Abstract
Context: Nasal mucosa is a desirable route for mucosal vaccine delivery. Mucosal co-administration of chitosan nanoparticles with absorption enhancers such as cross-linked dextran microspheres (CDM, Sephadex®) is a promising antigen delivery system.
Objective: In the current study, the chitosan nanospheres loaded with tetanus toxoid (CHT:TT NPs) was prepared and characterized. The immune responses against tetanus toxoid after nasal administration of CHT:TT NPs alone or mixed with CDM were also determined.
Materials and methods: Chitosan nanospheres were prepared by ionic gelation method. Particle size, releasing profile and antigen stability were evaluated by dynamic light scattering, diffusion chamber and SDS-PAGE methods, respectively. Rabbits were nasally immunized with different formulations loaded with 40 Lf TT. After three times immunizations with 2 weeks intervals, sera IgG titres and nasal lavage sIgA titres were determined.
Results: Mean size of CHT NPs and CHT:TT NPs were 205 ± 42 nm and 432 ± 85 nm, respectively. The release profile showed that 42.4 ± 10.5% of TT was released after 30 min and reached to a steady state after 1.5 h. Stability of encapsulated TT in nanospheres was confirmed by SDS-PAGE. The antibody titres showed that CHT:TT NPs-induced antibody titres were higher than TT solution. CHT NPs mixed with CDM induced the systemic IgG and nasal lavage sIgA titres higher than intranasal administration of TT solution (p < 0.001).
Discussion and conclusion: As the results indicated, these CHT:TT NPs when co-administered with CDM were able to induce more immune responses and have the potential to be used in mucosal immunization.
Keywords: Nasal immunization, dry powder formulation, immune responses, drug delivery
Introduction
Nasal immunization is a mucosal administration route which shows considerable potential for vaccine delivery. Nasal vaccination provides all the prerequisites for a successful needle-free vaccine delivery and also for better vaccine efficacy (Amorij et al. 2012). Nasal cavities are also common sites for entrance of pathogens. Furthermore, nasal administration of vaccines can lead to local production of antigen specific sIgA, which are able to prevent pathogens from colonizing at mucosal sites (Amorij et al. 2012). Protection of antigen from enzymatic digestion, the improvement of antigen uptake by relevant cells and immune stimulation are listed as other advantages of nasal vaccine delivery (Barhate et al. 2014).
For mucosal administration of antigens, mucoadhesive polymers, such as chitosan, could be used as delivery system/adjuvant. Encapsulation of antigens with these mucoadhesive polymers could protect them from degradative enzymes, increase their presence time in the nasal cavity because of their mucoadhesive properties, and increase their interaction and uptake by microfold cells (M cells) located in the nasal-associated lymphoid tissue (NALT) (Sajadi Tabassi et al. 2008). There are several studies on the application of chitosan in mucosal delivery of vaccines (Amin et al. 2009; Arthanari et al. 2016; Barhate et al. 2014; Khameneh et al. 2014). Despite the advantages of chitosan, this polymer suffers from low solubility at physiological pH (Elsabee et al. 2009). Different approaches were suggested to overcome these limitations such as derivatization or preparation of its nanoparticles (Yoksan & Chirachanchai 2008; Elsabee et al. 2009; Mohajer et al. 2014).
Cross-linked dextran microspheres (CDM, Sephadex®) have been successfully used as absorption enhancer for macromolecular drugs such as insulin (Chandler et al. 1991). They also could act as an absorption enhancer adjuvant to increase transepithelial absorption of antigens. However, there are limited reports on adjuvant potential of CDM (Sajadi Tabassi et al. 2008).
Dry powder form of vaccines have more chemical and microbiological stability, which could eliminate the need for cold chain and result in easier and more economical storage, distribution and mass vaccination, compared with liquid-based vaccines (Garmise et al. 2007; Tafaghodi & Rastegar 2010). Therefore, microspheres encapsulated with antigens are better to be used in dry powder form.
In the present study, chitosan nanospheres loaded with tetanus toxoid (CHT:TT NPs) were prepared and characterized. CDM powder as an absorption enhancer was added to the nanospheres. Rabbits were nasally immunized with this dry powder and mucosal and systemic immune responses against tetanus toxoid (TT) were evaluated.
Materials and methods
Materials
Tetanus toxoid (TT) solution (1700 Lf/mL) was from Razi Inc. (Hesarak, Iran). Anti-rabbit IgA was prepared from Bethyl Laboratories Inc. (Montgomery, TX). Anti-rabbit IgG was obtained from Sigma (St. Louis, MO). Chitosan was purchased from the Fluka (Singapore). All other used chemicals were of analytical grade. White albino rabbits weighing 1.5–2 kg were provided by Pasteur Institute (Tehran, Iran). All experiments were conducted according to the guidelines of ethics committee.
Preparation of blank and antigen-encapsulated CHT NPs
CHT NPs were fabricated using an ionic gelation method (Xu & Du 2003; Dehghan et al. 2013). Briefly, 5% w/v solution of tripolyphosphate (TPP) in purified water, as a hardening agent, was emulsified in paraffin oil containing Span 80 and Tween 80 (3% v/v, 1:1 ratio) as emulsifier. The ratio of TPP aqueous solution to oil solution was 3% v/v. Aqueous solution of chitosan (1% w/v) was prepared in acetic acid (0.1 M) and then was emulsified in paraffin oil. The ratio of chitosan solution to oil solution was 20% v/v. This emulsion was added dropwise into the first emulsion under gentle stirring. The nanospheres were formed by joining chitosan and TPP droplets and by solidification of chitosan droplets by TPP. After stirring for about 1 hour, nanospheres were rinsed with acetone and separated by centrifugation. Nanospheres were finally vacuum-dried. For the preparation of CHT:TT NPs, TT was added to the TPP solution before emulsification. The amount of antigen was adjusted to achieve 40 Lf of antigen/5 mg of powder.
Characterization of nanospheres
Particle size and distribution were evaluated by dynamic light scattering (DLS) method (Zetasizer nanoseries, Malvern, UK). To measure the amount of entrapped antigen in nanospheres, 5 mg of nanospheres were dissolved in 1 ml of a solvent mixture consisting of 1% (w/v) sodium nitrate and hydrochloric acid (1 N). After 1 h of incubation at 37 οC, the supernatant was analyzed by Bradford’s protein assay method.
The release profile of TT from nanospheres was studied with a diffusion chamber, which mimics the hydration conditions of the nasal mucosa (Tafaghodi et al. 2006b). The donor compartment contained air saturated with water vapour and the receiver contained 25 mL of PBS (pH 7.4), working at 37 οC. The nanopheres (25 mg) were laid on a filter paper in contact with the liquid phase of the receiver compartment. During 4 h, every 30 min, 400 μL samples were drawn from the receiver compartment and amount of the released TT was quantified. Each experiment was performed in triplicates.
The amount of antigens liberated from formulations in each time point was evaluated kinetically in order to find out the best-fit kinetic model of release profiles. To this end, the following models were used:
Zero-order model: Mt/M∞ = Kt
First order model: Mt/M∞ = 1−e(-kt)
Higuchi square root of time model: Mt/M∞ = kt0.5
Krosmeyer–Peppas kinetic model: Mt/M∞ = ktn
Where Mt is the released amount of the drug under investigation in a particular time, M∞ is the theoretical loaded amount of the drug, t is time and K is the rate constant of the release profile and n is the release exponent which explains characteristics and drug release mechanism.
The relative sizes of the sum of squared errors (SSE) shows how ‘good’ the regression is in terms of fitting the calibration data. The best regression is one in that the SSE value is closest to zero (Sadighi et al. 2012; Slane et al. 2014).
Antigen stability
The structural stability of released TT from nanospheres was detected by sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS–PAGE) and compared with native TT as perviosly described (Khodaverdi et al. 2015). Antigen samples were diluted with Tris-buffer (pH 6.8) with 2% SDS and electrophoresis of samples was performed using a Bio-RadMiNi-Protein II electrophoresis system at a constant voltage of 200 V in a Tris/glycine/SDS buffer. After migration, the protein bands were visualized by silver nitrate.
Nasal immunization studies
White albino rabbits weighing 1.5–2 kg (four animals per group) were nasally immunized with the following formulations in days 0, 14 and 28 of experiment:
Chitosan nanospheres mixed with 5 mg CDM (CHT NPs + CDM);
40 Lf TT solution (TT-Sol);
40 Lf TT in chitosan nanospheres mixed with 5 mg lactose, (CHT:TT NPs + Lac);
40 Lf TT in chitosan nanospheres mixed with 5 mg CDM, (CHT:TT NPs + CDM);
10 Lf alum adsorbed TT [used for parenteral immunization (IM)] (Alum-TT).
Animals were first sedated with 40 mg/kg ketamine HCl to prevent sneezing after administrations. Then, 10 mg of nanosphere (5 mg in each nostril, drown into polyethylene tubes) were nasally administered. The solutions (200 μL, 100 μL in each nostril) were administered using a pipetter. Each animal was bled in day 42. After the bleeding, nasal cavity was washed with 10 mL sterile normal saline. Sera and nasal lavages of each group were pooled and kept frozen until immunological assays. Anti-TT antibodies in the rabbit serum and nasal lavage were determined by end-point titration using an ELISA method (Amin et al. 2009). Serum and nasal lavages of unimmunized animals were used as negative control. End-point titres for IgG and IgA were determined as the highest serum or lavage dilution that resulted in an absorbance value (OD 450) equal to negative control.
Statistical analysis
One-way analysis of variance (ANOVA) statistical test with Tukey’s post test was performed using Graph-Pad InStat version 3.05 for Windows (GraphPad Software, San Diego) to determine the significance of the differences between various groups. p Values smaller than 0.05 were considered as significant.
Results
Characterization of TT-loaded chitosan nanospheres
The Z-average and zeta potential of blank CHT NPs were 205 ± 42 and 83.2 ± 4, respectively. These values for TT-loaded CHT NPS were 432 ± 85 and 71.6 ± 4, respectively. Encapsulation efficiency of TT in nanospheres was measured to be 39%.
Release profile of TT from chitosan nanosphere
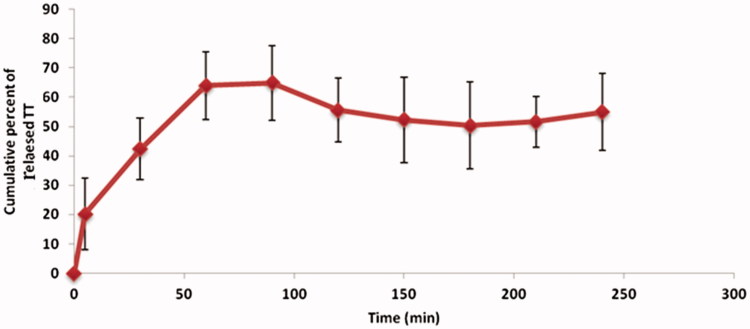
The TT was released from nanospheres with a burst release of 42.4 ± 10.6% within 30 min. This was followed by a slow and sustained release profile and after 90 min, it reached to plateau. After 4 h, 60 ± 13.1% of encapsulated TT was released (Figure 1).
Figure 1.
In vitro release profile of encapsulated TT from chitosan nanospheres.
The rate constants for each kinetic model were calculated and plotted against time, and the related square regressions (R2) and SSE values were computed (Table 1). It was observed that the R2 value from the Krosmeyer–Peppas model is the highest among other models and it is also the closest to 1. So that, in vitro release of antigen from chitosan nanospheres is best described by the Krosmeyer–Peppas model. The obtained value of n for antigen was 0.454 and lied between 0.43 and 0.85; which indicated that the release of antigen followed Fickian diffusion model from the formulations (Dehghan et al. 2013).
Table 1.
The rate constants (K), squared regression (R2) and sum of squared errors (SSE) for the investigated kinetic models.
| Zero-order | First-order | Higuchi | Krosmeyer–Peppas | |
|---|---|---|---|---|
| K | 0.6804 | 9.4864 | 7.5049 | 9.5753 |
| R2 | 0.8611 | 0.8967 | 0.9712 | 0.9926 |
| SSE | 431.1 | 320.6 | 89.3 | 65.7 |
The structural stability of the encapsulated TT was studied with SDS–PAGE method. The structural integrity of the TT was confirmed by this method.
Serum anti-TT IgG titres
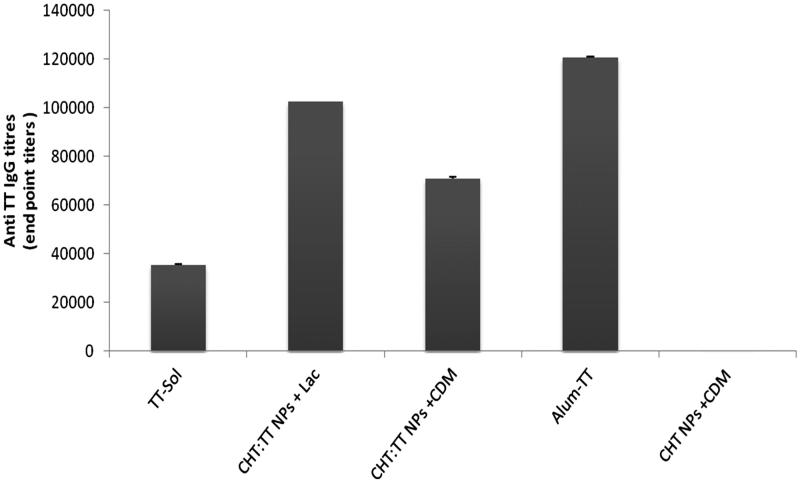
The highest IgG titres were observed in Alum-TT group (p < 0.001) (Figure 2). Nanoparticulate form of TT showed higher IgG titres than TT solution (p < 0.001). Among nanoparticulate formulations, more IgG titres were observed with nanospheres mixed with lactose powder (p < 0.001).
Figure 2.
Serum anti-TT IgG titers. Rabbits (n = 4) were nasally immunized at weeks 0, 14 and 28, and bled at week 6.
Nasal lavage anti-TT sIgA titres
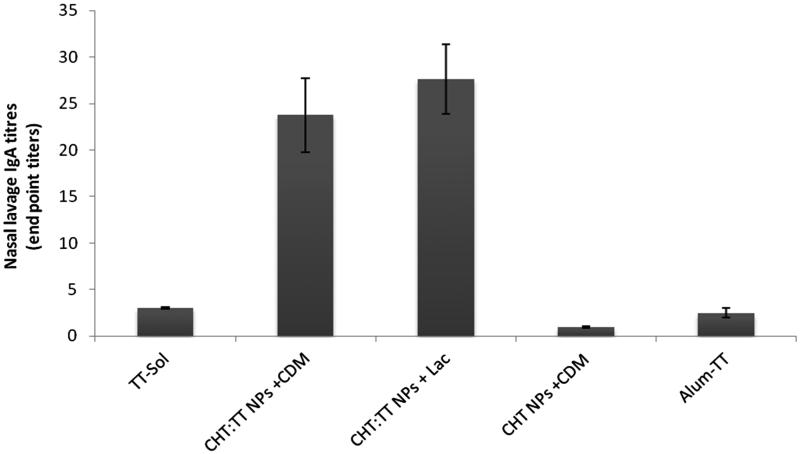
Among the immunized groups, the lowest sIgA titres were observed in TT-Sol group (p < 0.001). Both nanoparticulate formulations showed significantly higher sIgA titres than TT solution (p < 0.001). The sIgA titres induced with nanospheres mixed with lactose and CDM were not significantly different (p > 0.05) (Figure 3).
Figure 3.
Nasal lavage anti-TT IgA titers. Rabbits (n = 4) were nasally immunized at weeks 0, 14 and 28, and lavages were collected at week 6.
Discussion
Nasal delivery of vaccines has several advantages like induction of mucosal immune responses and escape from injections and their drawbacks. For a successful mucosal immunization, efficient adjuvant/delivery systems are required for the protection of antigens from mucosal enzymes, enhancement of antigen uptake by NALT microfold (M) cells and also improving the interaction of antigen with immune cells. At the present study, the model antigen (tetanus toxoid) was encapsulated with chitosan NPs, as a protective and mucoadhesive polymer. Cross-linked dextran microspheres (CDM) have also been added as mucosal penetration enhancer particles, with a good history for nasal delivery of macromolecules, such as insulin (Pereswetoff-Morath & Edman 1995). Formulations were prepared as dry powder form because of their stability, ease of administration and possible escape from the cold chain. Rabbits were chosen for the in vivo studies because of their similar nasal immune system to human and also possibility of correct administeration of dry powder formulations (Amin et al. 2009).
The size of particles plays a critical role in immunoadjuvant properties of the nanoparticles. It has been shown that by reducing the particle size to nano range, mucoadhesion property will be increased significantly (Khameneh et al. 2014). Jubeh et al. (2004) explored that by reducing the particle size from 800 nm to 100 nm, the mucoadhesion property increased significantly. The size of nanospheres prepared was approximatly 400 nm. It has also been shown that for efficient vaccine delivery from nasal route, the particle size must be less than 10 μm, therefore, the prepared nanopheres could efficiently interact with the immune cells and could have a good potential for mucoadhesion (Eldridge et al. 1991).
To study the release profile of encapsulated antigen, a diffusion cell model was used to simulate the nasal cavity situations in terms of temperature and humidity. This method shows several benefits over usual in vitro release studies, in which nanoparticles are immersed in the release medium. In this model, nanospheres are in contact with a wetted and warm surface (filter paper clamped in a Frantz diffusion cell) in a humid atmosphere, similar to nasal cavity. The release profile shows a primary burst release within the first hour and followed by a plateau until the fourth hour (Figure 1). It has been shown that the clearance half-life in human nose is about 15–20 min (Illum et al. 1987; Turker et al. 2004). Considering the mucoadhesive potential of the chitosan nanospheres, more prolonged presence in the nasal cavity could be expected. Based on the release profile, about 50% of loaded TT is in the encapsulated form during the presence of nanospheres in the nasal cavity. Therefore, it is expected that TT is interfered with nasal mucosa both in solution and nanoparticulate form. Similar release profile from nanospheres was described earlier (Agnihotri et al. 2004). These results were in agreement with previous published data (Tafaghodi et al. 2006b).
The antigen stability was also evaluated in the present study. The results indicated that during preparation processes, antigen integrity was preserved. These results were in agreement with earlier findings (Khameneh et al. 2014). Taken together, these observations reflected the proper physichochemical properties of lead formulations for vaccine delivery.
As shown in Figure 2, the highest sera IgG titres was observed in the sera of rabbits immunized intramascularly with alum adsorbed TT, the official vaccine (p < 0.001). This is quite expectable to attain more systemic responses after parenteral immunization.
TT is a large (150 kDa) protein antigen. After nasal administeration, TT solution could be sampled by microfold (M) cells located in nasal-associated lymphoid tissue (NALT) (Amin et al. 2009). TT is detoxified by formaldehyde, a cross linker that preserves the structure of TT and increase its stability. TT solution could induce systemic IgG titres, even though there is least responses among the studied groups (p < 0.001). Encapsulation of TT with chitosan nanospheres has induced the highest IgG titres (p < 0.001). This observation could be resulted from one or more of the following reasons:
In chitosan nanospheres, polymer matrix can protect antigen from the proteolytic enzymes.
Chitosan has shown good mucoadhesive potential and can increase the residence time of antigen in the nasal cavity, by which the chance of uptake of nanospheres by M cells will be increased (Gavini et al. 2006; Khameneh et al. 2014).
Chitosan has absorption enhancing potential, and can increase the absorption of antigen (Gavini et al. 2006).
Chitosan nanospheres give particulate nature to the antigen, and help in the uptake of more antigen by M cells (Gavini et al. 2006; Ghendon et al. 2008).
Cross-linked dextran microspheres (CDM), as dried hydrogel particles, can absorb water from epithelial cells and open up the intercellular tight junctions. This absorption enhancer effect have been frequently reported after co-administeration with proteins such as insulin (Chandler et al. 1991). We have also shown that co-administeration of CDM with PLGA nanospheres, loaded with TT, could increase serum IgG titres (Mohaghegh & Tafaghodi 2011). However, in this study, a reverse effect was observed and sera IgG titres after mixing of chitosan nanospheres with CDM was decreased (p < 0.001).
After nasal administration of formulations, the sIgA titres in nasal lavages were determined (Figure 3). Among the nasally immunized groups, immunization with nanoparticulate TT was significantly higher than TT solution (p < 0.001). It shows the potential of chitosan nanospheres for the induction of mucosal IgA against their encapsulated antigen. This could be attributed to mucoadhesion potential of chitosan nanospheres and their more prolonged presence in contact with nasal mucosa (Nakamura et al. 1996). This was frequently reported with chitosan nanospheres and other polymer-based nanoparticles (Nakamura et al. 1996; Illum et al. 2001; Dehghan et al. 2014). In spite of the negative impact of CDM on the systemic IgG titres, they didn’t show any significant effect on mucosal sIgA titres. We have also reported the similar results after mixing of CDM powder with TT powder (Tafaghodi & Eskandari 2012), TT-alginate microspheres (Tafaghodi et al. 2006a) and TT-PLGA nanospheres (Tafaghodi et al. 2010). It is now well established that the immunoadjuvant mechanisms of CDM for systemic IgG is not effective for the induction of mucosal sIgA.
Conclusions
Dry powder form of chitosan nanospheres was evaluated for nasal immunizations against a model antigen, TT. TT-chitosan nanosperes induced high systemic immune responses. This immunoadjuvant effect along with more stability and easier storage, transport and administeration are very good reasons for positive attitude towards this formulation. However, mixing of TT-chitosan powders with CDM powder could not enhance the systemic and mucosal immune responses and it is not therefore a good excipient for nasal dry powder form of vaccines composed from chitosan nanoparticles.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM.. 2004. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 100:5–28. [DOI] [PubMed] [Google Scholar]
- Amin M, Jaafari MR, Tafaghodi M.. 2009. Impact of chitosan coating of anionic liposomes on clearance rate, mucosal and systemic immune responses following nasal administration in rabbits. Colloids Surf B Biointerfaces. 74:225–229. [DOI] [PubMed] [Google Scholar]
- Amorij JP, Kersten GF, Saluja V, Tonnis WF, Hinrichs WL, Slutter B, Bal SM, Bouwstra JA, Huckriede A, Jiskoot W.. 2012. Towards tailored vaccine delivery: needs, challenges and perspectives. J Control Release. 161:363–376. [DOI] [PubMed] [Google Scholar]
- Arthanari S, Mani G, Peng MM, Jang HT.. 2016. Chitosan-HPMC-blended microspheres as a vaccine carrier for the delivery of tetanus toxoid. Artif Cells Nanomed Biotechnol. 44:517–523. [DOI] [PubMed] [Google Scholar]
- Barhate G, Gautam M, Gairola S, Jadhav S, Pokharkar V.. 2014. Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalized gold nanoparticles and botanical adjuvant: characterization, immunogenicity, and stability assessment. J Pharm Sci. 103:3448–3456. [DOI] [PubMed] [Google Scholar]
- Chandler SG, Ilium L, Thomas NW.. 1991. Nasal absorption in rats. II. Effect of enhancers on insulin absorption and nasal histology. Int J Pharm. 76:61–70. [Google Scholar]
- Dehghan S, Tafaghodi M, Bolourieh T, Mazaheri V, Torabi A, Abnous K, Tavassoti Kheiri M.. 2014. Rabbit nasal immunization against influenza by dry-powder form of chitosan nanospheres encapsulated with influenza whole virus and adjuvants. Int J Pharm. 475:1–8. [DOI] [PubMed] [Google Scholar]
- Dehghan S, Tavassoti Kheiri M, Tabatabaiean M, Darzi S, Tafaghodi M.. 2013. Dry-powder form of chitosan nanospheres containing influenza virus and adjuvants for nasal immunization. Arch Pharm Res. 36:981–992. [DOI] [PubMed] [Google Scholar]
- Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM.. 1991. Biodegradable and biocompatible poly(DL-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 59:2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabee MZ, Morsi RE, Al-Sabagh AM.. 2009. Surface active properties of chitosan and its derivatives. Colloids Surf B Biointerfaces. 74:1–16. [DOI] [PubMed] [Google Scholar]
- Garmise RJ, Staats HF, Hickey AJ.. 2007. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS Pharm Sci Tech. 8:E81. [DOI] [PubMed] [Google Scholar]
- Gavini E, Hegge AB, Rassu G, Sanna V, Testa C, Pirisino G, Karlsen J, Giunchedi P.. 2006. Nasal administration of carbamazepine using chitosan microspheres: in vitro/in vivo studies. Int J Pharm. 307:9–15. [DOI] [PubMed] [Google Scholar]
- Ghendon Y, Markushin S, Krivtsov G, Akopova I.. 2008. Chitosan as an adjuvant for parenterally administered inactivated influenza vaccines. Arch Virol. 153:831–837. [DOI] [PubMed] [Google Scholar]
- Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS.. 2001. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 51:81–96. [DOI] [PubMed] [Google Scholar]
- Illum L, Jørgensen H, Bisgaard H, Krogsgaard O, Rossing N.. 1987. Bioadhesive microspheres as a potential nasal drug delivery system. Int J Pharm. 39:189–199. [Google Scholar]
- Jubeh TT, Barenholz Y, Rubinstein A.. 2004. Differential adhesion of normal and inflamed rat colonic mucosa by charged liposomes. Pharm Res. 21:447–453. [DOI] [PubMed] [Google Scholar]
- Khameneh B, Momen-nejad M, Tafaghodi M.. 2014. In vivo evaluation of mucoadhesive properties of nanoliposomal formulations upon coating with trimethylchitosan polymer. Nanomed J. 1:147–154. [Google Scholar]
- Khodaverdi E, Heidari Z, Tabassi SA, Tafaghodi M, Alibolandi M, Tekie FS, Khameneh B, Hadizadeh F.. 2015. . Injectable supramolecular hydrogel from insulin-loaded triblock PCL-PEG-PCL copolymer and γ-cyclodextrin with sustained-release property. AAPS PharmSciTech. 16:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohaghegh M, Tafaghodi M.. 2011. Dextran microspheres could enhance immune responses against PLGA nanospheres encapsulated with tetanus toxoid and Quillaja saponins after nasal immunization in rabbit. Pharm Dev Technol. 16:36–43. [DOI] [PubMed] [Google Scholar]
- Mohajer M, Khameneh B, Tafaghodi M.. 2014. Preparation and characterization of PLGA nanospheres loaded with inactivated influenza virus, CpG-ODN and Quillaja saponin. Iran J Basic Med Sci. 17:722–726. [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Ohta R, Machida Y, Nagai T.. 1996. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int J Pharm. 134:173–181. [Google Scholar]
- Pereswetoff-Morath L, Edman P.. 1995. Dextran microspheres as a potential nasal drug delivery system for insulin-in vitro and in vivo properties. Int J Pharm. 124:37–44. [Google Scholar]
- Sadighi A, Ostad SN, Rezayat SM, Foroutan M, Faramarzi MA, Dorkoosh FA.. 2012. . Mathematical modelling of the transport of hydroxypropyl-β-cyclodextrin inclusion complexes of ranitidine hydrochloride and furosemide loaded chitosan nanoparticles across a Caco-2 cell monolayer. Int J Pharm. 422:479–488. [DOI] [PubMed] [Google Scholar]
- Sajadi Tabassi SA, Tafaghodi M, Jaafari MR.. 2008. Induction of high antitoxin titers against tetanus toxoid in rabbits by intranasal immunization with dextran microspheres. Int J Pharm. 360:12–17. [DOI] [PubMed] [Google Scholar]
- Slane JA, Vivanco JF, Rose WE, Squire MW, Ploeg HL.. 2014. The influence of low concentrations of a water soluble poragen on the material properties, antibiotic release, and biofilm inhibition of an acrylic bone cement. Mater Sci Eng C Mater Biol Appl. 42:168–176. [DOI] [PubMed] [Google Scholar]
- Tafaghodi M, Eskandari M.. 2012. The mucosal adjuvant potential of cross-linked dextran microspheres as dry powder. Iran J Basic Med Sci. 15:873–879. [PMC free article] [PubMed] [Google Scholar]
- Tafaghodi M, Rastegar S.. 2010. Preparation and in vivo study of dry powder microspheres for nasal immunization. J Drug Target. 18:235–242. [DOI] [PubMed] [Google Scholar]
- Tafaghodi M, Sajadi Tabasi SA, Jaafari MR.. 2006a. Formulation, characterization and release studies of alginate microspheres encapsulated with tetanus toxoid. J Biomater Sci Polym Ed. 17:909–924. [DOI] [PubMed] [Google Scholar]
- Tafaghodi M, Sajadi Tabassi S, Jaafari MR.. 2010. Nasal immunization by (PLGA) nanospheres encapsulated with tetanus toxoid and (CpG-ODN). Iran J Pharmaceut Res. 6:151–158. [Google Scholar]
- Tafaghodi M, Sajadi Tabassi SA, Jaafari MR.. 2006b. Induction of systemic and mucosal immune responses by intranasal administration of alginate microspheres encapsulated with tetanus toxoid and CpG-ODN. Int J Pharm. 319:37–43. [DOI] [PubMed] [Google Scholar]
- Turker S, Onur E, Ozer Y.. 2004. Nasal route and drug delivery systems. Pharm World Sci. 26:137–142. [DOI] [PubMed] [Google Scholar]
- Xu Y, Du Y.. 2003. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm. 250:215–226. [DOI] [PubMed] [Google Scholar]
- Yoksan R, Chirachanchai S.. 2008. Amphiphilic chitosan nanosphere: studies on formation, toxicity, and guest molecule incorporation. Bioorg Med Chem. 16:2687–2696. [DOI] [PubMed] [Google Scholar]