Abstract
Context:Sechium edule (Jacq.) Sw. (Cucurbitaceae) is used in ethnomedicine, but the diversity of the varietal groups of this species has not often been considered. This is important because we previously reported that different variety of species exhibit different activities across different tumor cell lines.
Objective: This study investigates the chemical composition and biological activities of extracts obtained from S. edule var. nigrum spinosum.
Materials and methods: The leukemia P388 cell line and mononuclear bone marrow cells (MNCBMs) were treated with the extract at a concentration ranging from 40 to 2370 μg/mL for cytotoxicity and viability assays. CD-1 mice were treated with 8–5000 mg/kg extract and monitored every hour for the first 24 h and subsequently for seven days for signs of toxicity (LD50). In addition, the chromatographic profile of the extract was determined by HPLC.
Results: The extract inhibits the proliferation of both P388 cells and MNCBMs, with IC50 values of 927 and 1911 μg/mL, respectively, but reduced the viability and induced the apoptosis of only leukemia cells. The LD50 was higher than 5000 mg/kg, and this concentration did not alter the blood chemistry or cell count but doubled the mitotic index in the bone marrow. The HPLC showed the presence of cucurbitacins, phloridzin, naringenin, phloretin, apigenin, and gallic, chlorogenic, vanillic, p-hydroxybenzoic, caffeic, and p-coumaric acids.
Discussion and conclusion:Sechium edule var. nigrum spinosum contains bioactive compounds that explain the antiproliferative and nutraceutical activities, and its lack of physiological side effects constitutes an added value to a widely consumed vegetable.
Keywords: GISeM, chayote, cytotoxicity, differential, HPLC
Introduction
Sechium edule (Jacq.) Sw. (Cucurbitaceae), which is also known as chayote, is a neotropical plant species that is endemic to Mexico. This species includes 12 varieties, some of which have high nutritional value. S. edule is used in traditional medicine for the treatment of different illnesses due to its hypotensive and vasodilator activities (Cambar et al. 1980; Cambar 1985). Its roots, stems and leaves are used as antimicrobial agents, and the leaves, when mixed with the seeds, can act as antioxidants (Ordoñez et al. 2003, 2006). The fruit has been reported to have anti-inflammatory and cardiotonic activities (Salama et al. 1986) and is thus used to treat arteriosclerosis (Gordon et al. 2000). The fruit can also function as a depressor of the central nervous system and an antiepileptic agent (Firdous et al. 2012).
Sechium edule fruit extract contains non-phenolic alkaloids, saponins, sterols, triterpenoids (Salama et al. 1986) and glycosylated flavonoids (Siciliano et al. 2004) that confer its anti-inflammatory (Salama et al. 1987), antihypertensive (Gordon et al. 2000), antimicrobial (Ordoñez et al. 2003), antioxidant (Ordoñez et al. 2006), antitumor (Cadena-Iñiguez et al. 2013; Aguiñiga-Sánchez et al. 2015), nephroprotective (Firdous et al. 2012), and hepatoprotective properties (Firdous et al. 2013); however, even though S. edule has been used for the treatment of various diseases, most studies have not considered the diversity of the varieties of this species. This consideration is important because Cadena-Iñiguez et al. (2013) reported that eight of the 12 varieties have differential activities across different tumor cell lines and genotypes. One variety, nigrum spinosum, exhibits greater antiproliferative activity, particularly on the murine macrophage leukemic P388 cell line.
Hematopoietic tissue is responsible for the generation of both leukemic and normal hematopoietic cells (Orkin & Zon 2008). Leukemic cells are highly sensitive to the presence of antitumor agents because these agents reduce the number of cells undergoing cell division, an effect that can be quantified as the mitotic index. As a side effect, the cytotoxic effect through the body (Yi et al. 2010; Alakoc & Eroglu 2011) results in a reduction in the number of generated hematopoietic cells, which compromises the patient’s well-being (Culakova et al. 2014). Therefore, the use of selective antineoplastic agents that can eliminate tumor cells without harming normal cells would be desirable (Setzer & Setzer 2003; Alexander-Bryant et al. 2013). An inedible hybrid of S. edule known as H387 07 has these features (Aguiñiga-Sánchez et al. 2015). However, whether the edible S. edule var. nigrum spinosum can selectively kill leukemia cells without harming normal hematopoietic cells remains unknown. This study compared the in vitro and in vivo cytotoxic effects of fruit extract from S. edule var. nigrum spinosum and analyzed the secondary metabolites of the extract to provide insights that could help explain the observed biological effects.
Materials and methods
Plant material and extract preparations
Fruits of S. edule var. nigrum spinosum at horticultural maturity, specifically 18 ± 2 days after anthesis, were collected by an Interdisciplinary Research Group of Sechium edule in Mexico, A.C. (GISeM) in the Bank of Germplasm of CRUO-UACh, Veracruz, Mexico, in 2011 and authenticated by the National Seed Inspection and Certification Service (SNICS; Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food, Mexico) by Cadena-Iñiguez J. A voucher specimen (Accession 354-06 nigrum spinosum) remains in the Bank of Germplasm of CRUO-UACh (19°49′N, 97°21′E, Veracruz, Mexico). Immediately after collection, the fruits were cleaned, cut into small pieces, dried at 45 °C to a moisture level of 10%, and ground to a standardized particle size of 2 mm (mesh No. 10). The plant material was then stored under aseptic conditions at room temperature until use.
Extraction procedure
Fifteen hundred grams of the stored plant material was extracted in batches with methanol (99.8%, ACS, Merck, Darmstadt, Germany) for 48 h at room temperature (20 ± 2 °C). The resultant alcoholic extract was filtered with Whatman cellulose filter paper (Sigma-Aldrich St. Louis, MO) for 30 passages, and the solvent was renewed until the macerated product did not show a colour. The solvent was then evaporated at 50 °C under reduced pressure (Buchi Rotavapor R-114, Flawil, Switzerland) until a crude extract, without organic solvent, was obtained.
Additionally, to analyze whether the presence of sugars could modify the extraction yields of compounds found at lower concentration, we performed continuously selective extraction with lower-, medium- and higher-polarity organic solvents, specifically hexane, dichloromethane and methanol, respectively. The solvents were then evaporated until an extract fraction without organic solvent was obtained.
Identification of compounds by HPLC
Preliminary tests were performed by thin-layer chromatography (TLC) on silica gel plates (Merck G60 70–230 mesh, Germany) with a mobile phase of acetic acid and methanol (95:5) and cucurbitacins B, D, E and I as the standards. Subsequently, the content and type of the metabolites in the extract were identified by high-performance liquid chromatography (HPLC). For the cucurbitacins, a symmetry shield column RP18 (4.6 × 250 mm, Waters Corporation, Milford, MA) was used, and an isocratic analysis was conducted using water:methanol:acetonitrile (50:30:20) with the following experimental parameters: flow, 1 mL/min; pressure, 179 bar; temperature, 20 °C; injection volume, 20 μL; wavelength, 235 nm; and analysis time, 50 min. For the flavonoids, the analyses were performed on a Hypersil ODS (125 × 40 mm) Hewlett Packard Column with a gradient of (A) H2O at pH 2.5 with TFA (trifluoroacetic acid) and (B) ACN (acetonitrile) and the following parameters: flow, 1 mL/min; temperature, 30 °C; variable injection volume; and analysis time, 25 min. The standards used were rutin, phloridzin, myricetin, quercetin, naringenin, phloretin, apigenin and galangin. For phenolic acid, a Nucleosil column (125 × 4.0 mm) from Macherey-Nagel was used with a gradient of (A) H2O at pH 2.5 with TFA (trifluoroacetic acid) and (B) ACN (acetonitrile). The other experimental parameters included the following: flow, 1 mL/min; temperature, 30 °C; variable injection volume; and analysis time, 25 min. Caffeic, gallic, chlorogenic, vanillic, p-hydroxybenzoic, p-coumaric, ferulic and syringic acids were used as the standards.
In vitro toxicity assays
Cell culture
The murine macrophage-like leukaemia P388 cell line was purchased from the American Type Culture Collection (ATCC: The Global Bioresource Center, Manassas, VA) and maintained in Iscove’s Modified Dulbecco’s Medium (IMDM) culture medium (GIBCO-BRL Invitrogen Grand Island, NY) supplemented with 10% deactivated foetal bovine serum (FBS) (Invitrogen GIBCO-BRL HyClone, Carlsbad, CA) in glass Petri dishes (Pyrex, US) at 37 °C in an incubator (Thermo Forma, Marietta, OH) with an atmosphere of 5% CO2 and 95% humidity. The cells (1 × 105 cell/mL) were seeded and split every 48 h once they reached 90% confluence. Total bone marrow cells from CD-1 mice were extracted from the femur and flushed with IMDM supplemented with 10% FBS. Mononuclear bone marrow cells (MNCBMs) were isolated from the total cells through gradient separation with Ficoll-Pacque (Amersham Biosciences AB, Uppsala, Sweden) at a density of 1.077 g/mL and were washed twice with PBS. MNCBMs were cultured for 120 h in IMDM supplemented with 15% (v/v) FBS, 5% (v/v) horse Equus ferus Linnaeus (Equidae) serum (Gibco-BRL, Carlsbad, CA) and 5 ng/mL recombinant mouse interleukin-3 (rmIL3; R&D System, Minneapolis, MN). The cells were cultured in a humidified atmosphere with 5% CO2 at 37 °C for a maximum duration of 120 h.
Proliferation, cell viability and Annexin-V in vitro assays
P388 cells and MNCBMs were grown in 96-well plates (Corning Costar, St. Louis, MO) at initial densities of 2 × 104 and 1 × 105, respectively, with or without the addition of different concentrations of the extract. To evaluate cell proliferation after growth, the cultures were then fixed with 1.1% glutaraldehyde and stained with crystal violet in 0.1% formic acid (Sigma-Aldrich St. Louis, MO). The dye was solubilized in 10% acetic acid, and the optical density at 570 nm was determined using a plate reader (Tecan Spectra, Grödig, Austria). The data were plotted, and the IC50 value was determined by linear regression. Trypan blue (Sigma, St. Louis, MO) exclusion assays were used to determine the number of viable cells in each treated culture as well as the IC50. The cell viability was determined by direct counting in a Neubauer chamber, and the non-stained cells were considered viable. The results are shown as the mean cell viability percentages ± standard deviations (SDs) from triplicate cultures. Finally, to identify the induction of apoptosis in the early stages, the cells were washed and labeled with an Annexin V-FITC kit (BD Biosciences, Franklin Lakes, NJ). After incubation, the cells were analyzed using a BD FACSAria II flow cytometer (BD Biosciences, US).
In vivo toxicity assays
Experimental animals
Both female and male 10- to 12-week-old syngeneic CD-1 mice were maintained in the animal facility of FES-Zaragoza with an ad libitum sterile standard powdered rodent diet. All experimental protocols were approved by the Ethics Committee of FES-Zaragoza and were performed in accordance with the research methodologies and evaluation protocols for traditional medicine, the ‘Guide for the Care and Use of Laboratory Animals, Eighth Edition’ published by the National Institutes of Health and the national regulations for the care and use of experimental animals (NOM-062-ZOO-1999).
Determination of acute toxicity (LD50)
The method used for the determination of acute toxicity was described by Lorke in 1983. Ten groups of five mice each (both male and female) were used, and a single dose of the extract (8, 16, 40, 160, 400, 800, 1600, 2900, 5000 and 0 mg/kg extract in PBS/methanol, 1:100 v/v vehicle) was intraperitoneally administered. The mice were monitored every 3, 6, 9 and 12 h for the first 24 h post-treatment for signs of toxicity and death and were then monitored once a day for seven days. The LD50 was then determined based on the obtained data.
Organic disorders and blood samples
Eight days after treatment (and prior to sacrifice), the mice were weighed, and the value was recorded. The organs, such as the spleen, thymus and liver, were carefully removed and weighed, and the organ index was calculated as the ratio of the organ weight to the total body weight. Peripheral blood was collected by cardiac puncture with and without EDTA as an anticoagulant. Whole blood was used for the analysis of total leukocytes (WCBs), total red blood cells (RCBs), hemoglobin (Hb), hematocrit (Hct), mean corpuscular hemoglobin (HCMC), mean corpuscular volume (MCV), platelets (PLTs) and differential counts of lymphocytes, granulocytes and monocytes with an automated hematology MICROS 60 instrument (HORIBA Medical, Kyoto, Japan). Serum was used to determine the blood biochemistry parameters, such as the concentrations of glucose, cholesterol, triglycerides, creatinine, urea, uric acid, aminotransaminase (AST), alanine aminotransaminase (ALT) and bilirubin using a clinical automatic biochemical analyzer (Hitachi 912, Boehringer Mannheim, Germany).
Mitotic index
To evaluate the in vivo toxicity of the extract, the number of cells undergoing cell division (mitosis) in the bone marrow (Antunes et al. 2000) was determined. Briefly, 24 h after the last inoculation of the extract, 300 μL of 0.1% colchicine (Sigma, St. Louis, MO) was intraperitoneally injected, and 2 h after injection, both femurs were obtained using a hypotonic solution with 0.047 M KCl. These samples were then incubated for 20 min at 37 °C, and the cells were fixed with a solution of methanol:acetic acid (3:1) and stained with Giemsa dye (Sigma, St. Louis, MO). One thousand cells were counted to obtain the percentage of dividing cells.
Statistical analysis
All analyses were performed using Microsoft Excel (Redmond, WA) and IBM SPSS Statistics (version 18; Armonk, NY). All values are expressed as means ± SDs. Analysis of one-way variance was performed following Dunnett’s comparison test. p ≤ 0.05 was considered statistically significant.
Results
Effects of the S. edule var. nigrum spinosum extract on the P388 leukemia cell line and normal mononuclear bone marrow cells
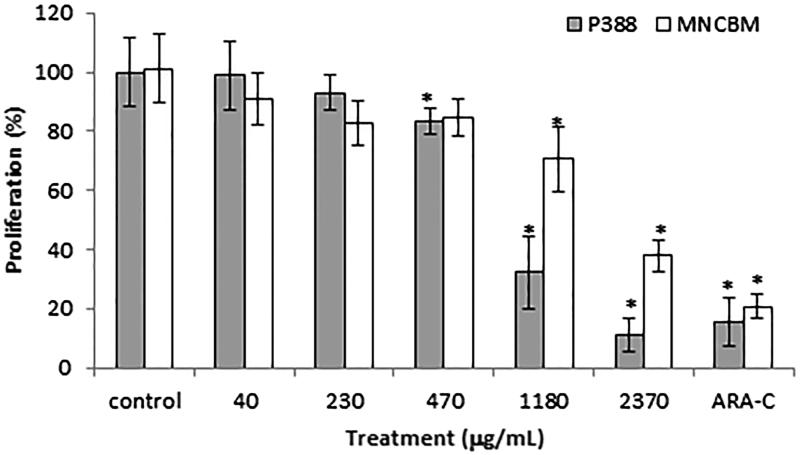
We recently showed that S. edule var. nigrum spinosum reduces the proliferation of the macrophage leukemic P388 cell line (Cadena-Iñiguez et al. 2013), but it remains unclear whether it also affects MNCBMs both in vitro and in vivo. The current results showed that the S. edule var. nigrum spinosum extract inhibited the proliferation of both leukemic P388 cells and normal cells, although the inhibition of proliferation was more marked in the leukemic cells (Figure 1). This observation was confirmed by the IC50 values of 927 and 1911 μg/mL obtained for the leukemic P388 cell line and MNCBMs, respectively. The extract at the IC50 concentration obtained for the leukemic line reduced the viability and induced the apoptosis of leukemic P388 cells but not the normal mononuclear bone marrow cells. Cytarabine damages both cell types equally (Table 1), as was previously shown for this antineoplastic agent (Stentoft 1990; Verstappen et al. 2003).
Figure 1.
In vitro effects of the S. edule var. nigrum spinosum extract on the proliferation of the P388 leukemia cell line and mononuclear bone marrow cells of healthy mice (MNCBMs). The cells were exposed to the S. edule var. nigrum spinosum extract, and the cellular proliferation was evaluated using a crystal violet assay. The data are presented as the means ± SDs (n = 4) and are representative of four independent experiments. The significance of the differences were determined by Tukey’s test (p ≤ 0.05).
Table 1.
Percentage of viability and apoptosis of P388 and bone marrow mononuclear cells from mice.
| Viability (%) |
Apoptosis (%) |
|||
|---|---|---|---|---|
| P388 | MNCBM | P388 | MNCBM | |
| CONTROL | 98.3 ± 5.8 | 95.9 ± 4.9 | 0.3 ± 0.2 | 40.4 ± 10.7 |
| S.e. nigrum spinosum | 39.2 ± 11.3* | 88.5 ± 14.0 | 95.5 ± 4.2* | 47.7 ± 8.7 |
| ARA-C | 24.6 ± 9.5* | 36.1 ± 11.4* | 99.1 ± 8.4* | 77.0 ± 7.9* |
The cells were treated in vitro with the IC50 value of the S. edule var. nigrum spinosum extract. The cell viability was determined using the Trypan blue exclusion assay in relation to the untreated population. The degree of apoptosis was estimated through an Annexin-V assay, and the cells were analyzed with a FACS Aria II flow cytometer. The data are presented as the means ± SDs (n = 4) and are representative of four independent experiments.
Indicates a significant difference (Tukey’s test, p ≤ 0.05).
We previously quantified the concentrations of sugars in the extract as 3.178 ± 0.3326 g of sugar/100 g of extract (Riviello 2015); however, we analyzed the extraction yield of the compounds found at lower concentrations (terpenes and flavonoids) in selective extract fractions and the biological activity of these individual fractions.
The selective extract fractions had yields of 0.52% in the hexane fraction (terpenes), 1.20% in the dichloromethane (terpene) fraction and 54.82% in the methanol fraction (flavonoids). The comparison of the biological activities of the crude extract and the three selective fractions for the proliferation of MNCBMs revealed that the crude extract exerted a significant antiproliferative effect at concentrations higher than 1180 μg/mL, whereas the fractions of hexane and methanol exerted effects at concentrations higher than 2370 μg/mL, and the dichloromethane fraction does not appear to have biological activity (data not shown). As a result, the biological activity of the crude extract is higher than those of the individual fractions.
Toxicity of the S. edule var. nigrum spinosum extract in healthy mice
Inspired by these findings and the fact that this variety of chayote eliminates leukemic cells by apoptosis without harming normal bone marrow cells (Aguiñiga-Sánchez et al. 2015), we evaluated the in vivo acute toxicity of the S. edule var. nigrum spinosum extract in accordance with Lorke (1983). The results indicated that the LD50 was higher than 5000 mg/kg, which indicated that the S. edule var. nigrum spinosum extract is safe according to Lorke (1983).
Literature data suggest that repeated doses should be administered if the dose is less than the LD50 (Akhila et al. 2007); therefore, 800 mg/kg was administered i.p. every 48 h for seven days. The data indicated that this dose does not induce changes in the spleen, liver or thymus index (Table 2), and alterations in these parameters indicate toxicity. The evaluation of the biochemical parameters of the blood plasma (Table 3) revealed that the S. edule var. nigrum spinosum extract only reduced the glucose levels, as previously published (Diré et al. 2006). Cytarabine, in contrast, increased the levels of uric acid and creatinine which indicatives kidney damage (Jesse 1982), and increased the levels of AST and total bilirubin which indicatis liver toxicity (Kumar et al. 2005; Karadeniz et al. 2011).
Table 2.
Spleen, liver and thymic indexes of healthy mice treated i.p. with the S.e. nigrum spinosum extract at a dose of 800 mg/kg.
| Index | Splenic | Hepatic | Thymic |
|---|---|---|---|
| CONTROL | 0.0048 ± 0.0004 | 0.0672 ± 0.0083 | 0.0013 ± 0.0002 |
| S.e. nigrum spinosum | 0.0059 ± 0.0011 | 0.0689 ± 0.0079 | 0.0012 ± 0.0002 |
| ARA-C | 0.0039 ± 0.0004* | 0.0629 ± 0.0056 | 0.0009 ± 0.0002* |
The organ indexes were determined as the ratio of the organ weights to the body weight. The data are represented as the means ± SDs and are representative of 20 mice per treatment.
Indicates a significant difference (Tukey’s test, p ≤ 0.05).
Table 3.
Blood biochemistry of healthy mice treated with the S.e. nigrum spinosum extract at a dose of 800 mg/kg.
| Control | S.e. nigrum spinosum | Ara-C | |
|---|---|---|---|
| Glucose (mg/dL) | 115.72 ± 16.6 | 72.27 ± 3.8* | 106.33 ± 25.8 |
| Cholesterol (mg/dL) | 69.73 ± 13.4 | 66.07 ± 13.6 | 68.91 ± 10.9 |
| Triglycerides (mg/dL) | 79.27 ± 16.2 | 88.9 ± 13.6 | 86.6 ± 22.9 |
| Uric acid (mg/dL) | 1.8 ± 0.9 | 2.1 ± 0.7 | 3.7 ± 0.6* |
| Urea (mg/dL) | 47.66 ± 6.7 | 45.81 ± 5.9 | 44.80 ± 2.0 |
| Creatinine (mg/dL) | 0.50 ± 0.1 | 0.48 ± 0.1 | 0.56 ± 0.2* |
| AST (U/L) | 120.67 ± 18.6 | 128.33 ± 16.7 | 248.00 ± 30.8* |
| ALT (U/L) | 59.00 ± 8.7 | 59.67 ± 13.5 | 58.00 ± 3.0 |
| Total bilirubin (mg/dL) | 0.35 ± 0.1 | 0.39 ± 0.1 | 0.23 ± 0.6* |
Serum was used to determine the biochemical parameters of the blood using a clinical automatic biochemical analyzer. The aminotransaminase (AST) and alanine aminotransaminase (ALT) levels were determined. The data are presented as the means ± SDs and representative of 20 mice per treatment.
Indicates a significant difference (Tukey’s test, p ≤ 0.05).
The same treatment revealed that the S. edule var. nigrum spinosum extract increased the WBC without altering the other parameters, whereas cytarabine significantly reduced the mean corpuscular volume (MCV) and increased the platelet levels (Table 4). These toxic effects were previously reported for cytarabine (Gobbi et al. 2009); thus, this dataset indicated that the S. edule var. nigrum spinosum extract does not induce systemic damage.
Table 4.
Blood count of healthy mice treated with the S.e. nigrum spinosum extract at a dose of 800 mg/kg.
| CONTROL | S.e. nigrum spinosum | ARA-C | |
|---|---|---|---|
| WBC (103/mm3) | 6.40 ± 1.40 | 10.53 ± 2.83* | 6.96 ± 2.23 |
| RBC (106/mm3) | 9.37 ± 0.83 | 9.82 ± 1.37 | 9.57 ± 1.28 |
| Hb (g/dL) | 13.87 ± 1.37 | 14.88 ± 1.02 | 16.05 ± 0.79 |
| Hct (%) | 47.79 ± 5.43 | 49.06 ± 4.55 | 50.08 ± 6.15 |
| HCMC (g/dL) | 31.43 ± 1.78 | 32.70 ± 2.17 | 29.35 ± 2.89 |
| VCM (mm3) | 51.38 ± 3.17 | 51.15 ± 3.33 | 53.56 ± 3.46 |
| HCM (pg) | 16.34 ± 1.18 | 16.10 ± 1.44 | 15.84 ± 0.41 |
| PLT (106/mm3) | 80.54 ± 19.54 | 89.93 ± 21.82 | 147.15 ± 35.71* |
Plasma was used to analyze the total leukocytes (WCBs), total red blood cells (RCBs), hemoglobin (Hb), hematocrit (Hct), mean corpuscular hemoglobin (HCMC), mean corpuscular volume (MCV), platelets (PLTs) and differential counts of lymphocytes, granulocytes and monocytes with an automated hematology MICROS 60 instrument. The data are presented as means ± SDs and are representative of 20 mice per treatment.
Indicates a significant difference (Tukey’s test, p ≤ 0.05).
Toxicity of the S. edule var. nigrum spinosum extract on mononuclear normal bone marrow cells
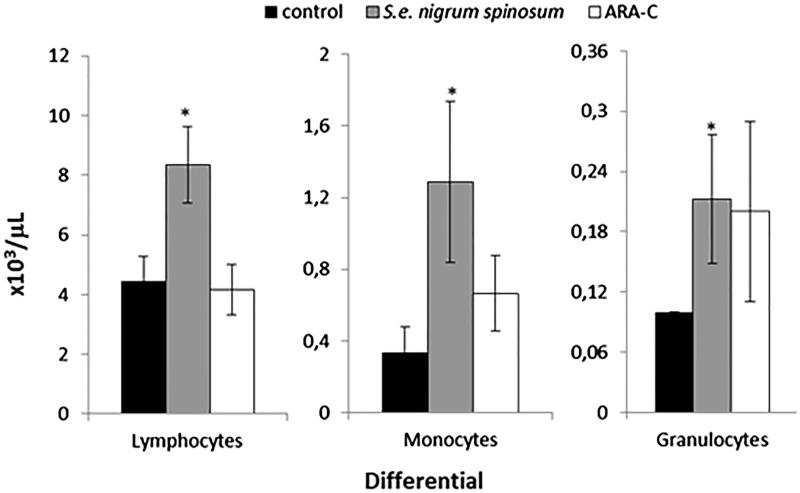
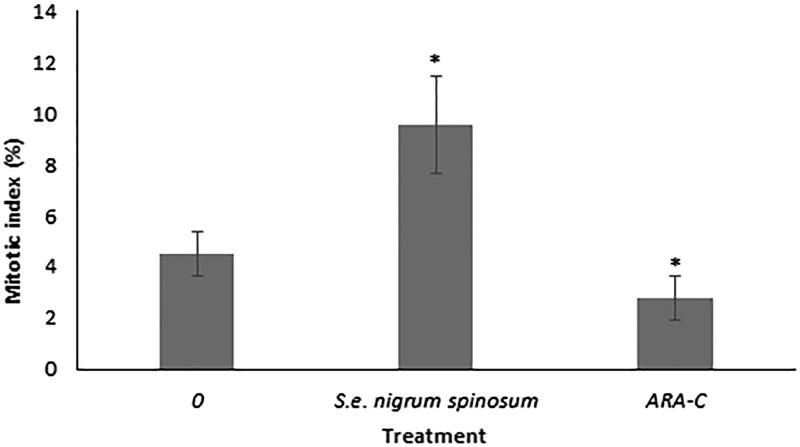
Based on the increased number of WBCs in mice treated with the S. edule var. nigrum spinosum extract, we evaluated the types of cell populations that were enhanced. The data indicated that the S. edule var. nigrum spinosum extract increased the levels of lymphocytes, monocytes and granulocytes, whereas cytarabine only increased the levels of monocytes and granulocytes (Figure 2). As the S. edule var. nigrum spinosum extract and cytarabine increased the levels of some but not all WBCs, we evaluated whether these increases are related to increased cell division in the bone marrow, an organ that is highly sensitive to cytotoxic agents (Scatena et al. 2010). We found that only cytarabine exhibited adverse effects, whereas the S. edule var. nigrum spinosum extract only increased the mitotic index in the bone marrow cells (Figure 3). It is known that cytarabine inhibits cell division in the bone marrow (Wei et al. 2013), and this finding was confirmed in this study.
Figure 2.
Differential peripheral blood counts of healthy mice treated with the S. edule var. nigrum spinosum extract at a dose of 800 mg/kg. n = 20 mice per treatment. The data are presented as the means ± SDs; *indicates a significant difference (Tukey’s test, p ≤ 0.05).
Figure 3.
Mitotic index (MI) of bone marrow cells from healthy mice treated with the S. edule var. nigrum spinosum extract at a dose of 800 mg/kg. n = 20 mice per treatment. The data are presented as the means ± SDs; *indicates a significant difference (Tukey’s test, p ≤ 0.05).
Identification of the chemical components of the S. edule var. nigrum spinosum extract
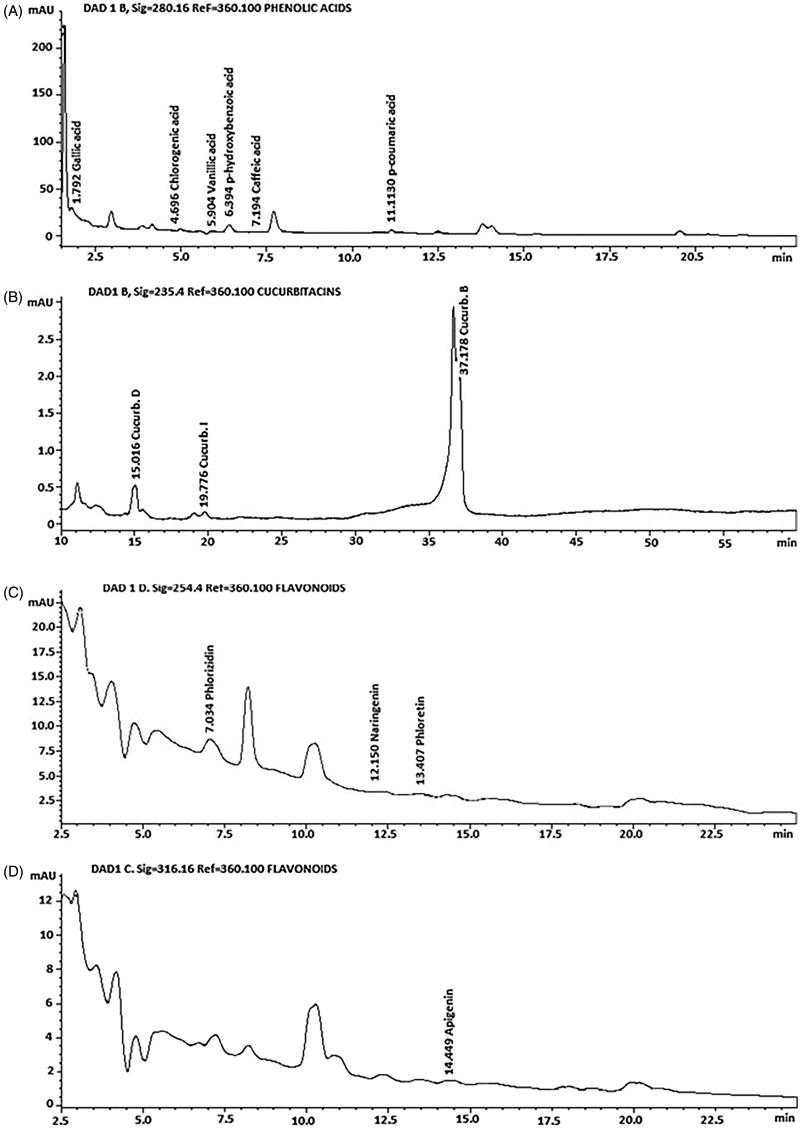
Chromatographic studies were performed to identify the secondary metabolites in the S. edule var. nigrum spinosum extract (Figure 4). Terpenes and flavonoids were the major components identified by colorimetric methods, and no alkaloids were identified in this study. In addition, an HPLC analysis indicated that the S. edule var. nigrum spinosum extract contains cucurbitacins B and D and traces of cucurbitacin I (1.008, 0.127 and 0.013 mg/g of extract, respectively), which are findings that have not been reported in the literature for this species and variety. Additionally, we found some phenolic acids, such as gallic, chlorogenic, vanillic, p-hydroxybenzoic, caffeic and p-coumaric acids (0.072, 0.823, 0.032, 0.020, 0.091 and 0.032 mg/g of extract, respectively). Flavonoids are represented in the S. edule var. nigrum spinosum extract by phloridzin, naringenin, phloretin and apigenin (0.005, 1.556, 0.018 and 0.292 mg/g of extract, respectively).
Figure 4.
Representative high-performance liquid chromatography (HPLC) chromatogram of the S. edule var. nigrum spinosum extract: (A) phenolic acids, (B) cucurbitacins C and D, and (C) flavonoids.
Discussion
Sechium edule var. nigrum spinosum is consumed by humans as food and is also used as an antitumour agent (Alonso-Castro et al. 2011). In this study, we show that the S. edule var. nigrum spinosum extract is used for treating leukemic cells, which is consistent with previous observations that showed normal cells are undamaged by the H387 07 chayote hybrid (Aguiñiga-Sánchez et al. 2015), even though S. edule var. nigrum spinosum has less potent biological activity than H387 07. In addition, these data indicate that this edible variety presents biological selectivity.
The increased mitotic index of the bone marrow in response to the S. edule var. nigrum spinosum extract explains the increase in lymphocytes, monocytes and granulocytes in the peripheral blood. In this sense, a nutraceutical formulation of blueberry extract, green tea extract, carnosine and vitamin D3, which is known as NT-020, promotes the proliferation of human hematopoietic stem cells in vitro and protects stem cells from oxidative stress when given chronically to mice in vivo (Shytle et al. 2010). In this study, we show that the S. edule var. nigrum spinosum extract of an edible vegetable promotes blood cell generation and can thus be considered a nutraceutical agent.
Our study of the secondary metabolites in the S. edule var. nigrum spinosum extract identified terpenes and flavonoids as the major components. These data correlate with the presence of saponins and sterols/triterpene in fruits collected from Colombia, where the presence of alkaloids has also been reported (Salama et al. 1987; Cadena-Iñiguez et al. 2007). We did not find alkaloids in this study, and this difference might be due to both the variety of the plant that was used and the growing conditions (Loraine & Mendoza-Espinoza 2010).
In addition, we found cucurbitacins B, D and I in the S. edule var. nigrum spinosum extract. These compounds belong to a group of terpenes, which are metabolites recognized as inhibitors of the proliferation of tumour and normal cells (Nelson & Falk 1992; Setzer & Setzer 2003; Shao et al. 2013; Li et al. 2015). Additionally, we found gallic, chlorogenic, vanillic, p-hydroxybenzoic, caffeic and p-coumaric acids, but only gallic and caffeic acids have been reported in S. edule leaves (Ordoñez et al. 2006).
Cucurbitacins B, D and I are extremely toxic (Setzer & Setzer 2003), but their enrichment with the other phytochemicals found in the extract of S. edule var. nigrum spinosum together maintains the effect of the extract on tumor cells but allows the protection of normal cells in vitro. It is also likely that this mechanism of action confers the nontoxic in vivo characteristics.
Interestingly, naringenin and apigenin have been found in the stems and leaves but not in the fruits. One possible explanation for this difference in the phytochemical content in fruits might result from the variety of fruit used, which was unfortunately not reported by Siciliano et al. (2004). All of the phytochemicals found in the S. edule var. nigrum spinosum fruit have been previously mentioned as possible agents with cytotoxic activity in leukaemia, lymphoma, and solid tumours (Nelson & Falk 1992; Wang et al. 2008; Shao et al. 2013; Spilioti et al. 2014).
Finally, we want to emphasize that caffeic acid is cytotoxic to SNU638 gastric cancer cells, AGS cells and HCT 116 colorectal carcinoma but not to normal cells (Wang et al. 2005; Kim et al. 2011; Watanabe et al. 2011). Furthermore, it has been found that gallic acid, caffeic acid, naringenin and phloridzin have antioxidant and anti-inflammatory activities (Hale et al. 2008; Wybranowski et al. 2014), which can help prevent cell damage (Pietta 2000) and might explain their cytoprotective activities on normal cells. In the future, it would be interesting to analyze the metabolic pathways that are activated in tumour cell lines relative to normal cells in order to explain the observed cytotoxicity or cytoprotection and thus, strengthen the therapeutic potential of the S. edule var. nigrum spinosum extract. These data indicate that consuming this product provides qualities beyond its nutritional value as food, such as antiproliferative and nutraceutical activities (based on the identified metabolites), and this characteristic provides an added value to a nontraditional vegetable that is widely consumed, particularly among socially disadvantaged people.
Conclusions
The S. edule var. nigrum spinosum extract is not cytotoxic to mononuclear bone marrow cells in vitro and in vivo when it is administered intraperitoneally at doses of 800 mg/kg every 48 h for seven days. The extract contains metabolites, such as flavonoids, phenolic acids and cucurbitacins, compounds that can eliminate tumour cells while protecting normal bone marrow cells. Therefore, the S. edule var. nigrum spinosum extract is an emerging natural agent that can be used for the treatment of various diseases without any harmful side effects.
Acknowledgements
The authors sincerely thank Adriana Altamirano Bautista, head of the Animal Facility of FES-Zaragoza, UNAM; Román Hernández Meza for his technical assistance with the animal care; Rubén San Miguel Chávez, a researcher in the Phytochemistry Area of the Colegio de Posgraduados, for handling the HPLC; and Taide Laurita Arista Ugalde and Ana Rocío Rivera Martínez for the technical assistance provided. Finally, we want to specially thank GISeM for the kind donation of S. edule var. nigrum spinosum.
Disclosure statement
There are neither any conflicts of interests nor any financial interests in this study from the part of the contributing authors that could inappropriately influence the work.
References
- Aguiñiga-Sánchez I, Soto-Hernández M, Cadena-Iñiguez J, Ruíz-Posadas L, Cadena-Zamudio J, González-Ugarte A, Weiss Steider B, Santiago-Osorio E.. 2015. Fruit extract from a Sechium edule hybrid induce apoptosis in leukaemic cell lines but not in normal cells. Nutr Cancer. 67:250–257. [DOI] [PubMed] [Google Scholar]
- Akhila J, Shyamjit D, Alawar M.. 2007. Acute toxicity studies and determination of median lethal dose. Curr Sci. 93:917–920. [Google Scholar]
- Alakoc C, Eroglu H.. 2011. Determining mitotic index in peripheral lymphocytes of welders exposed to metal arc welding fumes. Turk J Biol. 35:325–330. [Google Scholar]
- Alexander-Bryant A, Berg-Foels W, Wen X.. 2013. Bioengineering strategies for designing targeted cancer therapies. Adv Cancer Res. 118:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Castro A, Villarreal M, Salazar-Olivo L, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A.. 2011. Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol. 133:945–972. [DOI] [PubMed] [Google Scholar]
- Antunes L, Araújo M, Darin J, Maria de Lourdes P.. 2000. Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells. Mutat Res Genet Toxicol Environ Mutagen. 465:131–137. [DOI] [PubMed] [Google Scholar]
- Cadena-Iñiguez J, Arévalo-Galarza L, Avendaño-Arrazate C, SotoHernández M, Ruiz-Posadas L, Santiago-Osorio E, Acosta-Ramos M, Cisneros-Solano V, Aguirre-Medina J, Ochoa-Martínez D.. 2007. Production, genetics, postharvest management and pharmacological characteristics of Sechium edule (Jacq.) Sw In: Teixeira da Silva J, editor. Fresh produce. Vol. 1 Middlesex: Global Science Books; p. 41–53. [Google Scholar]
- Cadena-Iñiguez J, Soto-Hernández M, Torres-Salas A, Aguiñiga-Sánchez I, Ruiz-Posadas L, Rivera-Martinez A, Santiago-Osorio E.. 2013. The antiproliferative effect of chayote varieties (Sechium edule (Jacq.) Sw.) on tumour cell lines. J Med Plants Res. 7:455–460. [Google Scholar]
- Cambar J.1985. Algunos estudios farmacológicos de las plantas medicinales en Honduras. Rev Med Hondur. 53:190–196. [Google Scholar]
- Cambar P, Portillo P, Tabora E, Pineda L, Tovar O, Casco J, Alvarado C, Díaz G, Casco B, Cantillo L.. 1980. Estudio preliminar sobre las acciones farmacológicas de Sechium edule. Rev Med Hondur. 48:97–99. [Google Scholar]
- Culakova E, Thota R, Poniewierski M, Kuderer N, Wogu A, Dale D, Crawford J, Lyman G.. 2014. Patterns of chemotherapy‐associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med. 3:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diré G, Vasconcelos S, Siqueira P, Duarte R, Almeida M, Rodrigues J, Olveira J, Fernandes M, Bernardo-Filho M.. 2006. The analysis of the effect of a chayotte extract on the radiolabeling of blood elements in diabetic rats. Pak J Nutr. 5:269–273. [Google Scholar]
- Firdous S, Ahmed S, Dey S.. 2012. Antiepileptic and central nervous system depressant activity of Sechium edule fruit extract. Bangladesh J Pharmacol. 7:199–202. [Google Scholar]
- Firdous S, Sravanthi K, Debnath R, Neeraja K.. 2012. Protective effect of ethanolic extract and its ethylacetate and n-butanol fractions of Sechium edule fruits against carbon tetrachloride induced hepatic injury in rats. Int J Pharm Pharm Sci. 4:354–359. [Google Scholar]
- Firdous S, Pau lS, Kanti A.. 2013. Effect of Sechium edule on chemical induced kidney damage in experimental animals. Bangladesh J Pharmacol. 8:28–35. [Google Scholar]
- Gobbi P, Valentino F, Lambelet P, Perfetti V, Bergamaschi G, Girino M, Corazza G.. 2009. Shortened and intensified MJMA: an effective salvage therapy for relapsed and refractory lymphomas and a strong mobilizer of PBSCs. Bone Marrow Transplant. 44:19–25. [DOI] [PubMed] [Google Scholar]
- Gordon E, Guppy L, Nelson M.. 2000. The antihypertensive effects of the Jamaican Cho-Cho (Sechium edule). West Indian Med J. 49:27–31. [PubMed] [Google Scholar]
- Hale A, Reddivari L, Nzaramba M, Bamberg J, Miller J.. 2008. Interspecific variability for antioxidant activity and phenolic content among Solanum species. Am J Potato Res. 85:332–341. [Google Scholar]
- Jesse B.1982. Animal anatomy and hysiology. Reston (VA): Reston Publishing Company, Inc. [Google Scholar]
- Karadeniz A, Simsek N, Karakus E, Yildirim S, Kara A, Can I, Kisa F, Emre H, Turkeli M.. 2011. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid Med Cell Longev. 2011:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Hwang I, Lee J, Son K, Jeong C, Jung J.. 2011. Protective effect of Cimicifuga heracleifolia ethanol extract and its constituents against gastric injury. J Health Sci. 57:289–292. [Google Scholar]
- Kumar R, Gupta M, Mazumdar U, Rajeshwar Y, Kumar T, Gomathi P, Roy R.. 2005. Effects of methanol extracts of Caesalpinia bonducella and Bauhinia racemosa on hematology and hepatorenal function in mice. J Toxicol Sci. 30:265–274. [DOI] [PubMed] [Google Scholar]
- Li R, Feng Y, Chen J, Ge L, Xiao S, Zuo X.. 2015. Naringenin suppresses K562 human leukemia cell proliferation and ameliorates Adriamycin-induced oxidative damage in polymorphonuclear leukocytes. Exp Ther Med. 9:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loraine S, Mendoza-Espinoza J.. 2010. Las plantas medicinales en la lucha contra el cáncer, relevancia para México. Rev Mex Cienc Farm. 41:18–27. [Google Scholar]
- Lorke D.1983. A new approach to practical acute toxicity testing. Arch Toxicol. 54:275–287. [DOI] [PubMed] [Google Scholar]
- Nelson J, Falk R.. 1992. The efficacy of phloridzin and phloretin on tumor cell growth. Anticancer Res. 13:2287–2292. [PubMed] [Google Scholar]
- Ordoñez A, Gomez J, Cudmani N, Vattuone M, Isla M.. 2003. Antimicrobial activity of nine extracts of Sechium edule (Jacq.) Swartz. Microb Ecol Health Dis. 15:33–39. [Google Scholar]
- Ordoñez A, Gomez J, Vattuone M.. 2006. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 97:452–458. [Google Scholar]
- Orkin S, Zon L.. 2008. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 132:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietta P.2000. Flavonoids as antioxidants. J Nat Prod. 63:1035–1042. [DOI] [PubMed] [Google Scholar]
- Riviello M.2015. Evaluación de compuestos de importancia funcional en jugo de dos genotipos Sechium edule (Jacq.) Sw. Tesis de Maestría. Colegio De Postgraduados; p. 120. [Google Scholar]
- Salama A, Polo A, Contreras C, Maldonado L.. 1986. Análisis fitiquímico preliminar y determinación de las actividades antiinflamatoria y cardiaca de los frutos de Sechium edule. Rev Colomb Cienc Quím Farm. 15:79–82. [Google Scholar]
- Salama A, Achenbach H, Sánchez M, Gutiérrez M.. 1987. Aislamiento e identificación de glicósidos antiinflamatorios de los frutos de Sechium edule. Rev Colomb Cienc Quím Farm. 16:15–16. [Google Scholar]
- Scatena C, Kumer J, Arbitrario J, Howlett A, Hawtin R, Fox J, Silverman J.. 2010. Voreloxin, a first-in-class anticancer quinolone derivative, acts synergistically with cytarabine in vitro and induces bone marrow aplasia in vivo. Cancer Chemother Pharmacol. 66:881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer W, Setzer M.. 2003. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev Med Chem. 3:540–556. [DOI] [PubMed] [Google Scholar]
- Shao H, Jing K, Mahmoud E, Huang H, Fang X, Yu C.. 2013. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol Cancer Ther. 12:2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle D, Tan J, Ehrhart J, Smith A, Sanberg C, Sanberg P, Anderson J, Bickford P.. 2010. Effects of blue-green algae extracts on the proliferation of human adult stem cells in vitro: a preliminary study. Med Sci Monit. 16:BR1–BR5. [PubMed] [Google Scholar]
- Siciliano T, De Tommasi N, Morelli I, Braca A.. 2004. Study of flavonoids of Sechium edule (Jacq) Swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J Agric Food Chem. 52:6510–6515. [DOI] [PubMed] [Google Scholar]
- Spilioti E, Jaakkola M, Tolonen T, Lipponen M, Virtanen V, Chinou I, Kassi E, Karabournioti S, Moutsatsou P.. 2014. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PloS One. 9:e94860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stentoft J.1990. The toxicity of cytarabine.. Drug Safety. 5:7–27. [DOI] [PubMed] [Google Scholar]
- Verstappen C, Heimans J, Hoekman, Postma T.. 2003. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 63:1549–1563. [DOI] [PubMed] [Google Scholar]
- Wang D, Xiang D, He Y, Li Z, Wu X, Mou J, Xiao H, Zhang Q.. 2005. Effect of caffeic acid phenethyl ester on proliferation and apoptosis of colorectal cancer cells in vitro. World J Gastroenterol. 11:4008–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Chen L, Wu W, Long Y, Wang R.. 2008. Potential cytoprotection: antioxidant defence by caffeic acid phenethyl ester against free radical-induced damage of lipids, DNA, and proteins. Can J Physiol Pharmacol. 86:279–287. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Amarante M, Conti B, Sforcin J.. 2011. Cytotoxic constituents of propolis inducing anticancer effects: a review. J Pharm Pharmacol. 63:1378–1386. [DOI] [PubMed] [Google Scholar]
- Wei Y, Bai H, Sun Y, Bao S, Xi R, Liu L.. 2013. [Effect of salidroside on apoptosis of bone marrow mesenchymal stem cells induced by ara-C]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 21:1572–1577. [DOI] [PubMed] [Google Scholar]
- Wybranowski T, Ziomkowska B, Kruszewski S.. 2014. Antioxidant properties of flavonoids and honeys studied by optical spectroscopy methods. Med Biol Sci. 27:53–58. [Google Scholar]
- Yi M, Yi H, Li H, Wu L.. 2010. Aluminum induces chromosome aberrations, micronuclei, and cell cycle dysfunction in root cells of Vicia faba. Environ Toxicol. 25:124–129. [DOI] [PubMed] [Google Scholar]