of diabetic-treated rats. From the histopathological studies, the changes observed in the pancreas of diabetic rats were significantly reversed on treatment with geraniol and glyclazide. Our results are in agreement with Rathinam et al. (2014) who also observed that, a monoterpene, myrtenal shows similar results in histological changes of pancreas. Thus, the present study demonstrated the antidiabetic potential of geraniol in STZ-induced type-2 diabetic rats.
Conclusions
We concluded that geraniol ameliorates carbohydrate metabolism and restores glucose homeostasis by altering the activities of enzymes involved in glucose production and utilization. It also protects pancreatic β-cells and exhibits significant insulinotrophic activity in experimental diabetic rats. In addition, immunohistochemical staining of pancreas confirms insulin secretion from remnant pancreatic β-cells in geraniol-treated diabetic rats. This suggests that geraniol exerts significant antidiabetic properties; however, further studies are essential to understand the plausible molecular mechanism of geraniol in diabetic rats.
Disclosure statement
The authors report no declaration of interest.
References
- Baltrusch S, Schmitt H, Brix A, Langer S, Lenzen S.. 2012. Additive activation of glucokinase by the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and the chemical activator LY2121260. Biochem Pharmacol. 83:1300–1306. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE.. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 7:947–953. [DOI] [PubMed] [Google Scholar]
- Bisse E, Abraham EC.. 1985. New less temperature-sensitive microchromatographic method for the separation and quantitation of glycosylated hemoglobins using a non-cyanide buffer system. J Chromatogr. 344:81–91. [DOI] [PubMed] [Google Scholar]
- Brandstrup N, Kirk JE, Bruni C.. 1957. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol. 12:166–171. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Gabbay KH, Gallop PM.. 1978. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 200:21–27. [DOI] [PubMed] [Google Scholar]
- Ceriello AI.2005. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabet. 54:1–7. [DOI] [PubMed] [Google Scholar]
- Cernea S, Dobreanu M.. 2013. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med (Zagreb). 23:266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Viljoen AM.. 2010. Geraniol-A review of a commercially important fragrance material. S Afr J Bot. 76:643–651. [Google Scholar]
- Choi HS, Song HS, Ukeda H, Sawamura M.. 2000. Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-diphenyl-2-picrylhydrazyl. J Agric Food Chem. 48:4156–4161. [DOI] [PubMed] [Google Scholar]
- Colosia AD, Palencia R, Khan S.. 2013. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabet Metab Syndr Obes. 6:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa B, Anuradha CV.. 2011. Linalool, a plant derived monoterpene alcohol, rescues kidney from diabetic-induced, nephropathic changes via blood glucose reduction. Diabetol Croat. 3:40–44. [Google Scholar]
- Duncan RE, Lau D, Sohemy A, Archer MC.. 2004. Geraniol and beta-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem Pharmacol. 68:1739–1747. [DOI] [PubMed] [Google Scholar]
- Ellis HA, Kirkman HN.. 1961. A colorimetric method for assay of erythrocytic glucose 6-phosphate dehydrogenase. Proc Soc Exp Biol Med. 106:607–609. [DOI] [PubMed] [Google Scholar]
- Fatima SS, Rajasekhar MD, Kumar KV, Kumar MT, Babu KR, Rao CA.. 2010. Antidiabetic and antihyperlipidemic activity of ethyl acetate:isopropanol (1:1) fraction of Vernonia anthelmintica seeds in streptozotocin induced diabetic rats. Food Chem Toxicol. 48:495–501. [DOI] [PubMed] [Google Scholar]
- Gancedo JM, Gancedo C.. 1971. Fructose-1,6-diphosphatase, phospho-fructokinase and glucose-6-phosphate dehydrogenase from fermenting and none fermenting yeasts. Arch Micro. 76:132–138. [DOI] [PubMed] [Google Scholar]
- Gandhi GR, Sasikumar P.. 2012. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover JK, Yadav S, Vats V.. 2002. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 81:81–100. [DOI] [PubMed] [Google Scholar]
- Jainandunsing S, Ozcan B, Rietveld T, van Miert JN, Isaacs AJ, Langendonk JG, de Rooij FW, Sijbrands EJ.. 2015. Failing beta-cell adaptation in South Asian families with a high risk of type 2 diabetes. Acta Diabetol. 52:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Si M, Podnos Y, Imagawa DK.. 2002. Monoterpene geraniol prevents acute allograft rejection. Transplant Proc. 34:1418–1419. [DOI] [PubMed] [Google Scholar]
- Kaveeshwar SA, Cornwall J.. 2014. The current state of diabetes mellitus in India. Australas Med J. 7:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide H, Oda T.. 1959. Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin Chim Acta. 4:554–561. [DOI] [PubMed] [Google Scholar]
- Kondeti VK, Badri KR, Maddirala DR, Thur SK, Fatima SS, Kasetti RB, Rao CA.. 2010. Effect of Pterocarpus santalinus bark, on blood glucose, serum lipids, plasma insulin and hepatic carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 48:1281–1287. [DOI] [PubMed] [Google Scholar]
- Lakshmi BS, Sujatha S, Anand S, Sangeetha KN, Narayanan RB, Katiyar C, Kanaujia A, Duggar R, Singh Y, Srinivas K, et al. . 2009. Cinnamic acid, from the bark of Cinnamomum cassia, regulates glucose transport via activation of GLUT4 on L6 myotubes in a phosphatidylinositol 3-kinase independent manner. J Diabet. 1:99–106. [DOI] [PubMed] [Google Scholar]
- Lone J, Yun JW.. 2016. Monoterpene limonene induces brown fat-like phenotype in 3T3-L1 white adipocytes. Life Sci. 153:198–206. [DOI] [PubMed] [Google Scholar]
- Lorenzi V, Muselli A, Bernardini AF, Berti L, Pages JM, Amaral L.. 2009. Geraniol restores antibiotic activities against multidrug-resistant isolates from Gram-negative species. Antimicrob Agents Chemother. 53:2209–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malini P, Kanchana G, Rajadurai M.. 2011. Antibiabetic efficacy of ellagic acid in streptozotocin-induced diabetes mellitus in albino Wistar rats. Asian J Pharm Clin Res. 4:124–128. [Google Scholar]
- Matschinsky FM.2009. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 8:399–416. [DOI] [PubMed] [Google Scholar]
- Murali R, Saravanan R.. 2012. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed Prev Nutr. 2:269–275. [Google Scholar]
- Nain P, Saini V, Sharma S, Nain J.. 2012. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol. 142:65–71. [DOI] [PubMed] [Google Scholar]
- Nordlie RC, Foster JD, Lange AJ.. 1999. Regulation of glucose production by the liver. Annu Rev Nutr. 19:379–406. [DOI] [PubMed] [Google Scholar]
- Ozougwu JC, Obimba KC, Belonwu CD, Unakalamba CB.. 2013. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol. 4:46–57. [Google Scholar]
- Pattanayak SP, Sunita P, Jha S.. 2009. In vivo antitussive activity of Coccinia grandis against irritant aerosol and sulfur dioxide-induced cough model in rodents. J Pharmacol. 1:157–161. [Google Scholar]
- Prasath GS, Subramanian SP.. 2014. Antihyperlipidemic effect of fisetin, a bioflavonoid of strawberries, studied in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 28:442–449. [DOI] [PubMed] [Google Scholar]
- Prathapan A, Nampoothiri SV, Mini S, Raghu KG.. 2012. Antioxidant, antiglycation and inhibitory potential of Saraca ashoka flowers against the enzymes linked to type 2 diabetes and LDL oxidation. Eur Rev Med Pharmacol Sci. 16:557–652. [PubMed] [Google Scholar]
- Rakieten N, Rakieten ML, Nadkarni MV.. 1963. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 29:91–98. [PubMed] [Google Scholar]
- Ramachandran V, Saravanan R.. 2013. Efficacy of Asiatic acid, a pentacyclic triterpene on attenuating the key enzyme activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Phytomedicine. 20:230–236. [DOI] [PubMed] [Google Scholar]
- Rathinam A, Pari L, Chandramohan R, Sheikh BA.. 2014. Histopathological findings of the pancreas, liver, and carbohydrate metabolizing enzymes in STZ-induced diabetic rats improved by administration of myrtenal. J Physiol Biochem. 70:935–946. [DOI] [PubMed] [Google Scholar]
- Selvaraja W, William Ndyeabura A.. 2011. Antidiabetic activity of crude stem extracts of coscinium fenestratum on streptozotocin-induced type-2 diabetic rats. A J Pharma Clin Res. 4:47–51. [Google Scholar]
- Soumya D, Srilatha B.. 2011. Late stage complications of diabetes and insulin resistance. J Diabetes Metab. 2:167. [Google Scholar]
- Srinivasan K, Ramarao P.. 2007. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 125:451–472. [PubMed] [Google Scholar]
- Stanton RC.2012. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 64:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram R, Naresh R, Shanthi P, Sachdanandam P.. 2013. Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine. 20:577–584. [DOI] [PubMed] [Google Scholar]
- Sundaram R, Shanthi P, Sachdanandam P.. 2014. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine. 21:793–799. [DOI] [PubMed] [Google Scholar]
- Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP.. 1982. The effect of graded doses of insulin on total glucose uptake, glucose oxidation and glucose storage in man. Diabetes. 3:957–963. [DOI] [PubMed] [Google Scholar]
- Tiwari M, Kakkar P.. 2009. Plant derived antioxidants – geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol in Vitro. 23:295–301. [DOI] [PubMed] [Google Scholar]
- Trinder P.1969. Determination of glucose in blood using glucose oxidase with analternative oxygen acceptor. J Clin Pathol. 6:24–27. [Google Scholar]
- Turk Z.2010. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol Res. 59:147–156. [DOI] [PubMed] [Google Scholar]
- Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Suriyanarayanan S, Gunasekaran P, Sivasubramanian S, Ramkumar KM.. 2014. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and β-cell function in streptozotocin-induced diabetic rats. Environ Toxicol Pharmacol. 37:326–335. [DOI] [PubMed] [Google Scholar]
- Vinothkumar V, Manoharan S.. 2011. Chemopreventive efficacy of geraniol against 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Redox Rep. 16:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding JP.2014. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Met Clin Exp. 63:1228–1237. [DOI] [PubMed] [Google Scholar]
- Young PW, Cawthorne MA, Coyle PJ, Holder JC, Holman GD, Kozka IJ, Kirkham DM, Lister CA, Smith SA.. 1995. Repeat treatment of obese mice with BRL 49653, a new and potent insulin sensitizer, enhances insulin action in white adipocytes. Association with increased insulin binding and cell-surface GLUT 4 as measured by photoaffinity labeling. Diabetes. 44:1087–1092. [DOI] [PubMed] [Google Scholar]