Abstract
Context:Paramignya trimera (Oliv.) Burkill (Rutaceae) has been used to treat liver diseases and cancer. However, the anti-inflammatory effects of this medicinal plant and its components have not been elucidated.
Objective: This study investigated chemical constituents of the P. trimera stems and evaluated anti-inflammatory effects of isolated compounds.
Materials and methods: Cytotoxicity of isolated compounds (5–40 μM) toward BV2 cells was tested using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) for 24 h. Inhibitory effects of isolated compounds (5-40 μM) on nitrite and PGE2 concentrations were determined using Griess reaction and PGE2 ELISA kit, respectively (pretreated with the compounds for 3 h and then stimulated for 18 h with LPS). Inhibitory effects of compounds (5-40 μM) on iNOS and COX-2 protein expression were evaluated by Western blot analysis (pretreated with the compounds for 3 h and then stimulated for 24 h with LPS).
Results: Seven coumarins were isolated and identified as: ostruthin (1), ninhvanin (2), 8-geranyl-7-hydroxycoumarin (3), 6-(6′,7′-dihydroxy-3′,7′-dimethylocta-2′-enyl)-7-hydroxycoumarin (4), 6-(7-hydroperoxy-3,7-dimethylocta-2,5-dienyl)-7-hydroxycoumarin (5), 6-(2-hydroxyethyl)-2,2-dimethyl-2H-1-benzopyran (6), and luvangetin (7). Compounds 1–4 and 7 inhibited NO and PGE2 production in LPS-stimulated BV2 cells, with IC50 values ranging from 9.8 to 46.8 and from 9.4 to 52.8 μM, respectively. Ostruthin (1) and ninhvanin (2) were shown to suppress LPS-induced iNOS and COX-2 protein expression.
Discussion and conclusion: The present study provides a scientific rationale for the use of P. trimera in the prevention and treatment of neuroinflammatory diseases. Ostruthin and ninhvanin might have potential therapeutic effects and should be considered for further development as new anti-neuroinflammatory agents.
Keywords: Rutaceae; BV2 microglia; ostruthin; ninhvanin; 8-geranyl-7-hydroxycoumarin; 6-(6′,7′-dihydroxy-3′,7′-dimethylocta-2′-enyl)-7-hydroxycoumarin; 6-(7-hydroperoxy-3,7-dimethylocta-2,5-dienyl)-7-hydroxycoumarin; 6-(2-hydroxyethyl)-2,2-dimethyl-2H-1-benzopyran; luvangetin
Introduction
Paramignya trimera (Oliv.) Burkill (Rutaceae) is a woody shrub, mostly distributed in the Southern regions of Vietnam. In the Vietnamese traditional medicine, P. trimera is well known as a medicinal plant used to treat liver diseases and cancer (Cuong et al. 2015; Nguyen et al. 2015). Recent pharmacological studies have shown that P. trimera possesses antioxidant activity and its crude methanol extract and hexane fraction display moderate cytotoxic effects toward several cancer cell lines (Nguyen et al. 2013). Previous chemical investigation of the stems and roots of P. trimera demonstrated the presence of several coumarins (Cuong et al. 2013, 2015). In this study, we report the isolation and structural elucidation of seven coumarins (1–7) from the methanol extract of the stems of P. trimera.
Microglia are the resident immune cells in the central nervous system (CNS) that play an important role in response to neuronal damage and removal of the damaged cells. In response to extracellular stimuli, including LPS, microglia are activated and produce pro-inflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2) and cytokines (Napoli & Neumann 2009). However, prolonged and excessive production of pro-inflammatory mediators by microglia appear to contribute to neuronal cell death (Gonzalez-Scarano & Baltuch 1999). Uncontrolled or aberrant activation of microglia was shown to be the cause of severe neuronal disorders, including Alzheimer’s disease, Pakinson’s disease, and Huntington’s disease (Zindler & Zipp 2010). Therefore, downregulation of pro-inflammatory mediators in microglia could be considered as an important target for the therapeutic approach of neurodegenerative diseases.
NO and PGE2, the inflammatory products of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), are considered as the major factors in the inflammatory response. Excessive production of these pro-inflammatory mediators was shown to be the cause of the neurodegenerative diseases (Nathan & Hibbs 1991; Shi et al. 2012). In order to discover anti-neurodegenerative compounds, we are looking for natural products-derived biomaterial with inhibitory effects of NO and PGE2 production in LPS-stimulated BV2 microglia cells. In this paper, we report the inhibitory effects of compounds 1–4, 6, and 7 on NO and PGE2 production and iNOS and COX-2 protein expression in LPS-stimulated BV2 microglia cells. Furthermore, the protective effects of these compounds on glutamate-induced mouse hippocampal HT22 cell injury were also reported.
Materials and methods
General experimental procedures
1D and 2D NMR experiments (1H, 13C, HSQC, and HMBC) were recorded on a Bruker AM500 FTNMR spectrometer: 500 MHz (1H NMR), 125 MHz (13C NMR). The electrospray ionization (ESI) mass spectra were recorded on an AGILENT 1200 LC-MSD trap spectrometer. Column chromatography (CC) was performed using silica gel (0.040–0.063 mm, Merck) and RP-18 resins (30–50 μm, Fuji Silysia Chemical Ltd.). Thin layer chromatography (TLC) was performed on DC-Alufolien 60 F254 (1.05715, Merck) and RP18 F254s (Merck) plates.
Plant material
The stems of P. trimera were collected in Khanh Hoa province, Vietnam during December 2013, and identified by one of the authors, Dr. Luu Hong Truong. A voucher specimen (NCCT-TR.01) was deposited at the Herbarium of the Institute of Marine Biochemistry, VAST.
Extraction and isolation
The dried stems of Paramignya trimera (3 kg) were ground and extracted with MeOH under sonication at room temperature. After concentration under reduced pressure, the MeOH extract (150 g) was suspended in water and then partitioned successively with CHCl3 and EtOAc to give CHCl3-, EtOAc-, and water-soluble fractions. The CHCl3-soluble fraction (60 g) was subjected to fractionation over silica gel, eluting with EtOAc in n-hexane (1-100%, step-wise) to give fractions TR1A-E. Fraction TR1A was separated by column chromatography (CC) over silica gel, using CH2Cl2-MeOH (26:1, v/v) as eluent to yield 5 (8 mg). Fraction TR1C was subjected to silica gel CC and eluted with n-hexane-EtOAc (7:1, v/v) to provide 1 (50 mg) and 2 (20 mg). Fraction TR1E was chromatographed over a silica gel column, eluting with n-hexane-acetone (9:2, v/v), and further purified by silica gel CC, using n-hexane-acetone (3:1, v/v) as eluent to give 6 (8 mg) and 7 (8 mg). The EtOAc-soluble fraction (40 g) was subjected to reversed phase (RP) C18 CC, eluting with a gradient of acetone in water (1:3–2:1, v/v) to give fractions TR2A-C. Fraction TR2A was separated by silica gel CC, using CH2Cl2-MeOH (15:1, v/v) as eluent to provide 4 (8 mg). Fraction TR2B was separated by silica gel CC, eluting with n-hexane-acetone (3:1, v/v) to give 3 (10 mg).
Ostruthin (1)
White, amorphous powder; 1H NMR (CDCl3, 500 MHz): δ 6.23 (d, J = 9.5 Hz, H-3), 7.65 (d, J = 9.5 Hz, H-4), 7.20 (s, H-5), 7.07 (s, H-8), 3.39 (d, J = 7.0 Hz, H2-1′), 5.34 (t, J = 7.0 Hz, H-2′), 2.10 (m, H2-4′), 2.12 (m, H2-5′), 5.10 (t, J = 6.5 Hz, H-6′), 1.68 (s, H3-8′), 1.60 (s, H3-9′), 1.73 (s, H3-10′); 13C NMR (CDCl3, 125 MHz): δ 162.6 (C-2), 111.9 (C-3), 144.5 (C-4), 128.1 (C-5), 126.1 (C-6), 158.9 (C-7), 103.0 (C-8), 154.1 (C-9), 112.1 (C-10), 28.1 (C-1′), 120.9 (C-2′), 138.3 (C-3′), 39.7 (C-4′), 26.5 (C-5′), 123.9 (C-6′), 131.7 (C-7′), 25.7 (C-8′), 17.7 (C-9′), 16.1 (C-10′).
Ninhvanin (2)
White, amorphous powder; 1H NMR (CDCl3, 500 MHz): δ 6.22 (d, J = 9.5 Hz, H-3), 7.60 (d, J = 9.5 Hz, H-4), 6.95 (s, H-5), 3.37 (d, J = 7.5 Hz, H2-1′), 5.32 (t, J = 7.5 Hz, H-2′), 2.09 (m, H2-4′), 2.13 (m, H2-5′), 5.11 (t, J = 6.5 Hz, H-6′), 1.68 (s, H3-8′), 1.60 (s, H3-9′), 1.71 (s, H3-10′), 4.10 (s, 8-OCH3); 13C NMR (CDCl3, 125 MHz): δ 160.7 (C-2), 112.2 (C-3), 144.4 (C-4), 122.3 (C-5), 125.2 (C-6), 150.2 (C-7), 133.1 (C-8), 145.3 (C-9), 112.2 (C-10), 27.5 (C-1′), 120.8 (C-2′), 137.4 (C-3′), 39.6 (C-4′), 26.5 (C-5′), 124.0 (C-6′), 131.4 (C-7′), 25.6 (C-8′), 17.6 (C-9′), 16.0 (C-10′), 61.6 (8-OCH3).
8-Geranyl-7-hydroxycoumarin (3)
White, amorphous powder; 1H NMR (CD3OD, 500 MHz): δ 6.18 (d, J = 9.5 Hz, H-3), 7.85 (d, J = 9.5 Hz, H-4), 7.31 (d, J = 8.5 Hz, H-5), 6.83 (d, J = 8.5 Hz, H-6), 3.53 (d, J = 7.0 Hz, H2-1′), 5.26 (t, J = 7.0 Hz, H-2′), 1.98 (m, H2-4′), 2.06 (m, H2-5′), 5.03 (t, J = 7.0 Hz, H-6′), 1.57 (s, H3-8′), 1.53 (s, H3-9′) 1.85 (s, H3-10′); 13C NMR (CD3OD, 125 MHz): δ 164.0 (C-2), 111.4 (C-3), 146.6 (C-4), 127.6 (C-5), 113.8 (C-6), 161.1 (C-7), 117.0 (C-8), 154.8 (C-9), 113.1 (C-10), 22.6 (C-1′), 122.8 (C-2′), 136.5 (C-3′), 40.8 (C-4′), 27.5 (C-5′), 125.3 (C-6′), 132.0 (C-7′), 25.7 (C-8′), 17.6 (C-9′), 16.4 (C-10′).
6-(6′,7′-Dihydroxy-3′,7′-dimethylocta-2′-enyl)-7-hydroxycoumarin (4)
White, amorphous powder; HRESIMS: m/z 331.1571 [M-H]- (calcd. for C19H23O5, 331.1545); 1H NMR (CD3OD, 500 MHz): δ 6.17 (d, J = 9.5 Hz, H-3), 7.83 (d, J = 9.5 Hz, H-4), 7.30 (s, H-5), 6.72 (s, H-8), 3.34 (d, J = 7.5 Hz, H2-1′), 5.42 (t, J = 7.5 Hz, H-2′), 2.12 (m, H-4′a), 2.34 (m, H-4′b), 1.43 (m, H-5′a), 1.81 (m, H-5′b), 3.29 (dd, J = 1.5, 10.5 Hz, H-6′), 1.18 (s, H3-8′), 1.15 (s, H3-9′), 1.75 (s, H3-10′); 13C NMR (CD3OD, 125 MHz): δ 164.0 (C-2), 112.0 (C-3), 146.3 (C-4), 129.4 (C-5), 127.9 (C-6), 160.8 (C-7), 102.6 (C-8), 155.4 (C-9), 112.9 (C-10), 28.6 (C-1′), 123.1 (C-2′), 137.8 (C-3′), 37.8 (C-4′), 30.6 (C-5′), 78.9 (C-6′), 73.7 (C-7′), 25.7 (C-8′), 24.9 (C-9′), 16.2 (C-10′).
6-(7-Hydroperoxy-3,7-dimethylocta-2,5-dienyl)-7-hydroxycoumarin (5)
White, amorphous powder; 1H NMR (CD3OD, 500 MHz): δ 6.14 (d, J = 9.5 Hz, H-3), 7.83 (d, J = 9.5 Hz, H-4), 7.29 (s, H-5), 6.71 (s, H-8), 3.37 (d, J = 7.5 Hz, H2-1′), 5.42 (t, J = 7.5 Hz, H-2′), 2.80 (d, J = 3.5 Hz, H2-4′), 5.66 (overlapped, H-5′ and H-6′), 1.31 (s, H3-8′ and H3-9′), 1.73 (s, H3-10′); 13C NMR (CD3OD, 125 MHz): δ 164.2 (C-2), 111.5 (C-3), 146.3 (C-4), 129.3 (C-5), 128.0 (C-6), 162.0 (C-7), 102.7 (C-8), 155.6 (C-9), 112.6 (C-10), 28.6 (C-1′), 123.9 (C-2′), 136.6 (C-3′), 43.6 (C-4′), 129.4 (C-5′), 137.0 (C-6′), 82.4 (C-7′), 24.9 (C-8′ and C-9′), 16.2 (C-10′).
6-(2-Hydroxyethyl)-2,2-dimethyl-2H-1-benzopyran (6)
White, amorphous powder; 1H NMR (CD3OD, 500 MHz): δ 5.60 (d, J = 10.0 Hz, H-3), 6.28 (d, J = 10.0 Hz, H-4), 6.82 (d, J = 2.5 Hz, H-5), 6.94 (dd, J = 2.5, 8.5 Hz, H-7), 6.71 (d, J = 8.5 Hz, H-8), 2.75 (t, J = 6.5 Hz, H2-11), 3.79 (t, J = 6.5 Hz, H2-12), 1.42 (s, 2-(CH3)2); 13C NMR (CD3OD, 125 MHz): δ 76.0 (C-2), 130.9 (C-3), 122.1 (C-4), 126.7 (C-5), 130.4 (C-6), 129.4 (C-7), 116.3 (C-8), 151.5 (C-9), 121.2 (C-10), 38.3 (C-11), 63.7 (C-12), 27.9 (2-(CH3)2).
Luvangetin (7)
White, amorphous powder; 1H NMR (CDCl3, 500 MHz): δ 6.24 (d, J = 9.5 Hz, H-3), 7.57 (d, J = 9.5 Hz, H-4), 6.82 (s, H-5), 6.33 (d, J = 10.0 Hz, H-1′), 5.71 (d, J = 10.0 Hz, H-2′), 1.51 (s, H3-4′ and H3-5′), 3.98 (s, 8-OCH3); 13C NMR (CDCl3, 125 MHz): δ 160.6 (C-2), 113.0 (C-3), 143.5 (C-4), 113.2 (C-4a), 119.0 (C-5 and C-6), 149.3 (C-7), 135.6 (C-8), 148.3 (C-8a), 121.0 (C-1′), 131.2 (C-2′), 77.7 (C-3′), 28.2 (C-4′ and C-5′), 61.4 (8-OCH3).
Cell culture and viability assay
BV2 microglia cells were obtained from Prof. Hyun Park at Wonkwang University (Iksan, Korea) and mouse hippocampal HT22 cells were received from Dr. Inhee-Mook Seoul National University (Seoul, Korea). The cells were maintained at 5 × 105 cells/mL in 100-mm dishes in DMEM supplemented with 10% heat-inactivated FBS, penicillin G (100 units/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM), and were incubated at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. The effects of the various experimental modulations on cell viability were evaluated by determining the mitochondrial reductase function based on reduction of the tetrazolium salt, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to a formazan crystal (Berridge & Tan 1993). The formation of formazan is proportional to the number of functional mitochondria in the living cells. For the determination of cell viability, cells (2 × 104 cells/200 μL in each well of the 96-well plates) were incubated with MTT at a final concentration of 0.5 mg/mL for 4 h. The formazan formed was dissolved in acidic 2-propanol, and the optical density was measured at 590 nm. The optical density of the formazan formed in the control (untreated) cells was considered as 100% viability. For determination of cytoprotective effects, HT22 cells (5 × 105 cells/mL in DMEM medium) were treated with compounds in the presence of 20 mM glutamate and incubated for indicated times and cell viability by MTT assay.
Nitrite (NO production) determination
The production of nitrite, the stable end product of NO oxidation, was used as a measure of iNOS activity. The nitrite present in the conditioned media was determined by a method based on the Griess reaction (Titheradge 1998). Briefly, the cells were pretreated for 3 h with different concentrations of compounds and then stimulated for 18 h with LPS (1 μg/mL). Each cell supernatant (100 μL) was mixed with an equal volume of the Griess reagent (Solution A: 222488; Solution B: S438081, Sigma), and the absorbance of the mixture at 525 nm was measured using an ELISA plate reader. The natural product-derived anti-inflammatory agent, butein was used as the positive control.
PGE2 assay
BV2 microglial cells were cultured in 24-well plates, pre-incubated for 3 h with different concentrations of compounds, and then stimulated for 18 h with LPS (Sigma-Aldrich). Supernatant of the culture media (100 μL) was collected to determine the PGE2 concentration using an ELISA kit (R & D Systems). Butein was used as the positive control.
Western blot analysis
BV2 cells were lysed in 20 mM Tris-HCl buffer (pH 7.4) containing a protease inhibitor mixture (0.1 mM PMSF, 5 mg/mL aprotinin, 5 mg/mL pepstatin A, and 1 mg/mL chymostatin). The protein concentration was determined using the Lowry protein assay kit (P5626; Sigma). An equal amount of protein from each sample was resolved using 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then electrophoretically transferred to the Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA, USA). The membrane was blocked using 5% skim milk and subsequently incubated with the primary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and horseradish peroxidase-conjugated secondary antibody, followed by ECL detection (Amersham Corp., Arlington Heights, IL).
Statistical analysis
Data expressed as the mean ± standard deviation (SD) of at least three independent experiments. Three or more groups were compared using one-way analysis of variance followed by the Newman-Keuls post hoc test. Statistical analysis was performed using GraphPad Prism software, version 3.03 (GraphPad Software Inc, San Diego, CA).
Results and discussion
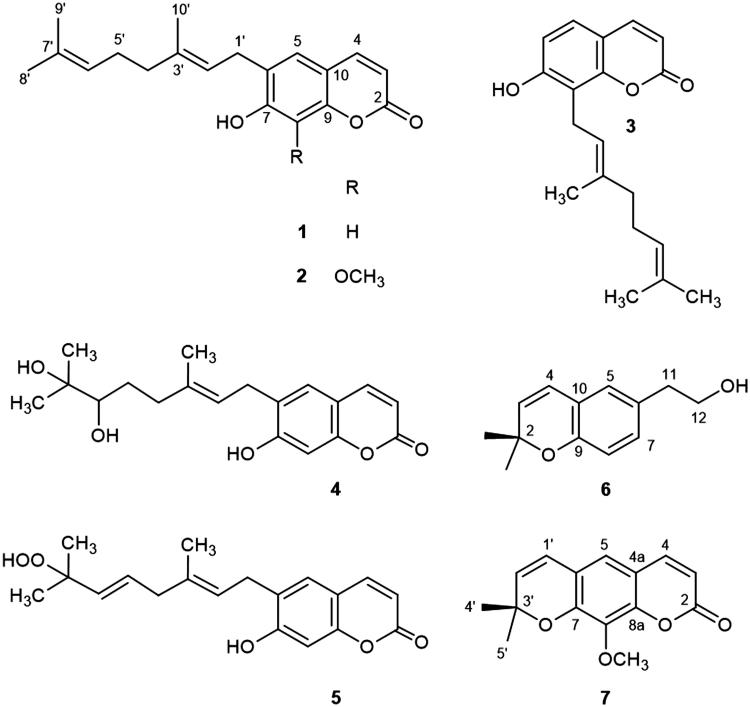
A MeOH extract of P. trimera was suspended in H2O and successively partitioned with CHCl3 and EtOAc to give CHCl3-, EtOAc-, and H2O-soluble fractions. The CHCl3- and EtOAc-soluble fractions were subjected to multiple chromatographic steps over silica gel and reversed phase C18, yielding compounds 1–7. The structures of these compounds were identified as: ostruthin (1) (Liu et al. 2005), ninhvanin (2) (Cuong et al. 2013), 8-geranyl-7-hydroxycoumarin (3) (Rashid et al. 1992), 6-(6′,7′-dihydroxy-3′,7′-dimethylocta-2′-enyl)-7-hydroxycoumarin (4) (Nguyen et al. 2016), 6-(7-hydroperoxy-3,7-dimethylocta-2,5-dienyl)-7-hydroxycoumarin (5) (Rashid et al. 1992), 6-(2-hydroxyethyl)-2,2-dimethyl-2H-1-benzopyran (6) (Wattanapiromsakul & Waterman 2000), and luvangetin (7) (Patra & Mitra 1981) by analysis of the 1D and 2D NMR spectroscopic data and comparison with the known values (Figure 1). It is noted that this is the first time to report the isolation of compounds 3–5 and 7 from the Paramignya genus and the NMR data for compound 4. Coumarin is a secondary phytochemical that belongs to the benzopyrone chemical class. Coumarin has been shown to possess various pharmacological properties such as anti-inflammatory, anticoagulant, antibacterial, antifungal, antiviral, anticancer, antihypertensive, antitubercular, anticonvulsant, antiadipogenic, antihyperglycemic, antioxidant, and neuroprotective activities (Venugopala et al. 2013). With the broad range of pharmacological effects, along with low toxicity and occurrence in many herbal remedies, coumarins appear as the promising target for discovering and developing novel therapeutic agents. Previous studies on the pharmacological effects of the isolated compounds have shown that ostruthin (1) is cytotoxic toward two human pancreatic cancer cell lines, including PANC-1 and PSN-1 cells in nutrient-deprived medium (Li et al. 2012), this compound was shown to inhibit vascular smooth muscle cell proliferation (Joa et al. 2011), inhibit acetylcholinesterase activity (Urbain et al. 2005), and display antimycobacterial activity (Schinkovitz et al. 2003); luvangetin (7) was shown to have significant protection against pylorus-ligated and aspirin-induced gastric ulcers in rats and cold restraint stress-induced gastric ulcers in rats and guinea pigs (Goel et al. 1997). However, there are no reports on the anti-inflammatory effects of the compounds 1–7 so far. Therefore, the anti-inflammatory effects of the isolated compounds were evaluated through inhibition of NO and PGE2 production and attenuation of iNOS and COX-2 protein expression in LPS-stimulated BV2 cells. Because compound 5 was decomposed, so it was excluded out of the evaluation of biological effects of the isolated compounds.
Figure 1.
Chemical structures of compounds 1–7 from P. trimera.
Microglia are important immune cells in the CNS because microglial activation initiate an inflammatory cascade and is known to trigger various neurodegenerative diseases. In this context, the control of microglial activation has been postulated as a putative target in the treatment of neurodegenerative diseases (Dheen et al. 2007). Therefore, we use LPS-stimulated BV2 microglia as a screening system to look for anti-neuroinflammatory natural compounds.
To exclude the possibility that our results could be misinterpreted by cytotoxic effects, we used the MTT assay to evaluate the viability of BV2 cells treated with various concentrations (5–40 μM) of isolated compounds. As the result, all of the tested compounds did not exhibit significant cytotoxicity in BV2 microglia cells at the tested concentrations (Figure S1, Supplementary material). Thus, for all subsequent experiments, the concentrations of the tested compounds were used within the range of 5–40 μM.
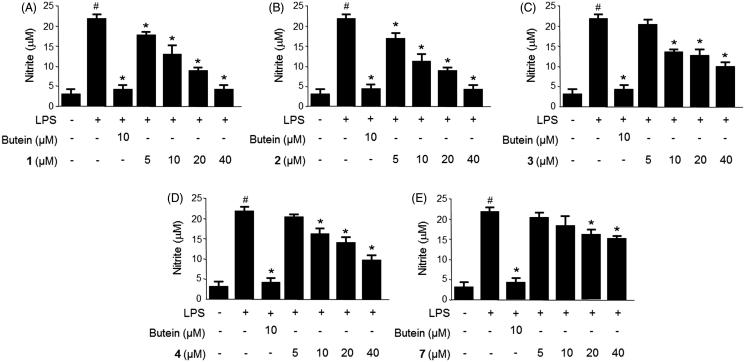
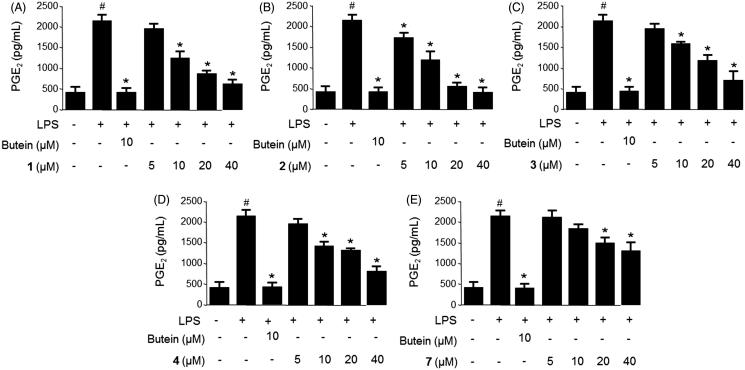
The microglia stimulants, including LPS were shown to induce neurotoxicity through the generation of pro-inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2) and pro-inflammatory cytokines (e.g. tumor necrosis factor-α), interleukins, and cytotoxic factors (Hammond et al. 1999; Brown & Neher 2010; Kang et al. 2012). Therefore, the levels of NO and PGE2 production in the LPS-stimulated migroglia may reflect the degree of inflammation and provide information to investigate the anti-inflammatory effects of the natural compounds (Chun et al. 2012). The anti-inflammatory effects of compounds 1–4, 6, and 7 were initially evaluated through inhibition of NO and PGE2 production in LPS-stimulated BV2 cells. BV2 cells were pretreated for 3 h with different concentrations (5–40 μM) of compounds 1–4, 6, and 7 followed by treatment with LPS (1 μg/mL) for 18 h. As shown in Figure 2, treatment of BV2 cells with LPS triggered an approximate seven-fold increase in nitrite concentration compared with that of the untreated group. When pretreated the cells with compounds 1–4, and 7 for 3 h, the production of NO, as indicated by the nitrite concentration, was decreased in a dose-dependent manner, with IC50 values ranging from 9.8–46.8 μM (Table 1). The PGE2 concentration was also increased approximately eight-fold compared with that of the untreated group when treating cells with LPS (Figure 3). However, pretreatment of the cells with compounds 1–4, and 7 for 3 h decreased the production of PGE2 in a dose-dependent manner, with IC50 values in the range of 9.4–52.8 μM (Table 1). Compound 6 displayed no inhibition on NO and PGE2 production at the tested concentration range. Considering the inhibitory effects of compounds 1–4, and 7 on the NO and PGE2 production, we next investigated the inhibitory effects of compounds 1 and 2 on iNOS and COX-2 protein expression in LPS-stimulated BV2 cells.
Figure 2.
Effects of compounds 1–4, and 7 on nitrite production in LPS-stimulated BV2 microglia (A−E). Cells were pretreated for 3 h with the indicated concentrations of the compounds, then stimulated for 18 h with LPS (1 μg/mL). The concentrations of nitrite were determined using a Griess reaction. Data represent the mean ± S.D. of three experiments. #p < 0.05, as compared with the control group; *p < 0.05, as compared with the group treated with LPS only. Butein was used as the positive control.
Table 1.
Inhibitory effects of compounds 1–4, 6, and 7 on NO and PGE2 production.
| IC50 (μM) |
||
|---|---|---|
| Compound | NO | PGE2 |
| 1 | 12.3 ± 0.6 | 13.4 ± 0.7 |
| 2 | 9.8 ± 0.5 | 9.4 ± 0.5 |
| 3 | 36.8 ± 1.8 | 34.7 ± 1.7 |
| 4 | 36.5 ± 1.8 | 32.1 ± 1.6 |
| 6 | >80a | >80a |
| 7 | 46.8 ± 2.3 | 52.8 ± 2.6 |
A compound is considered inactive with IC50 > 80 μM.
The values are mean ± SD (n = 3).
Figure 3.
Effects of compounds 1–4, and 7 on PGE2 production in LPS-stimulated BV2 microglia (A−E). Cells were pretreated for 3 h with the indicated concentrations of the compounds and then stimulated for 18 h with LPS (1 μg/mL). The concentrations of PGE2 were determined using a PGE2 ELISA kit. Data represent the mean ± S.D. of three experiments. #p < 0.05, as compared with the control group; *p < 0.05, as compared with the group treated with LPS only. Butein was used as the positive control.
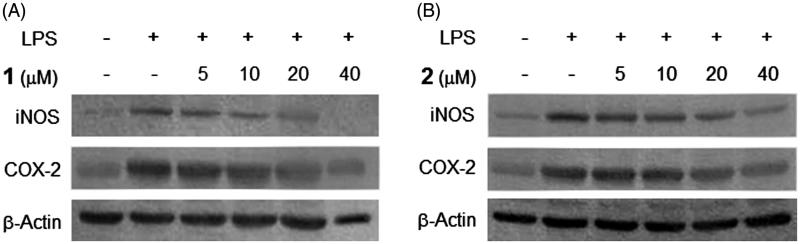
NO and PGE2 are pro-inflammatory mediators produced by their inducible enzymes iNOS and COX-2, respectively. These mediators play key roles in the activation of macrophages during the inflammation response (Korhonen et al. 2005; Zhou et al. 2008). Therefore, suppression of NO and PGE2 production by inhibition of iNOS and COX-2 protein expression could be considered as a therapeutic approach for anti-inflammatory diseases. The effects of compounds 1 and 2 on iNOS and COX-2 protein expression in BV2 cells were assessed by Western blot analysis. As shown in Figure 4, the expression of iNOS and COX-2 proteins was significantly up-regulated in response to LPS (1 μg/mL), however, compounds 1 and 2 suppressed the LPS-induced expression of iNOS and COX-2 in a concentration-dependent manner, respectively. These results suggest that these compounds inhibit pro-inflammatory mediators through suppressing iNOS and COX-2 protein expression in LPS-stimulated BV2 cells. The housekeeping protein, β-actin was shown to be unchanged by the presence of compounds 1 and 2 at the same concentrations.
Figure 4.
Effects of compounds 1 and 2 on iNOS and COX-2 protein expression in LPS-stimulated BV2 microglia. Cells were pretreated for 3 h with indicated concentrations of compounds 1 and 2, then stimulated for 24 h with LPS (1 μg/mL). Western blot analyses (A and B) were performed as described in ‘Materials and methods’ section.
NF-κB, a major transcription factor, plays a key role in the regulation of the inflammatory response. It is well known that NF-κB is mainly regulated by Toll like receptor 4 (TLR4) (Fitzgerald et al. 2003; Oeckinghaus et al. 2011). Down-stream of CD14, MyD88, TRIF, TRAF6, and TAK1 also conduct an important role in the regulation of phosphorylation of IκB and NF-kB heterodimers (p50 and p65) (Zhang & Ghosh 2001). Once stimulated by inflammatory signals such as LPS, IL-1β, and TNF-α, IκB is phosphorylated and degraded resulting in liberation of NF-κB (Karin & Ben-Neriah 2000; Lappas et al. 2002). Afterward, NF-κB p50/p65 heterodimers translocate to the nucleus and bind to the κB sites to control the transcription of the target genes, triggering expression of pro-inflammatory enzymes and cytokines such as iNOS, COX-2, TNF-α, IL-β, etc. Subsequently, NO and PGE2 are produced by iNOS and COX-2, respectively. Accordingly, when iNOS and COX-2 protein expression is inhibited by the tested compounds, production levels of NO and PGE2 are also decreased. In this study, we investigated the anti-inflammatory effects of the compounds via regulation of NO and PGE2 production. The mechanism of action of the compounds on NF-κB signalling may be conducted in a further study.
The cytoprotective effects of compounds 1–4, 6, and 7 were evaluated on the glutamate-induced toxicity in mouse hippocampal HT22 cells. However, all of the isolated compounds did not show any significant protection.
In summary, chemical investigation of the stems of P. trimera resulted in the isolation and identification of seven coumarin derivatives, including: ostruthin (1), ninhvanin (2), 8-geranyl-7-hydroxycoumarin (3), 6-(6′,7′-dihydroxy-3′,7′-dimethylocta-2′-enyl)-7-hydroxycoumarin (4), 6-(7-hydroperoxy-3,7-dimethylocta-2,5-dienyl)-7-hydroxycoumarin (5), 6-(2-hydroxyethyl)-2,2-dimethyl-2H-1-benzopyran (6), and luvangetin (7). Although some pharmacological effects of several coumarins have been reported as aforementioned, their anti-inflammatory effects in LPS-stimulated BV2 microglia and protective activity against glutamate-induced toxicity in HT22 cells have yet to be elucidated. In addition to the first report of compounds 3–5 and 7 from the Paramignya genus and the NMR data of compound 4, the present study reported the anti-inflammatory effects of the isolated coumarins for the first time. The results demonstrated that compounds 1–4, and 7 inhibited the NO and PGE2 production in LPS-stimulated BV2 cells in a dose-dependent manner. Furthermore, the inhibitory effects of compounds 1 and 2 on NO and PGE2 production were confirmed by the inhibition of LPS-induced iNOS and COX-2 expression. These results provide a scientific rationale for the use of P. trimera in the prevention and treatment of neuroinflammatory diseases and suggest that ostruthin (1) and ninhvanin (2) should be considered as lead compounds for further development as new anti-neuroinflammatory agents.
Supplementary Material
Disclosure statement
The authors report no declarations of interest.
References
- Berridge MV, Tan AS.. 1993. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 303:474–482. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ.. 2010. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 41:242–247. [DOI] [PubMed] [Google Scholar]
- Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, Lee DU, Kim YS.. 2012. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 14:375–383. [DOI] [PubMed] [Google Scholar]
- Cuong NM, Duc HV, Tai NV, Khanh PN, Ha VT, Huong TT, Nhat ND.. 2013. Initial research on chemical composition of Paramignya trimera. Tap Chi Hoa Hoc. 51:292–296. [Google Scholar]
- Cuong NM, Huong TT, Khanh PN, Tai NV, Ha VT, Son NT, Tai BH, Kim YH.. 2015. Paratrimerins A and B, two new dimeric monoterpene-linked coumarin glycosides from the roots and stems of Paramignya trimera. Chem Pharm Bull (Tokyo). 63:945–949. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA.. 2007. Microglial activation and its implications in the brain diseases. Curr Med Chem. 14:1189–1197. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT.. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 198:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel RK, Maiti RN, Manickam M, Ray AB.. 1997. Antiulcer activity of naturally occurring pyrano-coumarin and isocoumarins and their effect on prostanoid synthesis using human colonic mucosa. Indian J Exp Biol. 35:1080–1083. [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G.. 1999. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 22:219–240. [DOI] [PubMed] [Google Scholar]
- Hammond RA, Hannon R, Frean SP, Armstrong SJ, Flower RJ, Bryant CE.. 1999. Endotoxin induction of nitric oxide synthase and cyclooxygenase-2 in equine alveolar macrophages. Am J Vet Res. 60:426–431. [PubMed] [Google Scholar]
- Joa H, Vogl S, Atanasov AG, Zehl M, Nakel T, Fakhrudin N, Heiss EH, Picker P, Urban E, Wawrosch C, et al. . 2011. Identification of ostruthin from Peucedanum ostruthium rhizomes as an inhibitor of vascular smooth muscle cell proliferation. J Nat Prod. 74:1513–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CH, Jayasooriya RG, Dilshara MG, Choi YH, Jeong YK, Kim ND, Kim GY.. 2012. Caffeine suppresses lipopolysaccharide-stimulated BV2 microglial cells by suppressing Akt-mediated NF-κB activation and ERK phosphorylation . Food Chem Toxicol. 50:4270–4276. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y.. 2000. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E.. 2005. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 4:471–479. [DOI] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Georgiou HM, Rice GE.. 2002. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 67:668–673. [DOI] [PubMed] [Google Scholar]
- Li F, Okamura Y, Dibwe DF, Awale S, Kadota S, Tezuka Y.. 2012. Anti-austerity agents from Rhizoma et Radix Notopterygii (Qianghuo). Planta Med. 78:796–799. [DOI] [PubMed] [Google Scholar]
- Liu R, Sun Q, Shi Y, Kong L.. 2005. Isolation and purification of coumarin compounds from the root of Peucedanum decursivum (Miq.) Maxim by high-speed counter-current chromatography. J Chromatogr A. 1076:127–132. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H.. 2009. Microglial clearance function in health and disease. Neuroscience. 158:1030–1038. [DOI] [PubMed] [Google Scholar]
- Nathan CF, Hibbs JB. Jr.. 1991. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 3:65–70. [DOI] [PubMed] [Google Scholar]
- Nguyen MK, Pham TNH, Do TP.. 2013. Study on acute toxicity, hepatoprotective activity and cytotoxic activity of Paramignya trimera (Oliv.) Guillaum. Tap Chi Duoc Lieu. 1:18. [Google Scholar]
- Nguyen TP, Le TD, Minh PN, Dat BT, Pham NK, Do TM, Nguyen DT, Mai TD.. 2016. A new dihydrofurocoumarin from the fruits of Pandanus tectorius Parkinson ex Du Roi. Nat Prod Res. 30:2389–2395. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Bowyer MC, Vuong QV, Altena IAV, Scarlett CJ.. 2015. Phytochemicals and antioxidant capacity of Xao tam phan (Paramignya trimera) root as affected by various solvents and extraction methods. Ind Crops Prod. 67:192–200. [Google Scholar]
- Oeckinghaus A, Hayden MS, Ghosh S.. 2011. Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708. [DOI] [PubMed] [Google Scholar]
- Patra A, Mitra AK.. 1981. Carbon-13 NMR signals of some natural coumarins and their derivatives. Org Magn Resonance. 17:222–224. [Google Scholar]
- Rashid MA, Armstrong JA, Gray AI, Waterman PG.. 1992. Novel C-geranyl 7-hydroxycoumarins from the aerial parts of Eriostemon tomentellus. Z Naturforsch. 47b:284–287. [Google Scholar]
- Schinkovitz A, Gibbons S, Stavri M, Cocksedge MJ, Bucar F.. 2003. Ostruthin: an antimycobacterial coumarin from the roots of Peucedanum ostruthium. Planta Med. 69:369–371. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang Q, Johansson JU, Liang X, Woodling NS, Priyam P, Loui TM, Merchant M, Breyer RM, Montine TJ, et al. . 2012. Inflammatory prostaglandin E2 signaling in a mouse model of Alzheimer disease. Ann Neurol. 72:788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titheradge MA.1998. The enzymatic measurement of nitrate and nitrite. Methods Mol Biol. 100:83–91. [DOI] [PubMed] [Google Scholar]
- Urbain A, Marston A, Hostettmann K.. 2005. Coumarins from Peucedanum ostruthium as inhibitors of acetylcholinesterase. Pharm Biol. 43:647–650. [Google Scholar]
- Venugopala KN, Rashmi V, Odhav B.. 2013. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res Int. 2013:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanapiromsakul C, Waterman PG.. 2000. Flavanone, triterpene and chromene derivatives from the stems of Paramignya griffithii. Phytochemistry. 55:269–273. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S.. 2001. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Investigation. 107:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Shin EM, Guo LY, Youn UJ, Bae K, Kang SS, Zou LB, Kim YS.. 2008. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 586:340–349. [DOI] [PubMed] [Google Scholar]
- Zindler E, Zipp F.. 2010. Neuronal injury in chronic CNS inflammation. Best Pract Res Clin Anaesthesiol. 24:551–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.