Abstract
Context: Curcumin has been reported to have anti-inflammatory, antioxidant and hypoglycaemic properties, besides reducing mortality in sepsis.
Objective: This study evaluates the biological activities of a curcumin dispersion formulated by spray-drying in experimental sepsis.
Materials and methods: Male Wistar rats were subjected to sepsis by caecal ligation and puncture (CLP), controls were sham operated. The animals were treated with curcumin dispersion (100 mg/kg, p.o.) or water for 7 days prior to CLP and at 2 h after surgery. One group was used to analyze curcumin absorption through HPLC; another had the survival rate assessed during 48 h; and from a third group, blood was collected by decapitation to analyze metabolic and inflammatory parameters.
Results: The plasma curcumin levels reached 2.5 ng/mL at 4 h, dropped significantly (p < 0.001) at 6 h (1.2 ng/mL), and were undetectable at 24 h in both groups. Curcumin temporarily increased the survival rate of the septic rats by 20%. Moreover, it attenuated glycaemia (p < 0.05) and volemia (p < 0.05) alterations typically observed during sepsis, and decreased the levels of the proinflammatory cytokines IL-1β and IL-6 in plasma (p < 0.001) and peritoneal lavage fluid (p < 0.05) of septic rats. Serum HSP70 levels were decreased (p < 0.01) at 24 h after CLP.
Discussion and conclusion: Our results show that the curcumin dispersion dose employed was not detrimental to the septic rats. In fact, it temporarily increased their survival rate, improved important metabolic parameters, reduced proinflammatory cytokines and HSP70 production.
Keywords: CLP, HSP70, nitric oxide, glycaemia, volemia
Introduction
Sepsis is a syndrome or disease that can cause septic shock, multiple organ failure and death, especially when not diagnosed early and treated immediately (Bone et al. 1997; Vincent & Korkut 2008; Gonsalves & Sakr 2010).
In the early stage of sepsis, the pathogen–receptor interaction triggers a cascade of intracellular signalling pathways, wherein the nuclear factor kappa β (NF-κ-β) induces the expression of genes responsible for cytokine production (Nduka & Parrillo 2011). While this leads to the overproduction of tumour necrosis factor (TNF-α), interleukin 1beta (IL-1β) and nitric oxide (NO) increases the capacity of bacterial clearance, it is also responsible for certain complications, including myocardial cell depression, hypotension and organ failure (Szabo 1995; Kumar et al. 1996; Jean-Baptiste 2007;). The release of interleukin 6 (IL-6) induces fever and produces acute phase proteins by the liver, and it also has a strong correlation with sepsis-related mortality (Frink et al. 2009; Tschaikowsky et al. 2011; Faix 2013).
The application of curcumin, which is derived from the tropical plant Curcuma longa L. (Zingiberaceae), has previously been employed and tested in experimental sepsis protocols, where it was found to attenuate tissue damage, to decrease TNF-α expression, and to reduce sepsis-related mortality in rats (Siddiqui et al. 2006; Memis et al. 2008). Its structural configuration conveys the ability to inhibit lipid peroxidation in different tissues, to regulate intracellular concentrations of antioxidant enzymes, as well as to act as a scavenger of reactive oxygen species (ROS) (Sreejayan Rao 1994 ;Soobrattee et al. 2005; Singh et al. 2011). Furthermore, curcumin was reported to have antioxidant and anti-inflammatory properties in the brain (Vachharajani et al. 2010), lungs (Xiao et al. 2012), kidneys (Yilmaz Savcun et al. 2013), liver (Seehofer et al. 2010) and heart (Yang et al. 2013) of septic animals. Its anti-inflammatory effects are thought to be mediated by up-regulation of the peroxisome proliferator-activated receptor gamma (PPAR-γ). The increased expression of PPAR-γ by curcumin leads to the suppression of NF-κ-β and a consequent decrease in the release of pro-inflammatory mediators such as TNF-α, IL-1 and IL-6 (Siddiqui et al. 2006).
The mechanism of action of curcumin also seems to be associated with heat shock protein 70 (HSP70) (Dunsmore et al. 2001), as seen in studies on the relationship between serum HSP70 levels, oxidant status and sepsis outcome in patients (Gelain et al. 2011), but to the best of our knowledge there is no such report yet for experimental sepsis models.
Despite the great therapeutic potential of curcumin, its low solubility and stability in aqueous systems, as well as its rapid systemic metabolization and elimination, have limited its clinical applications (Anand et al. 2007). So as to increase the absorption of curcumin from the gastrointestinal tract, a solid dispersion consisting of curcumin/Gelucire®50/13- Aerosil® (SD17) has been formulated by spray drying (Teixeira et al. 2016). This dispersion was seen to reduce cisplatin-induced neurotoxicity in vitro (Mendonca et al. 2013) and to have antigenotoxic and anti-inflammatory activities in rats (Mendonca et al. 2015; Teixeira et al. 2016). Considering the reported activity of curcumin in experimental models of diseases characterized by the production of several inflammatory cytokines, the aim of this study was to investigate the effects of the SD17 curcumin preparation on immune and metabolic disorders seen in rats submitted to caecum ligation puncture (CLP)-induced sepsis.
Materials and methods
Animals
Male Wistar rats (250 ± 30 g) provided by the Animal Facility of the Campus of Ribeirão Preto, University of São Paulo, were housed in controlled temperature (25 ± 1 °C) and photoperiodic (12 h night/day cycle) conditions, with food (Nuvilab CR-1, NUVITAL) and tap water available ad libitum. All experimental protocols were approved and performed according to the guidelines of the Ethics Committee of the University of São Paulo – Campus Ribeirão Preto. Humane endpoints in shock research (Nemzek et al. 2004) were used as criterion to euthanize septic animals in high suffering, immediately before or soon after the studied time-points defined in this study.
Preparation and working solution of the SD17
The solid dispersion (SD17) prepared by the spray-drying technique and containing 338.4 mg of the substance per gram, has high solubility and stability (Teixeira et al. 2016). For the working solution used in this study, the SD17 preparation was dissolved in sterile water. The dose used (100 mg/kg) was based on previous reports of Basnet and Skalko-Basnet (2011) and Teixeira et al. (2016).
Experimental protocol
Following sham-operation or caecum ligation puncture (CLP) surgery (Rittirsch et al. 2009), the animals received by gavage a dose of SD17 (100 mg/kg) dissolved in water, or water as control for 7 days before the experiment, and at 2 h after surgery. In the first group of animals, the survival rate was assessed during 48 h. In the second group, the plasma curcumin levels were quantified by HPLC. Rats of the third group were decapitated at 4 or 6 h (early phase of sepsis), or at 24 h (late phase of sepsis) after surgery. Aliquots of whole blood, plasma or serum were used for the determination of blood glucose, cytokine (IL-6, IL-1β), NO and HSP70 levels. Peritoneal lavage fluid (PLF) was collected during each period for the quantification of cytokines (IL-1β, IL-6).
Quantification of plasma curcumin by high-performance liquid chromatography (HPLC)
HPLC was conducted in a Shimadzu instrument (Kyoto, Japan) equipped with an LC-10 AT VP pump and an SPD 10 A VP ultraviolet detector set at λ = 420 nm. Chromatographic separation was obtained isocratically at ambient temperature on a reverse phase column LiChrospher® 100 RP 18 (125 mm ×4 mm ×5 μm particle size – Merck, Darmstadt, Germany). The mobile phase used was a methanol/water/acetic acid mixture (68:30.4:1.6 v/v/v) at a flow rate of 0.7 mL/min. Acetonitrile (2 mL) was added to 1 mL plasma for protein precipitation. The mixture was agitated for 1 min and centrifuged for 5 min at 1495 g (Hitachi, himac CF8DL). The supernatant (2 mL) was transferred to a conical tube and dried by air flow at room temperature. Subsequently, the samples were reconstituted in 200 μL of mobile phase and 100 μL of n-hexane and vortexed for 1 min. After centrifugation (5 min, 1495 g), 100 μL of the mobile phase (lower) was injected into the chromatograph. Plasma curcumin levels were calculated by linear regression of the respective peaks in the samples against a standard calibration curve prepared with analytical grade curcumin with 94% purity (Sigma-Aldrich, St. Louis, MO).
Haematocrit determination and blood glucose measurement
Blood was collected in heparinized microcapillaries (Fisher Scientific, Hampton, NH) and haematocrit was measured by centrifugation (Centrimicro Mod. 211, FANEN). Glucose was measured in a calibrated portable blood glucose meter Accu-Chek Advantage (Roche Diagnostica Brasil Ltd, São Paulo, Brasil) using specific reactive tapes. Concentrations are given in mg/dL.
Determination of cytokines in plasma and peritoneal lavage fluid (PLF)
The IL-1β and IL-6 concentrations in plasma and PLF were determined using specific enzyme-linked immunosorbent assay (ELISA) kits for each cytokine (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The detection limits for IL-1β and IL-6 specific ELISA kits were 5 and 21 pg/mL, respectively.
Plasma nitrate determination
Total nitrate was determined using the purge system of a Sievers Instruments Nitric Oxide Analyzer (NOA model 280i, Boulder, CO). Plasma samples were deproteinized using cold absolute ethanol before injection into a reaction vessel containing vanadium trichloride (VCl3), which converts nitrate to NO. The NO produced was detected as ozone induced by chemiluminescence. Peak NO values of the samples were determined using a standard curve established with sodium nitrate solutions of various concentrations.
Heat shock protein 70 (HSP70) measurement
HSP70 serum levels were determined by western blot analysis using a mouse monoclonal antibody (Sigma, St. Louis, MO) diluted 1:1000, followed by incubation with a secondary anti-mouse IgG antibody conjugated to peroxidase (Santa Cruz Biotechnology, Dallas, TX) at 1:10,000 dilution. Detection was done using a chemiluminescence reaction kit (ECL, GE Healthcare, São Paulo, Brazil) followed by exposure to X-ray film. The films were photographed and the ECL-detected protein bands quantified by Gene Tools Software (Syngene, Cambridge, UK). The results were converted to arbitrary units of optical density and total plasma protein was used for normalization.
Statistical analysis
The data are presented as means ± SEM. Statistical significance was assessed by two-way analysis of variance (ANOVA) and a post hoc Student–Newman–Keuls (SNK) test, or one-way ANOVA followed by Tukey’s test. Survival analysis was performed by the Log Rank Mantel-Cox test. p values ≤0.05 were considered statistically different. The software used was GraphPad Prism (version 5.0).
Results
All animals subjected to CLP-induced experimental polymicrobial sepsis developed the typical early clinical signs of sepsis, such as lethargy, piloerection and diarrhoea. Sham-operated animals remained active in their cages, as expected.
Curcumin plasma concentration after SD17 administration
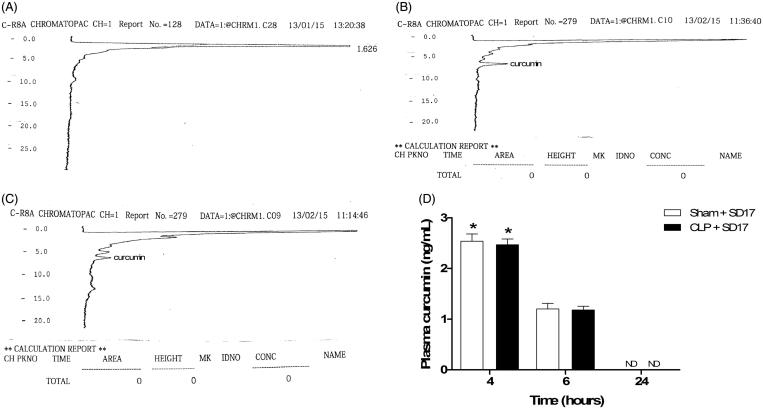
Representatives HPLC chromatograms are shown in Figure 1 (A–C). Following gavage administration of SD17, the plasma curcumin levels reached 2.49 ± 0.30 ng/mL at 4 h in the CLP group and 2.53 ± 0.34 ng/mL in sham animals before showing a significant drop at 6 h in both groups (F[2,32] = 445.701; p < 0.001). At 24 h, curcumin was undetectable in the plasma samples (Figure 1(D)).
Figure 1.
Curcumin plasma concentration after SD17 administration. Representative chromatograms of HPLC separations of (A) septic rat plasma blank, (B) septic rat plasma with curcumin standard and (C) septic rat plasma with SD17. (D) Curcumin levels of animals subjected to sham operation (Sham) or sepsis by caecal ligation and puncture (CLP), pretreated and treated by gavage with SD17 (100 mg/kg). The values shown are means ± SEM. *p < 0.001 compared to group 6 and 24 h. n = 4–7 animals per group. ND: not detectable.
Effect of SD17 treatment on the survival of septic animals
Survival rate was analyzed to assure that the dose of the curcumin dispersion employed in this study was not detrimental to the animals. Interestingly, we could in fact see a 20% improvement in survival rate at 24 h in the SD17 treated group. But, this improvement was apparently only temporary, as the Log Rank Mantel-Cox test did not indicate a statistical difference between the CLP groups treated with SD17 or vehicle at the 48 h time point (p = 0.48) (Figure 2).
Figure 2.
Effect of SD17 treatment on survival rate of septic animals. The animals were treated with SD17 by gavage (100 mg/kg) or water (vehicle) for seven consecutive days and at 2 h after the caecal ligation and puncture (CLP) surgery. Survival analysis was performed by the Log Rank Mantel-Cox test. The values are expressed as percentage of survival rate. The number of animals is shown in parentheses.
Effect of SD17 treatment on haematocrit and glycaemia
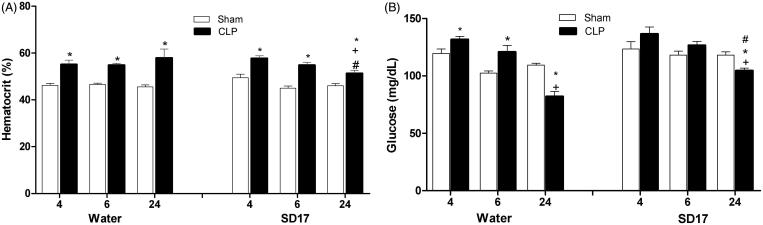
As expected, sepsis induction caused hypovolemia at each time point analyzed (F[2,65] = 2.477; p < 0.05). There were significant differences at 4, 6 and 24 h between the CLP and the Sham groups, which both had been treated with SD17. SD17 treatment caused a small improvement in hypovolemia, but only in the late phase of sepsis (F[3,65] = 2.447; p < 0.05) (Figure 3(A)).
Figure 3.
Effect of SD17 treatment on (A) haematocrit and (B) glycaemia levels at 4, 6 and 24 h after Sham or CLP operation. Values shown are means ± SEM. *p < 0.05 compared to the Sham group; +p < 0.05 compared to the same group (i.e., in comparison to the CLP + water and CLP + SD17 groups 4 and 6 h); #p < 0.05 compared to vehicle (water) treatment in the same period and same group (i.e., in comparison to the CLP + water group 24 h). n = 4–11 animals/group.
Sepsis induced hyperglycaemia at 4 and 6 h (early phase of sepsis) (F[2,79] = 28.436; p < 0.05) and hypoglycaemia in the late phase, at 24 h (F[2,79] = 28.436; p < 0.001). SD17 treatment abolished the hyperglycaemia condition in the early phase (4 h and 6 h), but had no effect on hypoglycaemia in the late phase (24 h) (Figure 3(B)).
Effect of SD17 treatment on the plasma and plasma lavage fluid (PLF) concentrations of IL-1β and IL-6
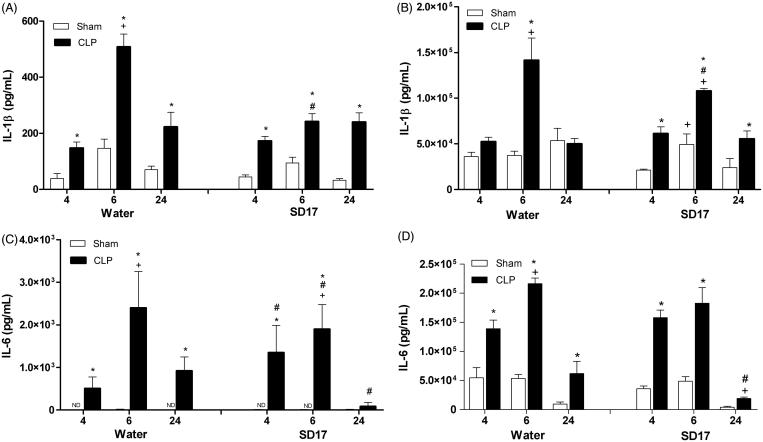
As expected, sepsis led to increased plasma and PLF cytokine levels already in the early phase of sepsis (F[2,112] = 42.578; p < 0.001) (Figure 4). Treatment with SD17 reduced the IL-1β concentration in plasma (F[3,112] = 58.321; p < 0.001) (Figure 4(A)) and in PLF (F[3,46] = 23.350; p = 0.039) at 6 h after sepsis induction (Figure 4(B)).
Figure 4.
Effect of SD17 treatment on plasma and peritoneal lavage fluid (PLF) concentrations of IL-1β and IL-6 at 4, 6 and 24 h after Sham or CLP surgery. Concentration of (A) IL-1β and (C) IL-6 in plasma and (B) IL-1β, and (D) IL-6 the PLF animals subjected to sham operation (Sham) or sepsis induced by caecal ligation and puncture (CLP), pretreated and treated by gavage with SD17 (100 mg/kg) or vehicle (water). The values shown are means ± SEM. *p < 0.001 compared to the Sham group; +p < 0.001 compared to the same group; #p < 0.05 compared to vehicle (water) treatment in the same period and same group. n = 4–11 animals/group. ND: not detectable.
A significant reduction was also seen for plasma IL-6 levels at 6 and 24 h (F[6,123] = 32.012; p < 0.001), although an increase had been seen at 4 h following CLP surgery (Figure 4(C)). For PLF, a reduction in IL-6 was observed only at 24 h after sepsis induction (F[6,46] = 6.338; p = 0.047) (Figure 4(D)).
Effect of SD17 treatment on plasma nitrate and serum HSP70 levels
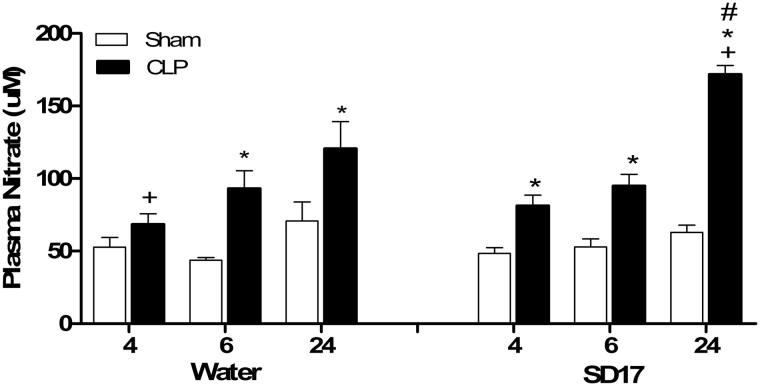
Sepsis caused an increase in plasma nitrate levels in the early (6 h) and late phase of sepsis (24 h) (F[2,76] = 29.446; p < 0.05). At 24 h after sepsis induction, the treatment with SD17 led to an even stronger increase in nitrate levels (F[6,76] = 7.598; p < 0.001) (Figure 5).
Figure 5.
- Effect of SD17 treatment on plasma nitrate levels at 4, 6 and 24 after Sham or CLP operation. Values shown are means ± SEM. *p < 0.05 compared to the Sham group; +p < 0.05 compared to the same group (i.e., in comparison to the CLP + water groups 6 and 24 hand CLP + SD17 groups 4 and 6 h); #p < 0.001 compared to vehicle (water) treatment in the same period and same group (i.e., in comparison to the CLP + water group 24 h); n = 3–10 animals/group.
HSP70 levels were increased at 6 h in both the CLP and sham groups. The SD17 treatment attenuated this increase in the sham animals only (F[6,63] = 2.427; p < 0.044), and a reduction in HSP70 levels was seen at 24 h in the CLP animals (F[6,63] = 2.427; p = 0.002) (Figure 6).
Figure 6.
Effect of SD17 treatment on serum HSP70 levels at 4, 6 and 24 h after Sham or CLP operation. (A) Representative western blots were analyzed for each group and time point. (B) HSP70 values shown are means ± SEM. *p < 0.05 compared to the Sham group; +p < 0.05 compared to the same group; #p < 0.01 compared to vehicle (water) treatment in the same period and group (i.e., in comparison to the Sham + water group 6 h and CLP + water group 24 h). n = 3–8 animals/group.
Discussion
Since it was important to show that the curcumin preparation used in the experimental protocol allowed good absorption from the gastrointestinal tract, we first confirmed this by HPLC analysis of the curcumin plasma concentrations. The results showed that curcumin was high at 4 h, significantly decreased at 6 h, and was undetectable at 24 h following surgery. Furthermore, there was no indication of a detrimental effect on animal health by the dosage used, as seen by survival rate analysis. In fact, the curcumin dispersion (SD17) treatment even improved survival of septic animals at 24 h.
Curcumin is reported to interact with different proteins, including albumin, which may act as carriers (Gupta et al. 2011). As the HPLC detection of free curcumin requires plasma deproteinization (Ma et al. 2007), it is not possible to conclude that it may no longer exert biological activity, even though curcumin concentrations were not detectable in plasma at 24 h. Rather, the rapid decline seen in curcumin plasma concentrations and its absence at 24 h suggests that various plasma proteins may rapidly distribute it to different tissue. This may maintain its bioactive properties and avoid drug metabolization or its rapid excretion (Tsai et al. 2011). We consider that this may explain the increase in survival rate of the SD17-treated CLP animals at 24 h after sepsis induction.
SD17 administration was seen to attenuate the altered metabolic parameters typically observed during sepsis, such as volemia and glycaemia. Hypovolemia in sepsis indicates plasma extravasation to interstitial spaces, due to elevated levels of inflammatory mediators, such as NO, cytokines, prostaglandins, complement and others, that cause damage to endothelial cells and increase vascular permeability (Fishel et al. 2003). Since we could not see a decrease in plasma NO levels, but only in cytokines, we consider that the decrease in the levels of the latter may explain the improvement in hypovolemia observed here in the late phase of sepsis.
Hyperglycaemia is typically seen in the initial, hyperdynamic phase of sepsis, when the body expends a large amount of energy. It is believed to be due to increased hepatic glycogenolysis and gluconeogenesis mediated by stress hormones, such as glucagon, catecholamines and epinephrine, which are all increased in this phase (Norbury et al. 2007). In the late phase of sepsis, hypoglycaemia may occur, primarily due to the decreased hepatic stocks of glycogen spent earlier in the hyperactive phase (Marik & Raghavan 2004). The SD17 treatment protocol used herein abolished the hyperglycaemia condition in the acute phase and attenuated hypoglycaemia in the late phase of sepsis, indicating that curcumin can improve harmful effects caused by glycaemia alterations. In fact, clinicians recommend the maintenance of basal levels of glycaemia during the septic state (Dellinger et al. 2008). Furthermore, a beneficial effect of curcumin on hyperglycaemia in diabetic rats has already been reported (Fujiwara et al. 2008). Though promising in terms of therapeutic applications, further studies are necessary to clarify the mechanism(s) of curcumin in the control of blood glucose during sepsis.
The results obtained on the temporal profile of IL-1β and IL-6 cytokine concentrations in plasma and peritoneal cavity fluid of septic animals treated with vehicle are similar to those already reported in previous studies (Figueiredo et al. 2012; Oliveira-Pelegrin et al. 2013). Here, we saw an increase in the levels of these cytokines at 4 and 6 h and a decrease at 24 h, representing, respectively, the early and late phases of sepsis. In the peritoneal lavage fluid (PLF), these cytokines showed essentially the same profile as in plasma, but since the peritoneum is the inflammation site, their concentrations were higher than in plasma at each time point studied.
Cytokines, such as IL-1β and IL-6, play important roles in the inflammatory process. They enhance microbicide activity of phagocytic cells, contribute to the recruitment of leucocytes to the infection site, and are also endogenous pyrogens, showing correlation with the increase in body temperature (Cavaillon et al. 2003; Figueiredo et al. 2012). Interleukin 1 is known to increase other cytokines, such as IL-6, IL-8 and TNF-α, besides augmenting leucotrienes, prostaglandins and reactive oxygen species. The increase seen in IL-6 during sepsis can be considered as an attempt of the organism to fight the infection by regulating the immune response, as this cytokine is known to stimulate the production of corticosterone (Salas et al. 1990) and of acute phase proteins produced in the liver (Faix 2013).
These cytokines are considered biomarkers for sepsis mortality, since they are elevated in non-survivor compared to survivor patients (Bozza et al. 2007). The observation that the curcumin dispersion diminished cytokine concentrations in plasma and PLF could indicate an anti-inflammatory effect that would be beneficial to the organism.
It has previously been shown that NF-κ-β activity is reduced by approximately 30% in muscle of rats treated with curcumin 1 h before and 8 and 15 h after the CLP septic stimulus (Poylin et al. 2008). Moreover, a systemic and myocardial reduction in these cytokines, and ultimately NF-κ-β, was also seen following administration of curcumin in Coxsackievirus-infected mice (Song et al. 2013). Although we did not analyze the NF-κ-B pathway in this study, we think that the biological effects of curcumin could be due to the inhibition of this transcription factor during the inflammatory process. This inhibition could occur through blocking the phosphorylation of its inhibitor Iκβ, which prevents the nuclear translocation of NF-κ-β (Jurenka 2009; Buhrmann et al. 2011).
We also evaluated the action of the SD17 curcumin dispersion on extracellular HSP70 protein, which is synthesized quickly by most cells in the body in response to various types of stresses, including inflammation and oxidative stress (Ziegler et al. 2005). We could see an increase in HSP70 protein levels both in sham and in septic rats, but only in the early phase of sepsis. We think that inflammation due to surgery was primarily responsible for the increase seen in this protein at 6 h.
The functions of this protein are dependent on whether it is localized intra- or extracellularly (Kregel 2002). Intracellular HSP70 has a protective function, and the increase of its expression reduces mortality and organ dysfunction in sepsis. It acts as a molecular chaperone suppressing several denatured proteins, regulating apoptosis, and inhibiting inflammatory cell activation (Chen et al. 2005). Its extracellular functions, though not yet clearly defined, can be considered as mediating the immune response. It binds to the Toll-like receptors types 2 and 4 (TLR2 and TLR4) on the surface of antigen-presenting cells (APCs), and through an NF-κ-β-dependent pathway it stimulates the production of proinflammatory cytokines. Moreover, it appears to provide a link between the innate and the adaptive immune systems, acting as a carrier of antigenic peptides to APCs (Joly et al. 2010). In inflammatory diseases, the serum level of HSP70 is directly linked to the inflammatory status of the organism (Njemini et al. 2004), increasing with the degree of inflammation (Njemini et al. 2011). Clinical studies showed that serum HSP70 levels are modulated according to the patient’s oxidant status, and its increased levels are associated with mortality in sepsis (Gelain et al. 2011). Hence, it is possible to use HSP70 as a biomarker of sepsis (Wong et al. 2012, 2014). In this study, curcumin administration was able to significantly decrease the levels of this protein at 24 h in septic rats, besides abolishing its increase in sham animals.
Therefore, our results would be the first to show an increase of this protein in septic rats. Moreover, the results suggest that curcumin may reduce the extracellular concentration of this protein, and thus may contribute to the small improvement in sepsis survival seen at 24 h after CLP. We believe that a longer and repeated treatment could enhance these biological effects of curcumin and significantly extend the survival of septic animals.
Several pathophysiological processes involve NO (Thiemermann 1997; Erusalimsky & Moncada 2007). A progressive increase in plasma nitrate, a stable metabolite used as an indicator of NO production, is seen in experimental sepsis induced by caecal ligation and puncture (Correa et al. 2007; Oliveira-Pelegrin et al. 2010; Wahab et al. 2015). Although well-known for its microbicide capacity, an overproduction of this gas contributes to a decrease in blood pressure and to the hyporesponsivity to vasoconstrictor agents in sepsis (Thiemermann 1997). This is thought to be responsible for worsening the polymicrobial sepsis conditions, since, when using a blocker of NO production, we found an increase in survival rate (Correa et al. 2007). Therefore, we were expecting to see a decrease in NO levels following the curcumin dispersion treatment. Surprisingly, this was not the case, as we did not see any modification in the early phase and even an increase in the late phase of sepsis.
The generally assumed antioxidant effect of curcumin and its beneficial effects have more recently been called into question (Mancuso & Barone 2009; Burgos-Moron et al. 2010). Under specific situations, high concentrations of curcumin were seen to be oxidant rather than antioxidant (Sandur et al. 2007). This would be in line with suggestions that the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which includes nitric oxide, by curcumin, could be responsible for its reported apoptotic effects that can prevent cancer development. However, most of these studies are based on in vitro experiments, and therefore there is a need to conduct in vivo studies to test which dosage levels of curcumin are oxidant or antioxidant.
Conclusions
Our study demonstrates that the curcumin dispersion formulation SD17 was able to decrease the production of pro-inflammatory cytokines and HSP70 and improved hypoglycaemia and hypovolemia conditions caused by sepsis. These results should stimulate further in vivo studies, needed to clarify the effect of curcumin, especially on NO production during sepsis.
Funding Statement
Financial support was provided by Fundação de Amparo á Pesquisa do Estado de São Paulo [grant number 11/14711-0].
Acknowledgements
The authors thank Vera Lúcia Lanchote for providing the HPLC system for measuring plasma curcumin, Evelin Capellari Cárnio for the Nitric Oxide analyzer, and Klaus Hartfelder for the English language revision. The technical support by Sonia Dreossi, Marcelo Batalhão and Nadir Fernandes is gratefully acknowledged. Financial support was provided by Fundação de Amparo á Pesquisa do Estado de São Paulo (FAPESP grant number 2011/14711-0).
Disclosure statement
The authors declare that they have no competing interests.
References
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB.. 2007. Bioavailability of curcumin: problems and promises. Mol Pharm. 4:807–818. [DOI] [PubMed] [Google Scholar]
- Basnet P, Skalko-Basnet N.. 2011. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 16:4567–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC, Grodzin CJ, Balk RA.. 1997. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 112:235–243. [DOI] [PubMed] [Google Scholar]
- Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT.. 2007. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 11:R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, Shakibaei M.. 2011. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 286:28556–28566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Moron E, Calderon-Montano JM, Salvador J, Robles A, Lopez-Lazaro M.. 2010. The dark side of curcumin. Int J Cancer. 126:1771–1775. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D.. 2003. Cytokine cascade in sepsis. Scand J Infect Dis. 35:535–544. [DOI] [PubMed] [Google Scholar]
- Chen HW, Kuo HT, Wang SJ, Lu TS, Yang RC.. 2005. In vivo heat shock protein assembles with septic liver NF-kappaB/I-kappaB complex regulating NF-kappaB activity. Shock. 24:232–238. [DOI] [PubMed] [Google Scholar]
- Correa PB, Pancoto JA, de Oliveira-Pelegrin GR, Carnio EC, Rocha MJ.. 2007. Participation of iNOS-derived NO in hypothalamic activation and vasopressin release during polymicrobial sepsis. J Neuroimmunol. 183:17–25. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. . 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 36:296–327. [DOI] [PubMed] [Google Scholar]
- Dunsmore KE, Chen PG, Wong HR.. 2001. Curcumin, a medicinal herbal compound capable of inducing the heat shock response. Crit Care Med. 29:2199–2204. [DOI] [PubMed] [Google Scholar]
- Erusalimsky JD, Moncada S.. 2007. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 27:2524–2531. [DOI] [PubMed] [Google Scholar]
- Faix JD.2013. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 50:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo MJ, Soares DM, Martins JM, Machado Rde R, Sorgi CA, Faccioli LH, Melo MC, Malvar Ddo C, Souza GE.. 2012. Febrile response induced by cecal ligation and puncture (CLP) in rats: involvement of prostaglandin E2 and cytokines. Med Microbiol Immunol. 201:219–229. [DOI] [PubMed] [Google Scholar]
- Fishel RS, Are C, Barbul A.. 2003. Vessel injury and capillary leak. Crit Care Med. 31:S502–S511. [DOI] [PubMed] [Google Scholar]
- Frink M, van Griensven M, Kobbe P, Brin T, Zeckey C, Vaske B, Krettek C, Hildebrand F.. 2009. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand J Trauma Resusc Emerg Med. 27:17–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hosokawa M, Zhou X, Fujimoto S, Fukuda K, Toyoda K, Nishi Y, Fujita Y, Yamada K, Yamada Y, et al. . 2008. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res Clin Pract. 80:185–191. [DOI] [PubMed] [Google Scholar]
- Gelain DP, de Bittencourt Pasquali MA, M Comim C, Grunwald MS, Ritter C, Tomasi CD, Alves SC, Quevedo J, Dal-Pizzol F, Moreira JC.. 2011. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock. 35:466–470. [DOI] [PubMed] [Google Scholar]
- Gonsalves MD, Sakr Y.. 2010. Early identification of sepsis. Curr Infect Dis Rep. 12:329–335. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB.. 2011. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 28:1937–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Baptiste E.2007. Cellular mechanisms in sepsis. J Intensive Care Med. 22:63–72. [DOI] [PubMed] [Google Scholar]
- Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C.. 2010. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2:238–247. [DOI] [PubMed] [Google Scholar]
- Jurenka JS.2009. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 14:141–153. [PubMed] [Google Scholar]
- Kregel KC.2002. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 92:2177–2186. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE.. 1996. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 183:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Shayeganpour A, Brocks DR, Lavasanifar A, Samuel J.. 2007. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed Chromatogr. 21:546–552. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Barone E.. 2009. Curcumin in clinical practice: myth or reality?. Trends Pharmacol Sci. 30:333–334. [DOI] [PubMed] [Google Scholar]
- Marik PE, Raghavan M.. 2004. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 30:748–756. [DOI] [PubMed] [Google Scholar]
- Memis D, Hekimoglu S, Sezer A, Altaner S, Sut N, Usta U.. 2008. Curcumin attenuates the organ dysfunction caused by endotoxemia in the rat. Nutrition. 24:1133–1138. [DOI] [PubMed] [Google Scholar]
- Mendonca LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi Mde L, Antunes LM.. 2013. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 34:205–211. [DOI] [PubMed] [Google Scholar]
- Mendonca LM, Machado Cda S, Teixeira CC, Freitas LA, Bianchi ML, Antunes LM.. 2015. Comparative study of curcumin and curcumin formulated in a solid dispersion: Evaluation of their antigenotoxic effects. Genet Mol Biol. 38:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nduka OO, Parrillo JE.. 2011. The pathophysiology of septic shock. Crit Care Nurs Clin North Am. 23:41–66. [DOI] [PubMed] [Google Scholar]
- Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG.. 2004. Humane endpoints in shock research. Shock. 21:17–25. [DOI] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T.. 2004. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 5:31–38. [DOI] [PubMed] [Google Scholar]
- Njemini R, Bautmans I, Onyema OO, Van Puyvelde K, Demanet C, Mets T.. 2011. Circulating heat shock protein 70 in health, aging and disease. BMC Immunol. 12:24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury WB, Jeschke MG, Herndon DN.. 2007. Metabolism modulators in sepsis: propranolol. Crit Care Med. 35:S616–S620. [DOI] [PubMed] [Google Scholar]
- Oliveira-Pelegrin GR, Aguila FA, Basso PJ, Rocha MJ.. 2010. Role of central NO-cGMP pathway in vasopressin and oxytocin gene expression during sepsis. Peptides. 31:1847–1852. [DOI] [PubMed] [Google Scholar]
- Oliveira-Pelegrin GR, Basso PJ, Soares AS, Martinez MR, Riester KD, Rocha MJ.. 2013. Cleaved caspase-3 expression in hypothalamic magnocellular neurons may affect vasopressin secretion during experimental polymicrobial sepsis. J Neuroimmunol. 258:10–16. [DOI] [PubMed] [Google Scholar]
- Poylin V, Fareed MU, O'Neal P, Alamdari N, Reilly N, Menconi M, Hasselgren PO.. 2008. The NF-kappaB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediators Inflamm. 2008:317851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA.. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 4:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas MA, Evans SW, Levell MJ, Whicher JT.. 1990. Interleukin-6 and ACTH act synergistically to stimulate the release of corticosterone from adrenal gland cells. Clin Exp Immunol. 79:470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB.. 2007. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med. 43:568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehofer D, Schirmeier A, Bengmark S, Cho SY, Koch M, Lederer A, Rayes N, Menger MD, Neuhaus P, Nüssler AK.. 2010. Curcumin attenuates oxidative stress and inflammatory response in the early phase after partial hepatectomy with simultaneous intraabdominal infection in rats. J Surg Res. 159:497–502. [DOI] [PubMed] [Google Scholar]
- Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, Hu M, Simms HH, Wang P.. 2006. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 34:1874–1882. [DOI] [PubMed] [Google Scholar]
- Singh U, Barik A, Singh BG, Priyadarsini KI.. 2011. Reactions of reactive oxygen species (ROS) with curcumin analogues: Structure-activity relationship. Free Radic Res. 45:317–325. [DOI] [PubMed] [Google Scholar]
- Song Y, Ge W, Cai H, Zhang H.. 2013. Curcumin protects mice from coxsackievirus B3-induced myocarditis by inhibiting the phosphatidylinositol 3 kinase/Akt/nuclear factor-κB pathway. J Cardiovasc Pharmacol Ther. 18:560–569. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T.. 2005. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 579:200–213. [DOI] [PubMed] [Google Scholar]
- Sreejayan Rao MN.1994. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 46:1013–1016. [DOI] [PubMed] [Google Scholar]
- Szabo C.1995. Alterations in nitric oxide production in various forms of circulatory shock. New Horiz. 3:2–32. [PubMed] [Google Scholar]
- Teixeira CC, Mendonça LM, Bergamaschi MM, Queiroz RH, Souza GE, Antunes LM, Freitas LA.. 2016. Microparticles containing curcumin solid dispersion: stability, bioavailability and anti-inflammatory activity. AAPS Pharm Sci Tech. 17:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C.1997. Nitric oxide and septic shock. Gen Pharmacol. 29:159–166. [DOI] [PubMed] [Google Scholar]
- Tsai YM, Chien C, Lin LC, Tsai TH.. 2011. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 416:331–338. [DOI] [PubMed] [Google Scholar]
- Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M.. 2011. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care. 26:54–64. [DOI] [PubMed] [Google Scholar]
- Vachharajani V, Wang SW, Mishra N, El Gazzar M, Yoza B, McCall C.. 2010. Curcumin modulates leukocyte and platelet adhesion in murine sepsis. Microcirculation. 17:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Korkut HA.. 2008. Defining sepsis. Clin Chest Med. 29:585–590. [DOI] [PubMed] [Google Scholar]
- Wahab F, Tazinafo LF, Cárnio EC, Aguila FA, Batalhão ME, Rocha MJ.. 2015. Interleukin-1 receptor antagonist decreases cerebrospinal fluid nitric oxide levels and increases vasopressin secretion in the late phase of sepsis in rats. Endocrine. 49:215–221. [DOI] [PubMed] [Google Scholar]
- Wong HR, Salisbury S, Xiao Q, Cvijanovich NZ, Hall M, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, et al. . 2012. The pediatric sepsis biomarker risk model. Crit Care. 16:R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HR, Weiss SL, Giuliano JS Jr, Wainwright MS, Cvijanovich NZ, Thomas NJ, Allen GL, Anas N, Bigham MT, Hall M, et al. . 2014. Testing the prognostic accuracy of the updated pediatric sepsis biomarker risk model. PLoS One. 29:e86242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Yang M, Sun D, Sun S.. 2012. Curcumin protects against sepsis-induced acute lung injury in rats. J Surg Res. 176:e31–e39. [DOI] [PubMed] [Google Scholar]
- Yang C, Su X, Liu A, Zhang L, Yu A, Xi Y, Zhai G.. 2013. Advances in clinical study of curcumin. Curr Pharm Des. 19:1966–1973. [PubMed] [Google Scholar]
- Yilmaz Savcun G, Ozkan E, Dulundu E, Topaloğlu U, Sehirli AO, Tok OE, Ercan F, Sener G.. 2013. Antioxidant and anti-inflammatory effects of curcumin against hepatorenal oxidative injury in an experimental sepsis model in rats. Ulus Travma Acil Cerrahi Derg. 19:507–515. [DOI] [PubMed] [Google Scholar]
- Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer P.. 2005. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med. 31:1079–1086. [DOI] [PubMed] [Google Scholar]