Abstract
Context:Helicteres vegae Cristóbal (Sterculiaceae) (Hv) and Heliopsis sinaloensis B.L. Turner (Asteraceae) (Hs) are endangered and poorly studied plant species; related plants have been used against chronic-degenerative and infectious diseases. Therefore, Hv and Hs could be sources of bioactive compounds against these illnesses.
Objective: To determine the chemical composition and biological activities (antioxidant, antimutagenic and antimicrobial) of Hv and Hs leaves (L) and stems (S).
Materials and methods: Methanol extracts (ME) of each plant/tissue were evaluated for their phytochemicals; phenolics (HPLC-DAD-ESI-MS); antioxidant activity (AA) (0.125–4 mg/mL) (DPPH, ABTS, ORAC and β-carotene discoloration); antimutagenicity (0.5 and 1 mg/plate) (Ames assay, tester strain Salmonella enterica serovar Typhimurium YG1024, 1-nitropyrene as mutagen); activity against human pathogens (1 mg/mL); and toxicity (0.01–2 mg/mL) (Artemia salina assay).
Results: All ME showed flavonoids and triterpenes/steroids. The ME-SHv had the highest content of total phenolics (TP) (2245.82 ± 21.45 mg GAE/100 g d.w.) and condensed tannins (603.71 ± 1.115 mg CE/100 g d.w.). The compounds identified were flavonoids (kaempferol 7-O-coumaroylhexoside, and two kaempferol 7-O-rhamnosylhexosides) and phenolics [rosmarinic acid, and 3′-O-(8″-Z-caffeoyl) rosmarinic acid]. The ME-LHs showed the highest content of flavonoids (357.88 mg RE/g d.w.) and phenolic acids (238.58 mg CAE/g d.w.) by HPLC. The ME-SHv showed the highest AA. All ME were strong antimutagens (63.3-85.7%). Only the Hs extracts were toxic (ME-LHs, LC50 = 94.9 ± 1.7 μg/mL; ME-SHs, LC50 = 89.03 ± 4.42 μg/mL).
Discussion and conclusions: Both Hv and Hs are potential sources of preventive and therapeutic agents against chronic-degenerative diseases.
Keywords: Phenolics, antimicrobial, antimutagenic, antioxidant, flavonoids, liquid chromatography, electrospray ionization, mass spectrometry, toxicity
Introduction
Chronic-degenerative diseases (CDD) represent serious public health problems worldwide. However, the pharmacological therapies available for the treatment of these illnesses are limited and most of them are inefficient. Plants have been traditionally used for the treatment of several diseases, and therefore they represent an important source of bioactive compounds against CDD. Phenolics are among these metabolites; they are associated with many biological properties (e.g., antioxidant and antimutagenic) of plant extracts (Cervellati et al. 2002; Zhu et al. 2014), and characterized from complex plant mixtures by liquid chromatography coupled with mass spectrometry (HPLC-DAD-ESI-MS) (Ablajan et al. 2006).
Mexico is ranked fifth in the world in terms of floristic diversity, but many of these plants are unknown and only few of those catalogued have been scientifically studied. Helicteres vegae Cristóbal (Sterculiaceae) and Heliopsis sinaloensis B.L. Turner (Asteraceae) are endemic plants to the state of Sinaloa, which is located in northwestern Mexico. These plant species have not been previously studied; however, other members of these genera have been used to treat CDD and infectious diseases (Molina-Torres et al. 1999; Varghese et al. 2012), and specific compounds have been associated with such biological activities (Arriaga-Alba et al. 2013).
In this study, we carried out a chemical characterization and evaluated the antioxidant, antimutagenic and antimicrobial activities of methanol extracts (ME) of Helicteres vegae and Heliopsis sinaloensis to demonstrate their potential use as novel sources of pharmaceutical compounds or supplements for the treatment and prevention of chronic-degenerative and infectious diseases.
Materials and methods
Plant material
Helicteres vegae was collected during September 2011 from ‘El Saladito’, municipality of Elota, Sinaloa (80 masl, N 23°51′24″, W 106°47′42″), and Heliopsis sinaloensis was obtained during July 2011 from ‘Imala’, municipality of Culiacan, Sinaloa (100 masl, N 24°51′37″, W 107°13′01″). Plant collectors were Vega-Aviña R, Delgado-Vargas F and Pío-León JF. Plant specimens were identified by Vega-Aviña R and deposited in the herbarium of the Agronomy School, Autonomous University of Sinaloa, with the assigned numbers 11805 (Helicteres vegae) and 11816 (Heliopsis sinaloensis). Leaves and stems were recovered from each plant, freeze-dried (freeze dryer VirTis 25EL, VirTis Co., Gardiner, NY), and milled to get a flour that passed through a number 40 sieve. Flours were stored at −20 °C in darkness until use.
Reagents and solvents
The reagents of analytical grade were purchased from Sigma/Aldrich (St. Louis, MO): β-carotene, 2,6-di-tert-butyl-4-methylphenol (BHT), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), Folin-Ciocalteu reagent, disodium fluorescein, 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH), chemical standards (gallic acid, rutin hydrate, caffeic acid, kaempferol, and catechin), 1-nitropyrene (1-NP), dimethyl sulfoxide (DMSO). HPLC grade organic reagents were from Baker Inc. (Philipsburg, PA).
Microorganisms
Tester strain YG1024 from Salmonella enterica serovar typhimurium was kindly provided by Dr. Takehiko Nohmi, Division of Genetics and Mutagenesis, Biological Safety Research Center, National Institute of Hygienic Science, Japan. The strain was maintained, propagated, routinely tested for genetic markers, and re-isolated whenever necessary. Eleven human pathogen bacterial strains were used for the antibacterial assay: four ATCC (Staphylococcus aureus 29213, Enterococcus faecalis 29212, Escherichia coli 25922, and Pseudomonas aeruginosa 27853) (DIFCO Laboratories, Detroit, MI); and seven clinical isolates (Streptococcus aureus, Streptococcus group A, Salmonella enterica serovar typhi, Shigella dysenteriae, E. coli A011, E. coli A019 and E. coli A055), which were provided by the Laboratory of Bacteriology of the National Institute of Pediatrics, Mexico City, Mexico. Giardia lamblia WB strain was provided by the Department of Experimental Pathology, Center for Research and Advanced Studies of the National Polytechnic Institute, Mexico City, Mexico. Artemia salina cysts were from a commercial trademark (Brine shrimp eggs, Bio-Marine Inc, Hawthorne, CA).
Preparation of ME
Flours were extracted with methanol (1:10 w/v) for three days at 150 rpm/room temperature; supernatant was recovered by filtration daily, and the residue was extracted again with fresh solvent. Supernatants were mixed and the solvent was removed with a rotavapor (Büchi Labortechnick AG, Flawil, Switzerland) at 40 °C; the residues were freeze-dried to obtain the ME of leaves (L) and stems (S) of Helicteres vegae (Hv) and Heliopsis sinaloensis (Hs). The extraction yields (%) of the ME were: ME-LHv 14.12, ME-SHv 12.73, ME-LHs 25.02, and ME-SHs 13.50. Extracts were stored at −20 °C in darkness until use.
Phytochemical analysis
Assays for phytochemicals in ME were carried out in test tubes or by thin-layer chromatography (silica gel matrix with fluorescent indicator 254 nm, Sigma/Aldrich) as follows: the Salkowski reaction for terpenes/sterols; the Shinoda test for flavonoids; reaction with 1.0% gelatin solution and quinine sulphate solution with FeCl3 for tannins; lather formation for saponins; yellow fluorescence by reaction with NaOH for coumarins; the Borträger reaction for free anthracenics; the reagents of Dragendorff and Mayer and Wagner for alkaloids; and the reagents of Baljet, Raymond-Marthoud, Keller-Killiani, Lieberman-Burchard, and Salkowski for cardiotonics (Harborne 1980; Yawalikar et al. 2014). The results were reported as the presence (+) or absence (−) of each family of compounds in the ME.
Phenolics determined by colorimetric methods
Total phenolics (TP) were measured by the Folin-Ciocalteu method as described by Ahumada-Santos et al. (2013). A 0.02 mL aliquot of ME (1–2 mg/mL), 1.58 mL of distilled water, and 0.1 mL of 2 N Folin-Ciocalteu reagent were mixed in a test tube. The mixture was left stand at 40 °C for 5 min, then added with 0.3 mL of Na2CO3 saturated solution, mixed, incubated at 40 °C in darkness for 30 min, and the absorbance was measured at 765 nm. A calibration curve of gallic acid (0–500 μg/mL in methanol) was used to report the TP as milligrams of Gallic Acid Equivalents per 100 g of flour on a dry weight basis (mg GAE/100 g d.w.).
Condensed tannins were quantified as described by Bae et al. (1993). In a test tube (12 × 75 mm), 0.5 mL of ME (5–10 mg/mL) was vortex mixed with 3 mL of butanol:HCl (95:5 v/v) and 0.1 mL of ferric reagent (2% of ferric ammonium sulfate in 2 N HCl, 50:50 v/v). The mixture was heated at 97–100 °C for 60 min, whereas that used as blank was kept at room temperature. The mixture was brought to room temperature before measuring the absorbance at 550 nm. A calibration curve of catechin (0–5 mg/mL in methanol) was used to report the content of condensed tannins as milligrams of Catechin Equivalents per 100 g of flour d.w. (mg CE/100 g d.w.).
Phenolic compounds determined by HPLC-DAD-ESI-MS
Quantitation was carried out by the internal standard method. The ME (20 mg) was mixed with 100 μL of caffeic acid (1 mg/mL), 350 μL of rutin (1 mg/mL), and 5 mL of deionized water. The mixture was sonicated for 50 min and the phenolic compounds were extracted three times with 5 mL of HPLC grade ethyl acetate. The solvent was removed with a rotavapor at 40 °C, whereas the residue was re-suspended in HPLC grade methanol (1:1 w/v), sonicated and passed through a syringe filter (PVDF membrane 0.45 μm, HPLC certified, Thermo Scientific, Darmstadt, Germany). A 5 μL aliquot was injected into the HPLC-DAD system (ACCELA, Thermo Scientific, Waltham, MA). The separation was carried out using a Fortis C18 HPLC column (3 μm, 50 × 2.1 mm) (Fortis Technologies Ltd, Neston, UK) with a linear gradient of 1% (v/v) formic acid (A) and acetonitrile (B), 0.5–60% of B in 35 min, and a total running time of 50 min.
The HPLC-DAD was coupled to a mass spectrometer with an electrospray ionization source (ESI) (Thermo Scientific, LTQ XL, US). The analysis was carried out in negative mode and full scan spectra were obtained in the m/z range of 50–1500. The parameters of the capillary tube were 35 V and 300 °C. Nitrogen and helium gases were used for drying and collision, respectively. The results were analyzed with the Xcalibur 2.2 software (Thermo Scientific, US).
Direct sample insertion was used for the MSn experiments, scanning was in negative mode and the selected ion was fragmented by collision induced dissociation applying 10–45 V.
The identification of phenolics was based on the UV-spectra, MS fragmentation, and by comparisons with MS data both generated with commercial standards and reported in the literature. Flavonoids were quantified as Rutin Equivalents (mg RE/g d.w.) and phenolics as Caffeic Acid Equivalents (mg CAE/g d.w.).
Antioxidant activity
DPPH method
Antioxidant activity by DPPH was measured according to Brand-Williams et al. (1995) with minor modifications. Aliquots of 0.2 mL of ME (0.25–4 mg/mL) or Trolox (0–75 μg/mL) and 1.8 mL of 150 μM DPPH were mixed in test tubes. The mixture was homogenized, left to stand at 37 °C/darkness for 30 min and the absorbance was measured at 515 nm. The results were reported as Trolox Equivalents Antioxidant Capacity (TEAC) per gram of flour on a dry weight basis.
ABTS method
The antioxidant activity by ABTS was measured according to Re et al. (1999). The radical (ABTS•+) was produced by mixing 5 mL of ABTS (14 mM in water) and 5 mL of potassium persulfate (4.9 mM); the mixture was left to stand at room temperature/darkness for 12–16 h. ABTS•+ was diluted with phosphate buffer saline (PBS) (pH 7.4, 23 mM) to reach an absorbance of 0.7 ± 0.02 at 734 nm. For evaluation, 0.05 mL of ME (0.125–4 mg/mL) or Trolox (0–0.4 mg/mL) was mixed with 1.95 mL of the ABTS•+ dilution; the mixture was left to stand at 37 °C for 10 min and the absorbance was measured at 734 nm. The results were expressed as TEAC per gram of flour on a dry weight basis.
ORAC method
Oxygen Radical Absorbance Capacity (ORAC) was measured as described by Huang et al. (2002). ME (2 mg/mL in methanol) was mixed with PBS (pH 7.4, 23 mM) to obtain 1:50, 1:100, 1:200 and 1:300 dilutions. Aliquots of 25 μL of the diluted ME or Trolox (0–100 μmol/L) were added to a 96 microwell plate and placed into a fluorescence spectrophotometer (Synergy HT, Bio-TEK Instruments, Winooski, VT). The equipment added 150 μL of fluorescein (0.1 μM in PBS), mixed the microplate at 1200 rpm for 20 s, and then added 25 μL of AAPH (0.207 g in 5 mL of PBS). The reaction was carried out at 37 °C and the fluorescence intensity (485 nm (ex)/538 nm (em)) was measured every minute up to 40 min. The area under the curve (AUC) was calculated as AUC =0.5 + f1/f0 +⋯+ fi/f0 +⋯+ f39/f0 + 0.5(f40/f0), where f0 is the fluorescence at 0 min and fi that measured at time i (i = 1, 2, 3, … 40 min). Results were expressed as TEAC per gram of flour on a dry weight basis.
β-Carotene discoloration
The β-carotene discoloration (βCD) method reported by Wang et al. (2006) was used with minor modifications. Briefly, the reaction mixture was prepared by homogenization of 50 mg of Tween 40, 6.25 μL of linoleic acid and 500 μL of β-carotene (2 mg/mL in CH2Cl2); the solvent was eliminated with a stream of N2(g) and the residue was vortex-mixed with 25 mL of 30% H2O2. A mixture without β-carotene was used as blank. The assays were carried out in 96 well flat bottom plates. For the blank, 50 μL of DMSO were mixed with 250 μL of the blank mixture; to evaluate the extracts, 50 μL aliquots of the ME or butylhydroxytoluene (BHT) (0.5 and 1 mg/mL in DMSO) or DMSO were mixed with 250 μL of the reaction mixture. Absorbances were determined at 490 nm with a microplate reader (Multiskan Bichromatic, Thermo Fisher Scientific, USA) at 0 min and after 2 h of incubation at 50 °C. The rate of β-carotene degradation (R) was determined as R = (ln [aS-C/bS-C])/t; where, aS-C is the absorbance of the sample (S) or control (C) at 0 min, and bS-C is the absorbance of S or C at 120 min. Antioxidant activity was calculated as percentage of inhibition of β-carotene discoloration: % AA = [(Rcontrol − Rsample)/Rcontrol] × 100.
Antimutagenic activity
Antimutagenicity was evaluated by the Ames microsuspension assay, as described by Cano-Campos et al. (2011), using the tester strain Salmonella enterica serovar typhimurium YG1024 and 1-nitropyrene (1-NP) as mutagen. The toxicity and mutagenicity of the ME were evaluated up to 1 mg/tube.
SalmonellaTyphimurium was grown overnight in a metabolic bath 3540 (Lab-Line Instruments, Inc, Melrose Park, IL) at 37 °C to reach about 1–2 × 109 cells/mL; the culture medium was a mixture of 50 mL of Oxoid nutritive broth number 2 (Oxoid Ltd, Hants, UK) with 157.5 μL of ampicillin (8 mg/mL). Cells were recovered by centrifugation at 4500 rpm/4 °C for 10 min and the pellet was re-suspended (1 × 1010 cells/mL) in PBS (0.15 M, pH 7.4). The culture medium used for the assay was prepared in a sterile test tube (12 × 75 mm) kept on ice, and the ingredients were added as follows: 0.095 mL of cocktail (38.5 μL of water, 50 μL of 0.2 M NAP buffer pH 7.4, 2 μL of a mixture of 0.4 M KCl and 1.65 M MgCl2, 4 μL of 0.1 M NADP+, and 0.5 μL of 1 M d-glucose-6-phosphate), 0.1 mL of bacteria (1 × 1010 cells/mL), 0.005 mL of 1-NP (100 ng/tube), and 0.01 mL of ME (0.5 and 1 mg/tube) or DMSO; 0.015 mL of DMSO was used for the negative control. The mixture was incubated at 150 rpm/37 °C for 90 min, then placed on ice, vortex-mixed with 2 mL of molten top agar supplemented with 90 nmol of biotin/histidine and poured into minimal glucose agar plates. Plates were incubated at 37 °C for 48 h and the colonies counted (SOL-BAT Model Q-20, SOL-BAT Co., Puebla, Mexico). The inhibition of the 1-NP mutagenicity was determined as % inhibition = (1–A/B) × 100; where A is the number of revertants in the presence of the extract and 1-NP and B is in the number of revertants when only 1-NP was present in the mixture.
Antimutagenic activity was classified based on the % inhibition as: 0–20% (negative), 20–40% (weak), 40–60% (positive or moderate), 60–90% (strong) and >90% (suspected toxicity) (Wall et al. 1988).
The toxicity or mutagenicity of ME was determined by using the mutagenic index (MI) at each concentration: MI=(Rs/Rws); where Rs and Rws are the average number of revertants/plate with and without the evaluated sample, respectively. The sample was considered mutagenic if MI ≥2 or cytotoxic if MI ≤0.6 (Maron & Ames 1983; Rosa et al. 2006).
Antimicrobial activity
Antibacterial activity was evaluated against Gram negative and Gram positive human pathogenic bacteria, four ATCC control and seven clinical isolates strains. Evaluation was carried by the microdilution method, as described by the Clinical and Laboratory Standards Institute (CLSI 2012), and using up to 1 mg/mL of ME. The assay was carried out in U-bottom 96 microwell plates, containing in each well 50 μL of inoculum (5 × 105 UFC/mL) and 50 μL of the ME (DMSO 2% v/v Mueller Hinton). The positive control contained gentamicin (0.25–16 μg/mL) instead of the ME. The microwell plate was incubated (37 °C/18–24 h) and the Minimal Inhibitory Concentration (MIC) was determined by visual examination.
Antiparasitary activity against Giardia lamblia trophozoites was carried out as reported by Calzada et al. (1998). In a disposable tube, 1 mg of ME was resuspended in 900 μL of TYI-S 33 modified medium with DMSO (0.5% v/v) and then mixed with 100 μL of inoculum (1 × 106 trophozoites/mL). Metronidazole (Sigma) was used as standard anti-giardiasis. After incubation for 24 h at 37 °C/5% CO2, cell viability was measured using a mitochondrial dehydrogenase activity assay with the substrate 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Viable cells reduce MTT and the absorbance of the formed product was measured at 540 nm (Multiskan Bichromatic, Thermo Fisher Scientific, US).
Toxicity
Artemia salina eggs (100 mg) were suspended in 250 mL of NaCl solution (38 g/L) and incubated at 25–30 °C for 48 h. Nauplii were recovered and placed in fresh saline solution. ME dissolved in DMSO (40 mg/1400 μL of 10% DMSO) was used to prepare serial dilutions (10–2000 ppm/tube) in saline solution. The evaluation was carried out in test tubes. Each tube was filled with 10 nauplii, 140 μL of each ME dilution or DMSO (10% v/v) and saline solution up to 2 mL. Tubes were incubated at room temperature (25–30 °C) for 24 h and the number of living nauplii/tube was counted (Meyer et al. 1982; Solis et al. 1993). Probit analysis was used to determine the values of the lethal concentration 50 (LC50). Toxicity scale in the A. salina assay was registered as high (0.1–100 μg/mL), moderate (100–300 μg/mL), low (300–640 μg/mL), minimal (>640 μg/mL) or nontoxic (>2000 μg/mL) (Meyer et al. 1982; Sanabria-Galindo et al. 1997).
Statistical analysis
Data were analyzed by one-way analysis of variance and the differences among means were established by the Fisher test (LSD, α = 0.05). Pearson correlation analysis was used to determine the association between variables. Statistical analyses were performed using the software STATGRAPHICS Centurion XVI (Statpoint Inc., Warrenton, VA). All evaluations were done at least by triplicate.
Results
Chemical composition
Phytochemical analysis
Five of the nine families of the secondary metabolites analyzed were found in the ME (Table 1). ME-SHs and ME-LHv showed the highest and the lowest diversity of metabolites, respectively. Colorimetric assay suggested tannins are highly abundant in the ME-SHv.
Table 1.
Secondary metabolites of methanol extracts (ME) from leaves (L) and stems (S) of Helicteres vegae (Hv) and Heliopsis sinaloensis (Hs)a.
| Family of compounds | ME-LHv | ME-SHv | ME-LHs | ME-SHs |
|---|---|---|---|---|
| Alkaloids | – | – | – | – |
| Reducing sugars | + | – | + | + |
| Cardiotonics | – | – | – | – |
| Volatile coumarins | – | – | – | – |
| Free anthracenics | – | – | – | – |
| Flavonoids | + | + | + | + |
| Saponins | – | + | – | + |
| Tannins | – | + | + | + |
| Triterpenes or steroids | + | + | + | + |
The metabolites are present (+) or absent (−) in the corresponding methanol extract.
Phenolics determined by colorimetric methods
Significant differences in TP content (mg GAE/100 g d.w.) were found among the analyzed ME; ME-SHv showed the highest content (2245.82 ± 21.45) followed by ME-LHs (1604.65 ± 118.25), ME-SHs (580.70 ± 30.68) and ME-LHv (571.85 ± 42.64). The colorimetric determination of condensed tannins was only possible in the ME of H. vegae stems (ME-SHv) (603.71 ± 1.115 mg CE/100 g d.w.).
Phenolic compounds determined by HPLC-DAD-ESI-MS
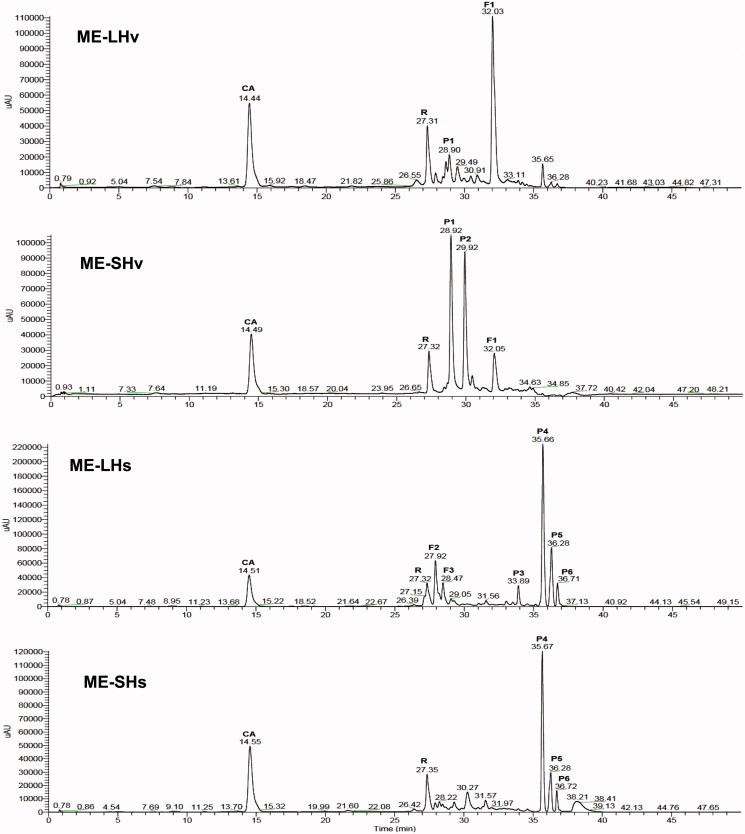
HPLC analyses showed that flavonoids (F) and phenolic acids (P) were the major compounds of the ME (Figure 1). The main phenolics of H. vegae and H. sinaloensis were different, and some of them were tissue specific: P2 was only found in the ME-SHv, whereas F2, F3 and P3 were specific for the ME-LHs. In addition, several phenolics were found in both tissues of the same plant but at different concentrations: P1 and F1 in H. vegae, and P4, P5 and P6 in H. sinaloensis (Figure 1).
Figure 1.
HPLC-DAD chromatograms of the methanol extracts (ME) from leaves (L) and stems (S) of Helicteres vegae (Hv) and Heliopsis sinaloensis (Hs). The identity of the major phenolics (flavonoids F and phenolic acids P) is shown in Table 1. Peaks for the commercial standards are CA (caffeic acid) and R (rutin).
The analysis of the retention times, absorption spectra, ESI-MS fragmentation patterns (Table 2; Figures 2, 1S, 2S and 3S) and ESI-MS data generated with commercial standards (i.e., flavonol aglycones and caffeic acid) allowed the identification of five phenolics: three kaempferol derivatives (kaempferol 7-O-coumaroylhexoside and two kaempferol 7-O-rhamnosylhexosides) and two phenolic acids [rosmarinic acid and 3′-O-(8″-Z-caffeoyl) rosmarinic acid] (Table 2).
Table 2.
HPLC-DAD-ESI-MS characterization of phenolics in methanol extracts (ME) from leaves (L) and stems (S) of Helicteres vegae (Hv) and Heliopsis sinaloensis (Hs)a.
| Peak | tR (min) | UV λmáx (nm) | [M – H]−m/z | ESI-MSn m/z (% relative abundance) | Tentative identification | Extract of plant/tissue |
|---|---|---|---|---|---|---|
| F1 | 32.05 | 316, 266, 242 | 593 | MS2 [M – H]-: 593→447 (12.2), 307 (5), 285 (100) MS3 [M – H]-: 593→285→267 (30), 257 (100), 255 (4), 241 (50), 229 (45), 213 (43), 169 (20), 151 (83), 107 (7.5) |
Kaempferol 7-O- coumaroylhexoside | ME-LHv, ME-SHv |
| F2 | 27.94 | 346, 265, 240 | 593 | MS2 [M – H]-: 593→447 (2), 327 (6), 285 (100), 257(6) MS3 [M – H]-: 593→285→285 (98), 267 (50), 257 (100), 255 (3), 229 (43), 213 (28), 197 (18), 195 (10), 169 (5), 163 (18), 151 (15) |
Kaempferol 7-O-rhamnosylhexoside | ME-LHs |
| F3 | 28.47 | 345, 265, 240 | 593 | MS2 [M-H]-: 593→447 (2), 285 (100), 283 (3) MS3 [M-H]-: 593→285→257 (100), 255 (4), 229 (41), 213 (22), 195 (7), 162 (15.8), 151 (12) |
Kaempferol 7-O-rhamnosylhexoside | ME-LHs |
| P1 | 28.93 | 328, 247, 232 | 359 | MS2 [M-H]-: 359→ 223 (20), 197 (30), 179 (30), 161 (100) MS3 [M-H]-: 359→197 (100), 135 (18) |
Rosmarinic acid | ME-LHv, ME-SHv |
| P2 | 29.94 | 322, 247, 234 | 537 | MS2 [M – H]-:537 → 493 (100), 417 (13), 399 (60), 357 (5), 298 (10) MS3 [M – H]-: 537 →493 →359 (100), 313 (15), 295 (38), 179 (5) MS4 [M – H]-:537 →493 →359→ 223 (20), 197 (30), 179 (18), 161 (100), 135 (5) |
3′-O-(8″-Z-caffeoyl)-rosmarinic acid | ME-SHv |
| P3 | 33.9 | 295, 241 | Unknown | ME-LHs | ||
| P4 | 35.68 | 329, 295, 247 | Unknown | ME-LHs ME-SHs |
||
| P5 | 36.2 | 335, 266, 243 | Unknown | |||
| P6 | 36.72 | 323,289, 245 | Unknown | ME-LHs ME-SHs |
Under the evaluated conditions, MSn fragmentation of P3, P4, P5 and P6 was not possible.
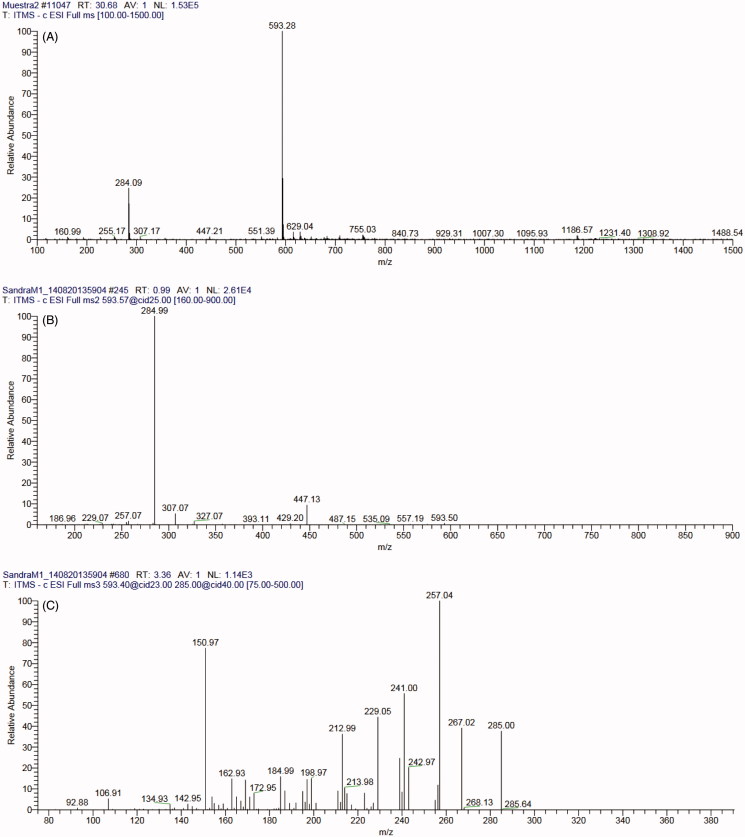
Figure 2.
ESI-MSn in negative mode of the compound F1. Full scan (A) and fragmentation of ion m/z 593, MS2 (B) and MS3 (C).
Based on the content of flavonoids (mg RE/g d.w.) and phenolic acids (mg CAE/g d.w.) determined by HPLC, we ordered the ME as ME-LHs (357.88) > ME-LHv (209.19) > ME-SHv (55.74) > ME-SHs (52.94) and ME-LHs (238.58) > ME-SHv (129.30) > ME-SHs (64.27) > ME-LHv (26.68), respectively.
Biological activities
Antioxidant activity
The ME of H. vegae stems (ME-SHv) showed the highest antioxidant activity estimated by the four methods, and its values were 3–14 times higher than those of the ME with the lowest activity. The extract of H. sinaloensis leaves (ME-LHs) was second in antioxidant activity measured by all assays, except for the β-carotene discoloration (Table 3). The antioxidant activity of the ME-SHv, expressed as IC50 values, was 218 μg/mL (DPPH) and 224.9 μg/mL (ABTS).
Table 3.
Antioxidant activity of ME from leaves (L) and stems (S) of Helicteres vegae (Hv) and Heliopsis sinaloensis (Hs)a.
| Evaluated by |
||||
|---|---|---|---|---|
| Methanol extract | DPPHb | ABTSb | ORACb | β-carotene discoloration[1 mg/mL]c |
| Helicteres vegae | ||||
| Leaves (ME-LHv) | 32.20 ± 2.43# | 102.96 ± 8.55# | 270.92 ± 19.45* | 22.77 ± 0.03& |
| Stems (ME-SHv) | 176.19 ± 12.55ξ | 304.67 ± 10.25ξ | 1336.6 ± 90.03& | 45.02 ± 0.02ξ |
| Heliopsis sinaloensis | ||||
| Leaves (ME-LHs) | 51.57 ± 2.85& | 286.07 ± 11.20& | 865.63 ± 13.79# | 3.24 ± 0.006* |
| Stems (ME-SHs) | 16.81 ± 0.83* | 90.92 ± 6.84* | 321.83 ± 32.71* | 13.32 ± 1.60# |
| BHT | 88.38 ± 2.06δ | |||
Results are the mean ± standard deviation of three independent replicates. Different symbols in the same column show significant differences, p < 0.0001 (LSD: DPPH = 7.904; ABTS = 11.273; ORAC = 59.44; β-carotene discoloration = 5.35).
μmoles of Trolox Equivalents/g of flour on a dry weight basis (μmol TE/g d.w.).
Percentage of antioxidant activity (% AA).
Antimutagenic activity
The ME evaluated up to 1000 μg/mL were neither toxic nor mutagenic against S.typhimurium YG1024 (0.6 ≤ MI ≤2.0) (Table 4). The number of induced and spontaneous revertants was about the same.
Table 4.
Mutagenicity index (MI) and antimutagenicity (% of inhibition) of the methanol extracts (MEs) from Leaves (L) and Stems (S) of Helicteres vegae (Hv) and Heliopsis sinaloensis (Hs). The mutagenic agent 1-nitropyrene (1-NP) and the tester strain Salmonella enterica serovar Typhimurium YG1024 were used in the assay.
| Methanol extract | Concentration [μg/plate] | MIa | Revertants/platea | % of inhibitiona,b |
|---|---|---|---|---|
| Helicteres vegae | ||||
| Leaves (ME-LHv) | 1000 | 1.08 ± 0.2 | 135 ± 25 | 85.7 ± 4.67ξ |
| 500 | 1.21 ± 0.17 | 203 ± 43 | 78.5 ± 7.6#,& | |
| Stems (ME-SHv) | 1000 | 1.27 ± 0.22 | 236 ± 52 | 74.9 ± 9.10# |
| 500 | 0.99 ± 0.06 | 351 ± 9 | 63.3 ± 4.46* | |
| Heliopsis sinaloensis | ||||
| Leaves (ME-LHs) | 1000 | 1.14 ± 0.16 | 158 ± 3 | 83.4 ± 2.76&,ξ |
| 500 | 0.16 ± 0.3 | 243 ± 22 | 74.4 ± 6.03# | |
| Stems (ME-SHs) | 1000 | 1.28 ± 0.46 | 170 ± 4 | 82.1 ± 3.05&,ξ |
| 500 | 1.26 ± 0.23 | 227 ± 15 | 76.4 ± 1.94# | |
| 1-NP [100 ng/plate] | 965 ± 142 |
Results are the mean ± standard deviation of two independent experiments per triplicate.
Inhibition of the 1-NP (100 ng/tube) mutagenicity by the corresponding ME. Different symbols show significant differences (LSD = 5.67 p < 0.0001). The number of colonies due to spontaneous reversion was 69 ± 16.
The 1-NP treatment of S.typhimurium YG1024 exhibited a dose–response effect. The chosen concentration of 1-NP for the antimutagenicity evaluation was 100 ng/tube, because the number of induced revertants at this concentration was five times higher than that of spontaneous revertants.
The ME showed strong inhibition of the 1-NP mutagenicity (63–86%) at both concentrations evaluated (500 and 1000 μg/plate), and their activities differed significantly between the plants and tissues (p < 0.05) (Table 4). The highest antimutagenic activity was observed in the extract of H. vegae leaves (ME-LHv) and the lowest in the extract of H. vegae stems (ME-SHv).
Antimicrobial activity
The ME (1 mg/mL) of H. vegae and H. sinaloensis were not active against the evaluated human pathogenic bacteria and Giardia lamblia.
Correlation between biological activities and phenolics content
The total phenolics content (TP) showed high positive correlations with the antioxidant activity by DPPH (r = 0.8798, p = 0.0040), ABTS (r = 0.9246, p = 0.0010) and ORAC (r = 0.9965, p < 0.0001), but no significant correlation was found with the AA obtained by using the β-carotene discoloration method (r = 0.4896, p = 0.2182). TP also showed a positive correlation with the antimutagenic activity, but in the limit of significance (r = 0.7030, p = 0.052). However, when the correlation analysis was done with the content of the main phenolics determined by HPLC, the biological activities were neither correlated with flavonoids (0.02 ≤ r ≤ 0.54) nor with phenolic acids (0.01 ≤ r ≤ 0.68); moreover, the sum of flavonoids and phenolics acids for each extract did not correlate with the biological activities (0.16 ≤ r ≤ 0.58).
Toxicity
In the A. salina assay, the ME-LHv showed minimal toxicity (LC50 = 807.11 ± 145.6 μg/mL), and the ME-SHv was non-toxic (LC50 > 2000 μg/mL). In agreement with these results, we observed during field work that Helicteres vegae plants were consumed by cattle. On the other hand, the ME of H. sinaloensis showed high toxicity (ME-LHs, LC50 = 94.9 ± 1.7 μg/mL; ME-SHs, LC50 = 89.03 ± 4.42 μg/mL).
Discussion
Phytochemical analysis
The phytochemicals found in the studied species were similar to those found in related plants. Heliopsis oppositifolia has flavonoids, tannins, triterpenes/steroids and alkaloids in the aerial parts (Sanabria-Galindo et al. 1997). The fruit of Helicteres isora contains carbohydrates, proteins, tannins, phenolics and steroids (Tambekar et al. 2008); whereas the root has tannins, phenolics, amino acids, carbohydrates, phytosterols, triterpenoids and alkaloids (Tiwari et al. 2010). The analysis of our ME did not show the presence of alkaloids, perhaps because they are absent or below the limit of detection.
Phenolics determined by colorimetric methods
The differences in TP found in our ME were partially associated with differences in the content of tannins; ME-SHv showed the highest content of condensed tannins (603.71 ± 1.11 mg CE/100 g d.w.) and TP (2245.82 ± 21.45 mg GAE/100 g d.w.). However, the TP content of the ME-SHv was lower than that reported for fruits of Helicteres isora (2600 mg TAE/100 g d.w., as tannic acid equivalents) (Loganayaki et al. 2013).
Phenolics determined by HPLC-DAD-ESI-MS
The flavonoid F1 (tR = 32.03 min) was only detected in H. vegae (ME-LHv and ME-SHv). Mass spectrometric data were: molecular ion 593 [M − H]−; MS2m/z 285 [(M − H) − 146 − 162]− (base peak) that corresponds to kaempferol aglycone ion [Y0 − H]−; and m/z 447 [(M − H) − 146]− identified as kaempferol 7-O-hexoside ion (Figure 2). A fragment of 162 U is characteristic of hexosides, whereas 146 U could be coumaroyl or rhamnosyl; coumaroyl was confirmed with the fragment m/z 307 [coumaroylhexoside − H]−. These data coincide with those previously published by Gouveia and Castilho (2010); likewise, MS3 of m/z 285 gave the fragments m/z 257, 255, 229, and 151 that have been reported for kaempferol (Gouveia & Castilho 2010); moreover, the identity of the kaempferol aglycone was validated with the MS data of the commercial standard. It is known that flavonols, such as kaempferol, usually present glycosyl substitutions in the 3-OH and 7-OH positions. In the MS2, the aglycone ion m/z 285 was more abundant than the aglycone radical ion m/z 284, and the spectra showed an intense peak for m/z 257 [Y0 − CO]−; these results agree with the data published by Ablajan et al. (2006), who mentioned that substitution is in 7-OH. Consequently, F1 was identified as kaempferol 7-O-coumaroylhexoside.
The flavonoids F2 (tR = 27.92 min) and F3 (tR = 28.47 min) of the H. sinaloensis leaves (ME-LHs) showed the same ion m/z 593 [M − H]−. MS2 showed fragments of m/z 447 [(M − H) − 146]− and m/z 285 [(M − H) − 146 − 162]− (Figure 1S) assigned to rhamnosyl and hexosyl units, respectively; rhamnosyl was assigned based on the absence of m/z 307 (Gouveia & Castilho 2010). Considering the fragmentation pattern, previous reported data (Gouveia & Castilho 2010), and the MS3 fragmentation of ion m/z 285, the results were similar to those of the commercial standard kaempferol. Therefore, F2 and F3 were identified as kaempferol 7-O-rhamnosylhexoside. The differences in the retention times and maxima of absorption of F2 and F3 could be associated with differences in the substituent sugars. This is the first report about the identification of phenolics in the genus Heliopsis.
The phenolic acid P1 (tR = 28.93 min) showed the following MS data: molecular ion m/z 359 [M − H]− (Figure 2S); MS2 produced fragments of m/z 223, 197, 179 and 161; and MS3 of m/z 197 showed an ion of m/z 135 that corresponded with the fragmentation of the commercial standard for caffeic acid. These data were compared with those in the literature, and P1 was identified as rosmarinic acid (Guan et al. 2014). This compound is present in Helicteres isora (Satake et al. 1999).
The phenolic acid P2 (tR = 29.94 min) was found in H. vegae stems (ME-SHv) and showed the following MS data: molecular ion m/z 537 [M − H]− (Figure 3S); MS2 produced a fragment of m/z 493 [(M − H) − 44]− that was associated with the loss of a carboxylic group, characteristic of phenolics (Nowacka et al. 2014); MS3 showed the ion m/z 359 [(M − H] − 178]−, characteristic of rosmarinic acid; and MS4 of m/z 359 (i.e., 223, 197, 179, 161, and 135) validated its identity (Guan et al. 2014). Taking into account the UV spectra, the MSn data and previous reports, P2 was tentatively identified as 3′-O-(8″-Z-caffeoyl) rosmarinic acid.
UV spectra of P3, P4, P5 and P6 suggested they are phenolics, but the MS analysis did not allow us to identify them (Table 2). They showed MS ions in the range of m/z 785–1369; however, a proper MSn fragmentation was not acquired under the analyzed conditions.
Antioxidant activity
The antioxidant activity (AA) of ME-SHv by DPPH was higher than that reported previously in ME of Helicteres isora fruits, whereas the opposite was observed for the AA by ABTS (Loganayaki et al. 2013). In addition, the AA of ME-SHv by the βCD method was similar to that of the aqueous extract of Helicteres isora fruits (Suthar et al. 2009). The high AA of the ME-SHv could be due to its content of TP and tannins. The correlation between AA and TP content has been previously reported, e.g., for flaxseed products (r2 = 0.963, p < 0.01) (Velioglu et al. 1998) and for three species of Echeveria (r = 1.00, p = 0.03) (López-Angulo et al. 2014). Our results showed a significant correlation between AA and TP determined by the Folin-Ciocalteu spectrophotometric method, but not between AA and the content of the main phenolics determined by HPLC. Thus, the AA could be due to compounds of other families, to phenolics in minor quantities in the ME, or to synergic effects.
Rosmarinic acid (P1) and its derivative (P3) have shown good AA (Cervellati et al. 2002; Zhu et al. 2014; Govindaraj & Sorimuthu Pillai 2015). The ME from the stem of H. vegae (ME-SHv) showed the highest content of these compounds and the highest AA. Therefore, rosmarinic acid and its derivative appear to have an important role in the AA of the ME of Helicteres vegae.
Antimutagenic activity
ME of the studied plants were strong antimutagens with an inhibition of 1-NP mutagenicity in the range of 60–90% (Wall et al. 1988). Compared with the antimutagenicity values reported for plants using the same strain and mutagen, our results are very promising; the mutagenicity inhibition by ME of H. vegae and H. sinaloensis were higher than those obtained for the aqueous (500 μg/plate, 53%) and ME (1000 μg/plate, 32%) of Randia echinocarpa fruits (Santos-Cervantes et al. 2007; Cano-Campos et al. 2011). Moreover, these values were also higher than those reported for affinin extracted from the roots of Heliopsis longipes (TA98 strain, 2-aminoanthracene at 25 and 50 μg/plate) (Arriaga-Alba et al. 2013). The YG1024 strain used in the present study is derived from the TA98 strain (Watanabe et al. 1994).
The antimutagenic activity of rosmarinic acid was previously determined using S.typhimurium TA-100 and the mutagens sodium azide and N-methyl-N′-nitro-N-nitrosoguanidine (Vattem et al. 2006); the antimutagenicity of this compound was associated with modulation of redox conditions in the bacteria. In another report, rosmarinic acid reduced the risk of chromosome damage induced by doxorubicin in the micronucleus assay in mice (Andrade et al. 2008). This suggests that rosmarinic acid is partially responsible for the antimutagenicity of the ME of H. vegae. However, ME-LHv and ME-SHv showed similar activities but different contents of this compound; additional studies are necessary to elucidate other compounds contributing to the antimutagenicity of H. vegae extracts.
Antimicrobial activity
ME (1 mg/mL) of Helicteres vegae and Heliopsis sinaloensis were not active against the evaluated human pathogens (bacteria and G. lamblia), because plant extracts with MIC values higher than 1 mg/mL (antibacterial assay) and IC50 values higher than 500 μg/mL (anti-Giardia assay) are considered inactive (Rios & Recio 2005; Amaral et al. 2006). Nevertheless, some researchers have reported antimicrobial activity for other species of Helicteres (Truiti et al. 2005; Tambekar et al. 2008; Gairola et al. 2013) and Heliopsis (Gutierrez-Lugo et al. 1996).
Toxicity
The toxicity of ME from Helicteres vegae was slight (ME-LHv) or null (ME-SHv) in the A. salina model, but it was high for H. sinaloensis (ME-LHs and ME-SHs). Previous studies showed that the ME of Heliopsis longipes roots was more cytotoxic (LC50 = 58.65 μg/mL) than our extracts in this assay (Gutierrez-Lugo et al. 1996). Artemia salina assay is commonly used to establish the toxicity of plant extracts and to screen for antitumoral extracts and compounds (Meyer et al. 1982; Ajoy & Padma 2013; Tulsi et al. 2014). Thus, ME of both plants have the potential of being antitumoral agents.
Conclusions
The ME of Helicteres vegae and Heliopsis sinaloensis showed high antioxidant and antimutagenic effects but no antimicrobial activity. The main phenolics in these plants were flavonoid derivatives of kaempferol and phenolic acid derivatives of caffeic acid (e.g., rosmarinic acid). The content of the main phenolics did not fully explain the antioxidant and antimutagenic activities, but the contribution of the rosmarinic acid derivatives could play an important role. In general, H. vegae extracts were non-toxic, whereas H. sinaloensis extracts showed selective toxicity: high toxicity was shown in the Artemia salina assay, and they were nontoxic in the Ames assay and against the evaluated microorganisms. Our results suggest that H. vegae and H. sinaloensis could be a source of novel therapeutics or supplements for the treatment and prevention of chronic-degenerative diseases.
Supplementary Material
Funding Statement
This work was supported by the CONACYT [grant number CB201 0-156645-Z] Mexico and PROFAPI (Autonomous University of Sinaloa) [grant number PROFAPI2014-062].
Acknowledgements
Authors acknowledge the technical assistance of Yesmi Patricia Ahumada-Santos (School of Chemical and Biological Sciences, Autonomous University of Sinaloa). As well as the language/grammar (US English) review by MVZ Nadia Gallardo Romero (Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, US).
Disclosure statement
The authors report no declarations of interest.
References
- Ablajan K, Abliz Z, Shang XY, He JM, Zhang RP, Shi JG.. 2006. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J Mass Spectrom. 41:352–360. [DOI] [PubMed] [Google Scholar]
- Ahumada-Santos YP, Montes-Avila J, Uribe-Beltrán MJ, Díaz-Camacho SP, López-Angulo G, Vega-Aviña R, López-Valenzuela JÁ, Heredia JB, Delgado-Vargas F.. 2013. Chemical characterization, antioxidant and antibacterial activities of six Agave species from Sinaloa, Mexico. Ind Crops Prod. 49:143–149. [Google Scholar]
- Ajoy G, Padma C.. 2013. Brine shrimp cytotoxic activity of 50% alcoholic extract of Croton bonplandianum Baill. Asian J Pharm Clin Res. 6:40–41. [Google Scholar]
- Amaral MF, Ribeiro SM, Babosa-Filho JM, Reis AS, Nascimento FR, Macedo RO.. 2006. Plants and chemical constituents with giardicidal activity. Braz J Pharmacogn. 16:696–720. [Google Scholar]
- Andrade FM, Fernandez AL, Andrade FR, Cunha WR, Tavares DC.. 2008. Antimutagenicity of rosmarinic acid in Swiss mice evaluated by the micronucleus assay. Mutat Res. 657:150–154. [DOI] [PubMed] [Google Scholar]
- Arriaga-Alba M, Rios MY, Deciga-Campos M.. 2013. Antimutagenic properties of affinin isolated from Heliopsis longipes extract. Pharm Biol. 51:1035–1039. [DOI] [PubMed] [Google Scholar]
- Bae HD, McAllister TA, Muir AD, Yanke LJ, Bassendowski KA, Cheng KJ.. 1993. Selection of a method of condensed tannin analysis for studies with rumen bacteria. J Agric Food Chem. 41:1256–1260. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C.. 1995. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci Technol. 28:25–30. [Google Scholar]
- Calzada F, Meckes M, Cedillo-Rivera R, Tapia-Contreras A, Mata R.. 1998. Screening of mexican medicinal plants for antiprotozoal activity. Pharm Biol. 36:305–309. [Google Scholar]
- Cano-Campos MC, Díaz-Camacho SP, Uribe-Beltrán MJ, López-Angulo G, Montes-Avila J, Paredes-López O, Delgado-Vargas F.. 2011. Bio-guided fractionation of the antimutagenic activity of methanolic extract from the fruit of Randia echinocarpa (Sessé et Mociño) against 1-nitropyrene. Food Res Int. 44:3087–3093. [Google Scholar]
- Cervellati R, Renzulli C, Guerra MC, Speroni E.. 2002. Evaluation of antioxidant activity of some natural polyphenolic compounds using the Briggs–Rauscher reaction method. J Agric Food Chem. 50:7504–7509. [DOI] [PubMed] [Google Scholar]
- CLSI 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard, 6th edition CLSI document M07-A9. Wayne (PA): Clinical and Laboratory Standards Institute; p. 68. [Google Scholar]
- Gairola S, Sharma J, Gaur RD, Siddiqi TO, Painuli RM.. 2013. Plants used for treatment of dysentery and diarrhoea by the Bhoxa community of district Dehradun, Uttarakhand, India. J Ethnopharmacol. 150:989–1006. [PubMed] [Google Scholar]
- Gouveia SC, Castilho PC.. 2010. Characterization of phenolic compounds in Helichrysum melaleucum by high-performance liquid chromatography with on-line ultraviolet and mass spectrometry detection. Rapid Commun Mass Spectrom. 24:1851–1868. [DOI] [PubMed] [Google Scholar]
- Govindaraj J, Sorimuthu Pillai S.. 2015. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic rats. Mol Cell Biochem. 404:143–159. [DOI] [PubMed] [Google Scholar]
- Guan Z, Li SC, Lin ZT, Yang RN, Zhao Y, Liu JC, Yang SM, Chen AL.. 2014. Identification and quantitation of phenolic compounds from the seed and pomace of Perilla frutescens using HPLC/PDA and HPLC-ESI/QTOF/MS/MS. Phytochem Anal. 25:508–513. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Lugo MT, Barrientos-Benitez T, Luna B, Ramirez-Gama RM, Bye R, Linares E, Mata R.. 1996. Antimicrobial and cytotoxic activities of some crude drug extracts from Mexican Medicinal Plants. Phytomedicine. 2:341–347. [DOI] [PubMed] [Google Scholar]
- Harborne JB.1980. Phytochemical methods a guide to modern techniques of plant analysis. London, UK: Chapman and Hall. [Google Scholar]
- Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL.. 2002. High-throughput assay of Oxygen Radical Absorbance Capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 50:4437–4444. [DOI] [PubMed] [Google Scholar]
- Loganayaki N, Siddhuraju P, Manian S.. 2013. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol Mysore. 50:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Angulo G, Montes-Avila J, Díaz-Camacho SP, Vega-Aviña R, Ahumada-Santos YP, Delgado-Vargas F.. 2014. Chemical composition and antioxidant, α-glucosidase inhibitory and antibacterial activities of three Echeveria DC. species from Mexico. Arabian J Chem. 10.1016/j.arabjc.2014.11.050. [DOI] [Google Scholar]
- Maron DM, Ames BN.. 1983. Revised methods for the Salmonella mutagenicity test. Mutat Res. 113:173–215. [DOI] [PubMed] [Google Scholar]
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL.. 1982. Brine shrimp: a convenient general bioassay for active plant constituents. J Med Plant Res. 45:31–34. [PubMed] [Google Scholar]
- Molina-Torres J, Garcı´a-Chávez A, Ramírez-Chávez E.. 1999. Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: affinin and capsaicin. J Ethnopharmacol. 64:241–248. [DOI] [PubMed] [Google Scholar]
- Nowacka N, Nowak R, Drozd M, Olech M, Los R, Malm A.. 2014. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT – Food Sci Technol. 59:689–694. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C.. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237. [DOI] [PubMed] [Google Scholar]
- Rios JL, Recio MC.. 2005. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 100:80–84. [DOI] [PubMed] [Google Scholar]
- Rosa RM, Melecchi MI, da Costa Halmenschlager R, Abad FC, Simoni CR, Caramao EB, Henriques JA, Saffi J, de Paula Ramos AL.. 2006. Antioxidant and antimutagenic properties of Hibiscus tiliaceus L. methanolic extract. J Agric Food Chem. 54:7324–7330. [DOI] [PubMed] [Google Scholar]
- Sanabria-Galindo A, López SI, Gualdrón R.. 1997. Estudio fitoquímico preliminar y letalidad sobre Artemia salina de plantas colombianas. Rev Colomb Cienc Quim Farm. 26:15–19. [Google Scholar]
- Santos-Cervantes ME, Ibarra-Zazueta ME, Loarca-Pina G, Paredes-Lopez O, Delgado-Vargas F.. 2007. Antioxidant and antimutagenic activities of Randia echinocarpa fruit. Plant Foods Hum Nutr. 62:71–77. [DOI] [PubMed] [Google Scholar]
- Satake T, Kamiya K, Saiki Y, Hama T, Fujimoto U, Kitanaka S, Kimura Y, Uzawa J, Endang H, Umar M.. 1999. Studies on the constituents of fruits of Helicteres isora L. Chem Pharm Bull. 47:1444–1447. [Google Scholar]
- Solis PN, Wright CW, Anderson MM, Gupta MP, Phillipson JD.. 1993. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 59:250–252. [DOI] [PubMed] [Google Scholar]
- Suthar M, Rathore GS, Pareek A.. 2009. Antioxidant and antidiabetic activity of Helicteres isora (L.) fruits. Indian J Pharm Sci 71:695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambekar DH, Khante BS, Panzade BK, Dahikar SB, Banginwar YS.. 2008. Evaluation of phytochemical and antibacterial potencial of Helicteres isora L. fruits against enteric bacterial pathogens. Afr J Tradit Complement Altern Med. 5:290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, Tiwari A, Madhavan V.. 2010. Preliminary phytochemical analysis, HPTLC studies and antipyretic activity of alcohol and aqueous extract of Helicteres isora L. root. Int J Pharm Pharm Sci. 2:74–79. [Google Scholar]
- Truiti MCT, Ferreira ICP, Zamuner MLM, Nakamura CV, Sarragiotto MH, Souza MC.. 2005. Antiprotozoal and molluscicidal activities of five Brazilian plants. Braz J Med Biol Res. 38:1873–1878. [DOI] [PubMed] [Google Scholar]
- Tulsi NKS, Manjunath K, Chandrashekaran S, Koppad AV.. 2014. Cytotoxic and antitumour activity of methanolic extracts of medicinally important plants. Int J Pharma Bio Sci. 5:344–353. [Google Scholar]
- Varghese E, Pappachen LS, Sathia SN.. 2012. Isolation and evaluation of antimicrobial properties of isolated phytoconstituents of fruits of Helicteres isora Linn. Res J Pharm Biol Chem Sci. 3:959–964. [Google Scholar]
- Vattem DA, JAng HD, Levin R, Shetty K.. 2006. Synergism of cranberry phenolics ellagic acid and rosmarinic acid for antimutagenic and DNA protection functions. J Food Biochem. 30:98–116. [Google Scholar]
- Velioglu YS, Mazza G, Gao L, Oomah BD.. 1998. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 46:4113–4117. [Google Scholar]
- Wall ME, Wani MC, Hughes TJ, Taylor H.. 1988. Plant antimutagenic agents, 1. General bioassay and isolation procedures. J Nat Prod. 51:866–873. [DOI] [PubMed] [Google Scholar]
- Wang M, Tsao R, Zhang S, Dong Z, Yang R, Gong J, Pei Y.. 2006. Antioxidant activity, mutagenicity/anti-mutagenicity, and clastogenicity/anti-clastogenicity of lutein from marigold flowers. Food Chem Toxicol. 44:1522–1529. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Igarashi T, Kaminuma T, Sofuni T, Nohmi T.. 1994. N-Hydroxyarylamine O-acetyltransferase of Salmonella typhimurium: proposal for a common catalytic mechanism of arylamine acetyltransferase enzymes. Environ Health Perspect. 102:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawalikar N, Bhowal M, Rudra J.. 2014. Preliminary phytochemical analysis of Pentapetes phoenicea L. IOSR J Pharm Biol Sci. 9:36–39. [Google Scholar]
- Zhu F, Asada T, Sato A, Koi Y, Nishiwaki H, Tamura H.. 2014. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J Agric Food Chem. 62:885–892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.