Abstract
Context: Orange Jessamine [Murraya paniculata L. (Rutaceae)] has been used worldwide in folk medicine as an anti-inflammatory, antibiotic and analgesic.
Objective: The objective of this study is to investigate the in vitro antioxidant, cytotoxic, antibacterial and antifungal activity and the time-kill curve studies of orange jessamine essential oil and β-caryophyllene, as well as the chemical composition of the essential oil.
Material and methods: The cytotoxic activity of M. paniculata and β-caryophyllene (7.8–500 μg/mL) was evaluated using the MTT assay on normal fibroblasts and hepatoma cells. The minimal inhibitory concentration and time–kill curves (24 h) were evaluated against those of Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Enterococcus faecallis, Aspergillus (niger, fumigates and parasiticum) and F. solani by the broth microdilution method. The antioxidant activity was measured by the DPPH and ABTS assays. Chemical composition was evaluated by GC/MS analyses.
Results: GC/MS analyses identified 13 compounds, with β-caryophyllene as the major compound. The oil exhibited moderate antibacterial activity (MIC <1.0 mg/mL) and strong antifungal activity. Time–kill curve studies showed that either the essential oil or β-caryophyllene presented rapid bacterial killing (4 h for S. aureus) and fungicidal effect (2-4 h for F. solani); however, both displayed weak free radical scavenger capacity. The cytotoxic activity exhibited a prominent selective effect against hepatoma cancer cells (IC50 value =63.7 μg/mL) compared with normal fibroblasts (IC50 value =195.0 μg/mL), whereas the β-caryophyllene showed low cytotoxicity.
Discussion and conclusion: The experimental data suggest that the activities of M. paniculata essential oil are due to the synergistic action among its components.
Keywords: Orange Jessamine, cytotoxicity, fungicidal, bactericidal
Introduction
The use of medicinal plants comprises an important alternative in the treatment of diseases. The World Health Organization (WHO) has recommended since 2002 that governments adopt phytotherapy, or herbal medicine, in primary health care programs, using available natural resources in their territories (WHO 2002). Murraya paniculata L (Rutaceae), commonly known as Orange Jessamine in Asia, has been widely used in phytotherapy in China, India and Indonesia (Zhang et al. 2011; Gautam et al. 2012b). Murraya paniculata is a part of ancient Indian medicine, and this specie is described in the Chinese pharmacopeia as anti-inflammatory, antibiotic and analgesic remedy (Zhang et al. 2011), while Indonesian folk medicine reports the use of bindings made from the leaves of M. paniculata in the treatment of bone fractures (Wu et al. 2010). Despite the reported uses and effects, the therapeutic use of the plant is not yet widespread in Brazil.
Current literature describes M. paniculata as having antihyperglycemic (Gautam et al. 2012a), antifungal (Sundaram 2011), antibacterial (Rodanant et al. 2015) analgesic (Fazal-ur-Rehman et al. 2014), antioxidant (Gautam et al. 2012a), antispasmodic, bronchodilating and vasodilating actions (Saqib et al. 2015). In addition, nephroprotective properties in diabetic nephropathy are also reported (Zou et al. 2014).
The diversity of the properties attributed to M. paniculata is mainly due to the variety chemical compounds in this plant. Detailed studies of the extracts of M. paniculata describe the presence of flavonoids, phenols, alkaloids, polysaccharides, and coumarins (Gautam et al. 2012a; Shah et al. 2014; Teshima et al. 2014; Wu et al. 2016). Few studies have been conducted with the essential oil of M. paniculata, and the majority of them have evaluated only the chemical composition, describing the presence of sesquiterpenes, such as β-caryophyllene, limonene, spathulenol and elemene (γ and δ), as major compounds (Chowdhury et al. 2008; Rodriguez et al. 2012; Lv et al. 2013). The only two studies in the literature describing the biological activity of the essential oil revealed antioxidant and antibacterial activities against Klebsiella pneumoniae and Bacillus subtilis (Rodriguez et al. 2012), and repellency against Diaphorina citri Kuwayama (Andrade et al. 2016). In addition, no data were found for the main isolated compounds as well. Therefore, the aim of the present work was to evaluate the effects of β-caryophyllene and Orange Jessamine essential oil in the murine hepatoma cells (Hepa 1c1c7) and in the bacteria and fungi 24-h time–kill curve studies, as well as to evaluate the composition of the essential oil and its antioxidant activity.
Material and methods
Cell lines, chemicals
Swiss 3T3 albino mouse fibroblasts (American Type Culture Collection – ATCC® CCL-92TM), murine hepatoma (Hepa 1c1c7) cells (ATTC® CRL-2026TM) (Cell Line Service, Rio de Janeiro, Brazil), were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 μg/mL streptomycin, at 37 °C, in a humidified atmosphere containing 5% CO2 (all Sigma, St. Louis, MO). The following materials were purchased from Sigma Aldrich (St. Louis, MO): the free radicals DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS [2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)], gallic acid, Folin–Ciocalteau reagent, sodium carbonate, 2,3,5-triphenyl tetrazolium chloride (TTC), β-caryophyllene, dimethylsulfoxide (DMSO), saturated alkanes (C7-C30), Tween 40, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), quercetin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and camptothecin (CPT). The brain–heart infusion broth (BHI) and Mueller–Hinton agar were purchased from the company Himedia (Mumbai, India).
Plant material
Murraya paniculata samples were collected in Vila Velha (ES, Brazil): Latitude: −20.3557 and longitude: −40.3142 from August to December 2013. A sample was taxonomically identified by the Professor Solange Zanotti Schneider and a voucher specimen deposited in the herbarium of the University of Vila Velha under the number: 2125UVVES.
Essential oil
The essential oil was obtained from fresh leaves of M. paniculata by hydrodistillation using a Clevenger extractor. The fresh leaves were triturated with ultra-pure water in a blender and transferred to the distillation flask (2 L). After extraction, the essential oil was transferred to a glass vial, and purification was performed by freezing the remaining water. The essential oil, which was kept in liquid state, was drained to another flask and then stored under refrigeration until analysis.
Essential oil composition by GC-MS
The identification of the essential oil’s components was carried out by GC-MS analysis. Identification of the chemical constituents was done in a gas chromatograph (TraceUltra, Thermo Scientific, Waltham, MA) coupled with mass spectrometer (DSQII, Thermo Scientific, Waltham, MA), and in a gas chromatograph coupled with FID detector for quantification (Focus, Thermo Scientific, Waltham, MA). The compounds were separated in a DB-5 fused silica capillary column 30 m × 0.25 mm ×0.25 μm film thickness (J&W Scientific, Folsom, CA). Helium was the carrier gas used, at a flow rate of 1.0 mL/min. The analyses were performed using splitless injection at 220 °C. The oven temperature program used was 60–240 °C at 3 °C/min, and the final temperature was held for 7 min. The GC–MS interface and FID detector were maintained at 240 °C and 250 °C, respectively. The oil was dissolved in hexane (2 mg/mL) for the analyses. The MS data were obtained in the scan mode (35–400 m/z) and EI mode operating at 70 eV. Kovats retention indices (KI) were determined by injection of standard hydrocarbon solutions (C7–C30). The components were identified by comparison with data from the literature (Adams 1995) and with the profiles from the NIST mass spectral library (version 2.0, 2005), and by injection of pure compounds, when available.
Antioxidant activity
DPPH free radical scavenging
Antioxidant activity of the samples was determined by the DPPH method, according to Scherer and Godoy (2009). Antioxidant activity of the essential oil and β-caryophyllene was compared with the synthetic antioxidants BHA and BHT, and the phenolic compound quercetin. The antioxidant capacity was expressed as antioxidant activity index (AAI), which classifies antioxidants as weak when AAI <0.5, moderate when 0.5 < AAI <1.0, strong when 1.0 < AAI <2.0 and very strong when AAI >2.0.
ABTS free radical scavenging
The antioxidant activity was determined according to Re et al. (1999) with modifications. ABTS radical cation (ABTS•+) was formed by reaction of 7.0 mM ABTS (50% ethanol) with 2.45 mM potassium persulfate (in distilled water). This reagent was stored under refrigeration for at least 24 h. Before use, the reagent was diluted with 50% ethanol until absorbance of 1.0 (± 0.02) at 734 nm. In 96-well microplates 250 uL ABTS•+ and 20 μL of each concentration of the compounds were added. The blank was 20 μL of ethanol. After 10 min of reaction in the dark, the reading was performed at 734 nm using a microplate reader (SpectraMax 190 Microplate Reader, Molecular Devices, Sunnyvale, CA). The radical scavenging activity was calculated as follows: I (%) = [(Abs0−Abs1)/Abs0] × 100, where Abs0 is the absorbance of the blank and Abs1 is the absorbance in the presence of the test compound at different concentrations. The results were expressed as IR50 (concentration capable of reducing 50% of free radicals), and calculated using a calibration curve in the linear range by plotting the final concentration of the extract versus the corresponding scavenging effect. The antioxidant activity of the essential oil and β-caryophyllene was compared to quercetin, BHA and BHT.
Antimicrobial activity
The essential oil and β-caryophyllene oil were tested against four strains of bacteria (Staphylococcus aureus ATCC25923, Escherichia coli ATCC8739, Salmonella typhimurium ATCC14028 and Enterococcus faecallis ATCC14506) and against four strains of fungi (Aspergillus niger ATCC40067, Aspergillus fumigatus ATCC40014, Aspergillus parasiticum ATCC 40100 and Fusarium Solani ATCC 40099) according to the standards described by the Clinical and Laboratory Standards Institute (CLSI 2005).
Determination of minimum inhibitory concentration (MIC)
To determine the minimum inhibitory concentrations (MIC) for the bacteria, the essential oil of M. paniculata and β-caryophyllene were prepared at a concentration of 4.0 mg/mL in 5% dimethylsulfoxide (DMSO) and immediately diluted with saline solution (0.85%). The final cell concentration was adjusted to there McFarland scale, and thereafter adjusted with Mueller Hinton broth so that each well of the microplate had 5 × 105 CFU/mL. In each well, 150 μL of inoculum (in Mueller Hinton broth), and 150 μL of essential oil or β-caryophyllene solution were added. All microplates included a positive control, a negative control and the antibiotic ampicilin. The essential oil and β-caryophyllene were evaluated at final concentrations ranging from 2.0 to 0.015 mg/mL. The microplates were incubated at 36 °C for 24 h, and then 50 μL CTT (solution of 2,3,5-triphenyl tetrazolium 0.5% in deionized water) were added. After 6 h of incubation, MIC was determined as the lowest concentration that inhibited visible growth of bacteria since dead cells are not stained by CTT (CLSI 2002). The determination of MIC for the fungi investigated was similar to that employed for the bacteria; however, the final cell concentration was adjusted so that each well had 2.5 × 103 CFU/mL, and the standard antifungal agent used was fluconazole.
Minimum bactericidal concentration (MBC)
After determination of the MIC values, 10 μL were transferred from the wells referencing the values of MIC value, 2 × MIC, and 4 × MIC to the culture plate with Mueller Hinton agar medium and were incubated in an oven at 36 °C for 24 h. The MBC was considered that which showed no growth after incubation.
Minimum fungicidal concentration (MFC)
The determination of MFC was carried out by transferring 10 μL wells referencing the values of MIC, 2 × MIC, and 4 × MIC to the culture plate with Sabouraud agar and incubating at 25 °C for 48 h. The MFC was considered that which showed no growth after incubation.
Time–kill curves studies
Bacteria
The studies were performed according to M26-A method described by the Clinical and Laboratory Standards Institute (CLSI 1999). The final cell concentration was adjusted to the McFarland scale, and thereafter adjusted with Mueller Hinton broth so that each tube had 5.0 × 105 UFC/mL. Assays were performed in 2 mL tubes by adding 500 μL of inoculum in growth medium (Mueller Hinton broth) together with 500 μL of the essential oil or β-caryophyllene to the final concentration of MIC and 2 × MIC or 500 μL of medium for blank, and incubated at 37 °C. For the experiments, a 10 μL aliquot was added in 990 μL of saline 0.85%, and then 100 μL of the diluted solution was spread on a plate with Muller Hinton Agar, and incubated for 24 h at 37 °C. The tests were performed for 0, 2, 4, 8, 12 and 24 h. The reduction of ≥3 log10 CFU compared with the control was defined as bactericidal effect (CLSI 1999). The experiments were carried out in duplicate.
Fungi
The studies were performed according to M38-A method described by the Clinical and Laboratory Standards Institute (CLSI 2002). The final cell concentration was adjusted to the McFarland scale, and thereafter adjusted with Mueller Hinton broth so that each tube had 2.5 × 103 UFC/mL. Assays were performed in 2 mL tubes by adding 500 μL of inoculum in growth medium (Mueller Hinton broth) together with 500 μL of the essential oil or β-caryophyllene to the final concentration of MIC and 2 × MIC or 500 μL of medium for blank, and incubated at 37 °C. For the experiments, a 10 μL aliquot was added in 990 μL of saline 0.85%, and then 100 μL of diluted was spread in a plate with Sabouraud Agar, and incubated for 25 °C up to positive growth in the control group, when all plates were counted. The tests were performed for 0, 2, 4, 8, 12 and 24 h. The experiment was carried out twice. The reduction of ≥3 log10 CFU compared with the control was defined as fungicidal effect. The experiment was carried out twice.
In vitro cytotoxicity
The cytotoxic activity of the essential oil of M. paniculata and β-caryophyllene was evaluated by the colorimetric MTT assay proposed by Mosmann (1983). Briefly, 3T3 fibroblasts or HEPA h1c1c7 cells were added in 96-well microplate at a final concentration of 7 × 105 cells/mL. After overnight incubation, cells were exposure to increasing concentrations of the essential oil of M. paniculata or β-caryophyllene (7.8–500 μg/mL), and incubated for 24 h. After incubation, 100 μL of MTT (1 mg/mL) were added to each well and incubated for additional 2 h. Then, 100 μL of DMSO were added to dissolve the formazan crystals. Camptothecin (100 μM) was used as a positive control. The absorbance of purple formazan, proportional to the number of viable cells, was measured at 595 nm using a microplate reader (Molecular Devices, Spectra Max 190, Sunnyvale, CA). The experiments were carried out at least in triplicate.
Statistical analysis
Analysis of variance (ANOVA) followed by Turkey post-test were used to determine whether there was a significant difference between the means (p < 0.05), using the Biostat 5.0 software (Mary Ann Liebert Inc, New Rochelle, NY).
Results
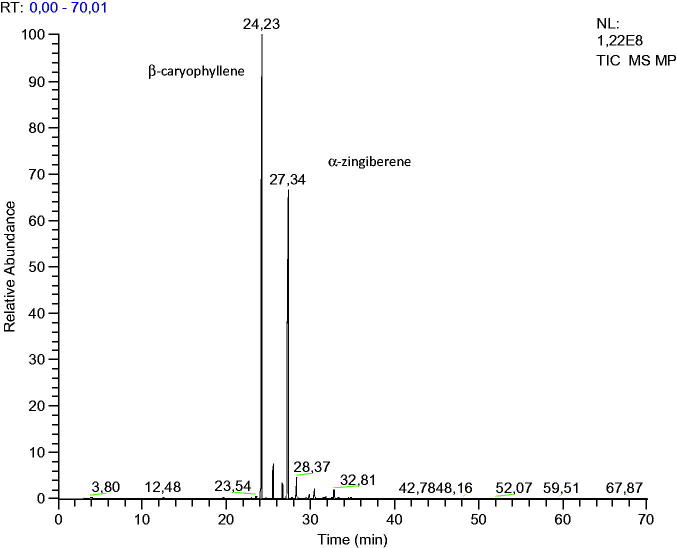
Fresh leaves (9 kg) were used for the extraction of essential oil resulting in 2.34 g of essential oil. Therefore, the yield of essential oil extraction by hydrodistillation was 0.026% ± 0.003. In the chemical composition analysis of the essential oil, 13 compounds were identified, representing a total of 99.5% of the essential oil, in which 99.3% were sesquiterpenes and 0.3% were monoterpenes. The major compound was β-caryophyllene (57.57%), followed by α-zingiberene (31.72%) (Figure 1) and α-caryophyllene (3.58%) (Table 1).
Figure 1.
GC chromatogram of essential oil of M. paniculata.
Table 1.
Chemical composition of the essential oil of M. paniculata.
| Compounds | n° CAS | RT | KI 1 | KI 2 | % |
|---|---|---|---|---|---|
| Limonene | 138-86-3 | 8.4 | 1032 | 1031 | 0.26 |
| Elemene (β) | 515-13-9 | 23.0 | 1388 | 1391 | 0.09 |
| Sesquithujene | 58319-06-5 | 23.5 | 1400 | 1405 | 0.23 |
| Caryophyllene (β) | 87-44-5 | 24.2 | 1418 | 1418 | 57.57 |
| Bergamotene (α) | 17699-05-7 | 24.7 | 1431 | 1434 | 0.08 |
| Caryophyllene (α) | 6753-98-6 | 25.6 | 1453 | 1454 | 3.58 |
| Curcumene (γ) | 28976-68-3 | 26.7 | 1478 | 1480 | 1.71 |
| Zingiberene (α) | 495-60-3 | 27.4 | 1495 | 1495 | 31.72 |
| Bisabolene (β) | 495-61-4 | 27.8 | 1505 | 1509 | 0.12 |
| Sesquiphellandrene (β) | 20307-83-9 | 28.4 | 1521 | 1524 | 1.89 |
| Nerolidol (trans) | 40716-66-3 | 29.8 | 1559 | 1561 | 0.41 |
| Caryophyllene oxide | 1139-30-6 | 30.5 | 1575 | 1581 | 1.02 |
| Cadinol (epi-α) | 481-34-5 | 32.8 | 1637 | 1640 | 0.77 |
| Total | 99.5 |
RT: retention time; KI 1: experimental; KI 2: literature (Adams, 1995).
Free radical scavenging capacity of the essential oil of M. paniculata and β-caryophyllene were evaluated by DPPH and ABTS methods, and the results are shown in Table 2. In the ABTS assay, the essential oil and β-caryophyllene showed no action even at the highest concentration tested (8 mg/mL). The antioxidant activity evaluated by DPPH method showed poor antioxidant activity according to the classification proposed by Scherer and Godoy (2009).
Table 2.
Antioxidant activity of the M. paniculata essential oil and β-caryophyllene by the DPPH and ABTS methods.
| DPPH |
ABTS | ||
|---|---|---|---|
| Compounds | IR50 (μg/mL) | IAA | IR50 (μg/mL) |
| Quercetin | 3.22 ± 0.2 | 16.83 ± 0.8a | 1.6 ± 0.1a |
| BHA | 5.28 ± 0.1 | 9.04 ± 0.4b | 3.1 ± 0.2b |
| BHT | 9.38 ± 0.2 | 4.24 ± 0.2c | 3.2 ± 0.2b |
| Essential oil | 238.26 ± 12 | 0.22 ± 0.01d | na |
| Caryophyllene (β) | 283.10 ± 18 | 0.18 ± 0.01d | na |
Different superscripts at the same column correspond to significant difference (p < 0.05). na: no activity.
The minimum inhibitory concentration and minimum bactericidal concentrations of the essential oil are presented in Table 3. According to the classification by Holetz et al. (2002), MIC values lower than 0.1 mg/mL represent strong antimicrobial action; values between 0.1 mg/mL and 0.5 mg/mL indicate moderate antimicrobial activity, values between 0.5 and 1.0 mg/mL indicate weak action, and values above 1.0 mg/mL inaction. Considering this classification, the essential oil showed weak antibacterial activity for S. aureus and moderate action for all other bacteria strains. The action of β-caryophyllene was similar for Gram-positive- and Gram-negative-tested bacteria and showed only weak activity in all bacterial strains investigated, suggesting that the moderate action found for the essential oil occurs by a synergistic effect among its compounds and not by action of its major compound.
Table 3.
Minimum Inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) of the essential oil of M. paniculata and β-caryophyllene.
| Essential oil (mg/mL) |
β-caryophyllene (mg/mL) |
|||||
|---|---|---|---|---|---|---|
| Microorganism | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC |
| S. aureus | 1.0 | 2.0 | 2.0 | 1.0 | 4.0 | 4.0 |
| S. typhimurium | 0.5 | 1.0 | 2.0 | 0.5 | 2.0 | 4.0 |
| E. coli | 0.5 | 2.0 | 4.0 | 1.0 | 2.0 | 2.0 |
| E. faecallis | 0.5 | 1.0 | 2.0 | 1.0 | 4.0 | 4.0 |
| |
MIC |
MFC |
MFC/MIC |
MIC |
MFC |
MFC/MIC |
| Aspergilus niger | 0.2 | 0.2 | 1.0 | 0.5 | 0.5 | 1.0 |
| Fusarium solari | 0.2 | 0.5 | 2.0 | 1.0 | 2.0 | 2.0 |
| Aspergilus fumigatus | 0.1 | 0.5 | 4.0 | 0.5 | 0.5 | 1.0 |
| Aspergilus parasiticum | 0.2 | 0.5 | 2.0 | 1.0 | 4.0 | 4.0 |
The minimum inhibitory concentration and minimum fungicidal concentration of the essential oil on the fungi are shown in Table 3. The MIC values of the essential oil demonstrate that it shows moderate to strong antifungal activity (MIC between 0.1 and 0.5 mg/mL), mainly on the fungus Aspergillus fumigatus. The mechanism of action of essential oils seems to involve the fungal membrane structures changing the micellar growth, but not affecting the germination of the spores (Abad et al. 2007). Similar to the antibacterial test, the MIC and MFC values for β-caryophyllene were higher than those found for the essential oil, indicating that the synergistic effect between the oil’s components is essential for the antifungal action. The ratio of the lethal concentration to the minimum inhibitory concentration (MBC/MIC or MFC/MIC) indicates that the compound exhibits bactericidal or bacteriostatic action, where values greater than 4.0 indicate bacteriostatic action, while values less than 4.0 indicate bactericidal action (Nkanwen et al. 2009). The values were all less than or equal to 4, indicating that the essential oil and β-caryophyllene showed bactericidal and fungicidal action (Table 3).
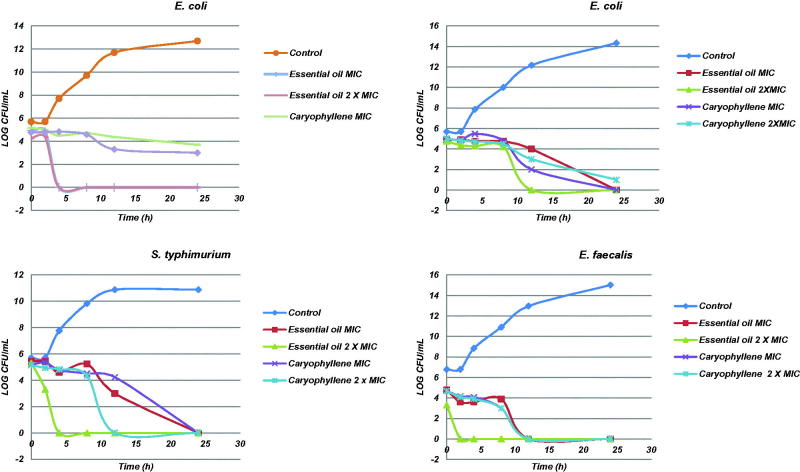
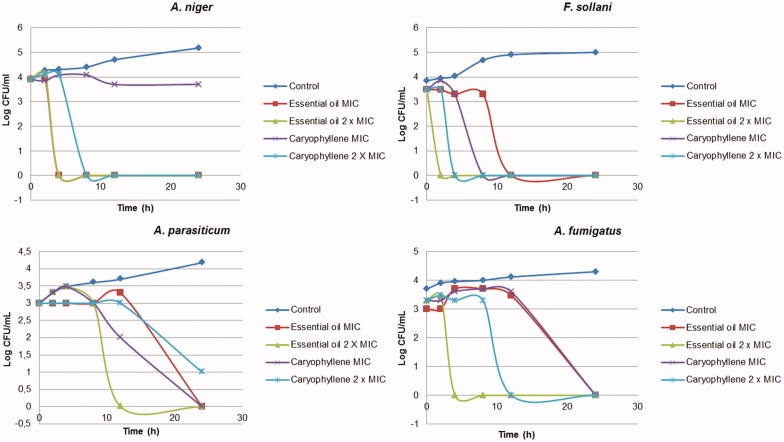
The results of time–kill curves for bacteria and fungi investigated are shown in Figures 2 and 3, respectively. Both essential oil and β-caryophyllene induced rapid bacterial killing with concentrations equal to or above the MIC for all bacteria, except for β-caryophyllene against S. aureus. The time–kill curve study showed that essential oil and β-caryophyllene exhibited a good and rapid fungicidal effect within 2–4 h for F. solani and 4–8 h for A. niger (Figure 3). The results showed a dose-dependent effect for all fungi, whereas the concentration of 2 × MIC led to a more rapid reduction in counts compared with the MIC concentration. This relationship was also observed in previous studies of filamentous fungi (Sumathy et al. 2014).
Figure 2.
Time–kill curve studies of M. paniculata essential oil and β-caryophyllene against S. aureus, E. coli, S. typhimurium and E. faecalis bacteria.
Figure 3.
Time–kill curve studies of essential oil of M. paniculata and β-caryophyllene against the fungi A. niger, F. sollani, A. parasiticum and A. fumigatus.
Figure 4 shows the results of the cytotoxic activity of essential oil of M. paniculata and β-caryophyllene in tumourous cells of hepatocytes and in normal fibroblast cells. On one hand, the essential oil was more cytotoxic on tumour cells than on normal cells, displaying an activity similar to that of camptothecin. On the other hand, β-caryophyllene showed lower activity in both investigated cell lines.
Figure 4.
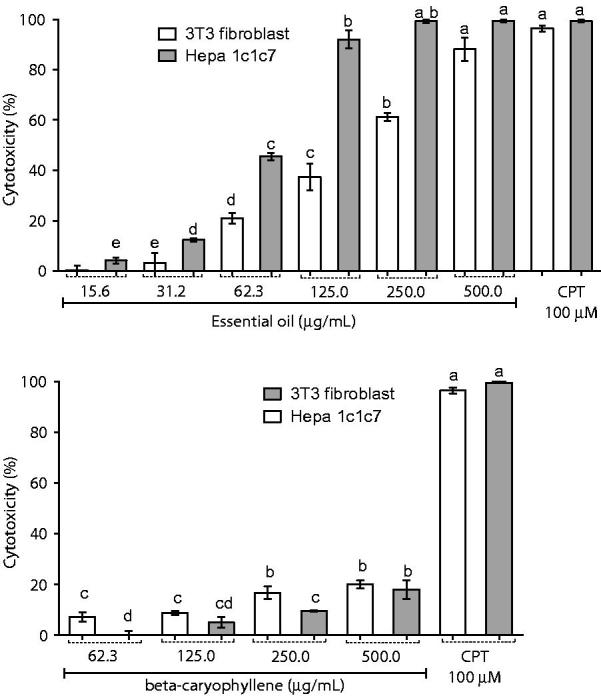
Cytotoxic activity of M. Paniculata essential oil and β-caryophyllene in 3T3 fibroblasts and Hepa 1c1c7, a human hepatoma cell line using the MTT assay. Data are presented as percentage of cell death from three independent experiments. Camptothecin was used as positive control. Different letters correspond to significant difference (p < 0.05); CPT: camptothecin.
Discussion
Similar results of the composition of the essential oil were found in India by Chowdhury et al. (2008) describing the presence of β-caryophyllene and α-caryophyllene among the main components, but not for α zingiberene. On one hand, another study, also made in India with the essential oil of M. paniculata leaves, found α-zingiberene and β-caryophyllene as major compounds representing 10% and 9.7%, respectively (Raina et al. 2006). On the other hand, the oil obtained in previous study does not show any similarity, since the major components were spathulenol (17.7%) and α-pinene (13.2%) (Li et al. 2010). Such similarities and differences may be related to differences in climatic conditions of the regions from which the leaves were collected since those studied by Chowdhury et al. (2008) and Raina et al. (2006) were collected in India, where the climate is tropical and humid subtropical, similar to the predominant climatic conditions in the region of Brazil where the samples of this study were collected. Furthermore, Li et al. (2010) collected the leaves in central China, where a dry continental climate dominates. A previous study reported factors that can alter the production or concentration of secondary metabolites in plants, such as seasonality, circadian rhythm, developmental stage and age, temperature, water availability, UV radiation, soil nutrients, altitude, atmospheric composition and tissue damage (Gobbo-Neto, 2007). Therefore, its understandable to be found differences in the composition of essential oils collected in different countries.
On one hand, the results of present study found poor antioxidant activity. On the other hand, Rodriguez et al. (2012) reported strong antioxidant activity by the essential oil of M. paniculata, in which β-caryophyllene was reported as the main constituent. The criteria used by Rodriguez et al. (2012) to classify the power of action of the essential oil as strong were not the same as those used in this work, which may have caused the discrepancy between the results. β-Caryophyllene showed weak antioxidant activity; thus, it is possible that the good antioxidant action found by Rodriguez et al. (2012) is due to the other constituents of the essential oil of M. paniculata cultivated in Cuba. There is a consensus in the literature that essential oils rich in monoterpenes with aromatic rings attached to hydroxyl groups (phenolics) have good antioxidant activity, while oils rich in sesquiterpene hydrocarbons have weak action. The main constituents of the essential oil obtained in our study are sesquiterpenes (99%), which is consistent with what is reported in the literature (Chowdhury et al. 2008). Antioxidant activity is directly related to the compound's ability to scavenge free radicals, so compounds with little or no chemical grouping with this ability (e.g. hydroxyl groups) will not be able to perform this function. The most abundant compounds in the essential oil of M. paniculata obtained in this experiment do not have hydroxyl groups, which can be the cause of weak antioxidant potential.
Previous studies indicate that Gram-positive bacteria are more susceptible to the action of essential oils due to the greater complexity of the dual cell wall of Gram-negative bacteria in contrast to the simple structure of the cell wall of Gram-positive bacteria (Rios & Recio 2005; Cos et al. 2006); however, the results of the present study indicate that the difference of the cell wall of Gram-positive and Gram-negative bacteria was not a major factor in determining the antimicrobial activity of the essential oil of M. paniculata. However, the characterization of an antimicrobial’s pharmacodynamics for dose optimization can be done using time–kill curve experiments, which provide much more information than a MIC value. While the MIC is a static, one-point in time measurement with a 2-fold variability, time–kill curves provide a profile of antimicrobial activity over time, offer information on the extent of kill and may even be used to detect the presence of resistant subpopulations. The graphs of death kinetics of bacteria have shown that Gram-positive bacteria count was reduced more quickly (4 h for S. aureus and 12 h for E. feacallis) than Gram-negative bacteria (24 h for E. coli and S. typhimurium) in MIC concentrations of essential oil and β-caryophyllene. This result shows a possible relationship between the difference of the cell wall and the mechanism of action of the essential oil and β-caryophyllene; however, this relationship was not observed in the results of the MIC and MBC. As cited before, time–kill curve experiments provide much more information than a MIC value.
Cytotoxicity on host cells is a very important criterion for assessing the selectivity of the observed pharmacological activities and must be always included in parallel (Cos et al. 2006). The essential oil exhibited an IC50 value of 63.73 μg/mL for tumourous cells of hepatocytes and IC50 value of 195.3 μg/mL for the fibroblasts, resulting in a selectivity index (IC50 non-tumourous cells/IC50 tumourous cells) equal to 3, which, according to the classification of Suffness and Pezzuto (1991), is good selectivity. The β-caryophyllene showed low cytotoxic effect at the highest concentration tested, 500 μg/mL, causing the death of about 20% of the fibroblasts and 18% of the hepatocytes. The mechanism of action of the essential oils against cancerous cells seems to be different from the mechanism of action of classical chemotherapeutic agent camptothecin presenting more selective cytotoxicity against cancer cells than on normal cells. Sesquiterpenes, such as elemene and α-bisabolol, are reported to have high selectivity against gliomas (Edris 2007). The essential oil of M. paniculata obtained in our work is rich in sesquiterpenes, which in previous studies showed antiproliferative action (Bardaweel et al. 2014). Furthermore, the essential oil presents high quantity of α-zingiberene, which is described as a compound of high antitumour activity by nucleosomal DNA fragmentation (Lee, 2016). The high selectivity index of the essential oil qualifies it to be investigated in more detail for use in the treatment of cancer, promoting death of cancer cells and preserving normal cells of death, which is a challenge for the current management of neoplasms (Bayala et al. 2014). Based on the obtained results in the MTT assay, it was possible to verify that the cytotoxic effect of the essential oil cannot be attributed solely to its main compound, β-caryophyllene, but may represent a synergistic effect among all the components of the oil.
Conclusions
Experimental data indicate β-caryophyllene and α-zingiberene as main compounds in the M. paniculata essential oil from Brazil and suggested that this plant can be used as a source for isolation of these two substances. The essential oil showed only weak free radical scavenging capacity; however, it exhibited the potential bactericidal and fungicidal activity for the microorganisms tested. In addition, the essential oil showed good selective cytotoxic activity against the hepatoma cells (Hepa 1c1c7). β-Caryophyllene, the major compound, showed significantly lower results than the essential oil in all analyses, indicating that the in vitro biological activities of the essential oil may be due to the synergistic effect of the components of the oil.
Acknowledgements
The authors also would like to thank Tommasi Laboratory for the cooperation in the chromatographic analysis.
Disclosure statement
The authors report no declarations of interest.
Funding
The Fundação de Amparo à Pesquisa do Espírito Santo (FAPES) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) are greatly acknowledged for the financial support.
References
- Abad MJ, Ansuategui M, Bermejo P.. 2007. Active antifungal substances from natural sources. Arkivoc. 7:116–145. [Google Scholar]
- Adams RP.1995. Identification of essential oils components by gas chromatography/quadrupole mass spectroscopy. Carol Stream, IL: Allured Publishing Corporation. [Google Scholar]
- Andrade MS, Ribeiro Ldo P, Borgoni PC, Silva MF, Forim MR, Fernandes JB, Vieira PC, Vendramin JD, Machado MA.. 2016. Essential oil variation from twenty two genotypes of citrus in Brazil-chemometric approach and repellency against Diaphorina citri Kuwayama. Molecules. 21:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardaweel SK, Tawaha KA, Hudaib MM.. 2014. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement Altern Med. 14:2971–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayala B, Bassole IHN, Gnoula C, Nebie R, Yonli A, Morel L, Figueredo G, Nikiema JB, Lobaccaro JM, Simpore J.. 2014. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One. 9:92122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury JU, Bhuiyan MNI, Mohammed Y.. 2008. Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangla J Pharmacol. 3:59–63. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26A; Approved standard, M26-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, M38-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) 2005. Performance standards for antimicrobial susceptibility testing, 15th informational supplement, M100-S15. Wayne, PA. [Google Scholar]
- Cos P, Vlietinck AJ, Berghe DV, Maes L.. 2006. . Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol. 106:290–302. [DOI] [PubMed] [Google Scholar]
- Edris AE.2007. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 21:308–323. [DOI] [PubMed] [Google Scholar]
- Fazal-ur-Rehman MFK, Khan I, Shareef H, Marwat SK.. 2014. Analgesic activity of carbazole alkaloid from Murraya paniculata Linn. (Rutaceae). World Appl Sci J. 32:1631–1636. [Google Scholar]
- Gautam MK, Gangwar M, Nath G, Rao CV, Goel RK.. 2012a. In-vitro antibacterial activity on human pathogens and total phenolic, flavonoid contents of Murraya paniculata Linn. leaves. Asian Pac J Trop Biomed. 2:1660–1663. [Google Scholar]
- Gautam MK, Gangwar M, Singh A, Rao CV, Goel RK.. 2012b. In-vitro antioxidant properties of Murraya paniculata (L.) leaves extract. Inventi Impact: Ethnopharmacol. 2012: 1–3. [Google Scholar]
- Gobbo-Neto LNP.2007. Plantas medicinais: fatores de influência no conteúdo de metabólitos secundários. Quim Nova. 30:374–381. [Google Scholar]
- Holetz FB, Pessini GL, Sanches NR, Cortez DA, Nakamura CV, Filho BP.. 2002. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz. 97:1027–1031. [DOI] [PubMed] [Google Scholar]
- Lee Y.2016. Cytotoxicity evaluation of essential oil and its component from Zingiber officinale Roscoe. Toxicol Res. 32:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Jiang CH, Chu SS, Zuo MX, Liu ZL.. 2010. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules. 15:5831–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv HN, Guo XY, Tu PF, Jiang Y.. 2013. Comparative analysis of the essential oil composition of Murraya paniculata and M. exotica. Nat Prod Commun. 8:1473–1475. [PubMed] [Google Scholar]
- Mosmann T.1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. [DOI] [PubMed] [Google Scholar]
- Nkanwen ER, Gatsing D, Ngamga D, Fodouop SP, Tane P.. 2009. Antibacterial agents from the leaves of Crinum purpurascens herb (Amaryllidaceae). Afr Health Sci. 9:264–269. [PMC free article] [PubMed] [Google Scholar]
- Raina VK, Verma SC, Dhawan SMK, Ramesh S, Singh SC, Yadav A, Srivastava SK.. 2006. Essential oil composition of Murraya exotica from the plains of northern India. Flavour Fragr J. 21:140–142. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C.. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radic Biol Med. 26:1231–1237. [DOI] [PubMed] [Google Scholar]
- Rios JL, Recio MC.. 2005. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 100:80–84. [DOI] [PubMed] [Google Scholar]
- Rodanant P, Khetkam P, Suksamrarn A, Kuvatanasuchati J.. 2015. Coumarins and flavonoid from Murraya paniculata (L.) Jack: antibacterial and anti-inflammation activity. Pak J Pharm Sci. 28:1947–1951. [PubMed] [Google Scholar]
- Rodriguez EJ, Ramis-Ramos G, Heyden YV, Simó-Alfonso EF, Lerma-García MJ, Saucedo-Hernández Y, Monteagudo U, Morales Y, Holgado B, Herrero-Martínez JM.. 2012. Chemical composition, antioxidant properties and antimicrobial activity of the essential oil of Murraya paniculata leaves from the mountains of Central Cuba. Nat Prod Commun. 7:1527–1530. [PubMed] [Google Scholar]
- Saqib F, Ahmed MG, Janbaz KH, Dewanjee S, Jaafar HZ, Zia-Ul-Haq M.. 2015. Validation of ethnopharmacological uses of Murraya paniculata in disorders of diarrhea, asthma and hypertension. BMC Complement Altern Med. 15:3191–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer R, Godoy HT.. 2009. Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food Chem. 112:654–658. [Google Scholar]
- Shah S, Saied S, Mahmood A, Malik A.. 2014. Phytochemical screening of volatile constituents from aerial parts of Murraya paniculata. Pak J Bot. 46:2051–2056. [Google Scholar]
- Suffness M, Pezzuto JM.. 1991. Assays related to cancer drug discovery In: Methods in plant biochemistry, Vol. 6. New York: Academic Press; p. 92. [Google Scholar]
- Sumathy V, Zakaria Z, Jothy SL, Gothai S, Vijayarathna S, Yoga LL, Chen Y, Sasidharan S.. 2014. In vitro and in vivo antifungal activity of Cassia surattensis flower against Aspergillus niger. Microb Pathog. 77:7–12. [DOI] [PubMed] [Google Scholar]
- Sundaram M.2011. Studies on in vitro antibacterial, antifungal property and antioxidant potency of Murraya paniculata. Pak J Nutr. 10:925–929. [Google Scholar]
- Teshima N, Manabe Y, Tada T, Arakawa R, Ban S, Okasyo F, Ju-ichi M, Furukawa H, Ito C.. 2014. Coumarins from the leaves of Murraya paniculata collected at Iriomote Island. Jpn. J Pharmacogn. 68:25–26. [Google Scholar]
- World Health Organization 2002. Traditional medicine strategy 2002–2005. Geneva: WHO. [Google Scholar]
- Wu J, Liu K, Shi X.. 2016. The anti-inflammatory activity of several flavonoids isolated from Murraya paniculata on murine macrophage cell line and gastric epithelial cell (GES-1). Pharm Biol. 54:868–881. [DOI] [PubMed] [Google Scholar]
- Wu L, Li P, Wang X, Zhuang Z, Farzaneh F, Xu R.. 2010. Evaluation of anti-inflammatory and antinociceptive activities of Murraya exotica. Pharm Biol. 48:1344–1353. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Li N, Che YY, Zhang Y, Liang SX, Zhao MB, Jiang Y, Tu PF.. 2011. Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J Pharm Biomed Anal. 56:950–961. [DOI] [PubMed] [Google Scholar]
- Zou J, Yu X, Qu S, Li X, Jin Y, Sui D.. 2014. Protective effect of total flavonoids extracted from the leaves of Murraya paniculata (L.) Jack on diabetic nephropathy in rats. Food Chem Toxicol. 64:231–237. [DOI] [PubMed] [Google Scholar]