Abstract
Context: Since antiquity, Pistacia lentiscus L. (Anacardiaceae) fruit oil (PLFO) has been used as a remedy for primary health care such as burn treatment.
Objective: This study assesses the healing effect of PLFO on CO2 laser fractional burn in a rat model.
Materials and methods: The study was carried out on 18 adult male Wistar rats. A second-degree laser burn (wound area = 2.2 cm2) was inflicted in the dorsal region by the application of CO2 fractional laser within the following parameters; Energy level: 25 MJ and Depth level: 4. After applying laser, the rats were divided into three groups: the first was treated with saline solution, the second with a reference cream ‘CYTOL BASIC®’ (0.13 μg/mm2) and the third with PLFO (0.52 μL/mm2). All treatments were topically administered for eight days. The healing effect was assessed using macroscopic, histological and biochemical parameters.
Results: After eight days, the higher percentage of wound healing contraction was observed among the PLFO-treated group (100%) followed by the ‘CYTOL BASIC®’ treated group (61.36%) and untreated group (32.27%). During the treatment, the PLFO-treated group showed less erythema, less crusting/scabbing, higher general wound appearance scores and a high content of collagen (220.67 ± 7.48 mg/g of tissue) than the other groups.
Discussion and conclusion: The current study has shown, for the first time, the healing effect of PLFO on CO2 laser fractional burn. Their wound healing effect could be attributed to their antimicrobial, anti-inflammatory and antioxidant effects.
Keywords: Wound contraction, collagen, fatty acids, tocopherol, sterol
Introduction
The fractional CO2 laser is a very common and effective aesthetic resurfacing technique of rejuvenation, designed to correct skin imperfections (Alexiades-Armenakas et al. 2008; Loesch et al. 2014). However, a successful result depends upon meticulous post-laser treatment care. The mainstay of post-operative care involves the application of a nonirritating moisturizing ointment to speed up the wound healing process, ensure a good result, and help avoid complications (Shah & Alam 2012).
Wound treatment after laser skin resurfacing is crucial for achieving a successful result (Batra 2004). There is no standard of care for post-laser resurfacing treatment of the face. The treatment of laser burn had began on the first postoperative day and continued daily until each burn was healed. A burn was considered to be healed when new epithelium completely covered the injury as judged by visual inspection (Chan et al. 1987). The healing from a fractional laser resurfacing often depends on the depth of the laser treatment and the density (or extend) that the skin was treated. Generally, the more extensive the treatment, the longer the recovery time. The perfect treatment should speed re-epithelialization and reduce downtime, with minimal irritation (Sarnoff 2011). Previous studies found that the post-laser resurfacing wound care is important to promote wound healing and prevent immediate and later complications. In fact, a combination of efficacy and safety of treatment is responsible for the final outcome of laser resurfacing care (Khatri et al. 2005).
So far, no evaluation has been reported about the healing effects, after laser application, of different natural products in the literature. On the one hand, many studies involve the use of extract or oil from medicinal plants to accelerate excision, incision and thermal burn wound healing. On the other hand, their uses for influencing wound healing after exposure to laser energy are not well elucidated. A single work published in 2014, showed that Arnebia euchroma L. (Boraginaceae) ointment has good healing effects on post-laser wounds in rats (Aliasl et al. 2014).
Natural products are becoming increasingly important as alternative medicines. Recently, there has been a call for the invention of drugs obtained from plants and animals including those with wound healing properties (Duke 2002).
Pistacia. lentiscus L. (Anacardiaceae) is one of the plants that possess a wound healing effect in the folk medicine. P. lentiscus tree is widely spread in the Mediterranean south-west basin of the Black Sea. It is known for its medicinal properties since antiquity (Palevitch et al. 1986). The fruit is a small drupe, from which fatty oil is extracted (Boulos 1983).
In the Mediterranean region, P. lentiscus with all its parts, the roots, the leaves, the fruits and the mastic possesses medicinal uses, which has been reported in many traditional pharmacopoeias. The medicinal benefits of the fruit’s fatty oil are particularly known in North Africa, in the eastern regions of Algeria and Tunisia. The fruit oil is recommended for respiratory allergies, externally to treat sore throats and locally applied for wounds and burns (Boukef & Souissi 1982; Boulos 1983). This oil is commonly used as an antiulcer treatment, a wound healer and an antiseptic (Mezghani 1992; Rejeb et al. 2006).
Other references reported on the traditional use of this oil, in the North West parts of Tunisia, not only as edible oil, but also as medicinal oil in the treatment of scabies and rheumatism as well as the manufacture of antidiarrhea pills (Trabelsi et al. 2012).
The PLFO is characterized by a high nutritional value. It contains a significant amount of unsaturated fatty acids (more than 70%) (Mezni et al. 2011; Trabelsi et al. 2012), a high level of phosphatidylinositol (Trabelsi et al. 2013), a remarkable amount of β-carotene and α-tocopherol (Mezni et al. 2014). The wound healing effect of PLFO was tested on rabbit burn model and demonstrated an increase in re-epithelialization (Djerrou et al. 2010). However, there are no similar studies about PLFO’s effect on post-laser wounds. In this respect, our study is the first research in this field.
This study assesses the wound healing efficacy of PLFO in fractional CO2 laser burn. The wound healing process was assessed according to a calorimetric assessment, the 3-point scale, histological evaluation and collagen density.
Materials and methods
Plant material
P. lentiscus fruits were collected from the region of El Kef, North Western Tunisia, in September 2014. The fruits of P. lentiscus were authenticated by Dr. Hammadi Ben Salah and the voucher sample was deposited at The National Botanical Research Institute Tunisia (INRAT). Following the local traditional procedures, the freshly-harvested fruits were initially ground by a stone grinder. The paste was then thoroughly mixed with hands or feet and left to rest overnight. The following day, cold water was added to the paste, and the upper part was removed and fire-heated till boiling. The liquid phase was separated from the oil meal using a tissue, and then reheated until complete evaporation of water. Finally, the oil was filtered and stored for subsequent assays. The oil had a clear green yellowish colour (Mezni et al. 2012).
The reference product is ‘CYTOL BASIC®’ which is a restoring and soothing skin cream based on a grape seed extract [Vitis venifera L. (Vitaceae)]. The extract of grape seeds, which is in synergic activity with the other compound of the product, allows ‘CYTOL BASIC®’ to soothe, restore and protect the irritated and stressed skin. This product is manufactured in Tunisia under the supervision of the French laboratory ‘Cytol nat’ and tested under dermatologic control. It has been demonstrated that grape seed oil facilitates significant wound healing. Its wound healing promoting effect could be due to a synergism of antimicrobial, anti-inflammatory and antioxidant activities, by the respective constituents or it could be due to the individual activity of active leucoanthocyanins, fatty acids and other polyphenols (Shivananda Nayak et al. 2011).
Animal model
Eighteen healthy adult male Wistar rats (weight, 240–280 g) of the same age (3 months) were included in the study. The rats were obtained from the local Central Pharmacy, Tunisia. Before the experiment, the animals were left to acclimatize in the animal housing for one week. They were kept in an environmentally controlled breeding room (temperature: 22 ± 2 °C; humidity: 60 ± 5%; 12 h dark/light cycle) and they were given free access to food and water. Those animals were housed in separate cages in order to prevent wounds from possible infections and wound areas from being bitten by other animals during the healing process.
All experimental animal procedures were reviewed and carried out in accordance with guidelines for animal care issued by the University of Sfax, Tunisia, and approved by the Committee of Animal Ethics (Protocol no. 94-1939).
Body weight
The study was conducted on adult male Wistar rats having being weighed before and after treatment using electronic scales to check the effect of experimental treatments on body weight (Fsting & Atman 2002).
Fractional CO2 laser burn creation
The laser was typically applied on fractional pulsed carbon dioxide (CO2) producing microscopic areas of thermal necrosis in the epidermis and the dermis using a grid pattern without damaging the viable tissue of the surrounding areas (Beylot et al. 2009).
Before burn creation, the rats were anesthetized by intramuscularly injecting 50 mg/kg of ketamine, along with 5 mg/kg Midazolan and shaved with an electrical clipper. Afterwards, the rats were exposed to a second-degree laser burn (wound area = 2.2 cm2), using fractional CO2 laser which was performed using a CO2 Fractional Laser System (DSE [Korea]) within the following set parameters: Density: (level: 20, line: 29 × 29, Dot: 0841); Energy level: 25 MJ; Depth level: 4.
Experimental design
After creating the CO2 laser burns, the18 animals were randomly divided into three groups of six each: PLFO was topically administered on burns at doses of 0.56 μL/mm2 (for tested group), the ‘CYTOL BASIC®’ cream (0.13 μg/mm2) was used as a reference drug and the control group only received the vehicle saline solution.
The treatment of the laser wounds was started on the first postoperative day and continued daily until the first group was completely healed (Al-Watban & Andres 2000). One investigator changed dressings, inspected the injuries, and measured the diameters of the burns. When the wounds of one of the groups were judged to be healed, the treatment was stopped and animals were sacrificed. A burn was considered to be healed when new epithelium completely covered the injury as judged by visual inspection (Chan et al. 1987).
Measurement of wound area and burn contraction
In order to assess the wound healing effect, all wounds were examined by digital photography every day, and the wound boundaries were traced manually on a transparent paper. The outlines of the wounds were scanned, uploaded to the computer and the wound surface areas were measured using Autodesk Auto CAD 2015 software application for designing and drafting (Bhedi et al. 2013; Bardaa et al. 2016).
The burn contraction rate was calculated according to the following equation:
Wound healing assessment
To monitor the wound healing progression, burns were photographed and documented for colorimetric analysis. Erythema, crusting/scabbing and the general wound appearance were assessed in all photographs observed by two blinded dermatologists. These wound healing parameters were evaluated using the 3-point scales for exploration, irritation and infection (Table 1) (Aliasl et al. 2014, 2015).
Table 1.
Grading score.
| Variable | Scale |
|---|---|
| Erythema | 0 = none or absent, 1 = mild, 2 = moderate, 3 = marked, 4 = severe |
| Crusting/scabbing | 0 = none, 1 = slight (up to 29%), 2 = moderate (30–59%), 3 = extensive (60–90%), 4 = almost complete (91–100%) |
| General wound appearance | 0 = poor, 1 = fair, 2 = good, 3 = very good, 4 = excellent |
Histological examination
On the eight day, the rats were sacrificed and the burn wound biopsies were collected for histopathological examination purposes. All samples were fixed in a 10% neutral buffered formalin solution, embedded in paraffin wax, cut into 5 mm-thick sections, and stained with hematoxylin-eosin. The slides were photographed with an Olympus U-TU1X-2 camera connected to an Olympus CX41 microscope (Olympus Inc., Tokyo, Japan).
Measurement of hydroxyproline and collagen
Collagen is the synthesis of the healing tissue. It is a constituent of growing cells and the major structural protein of extra-cellular tissue. The measurement of hydroxyproline was used as an index for collagen turnover (Nayak & Pinto Pereira 2006). Increase in the hydroxyproline content indicated increased collagen synthesis, which in turn led to enhanced wound healing.
Biopsies from sites from all animals were taken on the eighth day for hydroxyproline measurement. The hydroxyproline quantification was carried out according to the technique described by Bergman and Loxley (1963) which is based on oxidation by chloramine T. As a first step, tissue samples were desiccated at 60 °C. After that, specimens were hydrolyzed for 3 h with 6N HCl at 105 °C. Afterwards, the hydrolyzed specimens underwent chloramine T oxidation. Finally, the absorbance of the coloured adduct formed with Ehrlich reagent at 60 °C was measured at 557 nm using a ultraviolet?visible (UV?Vis) spectrophotometer (CE7200, CECIL, Bretagne, France). The standard calibration curve was plotted for pure hydroxyproline and used for the estimation of the test samples. The values were reported as mg/g dry weight of tissue.
As for the skin of the rat, the proportion of the amino acid hydroxyproline in collagen molecule was about 13%. For this reason, hydroxyproline was used as a means to determine the amount of collagen present in a tissue (Prockop & Juva 1995)
Statistical analysis
All the data were expressed as mean values ± standard deviation (S.D). The level of statistical significance was set at 0.05. SPSS program was employed for comparisons between all groups. In the case of multiple comparisons, repeated measurements of Analysis of Variance (ANOVA) were performed to compare the mean differences between and within groups, using Tukey tests. The average weight of rats before and after the experiment was compared by Student’s t-test.
Results
Body weight
Table 2 illustrated the body weight variations and proved that the growth of the rats was normal for all studied groups. The rats were comparative and homogenous.
Table 2.
Variation of the body weights of rats among theexperimental period.
| Body weight (g) |
||
|---|---|---|
| Groups | Before treatment | After treatment |
| Control group | 243.83 ± 25.60 | 282.83 ± 24.47 |
| Reference group | 238.01 ± 27.77 | 282.51 ± 20.91 |
| PLFO group | 245.33 ± 28.40 | 280.16 ± 29.49 |
Values are given as mean ± S.D (n = 6).
Morphological evaluation
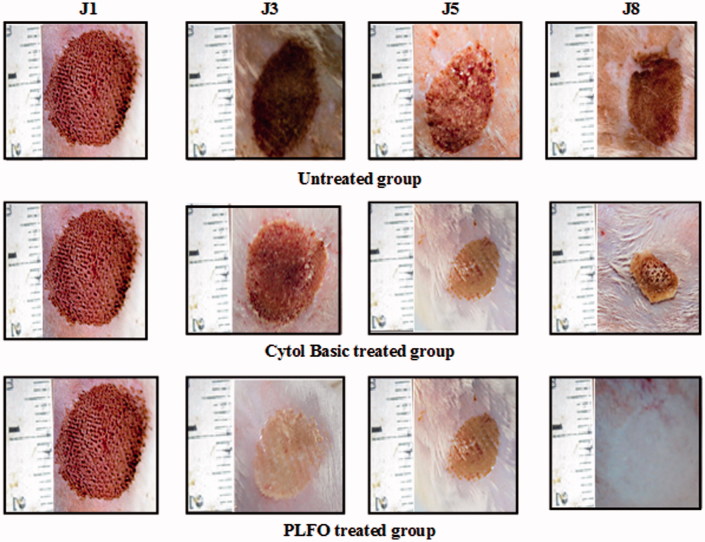
The wound healing effect was assessed according to a calorimetric assessment and the 3-point scale. Figure 1 displayed the representative sets of photographs taken on days 1, 3, 5 and 8 for all groups during the wound-healing period.
Figure 1.
Digital photographs of the wound surfaces in each group on 1st, 3rd, 5th and 8th days. A, B and C laser wounds treated with saline solution with 6.81%, 15.45% and 32.27% wound closure on 3rd, 5th and 8th days, respectively. D, E and F laser wound treated with ‘CYTOL BASIC®’ with 9.11%, 20% and 61.36% wound closure on 3rd, 5th and 8th days, respectively. G, H and I laser wound treated with PLFO with 41.81%, 60% and 100% wound closure on 3rd, 5th and 8th days, respectively.
By laser burn induction, all burns showed a similar dark red coloration. On the third day, we noticed a scab formation that was characterized by a brown color in all the groups. This scab started to disappear by the fifth day in the PLFO-treated group but persisted in the other groups. A pink coloration appeared once the scab fell off. Nevertheless, the majority of the animals from the untreated and the reference groups still showed no wound healing on the eight day.
Moreover, according to the 3-point scale, the evaluation revealed significantly less crusting during the experiment and high general wound appearance scores in the oil-treated group compared with the reference and control groups (Figure 2).
Figure 2.
Mean scores for the comparison of the three groups after 1, 3, 5 and 8 days using Duncan multiple range test. (A) Erythema mean score; with marked, moderate and absence of erythema on 8th day for untreated, reference and tested group, respectively. (B) Crusting/scabbing mean score; extensive, moderate and absence of Crusting/scabbing on 8th day for untreated, reference and tested group, respectively. (C) General wound appearance mean score; fair, good and excellent general wound appearance on 8th day for untreated, reference and tested group, respectively. Group 1: untreated (Saline solution), Group 2: ‘CYTOL BASIC®’ cream (Reference) Group 3: Pistacia lentiscus fruit oil (Tested oil) Values represent means ± S.D (n = 6) in each group. *p < 0.05, **p < 0.01 and ***p < 0.001. (a): compared to untreated group; (b): compared to reference group.
The morphological evaluation on day 8 demonstrated that the best result was obtained with PLFO. Biopsies from PLFO-treated group revealed a normal smooth skin similar to normal skin.
The healing time and the decrease of wound area
The burn wound area was used as an index for wound healing during the eighth day of the experimental period to assess the wound-healing potential of the PLFO and the reference cream.
The percentage of burn contraction on days 1, 3, 5 and 8 from all groups are illustrated in Table 3 and Figure 3, respectively.
Table 3.
Wound areas measurement of the different groups of rats.
| Days |
||||
|---|---|---|---|---|
| Burn wound area (cm2). | 1 | 3 | 5 | 8 |
| Control group | 2.2 | 2.05 ± 0.15b | 1.86 ± 0.21b | 1.49 ± 0.23c |
| Reference group | 2.2 | 1.94 ± 0.13b | 1.76 ± 0.19b | 0.85 ± 0.43b |
| PLFO group | 2.2 | 1.28 ± 0.12a | 0.88 ± 0.44a | 0.00 ± 0.00a |
Values are given as mean ± S.D (n = 6). Data with different letters for each column represent significant difference at p < 0.05.
Figure 3.
Percentage of burn wound contraction for the three studied groups. Untreated group: rats were treated with saline solution with 32.27% wound contraction on 8th day; reference group: rats were treated with reference drug ‘CYTOL BASIC’ with 61.36% wound contraction on 8th day; Tested group: rats were treated with PLFO with 100% wound contraction on 8th day, respectively. Values represent means ± S.D (n = 6) in each group. *p < 0.05, **p < 0.01 and ***p < 0.001. (a): compared to untreated group; (b): compared to reference group.
The PLFO-treated group exhibited a better performance than the reference and the control groups with regard to the decrease of wound area throughout the process of wound healing.
As far as treated oil groups were concerned, significant healing effects were observed starting from day 3 compared with the other groups. By day 8 of the experiment, the PLFO-treated group revealed a noticeable healing effect (0. 00 cm) as compared with the control and reference groups that still showed an open wound (1.49 and 0.85 cm2), respectively. At the end of the experiment, the control group wounds remained open. However, the wounds of two among six treated rats with the reference drug, healed. The wound contraction was, respectively, 100, 61.36 and 32.27% for PLFO, reference and control groups.
Histological evaluation
On the eight day of post burn induction, the biopsies of the burn areas were histologically assessed. The biopsies from completely healing burn-wounds from the treated and the untreated control group were shown in Figure 4.
Figure 4.
Histologic features of skin sections from burned area stained with hematoxyline–eosin after 8 d of burn induction; (A) untreated wound Gr 100; (B) Untreated wound Gr 200 with thickened and immature epidermis with debridement crust overlying the area of the wound; (C) reference drug ‘CYTOL BASIC’ treated wound Gr 100; (D) reference drug ‘CYTOL BASIC’ treated wound Gr 200 with reduced inflammation and enhanced wound contraction; (E) PLFO-treated wound Gr 100; (F) PLFO-treated wound Gr 200 with intact epidermis, re-epithelialization, regeneration of granulation tissue, angiogenesis and collagen deposition were detected in the treated wounds. Ep: epidermis; N: inflammatory nucleus.
Group 1 (control group): we monitored the persistence of an active inflammatory lesion with necrosis of the epidermis. The dermis revealed the presence an oedema and a pronounced hyperaemia of capillary blood vessels associated with inflammatory cell infiltration, in the underlying tissue Figure 4 (A) and (B).
Group 2 (reference group): after 8 days, the histological study demonstrated a complete epithelial regeneration manifested through a thick retracted sclerotic tissue with persistence of intra-dermal inflammation Figure 4 (C) and (D).
Group 3 (the PLFO-treated group): there was a complete tissue healing with epithelial regeneration and well organized dermis associated with a few inflammatory cells. We noticed also connective tissue characterized with a good collagen production as illustrated in Figure 4 (E) and (F).
Estimation of collagen content
In our study, biopsies of normal skin and healed burn tissues were assessed for their hydroxyproline content and provided an estimation of collagen concentration. The healing effect of PLFO was well attested. As presented in Table 4, collagen density in PLFO-treated group was found to be significantly higher (220.67 ± 7.48) than in the other groups. This collagen amount is comparable to the content of collagen in healthy skin (265.12 ± 12.89) used as control.
Table 4.
Collagen amount in different experimental animal groups.
| Groups | (collagen mg/g of tissue) |
|---|---|
| Control group | 124.02 ± 7.48a |
| Reference group | 209.33 ± 9.98b |
| PLFO group | 220.67 ± 7.48c |
| Normal skin (positif control) | 265.12 ± 12.89d |
All values are mean ± S.D (n = 6). Data with different letters for each column represent significant difference at p < 0.05.
Discussion
Fractional laser resurfacing is a relatively recent procedure that has been proven to be effective to correct skin imperfections of facial acne scars, wrinkles and photodamage (Nirmal et al. 2013; Marmon et al. 2014). This laser typically applies fractional pulsed carbon dioxide (CO2) operated at 1550 nm and generates light source of uniform frequency (wave length) which creates microscopic areas of thermal necrosis in the dermis in a grid pattern, without damaging the viable tissue of the surrounding areas (Khan et al. 2005). So far, there has been no standard of care for the treatment of laser resurfacing wounds. To optimize wound healing, a moist environment is required as it promotes cell migration, thereby re-epithelialization and preventing cell death (Krupashankar 2008).
The current study aimed to investigate the healing efficiency of PLFO on fractional CO2 laser burns in a rat model in comparison with that of ‘CYTOL BASIC®’. The assessment of the wound healing process was carried out through macroscopic observation of the general wound appearance and crusting, histological analysis and a biochemical quantification of collagen content. Our findings revealed that PLFO improved wound contraction and closure, and the effects were extremely significant compared to reference and control groups.
Comparing the general wound appearance and crusting of the PLFO-treated group with those of the untreated group and with the reference group, the PLFO showed greater improvements than the ‘CYTOL BASIC®’ treated group and the untreated group. Our results revealed that the tested oil, improved the formation of collagen and fibroblasts, and reduced the number of inflammatory cells, which enhanced effective wound healing.
The reduction of wound burn observed in the PLFO-treated group could be attributed to the barrier lipid formed by the tested oil. Indeed, the tested oil (PLFO) seems to provide a moist environment that enhances cell migration and wound healing. The accelerated healing was caused to a certain extent by faster contraction in wet wounds. It has been demonstrated that the wound healing process is faster in moist conditions than in dry conditions (Field & Kerstein 1994).
Furthermore, a previous photochemical analysis of Tunisian PLFO revealed the presence of a high amount of polyunsaturated fatty acids (73.44%), mainly fatty oleic (45.66%) and linolenic acids (24.21%) (Charef et al. 2008). The analysis also revealed that the fatty acids are able to reduce transepidermal water loss, increase skin hydration (Mcgaw et al. 2002) and suggest a relevant role and a potential therapeutic implication on wound healing (Cardoso et al. 2004). Moreover, linoleic and oleic acids have anti-inflammatory properties that play a major role in the recruitment of inflammatory cells at the inflammation site and accelerate the wound healing process.
Other research findings obtained from Magalhaes et al. (2008) proved a potential therapeutic involvement of linoleic acid. Indeed, linoleic acid is a precursor of arachidonic acid for wound healing. In fact, arachidonic acid generated inflammatory mediators such as prostaglandins, leukotrienes and thromboxanes. These mediators improved local neovascularization; extracellular matrix reorganization, cellular migration and fibroblastic differentiation (Corsi et al. 1994). As a matter of fact, the linoleic acid that was identified in the PLFO acted dramatically in the acceleration of the epidermis differentiation and the wound closing on the eight day.
The antioxidant effect may also speed up wound healing (Fitzmaurice et al. 2011). In fact, it has been determined that the fruit oil extracted from P. lentiscus growing in different regions of Tunisia is rich in antioxidants agents namely tocopherol (α-tocopherol and δ-tocopherol) and carotenoid species (lutein, zeaxanthin and β-carotene) (Mezni et al. 2014). The quantification of total tocopherol in PLFO has shown that α-tocopherol is the major tocopherol fraction (Mezni et al. 2014). Several previous studies demonstrated that α-tocopherol oral supplementation promotes the healing of chronic wounds (Gray 2003).
Besides, preliminary findings of phytochemical investigations of PLFO revealed the presence of phytosterols, such as β-sitosterol (90%), campesterol, cholesterol and stigmasterol (Trabelsi et al. 2012).
β-Sitosterol, which is the major constituent of PLFO, has a potent angiogenic activity (Choi et al. 2002) that could explain the extreme enhancement of wound healing activity of this oil observed in rats. These phytochemicals have been associated with several biological and pharmacological activities. Previous studies showed that oxidative, anti-inflammatory, and antimutagenic activities were associated with phytosterols (Moreno 2003; Park et al. 2003; Corbiere et al. 2003).
Further chemical investigations manifested the presence of the carotenoid in PLFO (varied from 5.8 to 10.57 mg/kg oil) especially β-carotene (Mezni et al. 2014). These components have been used as effective remedies for burn, frostbites, ulcers, skin cancers and various gynecological illnesses (Akulinin 1958). The β-carotene was used as an effective wound healing remedy (Gerber & Erdman 1982).
Antibacterial effects may serve to improve the wound healing process. However, the progress of the wound healing is potentially slowed down or prevented by a number of different events and conditions. One event that impedes the progression of the wound healing effect is the colonization of the wound bed by microorganisms (Robson 1997; Wright et al. 1998).
In the same context, all animals treated with oil have presented no microbial infection during the processing period of the treatment. These results can be attributed to the antibacterial properties of PLFO (Mezni et al. 2012).
Subsequently, the anti-inflammatory, the antioxidant and the antimicrobial proprieties of PLFO provide further evidence to support our findings about the specific pharmacological effects of oils on wound healing.
The ‘CYTOL BASIC®’ reference cream has shown a significant healing effect just on the 8th day compared to the control group. This observation reflected that the active principle might exert its effect on the late phase of wound healing. These findings corroborate previous studies showing that Vitis venifera L. (Vitaceae) extract exerted burn-wound healing activity in the late stage of wound healing (Fakhim et al. 2015). It has been demonstrated that Vitis venifera grape oil facilitates significantly wound healing. Its wound healing effect could be attributed to a combination of antimicrobial, anti-inflammatory, antioxidant activities and to its fatty acids as well as polyphenols compounds (Shivananda Nayak et al. 2011).
In this study, the PLFO-treated group demonstrated a significant increase in the hydroxyproline content, indicating an increase in collagen turnover. Thus, it seems that PLFO stimulated wound healing more potently and more effectively than the reference drug. These findings implied that PLFO promoted wound healing by influencing the proliferative and remodelling phases of wound healing. It does so via the pathways of angiogenesis, collagen deposition and wound contraction. These observations may explain why the wounds started to heal on the third day and the sustained positive effects that PLFO had on burns.
The wound contraction was remarkably faster and higher in the PLFO-treated group with a shorter epithelialization time than in the reference and the untreated groups (Figure 2). It may therefore be concluded that PLFO’s chemical compounds, contributed on a large scale to its burn-healing properties as depicted by the increment of the hydroxyproline content, speeding up wound contraction and shortening of the epithelialization time.
Conclusions
The healing effect of the PLFO reported in this experiment was proven. The wound-healing activity of this oil was better than the ‘CYTOL BASIC®’. These findings demonstrate that the PLFO could be considered as an effective treatment for second-degree burns and even for immediate post-operative management for laser resurfaced skin. These observations can extend the healing properties of this oil to a different type of trauma and pave the way for further future clinical applications.
Acknowledgments
The authors are grateful to Mrs Madiha Jallouli, English professor at the Faculty of Medicine of Sfax, for having proofread the manuscript. The authors would like to thank Miss Amal Hadrich and Miss Haifa Turki from the laser medical centre for their assistance.
Disclosure statement
The authors affirm that they do not have any financial or personal relationships with any people or organization that could inappropriately influence the information in this article.
Funding
This research was supported by the Tunisian Ministry of Higher Education and Scientific Research via Sfax University.
References
- Akulinin IA. 1958. Using sea buckthorn oil in treating burns. Sov Med. 11:137–138. [PubMed] [Google Scholar]
- Alexiades-Armenakas MR, Dover JS, Arndt KA. 2008. The spectrum of laser skin resurfacing: nonablative fractional and ablative laser resurfacing. J Am Acad Dermatol. 58:519–537. [DOI] [PubMed] [Google Scholar]
- Aliasl J, Barikbin B, Khoshzaban F, Naseri M, Sedaghat R, Kamalinejad M, Talei D, Emadi F, Akbari Z, Aliasl F, et al. 2015. Effect of Arnebia euchroma ointment on post-laser wound healing in rats. J Cosmet Laser Ther. 17:41–45. [DOI] [PubMed] [Google Scholar]
- Aliasl J, Khoshzaban F, Barikbin B, Naseri M, Kamalinejad M, Emadi F, Razzaghi Z, Talei D, Yousefi M, Aliasl F, et al. 2014. Comparing the healing effects of Arnebia euchroma ointment with petrolatum on the ulcers caused by fractional CO2 laser: a single-blinded clinical trial. Iran Red Crescent Med J. 16:16239–16239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Watban FA, Andres BL. 2000. Laser photons and pharmacological treatments in wound healing. Laser Therpy. 12:3–11. [Google Scholar]
- Bardaa S, Ben Halima N, Aloui F, Ben Mansour R, Jabeur H, Bouaziz M, Sahnoun Z. 2016. Oil from pumpkin (Cucurbita pepo L.) seeds: evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Batra R. 2004. Ablative laser resurfacing – postoperative care. Skin Therapy Lett. 9:6–8. [PubMed] [Google Scholar]
- Bergman I, Loxley R. 1963. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem. 35:1961–1965. [Google Scholar]
- Beylot C, Grognard C, Michaud T. 2009. Ablative and fractional lasers. Ann Dermatol Vener. 136:311–319. [DOI] [PubMed] [Google Scholar]
- Bhedi A, Saxena AK, Gadani R, Patel R. 2013. Digital photography and transparency-based methods for measuring wound surface area. Indian J Surg. 75:111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukef K, Souissi HR. 1982. Contribution à l’étude des plantes médicinales en médecine populaire en Tunisie. Rev. Soc. Pham. Tunisie. 2:34–35. [Google Scholar]
- Boulos L. 1983. Medicinal Plants of North Africa. Algonac, MI: Reference Publications. [Google Scholar]
- Chan P, Vincent JW, Wangemann RT. 1987. Accelerated healing of carbon dioxide laser burns in rats treated with composite polyurethane dressings. Arch Dermatol. 123:1042–1045. [PubMed] [Google Scholar]
- Cardoso CR, Souza MA, Ferro EA, Favoreto S, Pena JD. 2004. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 12:235–243. [DOI] [PubMed] [Google Scholar]
- Charef M, Yousfi M, Saidi M, Stocker P. 2008. Determination of the fatty acid composition of acorn (Quercus), Pistacia lentiscus seeds growing in Algeria. J Am Chem Soc. 85:921–924. [Google Scholar]
- Choi S, Kim KW, Choi JS, Han ST, Park YI, Lee SK, Chung MH. 2002. Angiogenic activity of beta-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta Med. 6:330–335. [DOI] [PubMed] [Google Scholar]
- Corbiere C, Liagre B, Bianchi A, Bordji K, Dauça M, Netter P, Beneytout JL. 2003. Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int J Oncol. 22:899–905. [PubMed] [Google Scholar]
- Corsi RCC, Pirana S, Muraco FAE, Jorge D. 1994. Cicatrizaçäo das feridas; revisäo da literatura. Rev Bras Cir. 84:17–24. [Google Scholar]
- Djerrou Z, Maameri Z, Hamdi-Pacha Y, Serakta M, Riachi F, Djaalab H, Boukeloua A. 2010. Effect of virgin fatty oil of Pistacia lentiscus on experimental burn wound's healing in rabbits. Afr J Tradit Complement Altern Med. 7:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke JA. 2002. Handbook of medicinal plants. France: CRC Press. [Google Scholar]
- Fakhim SA, Babaei H, Khansari Nia A, Ashrafi J. 2015. Wound healing effect of topical grape seed extract (Vitis vinifera) on rat palatal mucosa. Int J Curr Res Aca Rev. 3:477–489. [Google Scholar]
- Fsting MF, Altman DG. 2002. Guidelines for the design and statistical analysis of experiments using laboratory animals. Ilar J. 43:244–245. [DOI] [PubMed] [Google Scholar]
- Field FK, Kerstein MD. 1994. Overview of wound healing in a moist environment. Am J Surg. 167:2–6. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice SD, Sivamani RK, Isseroff RR. 2011. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol Physiol. 243:113–126. [DOI] [PubMed] [Google Scholar]
- Gerber LE, Erdman JRJW. 1982. Effect of dietary retinyl acetate, beta-carotene and retinoic acid on wound healing in rats. J Nutr. 112:1555–1564. [DOI] [PubMed] [Google Scholar]
- Gray M. 2003. Does oral supplementation with vitamins A or E promote healing of chronic wounds?. J Wound Ostomy Continence Nurs. 30:290–294. [DOI] [PubMed] [Google Scholar]
- Khan MH, Sink RK, Manstein D, Eimerl D, Anderson R. 2005. Intradermally focused infrared laser pulses: Thermal effects at defined tissue depths. Lasers Surg Med. 36:270–280. [DOI] [PubMed] [Google Scholar]
- Khatri KA, Margolis RJ, Bhatty RS, Garcia V. 2005. Comparison of two wound dressings after laser skin resurfacing. J Cosmet Laser Ther. 7:206–212. [DOI] [PubMed] [Google Scholar]
- Krupashankar DS., IADVL Dermatosurgery Task Force 2008. Standard guidelines of care: CO2 laser for removal of benign skin lesions and resurfacing. Indian J Dermatol Venereol Leprol. 74:61–67. [PubMed] [Google Scholar]
- Loesch MM, Somani AK, Kingsley MM, Travers JB, Spandau DF. 2014. Skin resurfacing procedures: new and emerging options. Clin Cosmet Investig Dermatol. 7:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes MS, Fechine FV, Macedo RN, Monteiro DLS, Oliveira CC, Brito GADC, Moraes MOD. 2008. Effect of a combination of medium chain triglycerides, linoleic acid, soy lecithin and vitamins A and E on wound healing in rats. Acta Cir Bras. 23:262–269. [DOI] [PubMed] [Google Scholar]
- Marmon S, Shek SY, Yeung CK, Chan NP, Chan JC, Chan HH. 2014. Evaluating the safety and efficacy of the 1,440-nm laser in the treatment of photodamage in Asian skin. Lasers Surg Med. 46:375–379. [DOI] [PubMed] [Google Scholar]
- Mcgaw LJ, Jager AK, Van Staden J, Houghton PJ. 2002. Antibacterial effects of fatty acids and related compounds from plants. S Afr J Bot. 68:417–423. [Google Scholar]
- Mezghani S. 1992. L'exploitation traditionnelle du maquis au nord de la Tunisie: Possibilités d'une meilleure utilization. Tunis: Office de l’elevage et des pâturages, p. 99–158. [Google Scholar]
- Mezni F, El Khorchani A, Boussaid M, Khouja ML, Khaldi A. 2011. Fatty acid composition and antioxidant activity of Pistacia lentiscus L. fixed oil. Planta Med. 77:207. PM-20845261 [Google Scholar]
- Mezni F, Maaroufi A, Msallem M, Boussaid M, Khouja ML, Khald A. 2012. Fatty acid composition, antioxidant and antibacterial activities of Pistacia lentiscus L. fruit oils. J Med Plant Res. 6:5266–5271. [Google Scholar]
- Mezni F, Khouja ML, Gregoire S, Martine L, Khaldi A, Berdeaux O. 2014. Effect of growing area on tocopherols, carotenoids and fatty acid composition of Pistacia lentiscus edible oil. Nat Prod Res. 28:1225–1230. [DOI] [PubMed] [Google Scholar]
- Moreno JJ. 2003. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic Biol Med. 35:1073–1081. [DOI] [PubMed] [Google Scholar]
- Nayak BS, Pinto Pereira LM. 2006. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement Altern Med. 6:41–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmal B, Pai SB, Sripathi H, Rao R, Prabhu S, Kudur MH, Nayak SU. 2013. Efficacy and safety of erbium-doped Yttrium aluminium garnet fractional resurfacing laser for treatment of facial acne scars. Indian J Dermatol Venereol Leprol. 79:193–198. [DOI] [PubMed] [Google Scholar]
- Palevitch D, Yaniv Z, Dafni A, Friedman J. 1986. Medicinal plants of Palestine: an ethnobotanical survey. In: Herbs, spices, and medicinal plants: recent advances in botany, horticulture, and pharmacology. USA. [Google Scholar]
- Park KY, Jung KO, Rhee SH, Choi YH. 2003. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat Res. 524:43–53. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Juva K. 1995. Synthesis of hydroxyproline in vitro by the hydroxylation of proline in a precursor of collagen. Proc Natl Acad Sci USA. 53:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeb MN, Khouja ML, Ghrabi Z, Chemli R, Albouchi A, Khaldi A, Dahman M. 2006. Guide des plantes médicinales et aromatiques. Tunis, Tunisie: Maghreb Editions. [Google Scholar]
- Robson MC. 1997. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 77:637–650. [DOI] [PubMed] [Google Scholar]
- Sarnoff DS. 2011. A comparison of wound healing between a skin protectant ointment and medical device topical emulsion after laser resurfacing of the perioral area. J Am Acad Dermatol. 64:36–43. [DOI] [PubMed] [Google Scholar]
- Shah S, Alam M. 2012. Laser resurfacing pearls In: Seminars in plastic surgery. New York, NY: Thieme Medical Publishers, p. 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivananda Nayak B, Dan Ramdath D, Marshall JR, Isitor G, Xue S, Shi J. 2011. Wound-healing properties of the oils of Vitis vinifera and Vaccinium macrocarpon. Phytother Res. 25:1201–1208. [DOI] [PubMed] [Google Scholar]
- Trabelsi H, Cherif OA, Sakouhi F, Villeneuve P, Renaud J, Barouh N, Mayer P. 2012. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 131:434–440. [Google Scholar]
- Trabelsi H, Renaud J, Herchi W, Khouja ML, Boukhchina S, Mayer P. 2013. LC–ESI–QTOF–MS, MS/MS Analysis of glycerophospholipid species in three Tunisian Pistacia lentiscus fruit populations. J Am Chem Soc. 90:611–618. [Google Scholar]
- Wright JB, Hansen DL, Burrell RE. 1998. The comparative efficacy of two antimicrobial barrier dressings: in vitro examination of two controlled release of silver dressings. Wounds. 10:179–188. [Google Scholar]