Abstract
Context: Ginkgo leaf tablets (GLTs) and losartan are often simultaneously used for the treatment of hypertension in Chinese clinics. However, the herb–drug interaction between GLT and losartan is still unknown.
Objective: This study investigates the effects of GLT on the pharmacokinetics of losartan and its metabolite EXP3174 in rats and its potential mechanism.
Materials and methods: The pharmacokinetic profiles of losartan and EXP3174 of orally administered losartan (10 mg/kg) with or without GLT pretreatment (80 mg/kg/day for 10 days) in Sprague–Dawley rats were determined. In vitro, the effects of GLT on the metabolic stability of losartan were investigated with rat liver microsomes.
Results: The Cmax (1.22 ± 0.25 vs 1.85 ± 0.37 μg/mL) and the AUC(0–t) (6.99 ± 1.05 vs 11.94 ± 1.79 mg·h/L) of losartan increased significantly (p < 0.05) with GLT pretreatment, while the Cmax (1.05 ± 0.19 vs 0.72 ± 0.12 μg/mL) of EXP3174 decreased significantly (p < 0.05) compared to the control. The t1/2 of losartan was prolonged significantly from 3.94 ± 0.62 to 4.75 ± 0.52 h (p < 0.05). The metabolic stability of losartan was increased from 37.4 min to 59.6 min with GLT pretreatment.
Discussion and conclusions: The results indicate that GLT might increase the plasma concentration of losartan and decrease the concentration of EXP3174 through inhibiting the metabolism of losartan.
Keywords: Herb–drug interaction, metabolism, CYP3A4
Introduction
Losartan is the first nonpeptide angiotensin II receptor blocker used in hypertension and diabetic nephropathy (Klishadi et al. 2015). Losartan can be absorbed quickly and transformed into its active metabolite EXP3174 after oral administration, and the clinical efficacy of EXP3174 is about 10-fold more potent than its parent drug (Rincon et al. 2015). Thus, the clinical hypotensive activity is predominantly mediated by the active metabolite EXP3174, although losartan itself exhibits good efficacy (Varshney et al. 2013). Because of its good anti-hypertension effect, losartan has been one of the anti-hypertension drug most frequently used for the prevention and control of hypertension in the clinic (Yasar et al. 2008; Yang et al. 2011). Losartan is mainly metabolized by cytochrome P450 enzymes CYP3A4 and CYP2C9, and therefore, modulation of CYP3A4 or CYP2C9 activities may cause significant changes in the pharmacokinetic profiles of losartan and its active metabolite EXP3174 (Li et al. 2009; Wang et al. 2016; Hu et al. 2017).
Ginkgo leaf tablet (GLT) is an effective traditional Chinese multi-herbal formula which is widely used in treating ischemic cerebrovascular disease in the clinic (Lin et al. 2003; Chung et al. 2006; Yang et al. 2017). The main components of GLT are ginkgo flavone glycosides, ginkgolides and bilobalides (Liu et al. 2009; Guan et al. 2014; Rao et al. 2014). Losartan and GLT are often simultaneously used for the treatment of hypertension in Chinese clinics. However, many herb–drug interactions resulting from concurrent use of herbal drugs with over-the-counter drugs may cause adverse reactions such as toxicity and treatment failure, and the herb–drug interaction between GLT and losartan are still unknown. Therefore, it is essential to investigate the effects of GLT on the pharmacokinetics of losartan and its potential mechanism.
This study investigates the effects of GLT on the pharmacokinetics of losartan and clarifies its main potential mechanism using rat liver microsome incubation systems.
Materials and methods
Chemicals and reagents
Standards of losartan (purity >98%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Losartan carboxylic acid (EXP3174) was purchased from Toronto Research Chemicals Inc., Canada. GLTs were purchased from Sichuan Kelun Pharmaceutical Co., LTD. β-Nicotinamide adenine dinucleotide phosphate (NADP) and lucifer yellow were provided by Sigma (St. Louis, MO). Rat liver microsomes were purchased from BD (Woburn, MA). Acetonitrile and methanol were purchased from Fisher Scientific (Fair Lawn, NJ). Formic acid was purchased from Anaqua Chemicals Supply Inc. Limited (Houston, TX). Ultrapure water was prepared with a Milli-Q water purification system (Millipore, Billerica, MA). All other chemicals were of analytical grade or better.
Instrumentation and conditions
The analysis was performed on an Agilent Series 1100 HPLC system and Agilent G1946 single quadrupole mass spectrometer (Palo Alto, CA) as previously reported (Li et al. 2016). The sample was separated on Waters Xbridge C18 column (100 × 3.0 mm, i.d.; 3.5 mm, USA) and eluted with an isocratic mobile phase: solvent A (H2O–HCOOH, 100:0.1, v/v) and solvent B (CH3OH) (55:45, v/v). The total analysis time was 8 min. The column temperature was 25 °C at a flow rate of 0.8 mL/min and injection volume of 5 μL, and the split ratio was 1:1.
The mass conditions were optimized as follows: capillary 4000 V, nebulizer pressure 40 psig, drying gas flowing rate 10 L/min, gas temperature 350 °C and fragmentor 80 ev. Select-ion-monitoring (SIM) in the positive ion mode was used. The ions of losartan, EXP3174, and irbesartan were recorded as m/z 423.1, m/z 437.1, and m/z 429.2 for the quantification, respectively.
Animal experiments
Male Sprague–Dawley rats weighing 250 ± 20 g were provided by the experimental animal center of the Taishan Medical University (Taishan, China). The rats were maintained in an air-conditioned animal quarter at 22 ± 2 °C and 50 ± 10% relative humidity. Water and food (laboratory rodent chow, Shanghai, China) were allowed ad libitum. The animals were acclimatized to the facilities for 5 days, and then fasted with free access to water for 12 h prior to each experiment. All experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Taishan Medical University and were in accordance with the National Institutes of Health guidelines regarding the principles of animal care.
In vivo pharmacokinetic study
Twelve rats were equally randomized to two groups (six rats in each group), including the losartan group (A) and the losartan + GLT group (B). Group B was pretreated with GLT solutions at a dose of 80 mg/kg/day for 10 days before the administration of losartan, and then losartan were orally administered to rats by gavage at a dose of 10 mg/kg. Blood samples (0.20 mL) were collected into a heparinized tube via the oculi chorioideae vein before drug administration and at 0.083, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 h after drug administration. The blood samples were centrifuged at 3500 rpm for 5 min. The plasma samples that were obtained were stored at −40 °C until analysis.
Effects of the main components in GLT on the metabolism of losartan in rat liver microsomes
The effects of GLT on the metabolic stability of losartan were investigated using rat liver microsome incubation systems. The assay conditions and reaction mixtures were similar as reported previously (Li et al. 2016). In brief, 10 μL rat liver microsomes (20 mg/mL), 4 μL losartan solution (100 μM, final concentration of 1 μM) and 366 μL PBS buffer were added to the centrifuge tubes on ice. The reaction mixture was incubated at 37 °C for 5 min and then NADPH-generating system (15 μL) was added. The effects of the above components on the metabolic stability of losartan were investigated by adding 4 μL GLT solution (350 μg/mL) to rat liver microsomes and preincubating them for 30 min at 37 °C, followed by the addition of losartan. Aliquots of 30 μL were collected from reaction volumes at 0, 1, 3, 5, 15, 30 and 60 min and 60 μL ice-cold acetonitrile containing IS was added to terminate the reaction, and then the concentration of losartan was determined using LC–MS. The half-life (t1/2) in vitro was obtained using equation: t1/2 = 0.693/k.
Data analysis
The pharmacokinetic parameters were calculated using the DAS 3.0 pharmacokinetic software (Chinese Pharmacological Association). The differences between the mean values were analyzed for significance using a one-way analysis of variance (ANOVA). Values of p < 0.05 were considered to be statistically significant.
Results
In vivo pharmacokinetic study
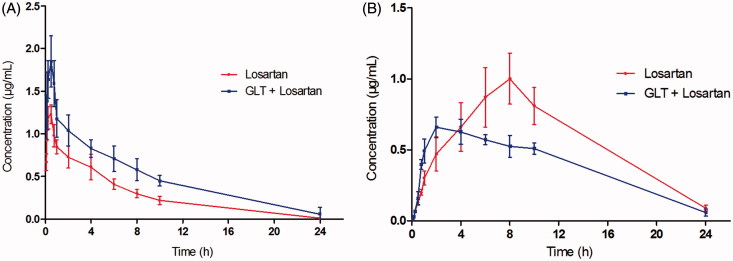
The pharmacokinetic parameters of losartan and EXP3174 were calculated using the noncompartmental method with DAS 3.0 pharmacokinetic software, and the pharmacokinetic parameters are shown in Table 1. The mean plasma concentration–time curves of losartan and EXP3174 are shown in Figure 1.
Table 1.
Pharmacokinetic parameters of losartan and EXP3174 in male Sprague–Dawley rats following oral administration of losartan alone (Group A) or both losartan and GLT (Group B).
| Parameter | Losartan |
EXP3174 |
||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Tmax (h) | 0.39 ± 0.08 | 0.51 ± 0.10* | 7.98 ± 1.02 | 3.34 ± 0.63* |
| Cmax (mg L−1) | 1.22 ± 0.25 | 1.85 ± 0.37* | 1.05 ± 0.19 | 0.72 ± 0.12* |
| t1/2 (h) | 3.94 ± 0.62 | 4.75 ± 0.52* | 4.42 ± 0.35 | 4.99 ± 0.51 |
| AUC(0–t) (mg h L−1) | 6.99 ± 1.05 | 11.94 ± 1.79* | 13.12 ± 2.52 | 7.28 ± 1.07* |
| Oral CL (L h−1 kg−1) | 1.43 ± 0.29 | 0.82 ± 0.21* | 0.75 ± 0.14 | 1.29 ± 0.25* |
*p < 0.05 indicates significant differences from the control.
Figure 1.
The mean concentration-time curves in rat plasma after oral administration of losartan or both GLT and losartan. (A) losartan; (B) EXP3174.
As shown in Table 1, the parameters Cmax and AUC(0–t) for losartan in the losartan and GLT (B) group were larger than those of losartan-only group (A), and the difference was significant (p < 0.05); However, Cmax and AUC(0–t) for EXP3174 in the losartan and GLT (B) group were smaller than those of losartan-only group (A), and the difference was also significant (p < 0.05). The results indicated that the plasma concentration of losartan increased in losartan and GLT group compared with the single losartan group, and however, the plasma concentration of EXP3174 decreased. The t1/2 of losartan was prolonged significantly with the pretreatment of GLT, and the oral clearance rate decreased significantly (p < 0.05). The results showed that the metabolism clearance of losartan was inhibited by GLT.
Effects of GLT on the metabolic stability of losartan in rat liver microsome incubation systems
As we know, the metabolism of losartan was mainly mediated by CYP450 enzyme, and therefore, in this research, the effects of GLT on the metabolic rate of losartan were investigated. The results showed that the metabolic stability of losartan was 32.5 min, while the metabolic rate was prolonged (46.8 min) in the presence of GLT. The results indicated that GLT could inhibit the metabolic stability of losartan.
Discussion
This is the first study to investigate the effects of GLT on the pharmacokinetics of losartan and its metabolite EXP3174 in rats, and the results indicated that GLT could increase the systemic exposure of losartan and while decrease the systemic exposure of EXP3174 in rats. The rat liver microsome incubation experiments revealed that GLT might affect the pharmacokinetics profiles of losartan and EXP3174 through inhibiting its metabolism in rat liver.
It is now well known that a drug can inhibit the metabolic stability of another drug, leading to a higher than intended plasma level (Wrighton and Stevens 1992; Zhang et al. 2007; Ye et al. 2011). The major clinical consequence of inhibitory drug–drug interactions is undesired drug toxicity (Gouws et al. 2012; Qi et al. 2013). For example, the antifungal ketoconazole, a potent inhibitor of CYP3A4, causes drug–drug interactions with drugs that are substrates of CYP3A4 (Xiaoyang et al. 2015). Losartan was primarily metabolized by CYP3A4 and CYP2C19 in liver, and therefore, herbs or drugs that could affect the activity of CYP3A4 or CYP2C19 might influence the pharmacokinetics profiles of losartan when they are co-administered. Some research articles have also indicated that the pharmacokinetic profiles of losartan was affected by co-administered herbs or drugs (Spanakis et al. 2013; Yuan et al. 2013; Li et al. 2016; Wang et al. 2016).
Previous studies have also reported that the GLT could affect the pharmacokinetic profiles of amlodipine, cilostazol, fexofenadine and clopidogrel when they are co-administered with GLT (Deng et al. 2016; Wang et al. 2016; Turkanovic et al. 2017). The main components in GLT are ginkgolides A, ginkgolides B, bilobalide, quercetin, and kaempferol, and several studies have indicated that the main components in could inhibit the activity of CYP3A4 enzymes (Etheridge et al. 2007; Zadoyan et al. 2012; Palle and Neerati 2017).
Therefore, we think that GLT might affect the pharmacokinetic profiles of losartan and EXP3174 through inhibiting the metabolism of losartan. The present study reminds us that the herb–drug interaction between GLT and losartan might happen, and further evaluation in clinical studies is also necessary.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chung SY, Cheng FC, Lee MS, Lin JY, Lin MC, Wang MF. 2006. Ginkgo biloba leaf extract (EGb761) combined with neuroprotective agents reduces the infarct volumes of gerbil ischemic brain. Am J Chin Med. 34:803–817. [DOI] [PubMed] [Google Scholar]
- Deng Y, Mo YF, Chen XM, Zhang LZ, Liao CF, Song Y, Xu C. 2016. Effect of Ginkgo niloba extract on the pharmacokinetics and metabolism of clopidogrel in rats. Phytother Res. 30:1886–1892. [DOI] [PubMed] [Google Scholar]
- Etheridge AS, Black SR, Patel PR, So J, Mathews JM. 2007. An in vitro evaluation of cytochrome P450 inhibition and P-glycoprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and Ginseng extracts and their constituents. Planta Med. 73:731–741. [DOI] [PubMed] [Google Scholar]
- Gouws C, Steyn D, Du Plessis L, Steenekamp J, Hamman JH. 2012. Combination therapy of Western drugs and herbal medicines: recent advances in understanding interactions involving metabolism and efflux. Expert Opin Drug Metab Toxicol. 8:973–984. [DOI] [PubMed] [Google Scholar]
- Guan H, Qian D, Ren H, Zhang W, Nie H, Shang E, Duan J. 2014. Interactions of pharmacokinetic profile of different parts from Ginkgo biloba extract in rats. J Ethnopharmacol. 155:758–768. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhou X, Shi H, Shi W, Ye S, Zhang H. 2017. The effect of tripterygium glucoside tablet on pharmacokinetics of losartan and its metabolite EXP3174 in rats. Biomed Chromatogr. 31:Epub [DOI] [PubMed] [Google Scholar]
- Klishadi MS, Zarei F, Hejazian SH, Moradi A, Hemati M, Safari F. 2015. Losartan protects the heart against ischemia reperfusion injury: sirtuin3 involvement. J Pharm Pharm Sci. 18:112–123. [DOI] [PubMed] [Google Scholar]
- Li H, Liu L, Xie L, Gan D, Jiang X. 2016. Effects of berberine on the pharmacokinetics of losartan and its metabolite EXP3174 in rats and its mechanism. Pharm Biol. 54:2886–2894. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang G, Wang LS, Zhang W, Tan ZR, Fan L, Chen BL, Li Q, Liu J, Tu JH, et al. 2009. Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica. 39:788–793. [DOI] [PubMed] [Google Scholar]
- Lin CC, Cheng WL, Hsu SH, Chang CM. 2003. The effects of Ginkgo biloba extracts on the memory and motor functions of rats with chronic cerebral insufficiency. Neuropsychobiology. 47:47–51. [DOI] [PubMed] [Google Scholar]
- Liu XP, Luan JJ, Goldring CE. 2009. Comparison of the antioxidant activity amongst Gingko biloba extract and its main components. Zhong Yao Cai. 32:736–740. [PubMed] [Google Scholar]
- Palle S, Neerati P. 2017. Enhancement of oral bioavailability of rivastigmine with quercetin nanoparticles by inhibiting CYP3A4 and esterases. Pharmacol Rep. 69:365–370. [DOI] [PubMed] [Google Scholar]
- Qi XY, Liang SC, Ge GB, Liu Y, Dong PP, Zhang JW, Wang AX, Hou J, Zhu LL, Yang L, et al. 2013. Inhibitory effects of sanguinarine on human liver cytochrome P450 enzymes. Food Chem Toxicol. 56:392–397. [DOI] [PubMed] [Google Scholar]
- Rao Z, Qin H, Wei Y, Zhou Y, Zhang G, Zhang F, Shao Y, Huang J, Wu X. 2014. Development of a dynamic multiple reaction monitoring method for determination of digoxin and six active components of Ginkgo biloba leaf extract in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 959:27–35. [DOI] [PubMed] [Google Scholar]
- Rincon J, Correia D, Arcaya JL, Finol E, Fernandez A, Perez M, Yaguas K, Talavera E, Chavez M, Summer R, et al. 2015. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 124:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanakis M, Vizirianakis IS, Batzias G, Niopas I. 2013. Pharmacokinetic interaction between losartan and Rhodiola rosea in rabbits. Pharmacology. 91:112–116. [DOI] [PubMed] [Google Scholar]
- Turkanovic J, Ward MB, Gerber JP, Milne RW. 2017. Effect of garlic, gingko, and St. John's wort extracts on the pharmacokinetics of fexofenadine: a mechanistic study. Drug Metab Dispos. 45:569–575. [DOI] [PubMed] [Google Scholar]
- Varshney E, Saha N, Tandon M, Shrivastava V, Ali S. 2013. Genotype-phenotype correlation of cytochrome P450 2C9 polymorphism in Indian National Capital Region. Eur J Drug Metab Pharmacokinet. 38:275–282. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang H, Sun S, Wang Y, Chai Y, Yuan Y. 2016. Effect of Ginkgo leaf tablets on the pharmacokinetics of amlodipine in rats. Eur J Drug Metab Pharmacokinet. 41:825–833. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang H, Wang Y, Yu X, Yuan Y. 2016. Effects of salvianolic acid B and tanshinone IIA on the pharmacokinetics of losartan in rats by regulating the activities and expression of CYP3A4 and CYP2C9. J Ethnopharmacol. 180:87–96. [DOI] [PubMed] [Google Scholar]
- Wrighton SA, Stevens JC. 1992. The human hepatic cytochromes P450 involved in drug metabolism. Critical Rev Toxicol. 22:1–21. [DOI] [PubMed] [Google Scholar]
- Xiaoyang L, Chenming N, Chengqing L, Tao L. 2015. Drug-drug interaction prediction between ketoconazole and anti-liver cancer drug Gomisin G. Afr Health Sci. 15:590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Cho YA, Choi JS. 2011. Effects of ticlopidine on pharmacokinetics of losartan and its main metabolite EXP-3174 in rats. Acta Pharmacol Sin. 32:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li Y, Wang J, Sun K, Tao W, Wang Z, Xiao W, Pan Y, Zhang S, Wang Y. 2017. Systematic investigation of Ginkgo biloba leaves for treating cardio-cerebrovascular diseases in an animal model. ACS Chem Biol. 12:1363–1372. [DOI] [PubMed] [Google Scholar]
- Yasar U, Babaoglu MO, Bozkurt A. 2008. Disposition of a CYP2C9 phenotyping agent, losartan, is not influenced by the common 3435C > T variation of the drug transporter gene ABCB1 (MDR1). Basic Clin Pharmacol Toxicol. 103:176–179. [DOI] [PubMed] [Google Scholar]
- Ye L, Wang T, Tang L, Liu W, Yang Z, Zhou J, Zheng Z, Cai Z, Hu M, Liu Z. 2011. Poor oral bioavailability of a promising anticancer agent andrographolide is due to extensive metabolism and efflux by P-glycoprotein. J Pharm Sci. 100:5007–5017. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhang H, Ma W, Sun S, Wang B, Zhao L, Zhang G, Chai Y. 2013. Influence of compound danshen tablet on the pharmacokinetics of losartan and its metabolite EXP3174 by liquid chromatography coupled with mass spectrometry. Biomed Chromatogr. 27:1219–1224. [DOI] [PubMed] [Google Scholar]
- Zadoyan G, Rokitta D, Klement S, Dienel A, Hoerr R, Gramatte T, Fuhr U. 2012. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 68:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Liu Y, Cheng J, Li W, Ma H, Liu HT, Sun J, Wang LM, He YQ, Wang Y, et al. 2007. Inhibition of human liver cytochrome P450 by star fruit juice. J Pharm Pharm Sci. 10:496–503. [DOI] [PubMed] [Google Scholar]