Abstract
Context: The search for bioactive compounds from botanical sources is attracting much interest. However, differences in chemical composition may occur within the same species depending on different geographical origins.
Objectives: We evaluated the properties on skin enzymes and cells of extracts from sulla legume crop Hedysarum coronarium L. (Fabaceae), collected at two Italian sites near Pisa and Ventimiglia, for possible dermatological and cosmetic applications.
Material and methods: Plant aerial portions were extracted in MTBE/ethyl acetate/acetone, obtaining two extracts named Pisa sulla extract (PSE) and Ventimiglia sulla extract (VSE). Extracts were subjected to chemical characterization, LC-MS/MS analysis and biological assays.
Results: PSE showed stronger antiradical scavenging and higher phenolic and flavonoid contents with respect to VSE. LC-MS/MS analysis revealed similar composition for the two extracts, but PSE was richer in condensed tannins and flavonoids, principally rhoifolin, quercetin, naringenin and derivatives. PSE induced stronger inhibition on collagenase and elastase by in vitro enzyme assays, possibly due to higher levels of condensed tannins and quercetin. ELISA bioassay on human dermal fibroblasts revealed stronger PSE induction of collagen production. Determination of glycerol release from adipocytes disclosed stronger stimulation of lipolysis by PSE, allegedly ascribed to higher charge of quercetin and derivatives. In summary, the higher richness in phenolics of PSE is strictly related to stronger bioactivity.
Discussion and conclusions: Data indicate that aerial H. coronarium material is suitable for the development of dermatological and cosmeceutical products, but the geographical origin is an important factor for maximally exploiting the biological properties of this species.
Keywords: 46BR.1N fibroblasts, adipocytes, collagenase, condensed tannins, elastase, flavonoids
Introduction
The search for bioactive secondary metabolites from botanical sources for health care purposes is attracting an ever-increasing interest due to the huge chemodiversity of plants. Such a tendency is further supported by the idea that many natural compounds may be more biocompatible and involve less adverse effects than synthetic or toxic drugs. However, significant differences in chemical composition may occur among closely related taxonomical entities, such as species, subspecies or varieties, but also within these taxonomical entities, depending on different geographical origins (Carmona et al. 2007). The variability in the phytocomplex of a plant species can be characterized in terms of chemotypes, suggesting that the geographical origin of plant materials is to be carefully considered in the search for new bioactive compounds (Kaiser et al. 2016).
In a survey of legume crops scarcely investigated for health and skin care potential (Pastorino et al. 2017), we focused here on Hedysarum coronarium L. [syn. Sulla coronaria (L.) Medik] (Fabaceae), commonly known as sulla or French honeysuckle. This is a short-lived, perennial legume present as both wild herb and cultivated forage throughout the Mediterranean (Annicchiarico et al. 2014). Aerial portions of the plant are known to contain good levels of protein, as well as condensed tannins ranging at about 20–50 mg/kg (Terrill et al. 1992). Phenolics reported to be present in this species include glycosylated derivatives of the flavonoids kaempferol, quercetin, genistein, formononetin and afromosin, and the anthocyanins peonidin 3-monoglucoside, peonidin 3,5-diglucoside and malvidin 3,5-diglucoside, as flower pigments (Chiki and Harborne 1983; Tibe et al. 2011).
The high content in tannins of sulla is thought to increase the performance of meat and milk in dairy sheep and cattle feeding on the plant, and in addition to induce resistance to gastrointestinal parasites (Molle et al. 2003; Hoste et al. 2006). Anthelmintic activity has been confirmed by in vitro experiments (Aïssa et al. 2015). Besides veterinary uses, sulla has also been consumed by humans as a vitaminic vegetable and as an astringent, anti-hypercholesterolaemic, laxative, refreshing and soothing herb (Lentini and Venza 2007). Scientific studies on medicinal properties are lacking, but its chemical composition rich in phenolic compounds suggests that the plant deserves attention for possible curative effects.
Our study was conducted on aerial portions of sulla collected at two different sites, located nearby the cities of Pisa and Ventimiglia. Pisa is in central Italy, well inside the geographic range of the species, while Ventimiglia is in western Italy, close to the boundary of the species range. Plant extracts obtained in methyl tert-butyl ether/ethyl acetate/acetone were chemically characterized by LC-MS and assayed for antioxidant power, total phenolics and flavonoid contents. Extracts were used for in vitro bioassays aimed at assessing modulatory effects on extracellular matrix turnover and on lipolysis. Positive correlation between phenolic content and bioactivity strength was observed.
Materials and methods
Materials
Cell culture reagents and other chemicals were from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated. Plant specimens of Hedysarum coronarium were collected in May 2015, during the flowering period, from two wild Italian populations, growing at the Hanbury Botanical Gardens, La Mortola, Ventimiglia (43°46′57″N 07°33′20″E), and in the southern surroundings of Pisa (43°41′32″N, 10°23′06″E). Aerial portions were cleaned, dried at air, and used for extraction procedure. Taxonomical identification was carried out by one of us (LC) and voucher specimens of plants from both geographical sites were deposited at the Herbarium of DISTAV, University of Genova (GE 20052015).
Extraction
Specimens of H. coronarium were air-dried in greenhouse at ∼25–30 °C. Aerial portions of dried material were cleaned, chopped and extracted according to Tibe et al. (2011). The dried material (25 g) was put in a beaker containing 100 mL methyl tert-butyl ether (MTBE), 70 mL ethyl acetate, 60 mL acetone and 2 mL distilled water at RT, sonicated for 15 min, cloth filtered, transferred to a Buchi Rotavapor R-114 (Buchi Italia s.r.l.) to remove organic solvents and then dried under nitrogen stream. Dried extracts were stored at −20 °C until use. For biological assays, dimethyl sulphoxide (DMSO) stock solutions of extracts were prepared in order to obtain a maximum DMSO concentration of 0.1% (v/v) at the highest extract incubation doses.
Radical scavenging assay
Radical scavenging activity of extracts was quantified by the DPPH (2,2-diphenyl-1-picrilidazide) assay, using the method described by Chung et al. (2002). Briefly, a volume of 0.5 mL DPPH dissolved in EtOH (40 μg/mL) was mixed with 0.5 mL of serial dilutions of extracts in EtOH, incubated for 30 min in the dark at RT and then read in an Agilent Cary 60 spectrophotometer (Agilent Technologies, Palo Alto, CA) at 517 nm. EtOH without DPPH was used as blank. Results were expressed as percent DPPH inhibition.
Quantification of total phenolics
The total phenolic content of extracts was determined by the common Folin–Ciocalteu method. Stock solutions were prepared by dissolving gallic acid or extracts in EtOH at 5 mg/mL. Aliquots of 2.4 mL distilled water, 0.15 mL Folin–Ciocalteu reagent and 0.45 mL Na2CO3 (20% in distilled water) were combined in 3 mL cuvettes with 30 μL of serial water dilutions of gallic acid or extract stock solutions. The contents of the cuvettes were mixed, incubated at 40 °C for 30 min and read at 765 nm in the spectrophotometer. A standard curve was obtained from gallic acid samples and total phenolic contents were expressed as mg gallic acid equivalents (GAE) per g of extract.
Determination of total flavonoids
The total flavonoid content of extracts was determined according to Zhishen et al. (1999). Stock solutions were prepared by dissolving quercetin or extracts in EtOH as above. Aliquots of 100 μL of serial water dilutions of extract stock solutions were placed in cuvettes with 6 μL of aqueous NaNO2 (1:20, w/v). After 5 min incubation, 6 μL of aqueous AlCl3 (1:10, w/v) was added and the mixture was vortexed, and then, after 1 min, 40 μL of 1 M NaOH was added. The absorbance was measured against a blank at 510 nm. A calibration curve was prepared using proper dilutions of a quercetin stock solution, and the total flavonoid content of samples was given as g quercetin equivalent per g of plant extract.
HPLC-MS
HPLC coupled with mass spectrometry analysis (HPLC-MS/MS) was performed using an Agilent 1100 HPLC-MSD Ion Trap XCT system, equipped with an electrospray ion source (HPLC-ESI-MS) (Agilent Technologies). Separations of extracts were performed on a Symmetry C18 column 1 × 150 mm with 3 μm particle size (Waters Corporation, Milford, MA). Eluents used were water (eluent A) and MeOH (eluent B), both added with 0.1% formic acid. The gradient employed was as follows: 50% eluent B for 3 min, then linear to 95% eluent B in 25 min and finally hold at 95% eluent B for other 15 min. The flow rate was set to 30 μL/min and the column temperature was set at 25 °C. The injection volume was 8 μL. Ions were detected in the positive and negative ion mode, in the 200–1000 m/z range and ion charged control with a target ion value of 200,000 and an accumulation time of 300 msec. A capillary voltage of 3300 V, nebulizer pressure of 15 psi, drying gas of 8 L/min, dry temperature of 325 °C and rolling averages 2 (averages: 5) were the parameters set for the MS detection. MS/MS analysis was conducted using amplitude optimized time by time for each compound.
Enzyme assays
Enzymatic assays were conducted in 96-well plates according to a previously described method (Pastorino et al. 2017). Collagenase (EC 3.4.24.3) inhibition was evaluated in a reaction mixture containing 50 mM tricine, pH 7.5, 10 mM CaCl2, 400 mM NaCl, 0.8 mM FALGPA (Sigma-Aldrich, F5135) as the enzyme substrate, 0.16 units/mL collagenase from Clostridium histolyticum (Sigma-Aldrich, C0130) and serial water dilutions of extract stock solutions in DMSO, as indicated. The mixture was incubated for 10 min at RT, and then plates were read at 345 nm in a Tecan Genios Pro plate reader (Tecan, Wien, Austria).
Elastase (EC 3.4.21.36) inhibition was measured in a mix containing 200 mM TRIS, pH 8.0, 10 mM Suc-Ala3-pNA (Sigma-Aldrich, S4760) as the enzyme substrate, 2 units/mL of elastase from porcine pancreas (Sigma-Aldrich, E1250) and extracts as above. After 15 min incubation, plates were read at 410 nm in the plate reader.
Cell culture
Stabilized human dermal fibroblasts (46BR.1N, Sigma-Aldrich) were grown in DMEM supplemented with 10% (v/v) FBS, 1% glutamine and 1% antibiotic, at 37 °C, in a 5% CO2, humidified atmosphere. Subcutaneous human preadipocytes (Zen-Bio Inc., Research Triangle Park, NC) were grown in preadipocyte medium (cat# PM-1, Zen-Bio Inc.) according to the manufacturer’s protocol.
Cell viability and proliferation assays
Effects of extracts on cell viability were determined on fibroblasts by the MTT assay, as previously reported by Pastorino et al. (2017). Cells were settled in 96-well plates, exposed for 48 h to increasing concentrations of extracts (1–1000 μg/mL), processed for MTT assay and read at 570 nm in a VMax microplate reader (Molecular Devices, Sunnyvale, CA). Absorbance values were used to obtain dose–response curves and IC50 values.
Collagen production
The effect of extracts on collagen type I production was evaluated by ELISA technique as previously reported (Pastorino et al. 2017). Briefly, fibroblasts were settled in 96-well plates, incubated with extracts for 48 h, fixed with 3.7% paraformaldehyde, blocked with BSA, probed with mouse anti-human collagen type I primary antibody (ab6308, Abcam, Cambridge, UK), then probed with HRP-conjugated rabbit anti-mouse IgG secondary antibody (ab97046, Abcam), incubated with Pierce 1-Step™ Ultra TMB ELISA Substrate Solution (Thermo Fisher Scientific, Waltham, MA), blocked with 2 M sulphuric acid and read at 620 nm in the microplate reader.
Lipolysis assay
The lipolytic activity of extracts was assessed on adipocytes by measuring glycerol released into the medium from triglyceride breakdown. Human subcutaneous pre-adipocytes were grown in preadipocyte medium (cat# PM-1, Zen-Bio Inc.) following the manufacturer’s protocol, settled in 96-well plates, differentiated into adipocytes for one week in adipocyte differentiation medium (cat# DM-2) and maintained for a further week in adipocyte medium (cat# AM-1). Thereafter, fully differentiated adipocytes were incubated for 3 h with extracts in adipocyte medium, and the conditioned medium was then assayed for glycerol using the Adipocyte Lipolysis Assay Kit (cat# LIP-1-L1; LIP-1-NCL1). The procedure involves glycerol phosphorylation by ATP, glycerol-1-phosphate oxidation by glycerol phosphate oxidase to dihydroxyacetone phosphate and H2O2, and peroxidase-catalyzed quinoneimine dye production, showing maximum absorbance at 540 nm. Samples were read in the microplate reader at 550 nm. The increase in absorbance is directly proportional to glycerol concentration in the sample.
Statistics
Data were analyzed with the R package, version 3.0.1 (http://www.r-project.org/foundation/), by using Student’s t-test with Bonferroni’s correction for multiple comparisons. The difference between two conditions was considered significant if p < 0.05. Inhibition of cell viability was determined using a logistic dose–response curve as reported by Ranzato et al. (2014).
Results
Chemical characterizations
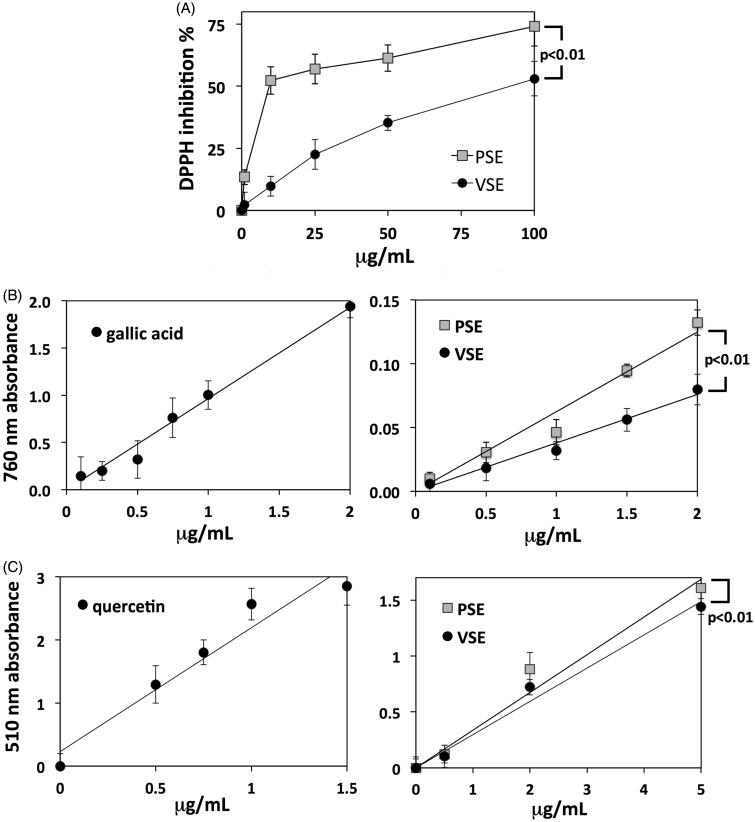
The extraction procedure, aimed at optimizing phenolic yield, allowed to obtain two dried extracts named Pisa sulla extract (PSE) and Ventimiglia sulla extract (VSE). Extracts were used for general chemical characterizations and chemical fingerprinting. The assay of DPPH scavenging activity was used as a measure of extract antioxidant power. Concentration-dependent curves of DPPH inhibition showed a significantly stronger activity of PSE with respect to VSE (Figure 1(A)).
Figure 1.
(A) Percentage inhibition of DPPH scavenging activity exerted by increasing concentrations of PSE and VSE. Data are means ± S.D. of n = 3 distinct determinations. (B) Folin–Ciocalteu assays of total phenolic content. Standard calibration curve obtained with gallic acid (left) and curves obtained with PSE and VSE (right). Data are means ± S.D. of n = 4 distinct determinations. In the right panel, statistical comparison between the two slope coefficients is shown. The phenolic contents of PSE and VSE expressed as GAE are reported in Table 1. (C) Assays of total flavonoids. Standard calibration curve obtained with quercetin (left) and curves obtained with PSE and VSE (right). Data and statistics as above. The flavonoid contents of PSE and VSE expressed as quercetin equivalents are reported in Table 1.
Total phenolic contents were determined by the Folin–Ciocalteu method, obtaining a gallic acid standard curve at 765 nm that allowed converting absorbance data into gallic acid equivalents (GAE). Thereafter, concentration-dependent curves at 765 nm were obtained for extracts, in order to compare their regression line slopes (Figure 1(B)). PSE showed a higher phenolic content than VSE (Table 1), while regression line comparison showed that the difference is statistically significant (Figure 1(B)).
Table 1.
Quantification of total phenolics and flavonoids in sulla extracts.
| Extract | Total phenolics | Total flavonoids |
|---|---|---|
| PSE | 62 ± 1 | 13 ± 8 |
| VSE | 38 ± 1 | 11 ± 4 |
Data are means ± S.E.M of triplicate determination. Total phenolics are expressed as gallic acid equivalents (mg/g). Total flavonoids are expressed as quercetin equivalents (mg/g).
A similar approach was used for the colorimetric quantification of total flavonoid content, by using quercetin as the reference standard (Figure 1(C)). Consistent with the analysis of total phenolics, PSE showed a higher flavonoid content than VSE (Table 1). Such a difference was smaller than that found for phenolics, but it resulted statistically significant according to regression line comparison (Figure 1(C)).
Compound identification
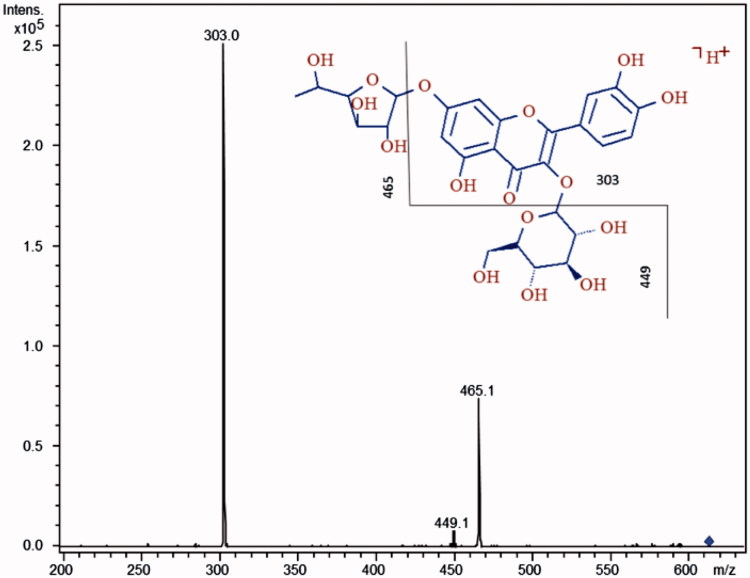
HPLC-MS/MS analysis of PSE and VSE extracts allowed the identification and relative quantification of different compounds. The MS/MS molecular masses obtained in our analyses were compared with online libraries (MassBank, http://www.massbank.jp/) and previously published MS data concerning different species of the genus Hedysarum, synonymous of Sulla (Dong et al. 2013). PSE and VSE showed similar composition in terms of major constituents, belonging to simple sugars, isoprenyl chalcones, coumestans, pterocarpenes, flavanones, flavones, flavonols, isoflavanes, isoflavones, flavan-3-ols, triterpenoids, phytosterols, fatty acids and esters (Table 2). However, a comparison of the relative abundances of these compounds showed that some molecules were significantly more abundant in PSE than in VSE, confirming the data obtained by general chemical characterizations. In Table 3, five flavonoid molecules are reported whose chromatographic peak areas were ∼20% (+), ∼40% (++) and ∼60% (+++), higher in PSE with respect to VSE. Each molecule was confirmed for its structure by mean of MS/MS analysis, and a typical spectrum thereof is reported in Figure 2.
Table 2.
LC-MS identification of major compounds in PSE and VSE extracts.
| Compound | aRT | b[M + H]+ | c[M − H]− |
|---|---|---|---|
| Paratocarpin E/hedysarumine B | 22.4 | 409.3 | |
| Naringenin-5,7-di-O-β-d-glucopyranoside | 22.0 | 599.3 | |
| Quercetin-3-O-d-glucopyranoside-7-O-α-rhamnofuranoside/rutin | 28.9 | 611.2 | |
| Isoquercitrin/hyperoside | 29 | 463.2 | |
| 3-Sitosterol | 29.9 | 415.4 | |
| Betulinic acid/ursolic acid | 29.9 | 457.3 | |
| Narcissin/isorhamnetin-3-O-rutinoside | 30.9 | 625.2 | |
| Galactose/glucose | 31.8 | 181.2 | |
| Hedysarimcoumestan G/hedysarimcoumestan H | 31.8 | 383.3 | |
| Quercetin | 32.7 | 303.4 | |
| Squasapogenol | 33.3 | 441.5 | |
| 1,3,9-Trimethoxycoumestan | 33.4 | 325.5 | |
| Sucrose | 33.4 | 343.9 | |
| Methyl-hedysarimcoumestan H | 33.4 | 394.7 | |
| (-)Vestitol/naringenin | 34.3 | 273.4 | |
| Epicatechin/catechin | 34.4 | 291.5 | |
| Prenylfurano-[3,2-g]-isoflavone | 34.7 | 376.6 | |
| Isoformononetin/formononetin | 34.8 | 269.4 | |
| Hedysarimcoumestan B/4',6-dimethoxy-7-hydroxy isoflavone/afrormosin | 34.8 | 299.4 | |
| Rhoifolin | 35.0 | 579.5 | |
| Hexadecanoic acid 2,3-dihydroxypropyl ester | 35.3 | 331.6 | |
| Oleic acid | 35.5 | 283.4 | |
| n-Tetracosanoic acid | 35.5 | 369.5 | |
| Kaempferide | 36.8 | 301.5 | |
| Hedysarimpterocarpene C | 36.8 | 627.5 | |
| Isorhamnetin | 37.1 | 317.6 | |
| Hexacosyl acetate | 37.1 | 425.5 | |
| Diosmin | 38.7 | 609.9 | 607.7 |
RT: retention time.
[M + H]+: m/z in positive ion mode.
[M − H]−: m/z in negative ion mode.
Table 3.
LC-MS relative quantification of major flavonoids from PSE and VSE.
| Compound | RA |
|---|---|
| Epicatechin/catechin | +++ |
| Rhoifolin | +++ |
| Naringenin-5,7-di-O-β-D-glucopyranoside | ++ |
| Quercetin | ++ |
| Quercetin-3-O-β-glucopyranoside-7-O-α-rhamnofuranoside | + |
RA: relative abundance of the compounds in PSE compared to VSE (+ ≈ 20%, ++ ≈ 40%, +++ ≈ 60%).
Figure 2.
MS/MS spectrum of quercetin-3-O-β-glucopyranoside-7-O-α-rhamnofuranoside. The hypothesized fragments and their m/z ratios are indicated with the broken line in the structure.
Effects of extracts on extracellular matrix
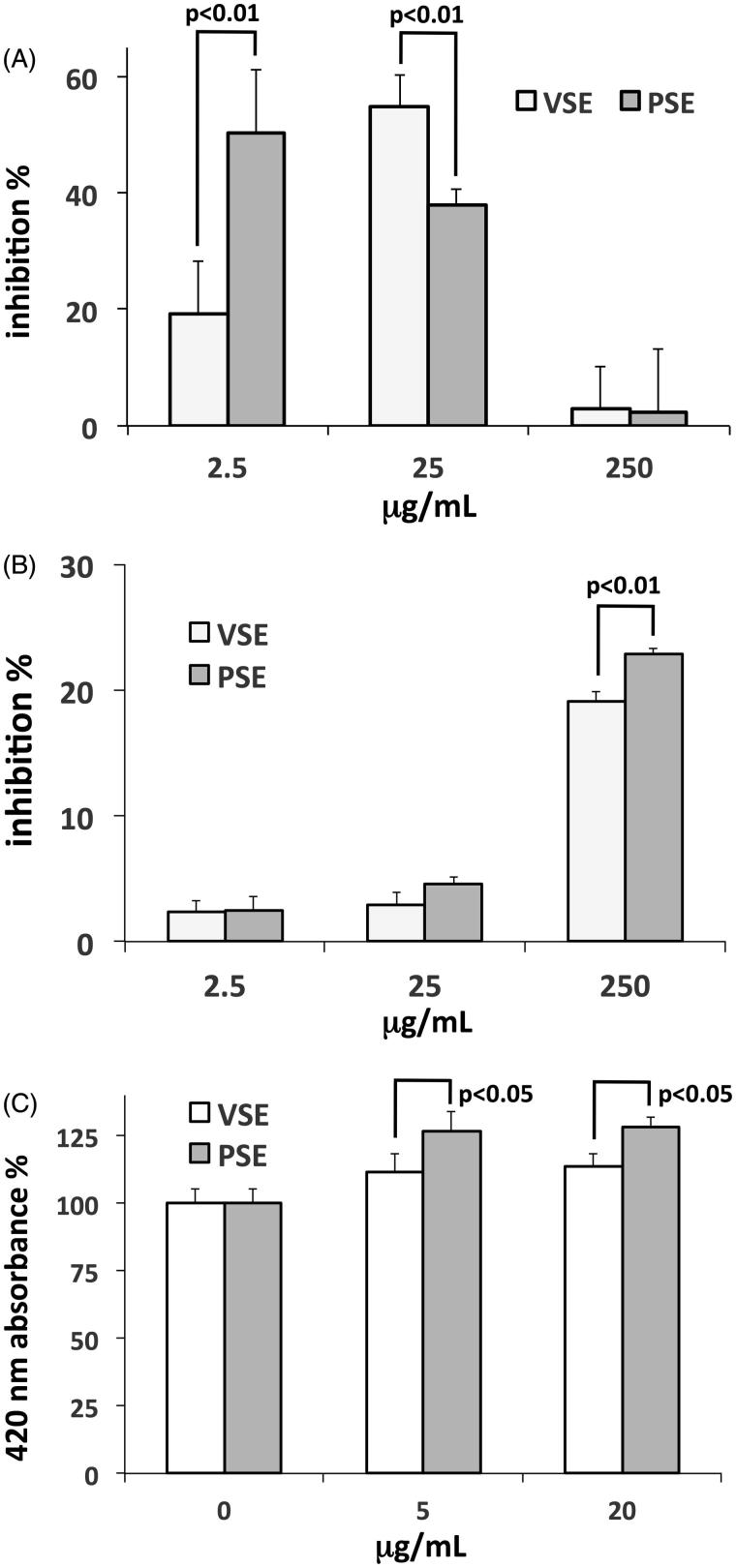
Extracellular matrix turnover is essential for the maintenance of a healthy skin dermal layer, involving both degradation and neosynthesis processes. We evaluated the in vitro inhibition of extracts on collagenase and elastase, two main dermal enzyme activities involved in the degradation of collagen and elastin, respectively. The effects on collagenase of the two extracts showed different patterns. VSE induced a biphasic effect, with inhibition rise at lower concentrations followed by a decrease in the effect at higher concentrations (Figure 3(A)). PSE showed strong inhibition at the lowest concentration and a progressive decline for higher doses (Figure 3(A)). The effect of the two extracts on elastase was inhibitory and significantly stronger for PSE than for VSE (Figure 3(B)).
Figure 3.
(A) Percent inhibition of Clostridium histolyticum collagenase activity exerted by PSE and VSE, determined by in vitro assay and expressed as means ± S.E.M (n = 3). (B) Percent inhibition of elastase from porcine pancreas by PSE and VSE. Data as above. (C) Induction of fibroblast collagen production by PSE and VSE, determined by ELISA after 48 h incubations. Data are expressed as means ± S.D (n = 6).
Before using extracts on in vitro grown fibroblasts, we made a check for possible deleterious effects on cell viability. The MTT assay showed low inhibition of cell viability for both extracts, with IC50 > 150 μg/mL at 48 h, warranting that any effect on cell viability in the tests conducted on cells was negligible. By using an ELISA assay, we measured the modulatory effect of extracts on collagen type I production in fibroblasts. The two extracts showed a dose-dependent stimulatory effect, and also in this case, the activity of PSE was stronger than that of VSE (Figure 3(C)).
Effects of extracts on lipolysis
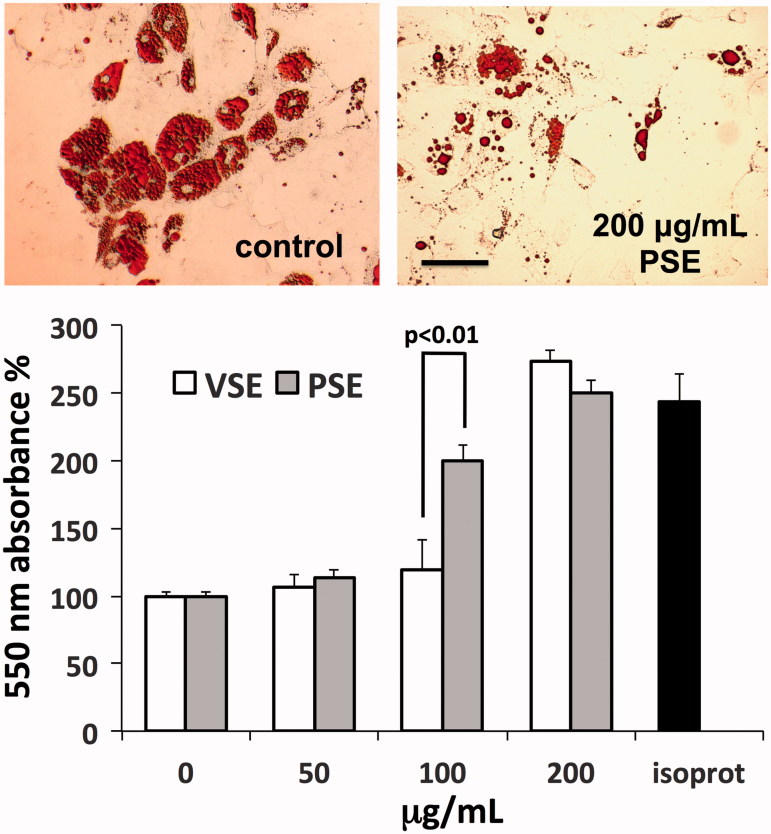
Fat tissue turnover is essential for the maintenance of the subdermal skin layer, while its impairment may concur to the development of cellulitis. We verified the effects of extracts on the lipolytic activity of in vitro cultured adipocytes (Figure 4, upper panel). Measurements of glycerol release from cells, used as an index of lipolytic activity, revealed a dose-dependent stimulatory effect induced by both extracts. However, a steeper rise in lipolysis was observed with PSE, showing maximum difference with VSE at 100 μg/mL, followed by an upward convergence of effects at 200 μg/mL, reaching the lipolytic effect of isoproterenol used as positive control (Figure 4, lower panel).
Figure 4.
Induction of lipolysis in human adipocytes by PSE and VSE. Upper panel: microscope views of adipocytes exposed or not to PSE, fixed with FineFix®, stained with Oil Red O and then photographed under an Olympus IX71 inverted microscope. Bar =100 μm. Lower panel: assay of glycerol released from adipocytes after exposure for 3 h to extracts or to 1 μM isoproterenol (isoprot) as positive control. Data are standardized as percent of control and expressed as means ± S.D (n = 6).
Discussion
Data of chemical characterizations consistently indicated a higher content of phenolic compounds in PSE with respect to VSE, allegedly linked to the stronger radical scavenging activity of the former extract. Moreover, the semiquantitative analysis conducted on LC-MS data indicated that the difference in phenolic contents is maximally accounted for by catechins. The identification of these compounds as major extract constituents could actually reflect the presence of condensed tannins, of which catechins are monomer units. Condensed tannins are known to be abundant in sulla and would undergo in-source collision-induced dissociation (CID) during MS analysis. Hence, the comparison of MS data from the two extracts suggests that the higher abundance of phenolics in PSE is primarily accounted for by tannins. However, total flavonoid content and MS data consistently showed that also the flavonoid component contributes to the difference in phenolic composition between the two extracts.
It is well known that phenolics, especially flavonoids, play an important role in determining the biological properties of herbal products. Therefore, a higher content of these compounds in a phytocomplex is predictive of stronger bioactivity. Our data are consistent with this view, as we found similar composition in the two sulla extracts and similar effects in different bioassay, but the higher content in phenolics of PSE went together with the stronger activity of this extract in all bioassays.
Inhibition of collagenase and elastase is generally considered to prevent unbalanced extracellular matrix turnover due to rapid breakdown of collagen type I in inflamed skin. Inhibitors are therefore sought for in the development of skin preserving and antiageing remedies. By using in vitro enzyme assays, we found biphasic effects of extracts on collagenase, characterized by inhibitory activities at very low concentration of PSE and at relatively low concentration of VSE. These results suggest the presence of strong inhibitors, more concentrated in PSE than in VSE, and of other compounds interfering with inhibition at higher extract doses. Based on the composition of our extracts, possible inhibitors could be condensed tannins and quercetin, previously found to exert inhibitory effects on collagenase (Lim and Kim 2007; Diaz-Gonzalez et al. 2012). Data of elastase inhibition revealed a weaker activity with respect to collagenase inhibition, but the effect of PSE was stronger. Also in this case, a role could be played by condensed tannins that have been previously shown to bind to pancreatic elastase, the enzyme used in our tests (Bras et al. 2010). However, the effect could be ascribed at least in part also to quercetin (Park et al. 2016).
The stimulatory effect of PSE on collagen synthesis is particularly interesting from pharmaceutical and pharmacological points of view, because it was observed at very low concentrations, suggesting the presence of very strong inductors in the phytocomplex. Condensed tannins, quercetin and other major polyphenols of sulla extracts are not known to induce this kind of effect. However, collagen induction has been reported for flavonoids and triterpenoids from other plants (Maquart et al. 1990; Pastorino et al. 2017). Therefore, it can be speculated that the effect observed with PSE could be due to compounds belonging to either of these classes.
The lipolytic effect of sulla extracts testifies their possible use in anticellulite products. This activity can be allegedly ascribed to quercetin and its derivatives, since these compounds are well represented in sulla extracts and their lipolytic effect has been pharmacologically characterized in rat adipocytes (Kuppusamy and Das 1994). It has also been shown in OP9 mouse stromal cells, which can differentiate into adipocytes, that quercetin prevents adipogenesis and stimulates lipolysis through the regulation of transcriptional factors and lipases (Seo et al. 2015). The stronger effect of PSE observed in our study is in line with these reports, because quercetin is a major compound accounting for the difference in polyphenols between PSE and VSE.
Conclusions
This study was aimed at verifying the possible use in dermatologic and cosmeceutical products of herbal extracts obtained from sulla. This species is a legume crop widely cultivated in the Mediterranean area and potentially available in amounts compatible with industrial use. Moreover, due to the known variability of plant phytocomplexes, even within a species or variety, depending on the geographical origin of plants, we have compared extracts from individuals sampled at two distant sites in the geographic range of the species.
Our data showed marked differences in total phenolic content between the two extracts, most likely depending on different abundances of condensed tannins and flavonoids, mainly rhoifolin, quercetin, naringenin and derivatives. In addition, the higher richness in phenolics of the PSE extract, deriving from Pisa, was in conjunction with a stronger bioactivity of this extract in all the conducted bioassays. These tests collectively showed that sulla plant material is suitable for the development of pharmaceutical and cosmeceutical products targeting major skin problems, such as inflammatory, degenerative and ageing processes, or the insurgence of cellulite. Hence, our data provide a strong indication that the legume crop H. coronarium can be exploited for these purposes, but they also show that the geographical origin of plants is an important factor to be considered for maximally exploiting the biological properties of the species.
Funding Statement
This work was granted by University of Genova, no. 100022-2015 FRA.
Acknowledgements
GP was recipient of a PhD scholarship from the Italian Ministry of University and Research (MIUR).
Disclosure statement
The authors report no declarations of interest.
References
- Aïssa A, Manolaraki F, Ben Salem H, Kraiem K, Hoste H.. 2015. In vitro anthelmintic activity of Tunisian Fabacae (Hedysarum coronarium L, ecotype Bikra 21) against haemonchus contortus. Int J Agron Agr Res. 7:103–110. [Google Scholar]
- Annicchiarico P, Ruisi P, Di Miceli G, Pecetti L.. 2014. Morpho-physiological and adaptive variation of Italian germplasm of sulla (Hedysarum coronarium L.). Crop Pasture Sci. 65:206–213. [Google Scholar]
- Bras NF, Goncalves R, Fernandes PA, Mateus N, Ramos MJ, de Freitas V.. 2010. Understanding the binding of procyanidins to pancreatic elastase by experimental and computational methods. Biochemistry. 49:5097–5108. [DOI] [PubMed] [Google Scholar]
- Carmona M, Sanchez AM, Ferreres F, Zalacain A, Tomas-Barberan F, Alonso GL.. 2007. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: comparative study of samples from different geographical origins. Food Chem. 100:445–450. [Google Scholar]
- Chiki A, Harborne JB.. 1983. Anthocyanins of Hedysarum coronarium and their contribution to flower colour variation. Phytochemistry. 22:2322–2323. [Google Scholar]
- Chung YC, Chang CT, Chao WW, Lin CF, Chou ST.. 2002. Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J Agric Food Chem. 50:2454–2458. [DOI] [PubMed] [Google Scholar]
- Diaz-Gonzalez M, Rocasalbas G, Francesko A, Tourino S, Torres JL, Tzanov T.. 2012. Inhibition of deleterious chronic wound enzymes with plant polyphenols. Biocatal Biotransfor. 30:102–110. [Google Scholar]
- Dong Y, Tang D, Zhang N, Li Y, Zhang C, Li L, Li M.. 2013. Phytochemicals and biological studies of plants in genus Hedysarum. Chem Cent J. 7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO.. 2006. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 22:253–261. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Carvalho AR, Pittol V, Dietrich F, Manica F, Machado MM, de Oliveira LF, Oliveira Battastini AM, Ortega GG.. 2016. Genotoxicity and cytotoxicity of oxindole alkaloids from Uncaria tomentosa (cat's claw): Chemotype relevance. J Ethnopharmacol. 189:90–98. [DOI] [PubMed] [Google Scholar]
- Kuppusamy UR, Das NP.. 1994. Potentiation of beta-adrenoceptor agonist-mediated lipolysis by quercetin and fisetin in isolated rat adipocytes. Biochem Pharmacol. 47:521–529. [DOI] [PubMed] [Google Scholar]
- Lentini F, Venza F.. 2007. Wild food plants of popular use in Sicily. J Ethnobiol Ethnomed. 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Kim HP.. 2007. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 73:1267–1274. [DOI] [PubMed] [Google Scholar]
- Maquart FX, Bellon G, Gillery P, Wegrowski Y, Borel JP.. 1990. Stimulation of collagen synthesis in fibroblast cultures by a triterpene extracted from Centella asiatica. Connect Tissue Res. 24:107–120. [DOI] [PubMed] [Google Scholar]
- Molle G, Decandia M, Fois N, Ligios S, Cabiddu A, Sitzia M.. 2003. The performance of Mediterranean dairy sheep given access to sulla (Hedysarum coronarium L.) and annual ryegrass (Lolium rigidum Gaudin) pastures in different time proportions. Small Ruminant Res. 49:319–328. [Google Scholar]
- Park CH, Ahn MJ, Hwang GS, An SE, Whang WK.. 2016. Cosmeceutical bioactivities of isolated compounds from Ligularia fischeri Turcz leaves. Appl Biol Chem. 59:485–494. [Google Scholar]
- Pastorino G, Marchetti C, Borghesi B, Cornara L, Ribulla C, Burlando B.. 2017. Biological activities of the legume crops Melilotus officinalis and Lespedeza capitata for skin care and pharmaceutical applications. Ind Crops Prod. 96:158–164. [Google Scholar]
- Ranzato E, Magnelli V, Martinotti S, Waheed Z, Cain SM, Snutch TP, Marchetti C, Burlando B.. 2014. Epigallocatechin-3-gallate elicits Ca2+ spike in MCF-7 breast cancer cells: essential role of Cav3.2 channels. Cell Calcium. 56:285–295. [DOI] [PubMed] [Google Scholar]
- Seo YS, Kang OH, Kim SB, Mun SH, Kang DH, Yang DW, Choi JG, Lee YM, Kang DK, Lee HS, et al. . 2015. Quercetin prevents adipogenesis by regulation of transcriptional factors and lipases in OP9 cells. Int J Mol Med. 35:1779–1785. [DOI] [PubMed] [Google Scholar]
- Terrill TH, Douglas GB, Foote AG, Purchas RW, Wilson GF, Barry TN.. 1992. Effect of condensed tannins upon body growth, wool growth and rumen metabolism in sheep grazing sulla (Hedysarum coronarium) and perennial pasture. J Agric Sci. 119:265–273. [Google Scholar]
- Tibe O, Meagher LP, Fraser K, Harding DR.. 2011. Condensed tannins and flavonoids from the forage legume sulla (Hedysarum coronarium). J Agric Food Chem. 59:9402–9409. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W.. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559. [Google Scholar]