Abstract
Context: Urtica urens L. (Urticaceae) is an important and commonly used plant for its medicinal and pharmacological properties.
Objective: We analyzed the antioxidant and antimicrobial activities of the leaves of Urtica urens in ethanol (EtOH) and water (WA) solvents, employing standard analytical methods.
Materials and methods: Polyphenol, flavonoid and tannin content of Urtica urens leaves were determined, after their extraction, using EtOH (70%) and WA extracts as well as the antioxidant (DPPH, ABTS, β-carotene and FRAP) and the antibacterial (via the method of dilution tests) activities of EtOH and WA extracts.
Results: The 70% EtOH of Urtica urens showed the highest values of total phenolic (31.41 mg GAE/g DW), flavonoids (6.81 mg quercetin/g DW), tannin (8.29 mg GAE/g DW) and TEAC (560 mmol Trolox/g DW), compared to the WA. The results of DPPH for EtOH (95.56%) were higher than that of WA (64.56%) at a concentration of 40 mg/L. The extracts displayed a FRAP 106.23 for EtOH and 30.55 μmol Fe(II)/g DW for WA. The results clearly indicated that EtOH was the strongest radical scavenger (IC50 = 245.65 ± 10.2 μg/mL). Ethanol was the most effective with minimum inhibitory concentration (MIC) < 250 μg/mL. WA has no antibacterial activity.
Discussion and conclusion: The results indicate that leaves of Urtica urens could be used as natural antioxidant and antimicrobial agents.
Keywords: Flavonoids, MIC, Polyphenols
Introduction
The world is presently over-dependent on a few plant species (Correa et al. 2013). Diversification of production and consumption habits to include a broader range of plant species, particularly those currently identified as under-utilized, could significantly contribute to improve health and nutrition, livelihoods and ecological sustainability. Wild plants have played a significant role in supplementing staple foods by supplying trace elements, vitamins, and minerals in order to obtain a balanced diet, and they may do so again in the future. Their interest as a source of nutraceuticals has been highlighted in recent studies (Leonti 2012). Several epidemiological studies suggest that a high intake of foods rich in natural antioxidants reduces the risk of some cancers, heart, and degenerative diseases. Urtica urens L. (Urticaceae) leaves, have a relatively high level of protein (66%), which is of better quality if compared with the proteins of other leafy vegetables (Hughes et al. 1980). The leaves of nettle are good sources of different significant minerals and vitamins (Adamski & Bieganska 1980; Kukric et al. 2012). Nettles contain flavonoids, fatty acids, terpenes, protein, vitamins, and minerals. Stinging nettle leaves are rich in vitamin C, B groups vitamins, vitamin K, and some minerals mainly calcium, iron, magnesium, phosphorus, potassium, and sodium (Upton 2013). Nettle leaves contain nine carotenoids: Lutein, lutein isomers and β-carotene are the basic carotenoids (Guil-Guerrero et al. 2003).
Urtica urens is widely distributed in Tunisia and traditionally used as an herbal medicine for a wide variety of purposes. Other species of Urtica has been studied the microbial and antioxidant activities (Gulcin et al. 2004; Kukric et al. 2012; Sidaoui et al. 2015). But, in our knowledge, the antimicrobial and antioxidant activities of the species of Urtica urens from Tunisia have not yet studied.
The use of such antioxidant and antimicrobial compounds from natural sources has been considerable interest not only for the preservation of foods and improving the shelf life of food products, but also for increasing the stability of fats and oils and to control the human and plant diseases of microbial origin (Benkeblia 2004).
This study evaluates the total phenolic compounds, total flavonoid compounds, total tannin contents, vitamins (D, E and C), the antioxidant properties (DPPH, ABTS, β-carotene and FRAP), and the in vitro antimicrobial activities of ethanol and aqueous extracts of leaves of Urtica urens against strains of bacteria which known as multi-resistant organisms or involved in diverse pathology.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), α-tocopherol, β-carotene, and linoleic acid were purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals, namely l-ascorbic acid, Folin-Ciocalteu reagent (mixture of phosphomolybdate and phosphotungstate, Sigma) and other solvents were of analytical grade. All solutions were freshly prepared in distilled water.
Plant material
Aerial parts of Urtica urens were collected from Sfax, Tunisia, during December 2014 and identified by Professor Mohamed Chaieb from the Faculty of Sciences of Sfax (Laboratory of Biology and Vegetable Ecophysiology, Faculty of Science, Sfax, Tunisia). The voucher sample was deposited at The National Botanical Research Institute Tunisia (INRAT). It was washed with distilled water and then dried at room temperature for two days, afterwards, crushed, milled in a knife mill to obtain 100 g of Urtica urens powder and subsequently stored in glass bottles at room temperature.
Preparation of plant extract
The dried powder (50 g) was extracted (by a maceration method) by adding ethanol (70%) at 37 °C for 72 h. The extract was filtered through Whatman No.1 filter paper in a Buchner funnel. The filtrate was evaporated to dryness under reduced pressure in a rotatory vacuum evaporator (EYELA N1000, Tokyo, Japan) (Nassiri-Asl et al. 2009).
The other dried powder (50 g) was subjected to an extraction with water for 20 min by infusion (1/10, plant/solvent). After the treatment, the aqueous filtrate was concentrated by rotatory evaporator in order to obtain the aqueous extract of Urtica urens in powder form.
The dried sample of each extract was weighed and the yield of soluble constituents was determined. The dried extracts were kept in dark at +4 °C until further analyses. The yield of the extract under study was calculated by the following equation:
Where A1 corresponds to the Urtica urens concentrated by evaporator and A2 corresponds to the powdered dried plant material used for extraction with ethanol or water.
Total phenolic compound
The total phenolic compound (TPC) of extracts was determined by the Folin-Ciocalteu method (Singleton & Rossi 1965). TPC was expressed as mg gallic acid equivalents (GAE) per gram extract. Values presented are the average of three measurements.
Total flavonoids compound
The total flavonoid content of Urtica urens extracts was determined by the method described by Zhishen et al. (1999). Flavonoid content was expressed as mg quercetin equivalent (QE)/g dried extract. Values presented are the average of three measurements.
Determination of hydrolyzable tannin
The hydrolyzable tannin content was determined from AECS using the potassium iodide test (Saad et al. 2012). The analysis was performed in triplicate and the results were expressed in mg equivalent of gallic acid per gram of dried extract (mg GAE/g DW) using the calibration curve of gallic acid (y = 0.121x + 0.011, r2 = 0.9819).
Antioxidant activity
DPPH assay
The DPPH radical-scavenging activity of Urtica urens extracts was determined using the method described by Kirby and Schmidt (1997) with some modifications. The DPPH scavenging percentage was calculated as follows:
Tests were carried out in triplicate. The free radical scavenging activity of each extract was expressed in terms of Trolox equivalents (TE) (mg TE/g extract) and at the extract concentration where 50% of DPPH• was attenuated (IC50).
Antioxidant capacity by ABTS•+ method
The 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid free radical (ABTS•+) neutralization was determined using a spectrophotometric, 96-well microplate method described by Re et al. (1999) with minor modifications. The antioxidant capacity of each extract was expressed quantitatively in terms of trolox equivalents (TE) (mg TE/g extract) and at the extract concentration where 50% of ABTS•+ was neutralized (IC50).
β-Carotene bleaching by linoleic acid assay
The ability of Urtica urens extract to prevent bleaching of β-carotene was assessed as described by Koleva et al. (2002).
Ferric reducing antioxidant power (FRAP assay)
A modified method of Benzie and Strain (1999) was adopted for the FRAP assay. Results are expressed in μM Fe (II)/g dry mass (DW).
Estimation of vitamin D
Vitamin D levels was measured by colorimetric methods using commercial reagent kits (Ref: 20151 and 20091), respectively, purchased from Biomaghreb (Ariana, Tunis, Tunisia).
Estimation of vitamin E
The extraction of vitamin E from the method has been described by both Katsanidis and Addis (1999).
Estimation of vitamin C
Ascorbic acid was determined as described by Jacques-Silva et al. (2001).
Antimicrobial activity
Microbial strains
Antimicrobial activities of Urtica urens extracts were tested against eight strains of bacteria: Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27893), Bacillus subtilis (JN 934392), Salmonella enteritidis, Escherichia coli (ATCC 25922), Staphylococcus epidermidis (MTCC 3615), Micrococcus luteus (ATCC 4698) and Enterococcus faecalis (ATCC 29212). These strains were kindly provided by the Centre of Biotechnology of Sfax, Tunisia.
Agar diffusion method
Antimicrobial activity was performed according to the method described by Vanden Berghe and Vlietinck (1991).
Determination of the minimal inhibitory concentration (MIC)
MIC values, which represent the lowest plant extracts concentration that completely inhibits the growth of microorganisms, were determined based on a micro-well dilution method (1998).
Determination of minimum bactericidal concentration (MBC)
To determine the minimum bactericidal concentration (MBC), a loop full from those tubes, which did not show any visible growth in MIC assay, was cultured on MHA and incubated at 37 °C for 18 to 24 h. The highest dilution that did not yield colony formation on MHA was considered as MBC (Testai et al. 2002).
Statistical analysis
Values were expressed as mean ± standard error of the mean (SEM) of many parallel measurements. Differences at p ≤ 0.05 were considered statistically significant. Database management and statistical analysis were performed using SPSS (Microsoft Corporation) statistical software package (SPSS Inc, Chicago, IL).
Results
Extraction yield and polyphenol, flavonoid and hydrolyzable tannin contents
The yield of extractable components relative to the weight of dried plant material ranged from 4.768% for EtOH extract and 0.87% for WA extract. The results of the phytochemical analysis evinced the quantitative values of EtOH extract: 31.41 ± 0.31 mg GAE/g E to phenolic compound, 6.81 ± 1.72 mg R/g E to flavonoids and 8.29 ± 0.3 mg GAE/g E to hydrolyzable tannin (Table 1). For WA extract; 5.34 ± 0.21 mg GAE/g E to total phenolic compound, 29.56 ± 1.56 mg R/g E to flavonoids and 4.05 ± 0.52 mg GAE/g E to hydrolyzable tannin (Table 1).
Table 1.
Extract yield and total flavonoids, phenolic and tannin contents from Urtica urens extracts.
| Sample | Yield (%) | Flavonoids (mg quercetin/g DW) | Phenolics (mg GAE/g DW) | Tannins (mg GAE/g DW) |
|---|---|---|---|---|
| Ethanol | 4.768 | 6.81 ± 1.72 | 31.41 ± 0.31 | 8.29 ± 0.3 |
| Aqueous | 0.87 | 5.34 ± 0.21* | 29.56 ± 1.56* | 4.05 ± 0.52* |
Values are expressed as mean ± SEM of three independent determinations. *p < 0.05.
DW: dry weight.
Antioxidant capacity by DPPH• method
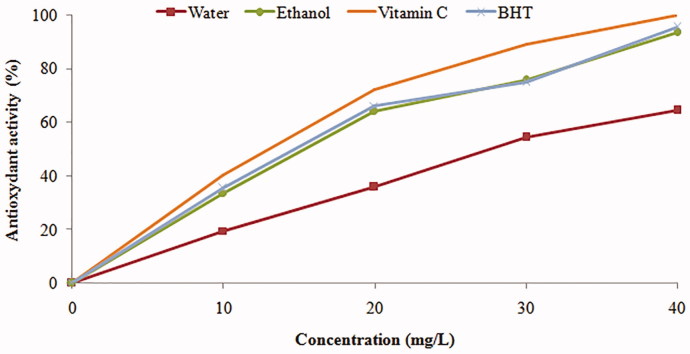
The DPPH method has been widely applied for estimating antioxidant activity in recent years. DPPH, a stable free radical with a purple colour, changes into a stable yellow compound upon reacting with an antioxidant. In brief, the reduction capacity of DPPH was determined by the decrease in its absorbance at 517 nm, which is reduced by the antioxidant (Duh 1998). As can be seen in Figure 1, the results clearly indicated that ethanol extract, which contained the highest amount of phenolics and flavonoids compounds, was the strongest radical scavenger (IC50 = 245.65 ± 10.2 μg/mL). The WA extract displayed free radical scavenging activity (IC50 = 142.94 ± 10.54 μg/mL) (Table 2).
Figure 1.
Antioxidant capacity of ethanol and aqueous extracts of Urtica urens by the DPPH• method at different concentrations. BHT and ascorbic acid (Vitamin C) were used as positive control. Values are means ± SEM (n = 3).
Table 2.
Antioxidant activity of Urtica urens extracts (ABTS•+, DPPH•).
| Sample | DPPH• scavenging (IC50, μg/mL) | ABTS•+ scavenging (IC50, μg/mL) | DPPH• scavenging (mg TE/g extract) | ABTS•+ scavenging (mg TE/g extract) |
|---|---|---|---|---|
| Ethanol | 245.65 ± 10.2 | 30.88 ± 3.03 | 65.33 ± 10.72 | 560.33 ± 29.45 |
| Aqueous | 142.94 ± 10.54*** | 14.65 ± 1.09*** | 45.67 ± 10.21*** | 350.33 ± 18.73*** |
Values are expressed as mean ± SEM of three independent determinations. ***p < 0.001.
TE: Trolox equivalents; IC: Inhibition Concentration.
The highest inhibition effect of WA and EtOH extracts of Urtica urens were 64.56 and 93.56%, respectively, compared with ascorbic acid with 99.83% and BHT with 95.56% at concentration of 40 mg/L. However, in our results further increase in the concentration of the Urtica urens extracts indicated increasing inhibition of DPPH radical (Figure 1).
Antioxidant capacity by the method of ABTS•+
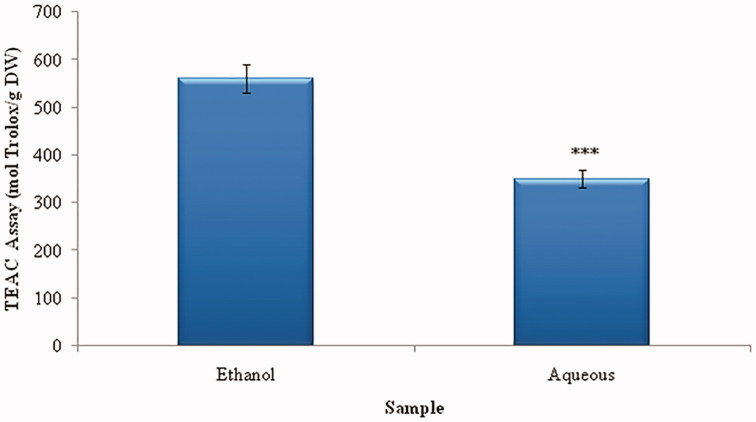
The free radical scavenging ability of Urtica urens phenolics was determined using ABTS radical cation, too. ABTS radical cation has often been used in the evaluation of antioxidant activity of single compounds and complex mixtures of various origins (body fluids, foods, beverages, plant extracts). In this assay, ABTS radical cation was directly generated in stable form using potassium persulfate. Generation of radicals before the antioxidants are added to prevent the interference of compounds, which affect radical formation. This modification makes the assay less susceptible to artifacts and inhibits overestimation of antioxidant capacity. EtOH extract had an activity of 560.33 ± 29.45 mol Trolox/g DW versus WA extract had much less activity compared to EtOH, 350.33 ± 18.73 mol Trolox/g DW (Figure 2).
Figure 2.
ABTS (TEAC) activities of Urtica urens extracts. Values are means ± SEM (n = 3). ***p < 0.001.
β-carotene bleaching by linoleic acid assay
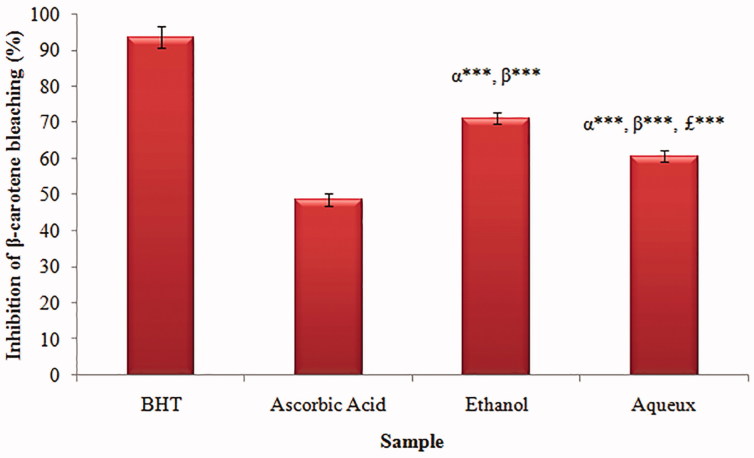
The β-carotene scavenging activity of the two extracts at different concentrations was compared with that of butylated hydroxytoluene (BHT), as shown in Figure 3. In this study, the addition of Urtica urens extracts and BHT prevented bleaching of the β-carotene at different degrees. The WA and EtOH extracts of Urtica urens demonstrated high ability to prevent bleaching of the β-carotene. Interestingly, they exhibited a strong inhibition of the β-carotene bleaching up to 71.12% and 60.52%, respectively, at concentrations up to 500 μg/mL.
Figure 3.
β-Carotene bleaching percentage of Urtica urens extracts. Values are means ± SEM (n = 3). ***p < 0.001. α: compared to BHT (control); β: compared to Ascorbic Acid; £: compared to Ethanol.
Ferric reducing antioxidant power ability
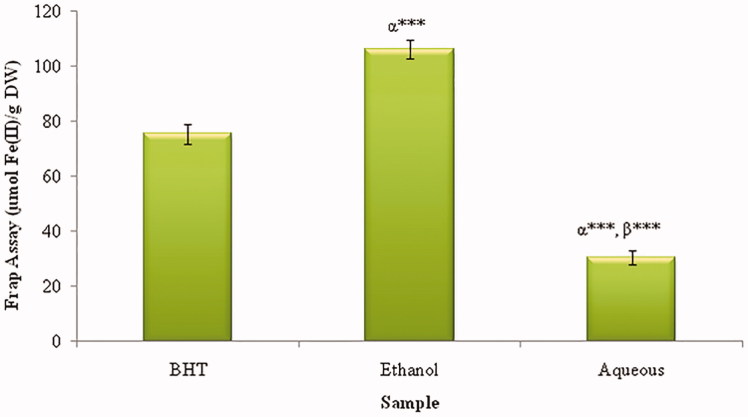
In this study, the ferric reducing power of plant extracts is dose-dependent as illustrated in Figure 4. The strongest activity of the reducing power is exhibited by the EtOH extract (106.23 ± 3.45 μmol Fe (II)/g DW). This is followed by WA extract (30.55 ± 2.67 μmol Fe(II)/g DW). This activity of the aqueous extracts is significantly lower than the EtOH and BHT (75.56 ± 3.57 μmol Fe (II)/g DW).
Figure 4.
FRAP activities in ethanol and water extracts of Urtica urens. Values represent means ± SEM (n = 3). ***p < 0.001. α: compared to BHT; β: compared to Ethanol.
Antibacterial activity
The antibacterial activity of the various Urtica urens extracts was assessed against Gram-positive (B. subtilis, S. epidermidis, S. aureus, M. luteus and E. faecalis) and Gram-negative (E. coli, P. aeruginosa and S. enteritidis) bacteria. The evaluation of the antibacterial activity was realized by the determination of MIC and MBC values. Table 3 reveal that all extracts vary the degrees of antibacterial activity against most of the tested Gram-positive and Gram-negative bacteria, and the EtOH extract was found to be the most effective. In fact, it displayed a large antimicrobial spectrum and exerted a major antibacterial effect against both tested Gram-positive and Gram-negative bacteria. While the most vulnerable bacteria for the EtOH extract were for Gram-positive (Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus and Staphylococcus epidermidis), and two Gram-negative bacteria (Salmonella enteritidis and Pseudomonas aeruginosa) at concentration of 150 μg/mL. However, the WA extract did not show any effect on almost all the tested bacteria.
Table 3.
Rapport MBC/MIC.
| Ethanol |
Aqueous | |||
|---|---|---|---|---|
| Stains | MBC/MIC | MBC/MIC | ||
| Gram+ | Bacillus subtilis | 2 | bactericidal | – |
| Staphylococcus aureus | 2 | bactericidal | – | |
| Micrococcus luteus | 2.01 | bactericidal | – | |
| Staphylococcus epidermidis | 2 | bactericidal | – | |
| Enterococcus faecalis | – | – | – | |
| Gram− | Escherichia coli | – | – | – |
| Salmonella enteritidis | 1.93 | bactericidal | – | |
| Pseudomonas aeruginosa | 2 | bactericidal | – | |
| Rapport MBC/MIC Canillac & Mourey 2001 | MBC/MIC ≥4 | bacteriostatic | ||
| MBC/MIC <4 | bactericidal | |||
Vitamins D, E and C
In our results (Table 4), we noted that the concentrations of vitamin D, vitamin C and E were 1.45 ± 0.14 mg/100 g; 238 ± 2.95 mg/100 g; 356 ± 0.15 mg/100 g, respectively, in EtOH extract of Urtica urens and of water extract 0.23 ± 0.04; 160.55 ± 3.09; 2.3 ± 0.01 mg/100 g, respectively.
Table 4.
Levels of vitamins D, C and E in Urtica urens leaves.
| Sample | Vitamin D (mg/100 g) | Vitamin C (mg/100 g) | Vitamin E (mg/100 g) |
|---|---|---|---|
| Ethanol | 1.45 ± 0.14 | 238 ± 2.95 | 356 ± 0.15 |
| Aqueous | 0.23 ± 0.04 | 160.55 ± 3.09α*** | 2.3 ± 0.01α*** |
Values are expressed as mean ± SEM of three independent determinations. ***p < 0.001.
α: compared to Ethanol.
Discussion
The total polyphenol, flavonoid and tannin content in the EtOH and WA extracts were determined. It is well known that phenolic and flavonoid compounds contribute directly to the biological activity of plant materials (Rice-Evans et al. 1996).
With 4.768% of yield in the EtOH extract, the results of the phytochemical analysis exhibited the quantitative values of Urtica urens 31.41 ± 0.31 mg GAE/g E to phenolic compound, 6.81 ± 1.72 mg R/g E to flavonoids and 8.29 ± 0.3 mg GAE/g E to hydrolyzable tannin. These results demonstrate that the Urtica urens has high quantities of phenolic compounds. Other studies performing quantification of the phenolic compounds and flavonoids in EtOH extract of leaves showed their presence in large quantity as compared with our results for the phenolic compounds and flavonoids (Manu Kumar et al. 2013).
The content of phenolic compounds found in plants may vary during processing steps such as growing, harvesting, storage and technological procedures used (Lombardo et al. 2010). The phenolic compounds present in plants have received great attention because of their antioxidant properties and they can potentially interact with biological systems and play an important role in anticancer, anti-inflammatory, and antimicrobial activity (Wang et al. 2003; Abu-Reidah et al. 2013). The antioxidant properties are attributed to flavonoids due to their hydroxyl groups that can act as free radical scavengers, reducing agents and metal chelation (Agati et al. 2012). It has been reported that free radical-scavenging activity is greatly influenced by the phenolic composition of the sample (Cheung et al. 2003). The antioxidant activity of the WA and EtOH extracts may be attributed to their phenolic and flavonoid contents.
The antiradical activity assay is based on the reduction of 1,1-diphenyl-2-picrylhydrazyl (DPPH). The ability of the extracts and standard ascorbic acid to scavenge free radicals and pair off the odd electron was shown in this assay. It was observed that WA and EtOH extracts of Urtica urens are a similar scavenger of DPPH radical compared with the standard BHT. The highest inhibition effects of WA and EtOH extracts of Urtica urens were 64.56% and 93.56%, respectively, compared with ascorbic acid with 99.83% and BHT with 95.56% at concentration of 40 mg/L. However, in our results, further increase in the concentration of the Urtica urens extracts displayed increasing inhibition of DPPH radical.
According to Manu Kumar et al. (2013), using DPPH to measure the results of radical scavenging activities, Urtica urens leaf extract by methanol showed higher activity (78% at 0.5 mg/mL) among the other solvents and ethyl acetate displayed lower activity compared to ethanol, water and acetone.
As is well known, the antioxidant activity of plant extracts containing polyphenol components is assigned to their capacity to be donors of hydrogen atoms or electrons and to capture the free radicals (Shon et al. 2003). The DPPH radical-scavenging assay is a widely used method to evaluate the ability of plant extracts to scavenge free radicals generated from DPPH reagent (Chung et al. 2006).
Like DPPH•, the method of ABTS•+ or TEAC (Trolox Equivalent Antioxidant Capacity) is widely used to evaluate the antioxidant capacity of a variety of substances including plant extracts.
The TEAC value takes into account the capacity of a substance to react with ABTS•+ radical (Arts et al. 2004). The water extract caused a greater attenuation of ABTS•+ than DPPH•. This could be attributed to the fact that the ABTS•+ assay is aqueous based, favouring hydrophilic compounds; whereas the DPPH• assay is an organic based assay, favouring hydrophobic phytoconstituents (Schlesier et al. 2002).
On the other hand, EtOH extract was ineffective regarding cellular-ROS neutralization. Instead, it caused a significant overproduction of intracellular ROS. It has been shown that Urtica urens can have an antioxidant or pro-oxidant effect depending on their concentration and/or structural properties.
The antioxidant activity increased with increasing extract concentration. The EtOH extract of Urtica urens displayed a significant (p < 0.001) cell-free (IC50 = 30.88 ± 3.03 μg/mL of ABTS•+) as comparison with WA extract (Table 2). This could be ascribed to patuletin, an abundant and potent aglycon flavonol extracted from Urtica urens (Ataa et al. 1995). This phytochemical is lipophilic and readily crosses the cell membrane, making it easily available in the cytosol to exert its protective antioxidant action (Abdel-Wahhab et al. 2005).
The β-carotene bleaching method is based on the loss of the orange colour of β-carotene due to its reaction with radicals formed by linoleic acid oxidation in an emulsion. The rate of β-carotene bleaching can be slowed down in the presence of antioxidants (Kulisic et al. 2004). Interestingly, the WA and EtOH extracts exhibited strong inhibition of β-carotene bleaching comparable to BHT and ascorbic acid. The control, without addition of sample, oxidized rapidly and the absorbance at 470 nm tends to zero. Therefore, the higher antioxidant activity of compounds from the Urtica urens extracts in this assay suggests a possible biological functionality in preventing the oxidative degradation of membrane lipids.
In this study, we used FRAP assay because it is quick and simple to perform, and its reaction is reproducible and linearly related to the molar concentration of the antioxidant(s) present. This method was initially developed to assay plasma antioxidant capacity, but could be used to measure the antioxidant capacity from a wide range of biological samples and pure compounds to fruits, wines, and animal tissues. In our study, the EtOH extract had higher ferrous reducing antioxidant power compared to standard BHT which had 75.56 ± 3.57 μmol Fe (II)/g DW (Figure 4). FRAP assay demonstrated that EtOH extract had higher activity (106.23 ± 3.45 μmol Fe (II)/g DW) than that of BHT. Based on this background, this study concludes that Urtica urens is considered as a potential source of natural antioxidants. The presence of general phytochemicals and specific active compounds might be responsible for their therapeutic effects. Their reducing power is probably attributed to the presence of polyphenols that may act in a similar way as reductones by donating the electrons and reacting with free radicals to convert them into more stable products and terminate the free radical chain reaction (Siddhuraju & Becker 2007). Reductones also react with certain precursors of peroxide, thus preventing the formation of the latter (Matsushige et al. 1996). The antioxidant activity has confirmed the medicinal importance of plants as naturally-occurring antioxidants (Venkatachalam & Muthukrishnan 2012).
According to Manu Kumar et al. (2013), FRAP assay exhibited ethanol fraction activity of 80 μmol Fe(II)/g DW. The antibacterial activity of Urtica urens extracts was evaluated against Gram-positive (B. subtilis, S. epidermidis, S. aureus, M. luteus and E. faecalis) and Gram-negative (E. coli, P. aeruginosa and S. enteritidis) bacteria. These strains are available in the laboratory. The antibacterial activity was assessed by the determination of MIC and the minimal bactericidal concentration (MBC) (the minimum concentration that inhibits all visible growth of microorganisms for 48 h at 37 °C) (Diao et al. 2014). As can be seen in Table 3, Urtica urens showed varying degrees of antibacterial activity against most of the Gram-positive and Gram-negative bacteria tested. The aqueous extract was found to be not active against all tested bacteria. Ethanol extract exhibited antibacterial activity (bactericidal) against Gram-positive (Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus and Staphylococcus epidermidis), and two Gram-negative bacteria (Salmonella enteritidis and Pseudomonas aeruginosa) at concentration of 150 μg/mL.
The reason for the difference in sensitivity between Gram-positive and Gram-negative bacteria might be ascribed to the differences in morphological constitutions between these microorganisms; Gram-negative bacteria having an outer phospholipidic membrane carrying the structural lipopolysaccharide components. This makes the cell wall impermeable to antimicrobial chemical substances. On the other hand, Gram-positive bacteria are more susceptible, having only an outer peptidoglycan layer which is not an effective permeability barrier. Therefore, the cell walls of Gram-negative organisms are more complex in lay out than Gram-positive ones acting as a diffusional barrier and making them less susceptible to the antimicrobial agents than Gram-positive bacteria (Kaushik et al. 2015).
The most susceptible bacteria for the EtOH extract were S. aureus, M. luteus, S. enteritidis, S. epidermidis, P. aeruginosa and B. subtilis with a rapport MBC/MIC values of 2, 2.01, 1.93, 2 and 2, respectively.
These results are of great importance, particularly in the case of S. aureus which is well-known for being resistant to a number of antibiotics and for the production of several types of enterotoxins that cause many enteritis types and septicemia (Kaushik et al. 2015). P. aeruginosa is a naturally resistant Gram-negative bacteria that causes various infections in humans, and is rather insusceptible to herbal extracts (Kukric et al. 2012). Interestingly, EtOH extract showed antibacterial activity against these bacteria.
These results are in agreement with the report of Steenkamp et al. (2004) who confirmed that the aqueous extract have no activity against Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli and Pseudomonas aeruginosa at concentration of 100 μg/mL. However, they reported no inhibitory activity. Moreover, according to Kaushik et al. (2015) WA extract of Urtica urens did not show any activity against the three tested microorganisms (Staphylococcus aureus, Candida albicans, Escherichia coli).
The antibacterial activity of EtOH extract of Urtica urens were apparently related to its terpenes and flavonoids type components, respectively, since phytochemical analysis revealed that these extracts had violet and yellow-orange spots indicating possible presence of triterpenes and flavonoids, respectively. These results support the fact that Urtica urens, investigated in this study, also displayed antibacterial activity.
Valuable plant source of vitamins is common nettle which is used in traditional and officinal medicine as haemostatic and vitamin substances (Dar et al. 2013). The nettle leaves are utilized mostly for the preparation of infusions. Nettle leaves contain ascorbic acid (270 mg/100 g), carotenoids (50 mg/100 g), vitamins B, D (200 mg/100 g) and E.
It was established that leaves and aqueous extract of Urtica dioica contain 36.402 ± 0.017 mg/100 g and 0.032 ± 0.001 mg/100 g of vitamin C, respectively (Tatyana & Valentina 2013). According to Daovy (2009), Urtica is a crucial source of vitamin C (20 to 60 mg/100 g), vitamins B1, B2, B5, B9, E and D. From our results, Urtica urens extracts are a substantial source of vitamins C, E and D.
Conclusion
The results of this study concluded that the leaves of Urtica urens contain appreciable number of flavonoids, polyphenols and tannins. The extracts seem to present a real interest and potential through their antioxidant activities that have been made by the different tests (DPPH, ABTS, β-carotene and FRAP).
Their antioxidant activities further lend credence to the biological value of this plant. The EtOH extract shows activity against bacteria. This may be due to high phenolic content and the presence of active compounds such as tannins.
Funding Statement
This research was supported by the Tunisian Ministry of Higher Education and Scientific Research via Sfax University.
Acknowledgements
This research was supported by the Tunisian Ministry of Higher Education and Scientific Research.
Disclosure statement
The authors declare no conflict of interest.
References
- Abdel-Wahhab MA, Said A, Huefner A.. 2005. NMR and radical scavenging activities of patuletin from Urtica urens against aflatoxin B1. Pharm Biol. 43:515–525. [Google Scholar]
- Abu-Reidah IM, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A.. 2013. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC-DAD-ESI-QTOF-MS. Food Chem. 141:2269–2277. [DOI] [PubMed] [Google Scholar]
- Adamski R, Bieganska J.. 1980. Studies of chemical substances present in Urtica dioica leaves. Part I, Trace element. Herba Polonica. 26:177–180. [Google Scholar]
- Agati G, Azzarello E, Pollastri S, Tattini M.. 2012. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 196:67–76. [DOI] [PubMed] [Google Scholar]
- Arts MJTJ, Sebastiaan Dallinga J, Voss HP, Haenen GRMM, Bast A.. 2004. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 88:567–570. [Google Scholar]
- Ataa S, Wafaa E, Youssry A.. 1995. Flavonoids of Urtica urens L. and biological evaluation. Egypt J Pharm Sci. 36:415–427. [Google Scholar]
- Benkeblia N. 2004. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT-Food Sci Technol. 37:263–268. [Google Scholar]
- Benzie IFF, Strain JJ.. 1999. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 299:15–27. [DOI] [PubMed] [Google Scholar]
- Canillac N, Mourey A.. 2001. Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Food Microbiol. 18:261–268. [Google Scholar]
- Cheung LM, Cheung PCK, Ooi VEC.. 2003. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 81:249–255. [Google Scholar]
- Chung YC, Chien CT, Teng KY, Chou ST.. 2006. Antioxidative and mutagenic properties of Zanthoxylum ailanthoides Sieb & Zucc. Food Chem. 97:418–425. [Google Scholar]
- Correa SC, Rezznde ML, Ferreira EB, Azevedo L.. 2013. Marolo (Annona crassiflora Mart.): a study of value chain and processing. Food Sci Technol Campinas. 33:362–368. [Google Scholar]
- Daovy A. 2009. L’ortie dioïque. Actualities Pharmaceutiques. 490:53–55. [Google Scholar]
- Dar SA, Ganai FA, Yousuf AR, Balkhi MU, Bhat TM, Sharma P.. 2013. Pharmacological and toxicological evaluation of Urtica dioica L. Pharm Biol. 51:170–180. [DOI] [PubMed] [Google Scholar]
- Diao WR, Hua QP, Zhang H, Xu JG.. 2014. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill). Food Control. 35:109–116. [Google Scholar]
- Duh PD. 1998. Antioxidant activity of Burdock (Arctium lappa Linne): its scavenging effect on free-radical and active oxygen. J Am Oil Chem Soc. 75:455–461. [Google Scholar]
- Guil-Guerrero JL, Rebolloso-Fuentes MM, Torija-Isasa ME.. 2003. Fatty acids and carotenoids from stinging nettle (Urtica dioica L). J Food Compos Anal. 16:111–119. [Google Scholar]
- Gulcin I, Kufrevioglu OI, Oktay M, Buyukokuroglu ME.. 2004. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 90:205–215. [DOI] [PubMed] [Google Scholar]
- Hughes RE, Ellery P, Harry T, Jenkins V, Jones E.. 1980. The dietary potential of the common nettle. J Sci Food Agric. 31:1279–1286. [DOI] [PubMed] [Google Scholar]
- Jacques-Silva MC, Nogueira CW, Broch LC, Flores EM, Rocha JB.. 2001. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol. 88:119–125. [DOI] [PubMed] [Google Scholar]
- Katsanidis E, Addis PB.. 1999. Novel HPLC analysis of tocopherols, tocotrienols, and cholesterol in tissue. Free Radic Biol Med. 7 :1137–11340. [DOI] [PubMed] [Google Scholar]
- Kaushik A, Aron A, Mihreteab S, Mohamedkassm N, Michael Kidane E.. 2015. Phytochemical screening and antimicrobial activity of medicinal plants used by eritrean traditional healers. World J Pharm Res. 4:501–509. [Google Scholar]
- Kirby AJ, Schmidt RJ.. 1997. The antioxidant activity of Chinese herbs for eczema and of placebo herbs-I. J Ethnopharmacol. 56:103–108. [DOI] [PubMed] [Google Scholar]
- Koleva II, van Beek TA, Linssen JPH, de Groot A, Evstatieva LN.. 2002. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 13:18–17. [DOI] [PubMed] [Google Scholar]
- Kukric ZZ, Topalic-Trivunovic LN, Kukavica BM, Matoš SB, Pavičic SS, Boroja MM, Savič AV.. 2012. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Apteff. 43:259–272. [Google Scholar]
- Kulisic T, Radonic A, Katalinic V, Milos M.. 2004. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 85:633–640. [Google Scholar]
- Leonti M. 2012. The co-evolutionary perspective of the food-medicine continuum and wild gathered and cultivated vegetables. Genet Resour Crop Evol. 59:1295–1302. [Google Scholar]
- Lombardo S, Pandino G, Mauromicale G, Knödler M, Carle R, Schieber A.. 2010. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 119:1175–1181. [Google Scholar]
- Manu Kumar HM, Prathima VR, Sowmya S, Thribhuvan KR.. 2013. Study of nutritional quality, phytochemical constituents and antioxidant activities by different solvents of nettle (Urtica urens) from madikeri-karnataka state. Int Res J Pharm App Sci. 3:112–119. [Google Scholar]
- Matsushige K, Basnel P, Kadola S, Namba T.. 1996. Potent free radicals scavenging of dicaffeoyl quinic acid derivatives from propolis. J Trad Med. 13:217–228. [Google Scholar]
- Nassiri-Asl M, Zamansoltani F, Abbasi E, Daneshi MM, Zangivand AA.. 2009. Effects of Urtica dioica extract on lipid profile in hypercholesterolemic rats. J Chin Integr Med. 7:428–433. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C.. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G.. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Radic Biol Med. 20:933–956. [DOI] [PubMed] [Google Scholar]
- Saad H, Bouhtoury FC, Pizzi A, Rode K, Charrier B, Ayed N.. 2012. Characterization of pomegranate peels tannin extractives. Ind Crop Prod. 40:239–246. [Google Scholar]
- Schlesier K, Harwat M, Böhm V, Bitsch R.. 2002. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 36:177–187. [DOI] [PubMed] [Google Scholar]
- Shon MY, Kim TH, Sung NJ.. 2003. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetoceae) extracts. Food Chem. 82:593–597. [Google Scholar]
- Sidaoui F, Belghith Igueld S, Barth D, Trabelsi-Ayadi M, Cherif JK.. 2015. Study of Tunisian nettle leaves (Urtica dioica L.): mineral composition and antioxidant capacity of their extracts obtained by maceration and supercritical fluid extraction. Ijppr. 7:707–713. [Google Scholar]
- Siddhuraju P, Becker K.. 2007. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 101:10–19. [Google Scholar]
- Singleton VL, Rossi JA.. 1965. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic. 16:144–158. [Google Scholar]
- Steenkamp V, Mathivha E, Gouws MC, van Rensburg CE.. 2004. Studies on antibacterial, antioxidant and fibroblast growth stimulation of wound healing remedies from South Africa. J Ethnopharmacol. 95:353–357. [DOI] [PubMed] [Google Scholar]
- Tatyana AS, Valentina OR.. 2013. Leaves of common nettle (Urtica dioica L.) as a source of ascorbic acid (vitamin C). World Appl Sci J. 28:250–253. [Google Scholar]
- Testai L, Chericoni S, Calderone V, Nencioni G, Nieri P, Morelli I, Martinotti E.. 2002. Cardiovascular effects of Urtica dioica L. (Urticaceae) roots extracts: in vitro and in vivo pharmacological studies. J Ethnopharmacol. 81:105–109. [DOI] [PubMed] [Google Scholar]
- Upton R. 2013. Stinging nettles leaf (Urtica dioica L.): extraordinary vegetable medicine. J Herb Med. 3:39–38. [Google Scholar]
- Vanden Berghe DA, Vlietinck AJ.. 1991. Screening methods for antibacterial and antiviral agents from higher plants. Meth Plant Biochem. 6:47–68. [Google Scholar]
- Venkatachalam U, Muthukrishnan S.. 2012. Free radical scavenging activity of ethanolic extract of Desmodium gangeticum . J Acute Med. 2:36–42. [Google Scholar]
- Wang M, Simon JE, Aviles IF, He K, Zheng QY, Tadmor Y.. 2003. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J Agric Food Chem. 51:601–608. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W.. 1999. The determination of flavonoids contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559. [Google Scholar]