Abstract
Context: Numerous etiological studies have established positive clinical association between hypertension and vascular dementia (VaD). Lactobacillus paracasei subsp. paracasei NTU 101-fermented products have been shown to decrease vascular risk factors such as hypertension, atherosclerosis, hyperlipidemia and obesity.
Objective: This study investigated the effect of ethanol extract of Lactobacillus paracasei subsp. paracasei NTU 101-fermented products (NTU101F) in hypertension-induced VaD in rats.
Materials and methods: Hypertension was promoted by subcutaneous injection of deoxycorticosterone acetate (DOCA, 25 mg/kg body weight/day, twice a week) and substitution of drinking water with 1.0% NaCl and 0.2% KCl. The NTU101F groups (0.5, 1.0, and 5.0) administered NTU101F at the concentrations 11, 22, and 110 mg/kg body weight/day, respectively, starting from day 51 day of DOCA-salt treatment. Morris water maze (MWM) was used for testing learning and memory. Different biochemical estimations were used to assess oxidative stress and inflammatory response in hippocampus.
Results: Oral administration of NTU101F in DOCA-salt hypertension-induced VaD rats resulted in a significant decrease in blood pressure by 18.3–23.2% (p < 0.001), which was regulated by increasing eNOS density (about 3-fold) in the aorta, promoting NO production, and decreasing of matrix metallopeptidase 9 activity (about 2-fold) in the hippocampus, in addition to improve the kidney function and structure, decrease escape latency and increase the times spent in the target quadrant by 23.5–27.8% (p < 0.05).
Conclusion: Overall, our findings suggest that NTU101F could exert neuroprotection in the brain and attenuate hypertension-induced VaD.
Keywords: Fermentation, deoxycorticosterone acetate, vascular dementia, hypertension
Introduction
Hypertension is by far the most important risk factor of heart disease and stroke (Johansson 1999). The long-standing effect of hypertension on arterial luminal caliber accounts for hypoperfusion to vital areas of the brain controlling memory function (Chiang et al. 2012). Although the knowledge of this field is accumulating, the mechanisms underlying the negative effects of high blood pressure on cognitive abilities that including attention, learning, and memory are still illusive (Vaitkevicius et al. 1993). Currently, the possible mechanisms by which hypertension could increase the risk of dementia include compromised vasoreactivity and hypoperfusion, damage to the blood–brain barrier, oxidative stress, stroke, occlusion and degeneration of cerebral capillaries (Gentile et al. 2009). Vascular dementia (VaD), the second leading cause of dementia in the world after Alzheimer’s disease, occurs as a result of stroke and is characterized by the impairment in cognitive function due to vascular lesion and infarction (Al-Qazzaz et al. 2014). Clinical studies have shown that primary aldosteronism, i.e. increased production of aldosterone, which leads to a decrease in the renin to aldosterone ratio and potassium levels in the blood, could be a significant cause of hypertension (Krishnan & MacDonald 2004; Kotlyar et al. 2006; Tomaschitz et al. 2010). Mineralocorticoids such as deoxycorticosterone acetate (DOCA) have long been investigated as hypertension-inducing compounds (Berecek et al. 1982; Fernandes et al. 2002). Mineralocorticoids promote sodium retention in the kidneys, leading to volume expansion and increased arterial blood pressure, which facilitates sodium excretion (Cowley et al. 1986; DiBona 1994). The treatment of rats with DOCA in combination with sodium chloride induces hypertension and mimics most of the changes characteristic for chronic cardiovascular remodelling in humans, including cardiac and vascular hypertrophy, fibrosis, and impaired electrical conductivity (Weber et al. 1993, 1996). Therefore, DOCA-salt-treated rats provide a reliable model for studying hypertension-induced physiological changes and testing the effects of natural and synthetic materials with beneficial antioxidant and anti-hypertensive activities on cardiovascular repair (Sun 2009; Iyer et al. 2010).
Lactobacillus paracasei subsp. paracasei NTU 101 (NTU 101) has been isolated from faeces of a new born infant in Taiwan (Lin et al. 2004). Our previous studies indicated that the NTU 101-fermented products decreased vascular risk factors such as hypertension, atherosclerosis, hyperlipidemia and obesity (Chiu et al. 2006; Tsai et al. 2009, 2014; Liu et al. 2011; Lee et al. 2013; Cheng et al. 2015). NTU 101 has also demonstrated potent antioxidant activity in vitro (Liu & Pan 2010), while its fermentation products and hydrolysates have been effective in preventing hyperlipidaemia-induced oxidative stress and modulating the innate and adaptive immune systems and inflammatory response in animal models (Tsai et al. 2009; Chiang et al. 2012).
Gut microbiota and probiotics secrete signalling molecules that can regulate important functional mechanisms involved in brain activity, the mucosal immune system, and enterochromaffin cell-mediated vagal activation (Rhee et al. 2009; Cryan & Dinan 2012; Nicholson et al. 2012; Tillisch et al. 2013). Bercik et al. (2011) found that Bifidobacterium longum-fermented medium modifies excitability of enteric neurons and alleviate anxiety-like behaviour in mice, suggesting that probiotic fermentation products may signal to the central nervous system (Bercik et al. 2011). Chronic ingestion of a probiotic-containing fermented milk product could modulate the activity of affected brain regions controlling emotional responsiveness in women (Tillisch et al. 2013). These results suggest that probiotic bacteria, through their fermentation-derived bioactive compounds, could exert significant beneficial effects on the nervous system.
In view of these data, we conducted this study to investigate the potential of NTU101F on hypertension-induced VaD using the DOCA-salt rat model.
Materials and methods
Adult male Wistar rats weighing 200–250 g (BioLasco Co., Taipei, Taiwan) were individually housed in stainless steel screen-bottomed cages with free access to water and standard laboratory pellet chow (Ralston Purina, St. Louis, MO). The animals were maintained in the conditions of 12 h light/dark, relative humidity of 60%, and temperature of 25 °C, according to the guidelines of the Animal Protection Law amended on 29 June 2011 Hua-Zong-(1)-Yi-Tzi 10000136211, Council of Agriculture, Executive Yuan, Taiwan, ROC. The animals were acclimatized to the laboratory conditions for one week prior to behavioural testing. The protocol of the study was reviewed and approved by the Animal Care and Research Ethics Committee of Fu Jen Catholic University (IACUC approval No: A10384).
Preparation and extraction of NTU101F
Reconstituted skimmed milk (25%, w/v, Anchor, Auckland, New Zealand) supplemented with 5% (w/v) filter-sterilized glucose was inoculated with NTU 101 and incubated at 38 °C for 5 days. Fermentation products were freeze-dried (SDF-25 freeze-dryer, Chang Jung Business Co., Feng-Jen, Taiwan), extracted with ethanol in a rotary shaker at 150 rpm for 1 h at 37 °C, and filtered through Whatman No. 42 filter paper (Fisher Scientific, Loughborough, UK). NTU101F was concentrated to remove residual solvent and kept refrigerated until use.
DOCA-salt hypertension-induced vascular dementia model
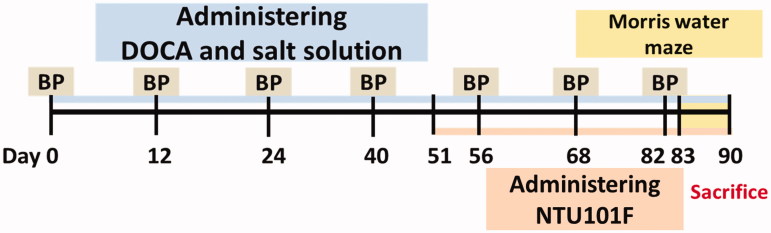
DOCA (Sigma Aldrich, St. Louis, MO) was mixed with olive oil and administered subcutaneously as previously described (Sharma & Singh 2012a,b, 2013); the experimental schedule is shown in Figure 1. Hypertension and subsequent VaD in rats were induced by DOCA injected at the dose of 25 mg/kg twice weekly and salt delivered by replacing drinking water with 1.0% NaCl and 0.2% KCl, for 90 days (Bockman et al. 1992; Sharma & Singh 2013), hypertension was observed in approximately 50 days. After 86 days, the animals were subjected to biochemical and behavioural tests. Mean arterial blood pressure was measured by tail-cuff method (BP-2000 Blood Pressure Analysis, Visitech System, Inc., Apex, NC).
Figure 1.
Experimental schedule of DOCA-salt-induced hypertension and vascular dementia. BP: blood pressure; DOCA-salt: deoxycorticosterone acetate and salt solution; NTU101F: ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented products.
To investigate the effects of NTU101F on hypertension-induced VaD, it was administered orally by a canulla for the last 40 days of DOCA-salt treatment. Rats were divided into five groups (n = 6): the normal control (NC) group treated with 0.9% saline; the hypertension group, receiving DOCA and salt for 90 days; and the NTU101F groups (0.5, 1.0 and 5.0) administered NTU101F at the concentrations 11, 22 and 110 mg/kg body weight/day, respectively, starting from day 51 of DOCA-salt treatment. All the reagents were freshly prepared before use.
Biochemical and histological assessment
Plasma aldosterone level was evaluated using a Wallac 2470 WIZARD2 gamma counter (PerkinElmer Life and Analytical Sciences, Waltham, MA).
For histopathological examination, the kidneys were weighed, grossly examined, fixed in 10% neutral-buffered formalin for one week, embedded in paraffin, and cut into 2 μm sections. The sections were stained with hematoxylin-eosin and Masson trichrome, respectively, and evaluated by light microscopy. For semi-quantitative grading, the severity of lesions was graded based on the standard criteria (Shackelford et al. 2002). 1 = minimal (< 10% of lesion); 2 = slight (11–25%); 3 = moderate (26–50%); 4 = moderate severe (51–75%); and 5 = severe high (76–100%).
To analyze vascular elastin distribution and nitric oxide synthase (eNOS) protein expression, kidney and aorta samples were embedded in paraffin, sectioned and stained with Verhoff’s stain or processed by immunohistochemistry (Puchtler & Waldrop 1979; Lee et al. 2000; Liu et al. 2015), respectively, and examined under a Nikon TS-100 microscope (Tokyo, Japan). The Motic Images 2000 software (Xiamen, China) was used to assess hypertensive changes in tissues.
Morris water maze test
Morris water maze is one of the most commonly used tests to assess hippocampal-dependent spatial learning and memory (Morris 1984; Sharma & Singh 2013). Experimental setting consisted of a black circular water tank (diameter, 140 cm; height, 45 cm) partially filled with water (25 °C) where a movable escape platform (diameter, 10 cm; height, 25 cm) was placed. The tank was divided into four quadrants where the points equally distant from the centre and edges of the platform were marked (Lee et al. 2010).
Reference memory testing was performed from day 83 to day 86 and included four continuous trials per day; probe testing was conducted after the 12th training trial. Experimental memory testing was performed from day 88 to day 90 and consisted of five trails per day (Lee et al. 2009). The end points were escape latency and swimming speed analyzed with the Target/2 system (Neuroscience Inc., Tokyo, Japan).
Brain preparation and biochemical analysis
After the completion of behavioural testing, rats were anesthetized by carbon dioxide inhalation and sacrificed. Blood was collected by cardiac puncture, and serum was obtained by allowing the blood to clot in serum separation tubes and centrifuging at 3000 g for 10 min. Plasma was obtained by collecting blood with syringes containing 5% heparin and 2% sodium citrate and centrifuging at 3000 g for 15 min. The aorta, kidney, and left part of the brain were cut and immediately fixed in 10% neutral-buffered formalin for histological analysis. The hippocampus was separated from the brain on ice. The blood and extraneous fragments were gently removed and hippocampus was flash-frozen in liquid nitrogen, and stored at −80 °C until use. Hippocampus tissue (100 mg) was homogenized using the FastPrep System (MP Biomedicals, Santa Ana, CA), suspended in 1 mL of ice-cold 0.1 M phosphate-buffered saline (PBS; pH 7.4) and centrifuged at 12,000 g for 30 min; the supernatant was collected and stored at −80 °C until use for measurement of cytokines and antioxidant enzymes.
The intensity of matrix metallopeptidase 9 (MMP-9) was determined by gelatin zymography. Equal amounts of sample proteins were loaded onto 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gels containing gelatin (Sigma) at 1 mg/mL. Gels were washed three times in 50 mM NaCl (pH 7.6), 10 mM CaCl2, and 5 mM ZnCl2 for more than 12 h at 37 °C without shaking before being subjected to Coomassie brilliant blue staining and destaining (Hiratsuka et al. 2002; Lee et al. 2007).
The activity of glutathione peroxidase (GPx) and catalase (CAT) were determined by a commercially available kit (Cayman, Ann Arbor, MI). The activity of superoxide dismutase (SOD) was measured using the SD125 kit (Randox Laboratories Ltd., Crumlin, UK). The concentration of malondialdehyde (MDA) was determined as an index of lipid peroxidation (Yagi 1976).
Interleukin-1β (IL-1β) and IL-6 levels were measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits from Peprotech (Rocky Hill, NJ) and BioLegend (San Diego, CA), respectively.
Statistical analysis
The data were presented as the means ± standard deviation (SD). Statistical significance of the results was determined by one-way analysis of variance (ANOVA) followed with Duncan’s test using the general linear model of the Statistical Package for Social Sciences (SPSS, version 19.0; Chicago, IL). The difference was calculated relative to the DOCA-salt group or normal control group, and p < 0.05 was considered statistically significant.
Results
Effect of NTU101F on arterial blood pressure
The treatment with DOCA-salt progressively elevated the tail-cuff pressure, which reached 168.7–176.1 mm Hg in hypertensive rats versus 121.5 mm Hg in control animals after eight weeks of treatment (p < 0.001; Table 1). However, oral administration of NTU101F (0.5, 1.0 and 5.0) reversed DOCA-salt-induced increase of blood pressure by 18.4, 18.3 and 23.2%, respectively, compared to the DOCA-salt group at 12-week (p < 0.001).
Table 1.
The effect of ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented product on arterial blood pressure in rats with hypertension-induced vascular dementia.
| Feeding time | Arterial blood pressure (mm Hg; mean ± SD, n = 6) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Week) | NC | DOCA-salt | NTU101F 0.5 | NTU101F 1.0 | NTU101F 5.0 | |||||
| 0 | 112.0 ± 6.8Aa | 112.4 ± 3.8Ac | 105.7 ± 8.0Ad | 112.2 ± 2.9Ae | 109.2 ± 5.4Ad | |||||
| 2 | 113.4 ± 7.0Aa | 129.7 ± 13.6Ac | 126.4 ± 5.0Ac | 131.9 ± 11.2Ade | 126.7 ± 5.1Ac | |||||
| 4 | 121.1 ± 11.1Aa | 153.9 ± 10.5Ab | 145.5 ± 5.8Abc | 147.1 ± 6.1Acd | 143.6 ± 3.8Abc | |||||
| 6 | 115.9 ± 6.4Ba | 173.2 ± 11.5Aab | 168.4 ± 9.7Aa | 171.0 ± 7.5Aab | 170.0 ± 10.6Aa | |||||
| 8 | 121.5 ± 5.4Ba | 176.1 ± 9.5Aa | 168.7 ± 11.5Aa | 173.8 ± 6.0Aa | 169.7 ± 3.8Aa | |||||
| 10 | 120.2 ± 5.7Ca | 185.0 ± 9.1Aa | 161.9 ± 15.0ABab | 158.4 ± 12.2Babc | 153.7 ± 11.4Bab | |||||
| 12 | 124.1 ± 5.6Ca | 185.1 ± 4.3Aa | 151.0 ± 10.2Bab | 151.2 ± 17.3Bbcd | 142.2 ± 11.5BCbc | |||||
NC: normal control rats; DOCA-salt: rats receiving subcutaneous injection of deoxycorticosterone acetate (DOCA, 25 mg/kg BW/day), and 1.0% NaCl and 0.2% KCl in drinking water; NTU101F 0.5, 1.0, and 5.0: DOCA-salt rats treated with 11, 22, and 110 mg/kg BW/day of ethanol extract of NTU 101-fermented product. Different lowercase letters in the same column and uppercase letters in the same row indicate significant difference (p < 0.001) according to Duncan’s multiple range test.
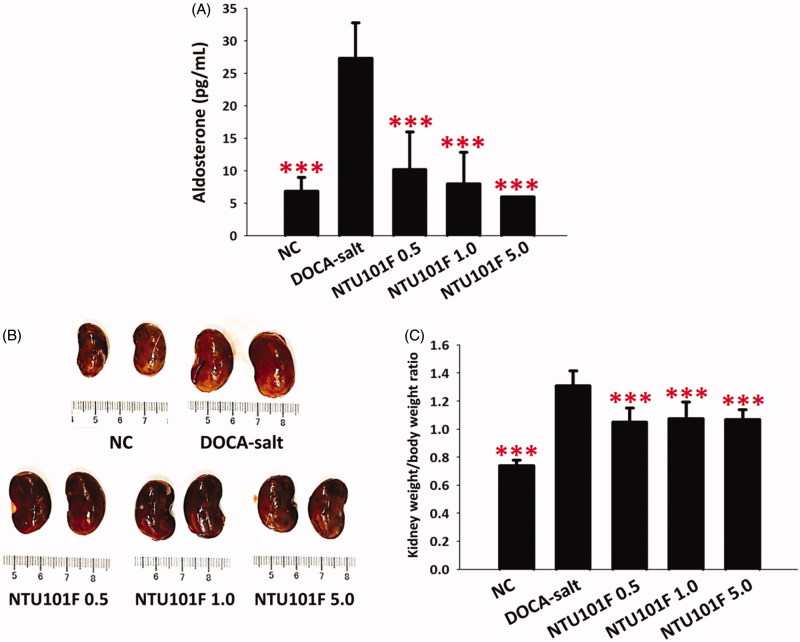
Effect of NTU101F on plasma aldosterone levels
Aldosterone levels in plasma of rats treated with DOCA-salt for 90 days were significantly (about 4-fold) increased in parallel with hypertension (Figure 2(A)). However, the treatment with NTU101F attenuated the increase in aldosterone levels compared to the DOCA-salt group (p < 0.001).
Figure 2.
The effect of ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented products on kidney function in rats with hypertension-induced vascular dementia. (A) Plasma aldosterone levels. (B) Representative images of the kidney. (C) Kidney weight relative to body weight (BW). NC: normal rats; DOCA-salt: rats receiving subcutaneous injection of deoxycorticosterone acetate (DOCA, 25 mg/kg BW/day), and 1.0% NaCl and 0.2% KCl in drinking water; NTU101F 0.5, 1.0, and 5.0: DOCA-salt rats treated with 11, 22, and 110 mg/kg BW/day, respectively, of ethanol extract of NTU 101-fermented products. The data are presented as the mean ± SD (n = 6). ***p < 0.001 versus DOCA-salt according to Duncan’s multiple range test.
Effect of NTU101F on kidney function and structure
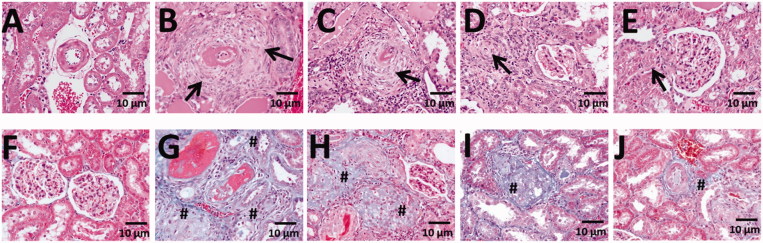
DOCA-salt administration resulted in significant increase of rat kidney weight (normalized to body weight), indicating hypertrophy of renal tissues (Figure 2(B) and (C)). However, NTU101F treatment inhibited DOCA-salt-induced kidney hypertrophy (p < 0.001), suggesting protective effects. Pathological analysis is summarized in Table 2. All kidneys from control rats were normal, while those from the DOCA-salt group showed arteriolopathy, accumulation of proteinaceous cast material in the tubular lumen, glomerulosclerosis and infiltration of inflammatory cells. The administration of NTU101F in all tested concentrations significantly reduced arteriolopathy, cast material in the tubular lumen, and fibrosis (p < 0.05); moreover, NTU101F at the highest concentration also alleviated glomerulosclerosis and inflammation (p < 0.05; Table 2, Figure 3).
Table 2.
Pathological scores of the kidneys from rats with hypertension-induced vascular dementia.
| Lesions | NC | DOCA-salt | NTU101F 0.5 | NTU101F 1.0 | NTU101F 5.0 |
|---|---|---|---|---|---|
| Arteriolopathy, focal | 0.0 ± 0.0c | 3.2 ± 0.4d | 2.0 ± 1.3a | 1.2 ± 1.0c | 1.2 ± 0.8c |
| Cast, hyaline, tubule, focal | 0.0 ± 0.0c | 3.0 ± 1.3d | 1.8 ± 1.0a | 1.5 ± 0.8b | 1.2 ± 0.4b |
| Glomerulosclerosis, focal | 0.0 ± 0.0c | 2.3 ± 0.5d | 1.7 ± 1.4 | 1.3 ± 1.2 | 1.0 ± 0.0a |
| Inflammation, focal | 0.0 ± 0.0c | 3.0 ± 0.6d | 2.2 ± 1.5 | 2.0 ± 1.4 | 1.5 ± 0.5a |
| Fibrosis, interstitial, focal | 0.0 ± 0.0c | 2.3 ± 0.5d | 1.2 ± 1.0a | 1.0 ± 1.3b | 0.3 ± 0.5c |
The meaning of abbreviations was shown in Table 1. Degree of lesions was graded from 1 to 5 depending on severity: 1 = minimal (< 1%); 2 = slight (1–25%); 3 = moderate (26–50%); 4 = moderate severe (51–75%); 5 = severe high (76–100%). The data are presented as the mean ± SD (n = 6).
p < 0.05.
p < 0.01.
p < 0.001 versus DOCA-salt.
p < 0.001 for DOCA-salt versus NC by Duncan’s multiple range test.
Figure 3.
The effect of ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented products on kidney structure in rats with hypertension-induced vascular dementia. (A–E) Hematoxylin-eosin-stained sections of the kidneys from rats treated with saline (A), DOCA-salt (B), NTU101F 0.5 (c), NTU101F 1.0 (D), and NTU101F 5.0 (E). (F–J) Masson’s trichrome-stained sections demonstrating collagen deposition (blue) in the kidneys from control (F), DOCA-salt (g), NTU101F 0.5 (H), NTU101F 1.0 (I), and NTU101F 5.0 (J) rats. Arrows and # mark inflammation and fibrosis, respectively (magnification, ×400). The meaning of abbreviations was shown in Figure. 1.
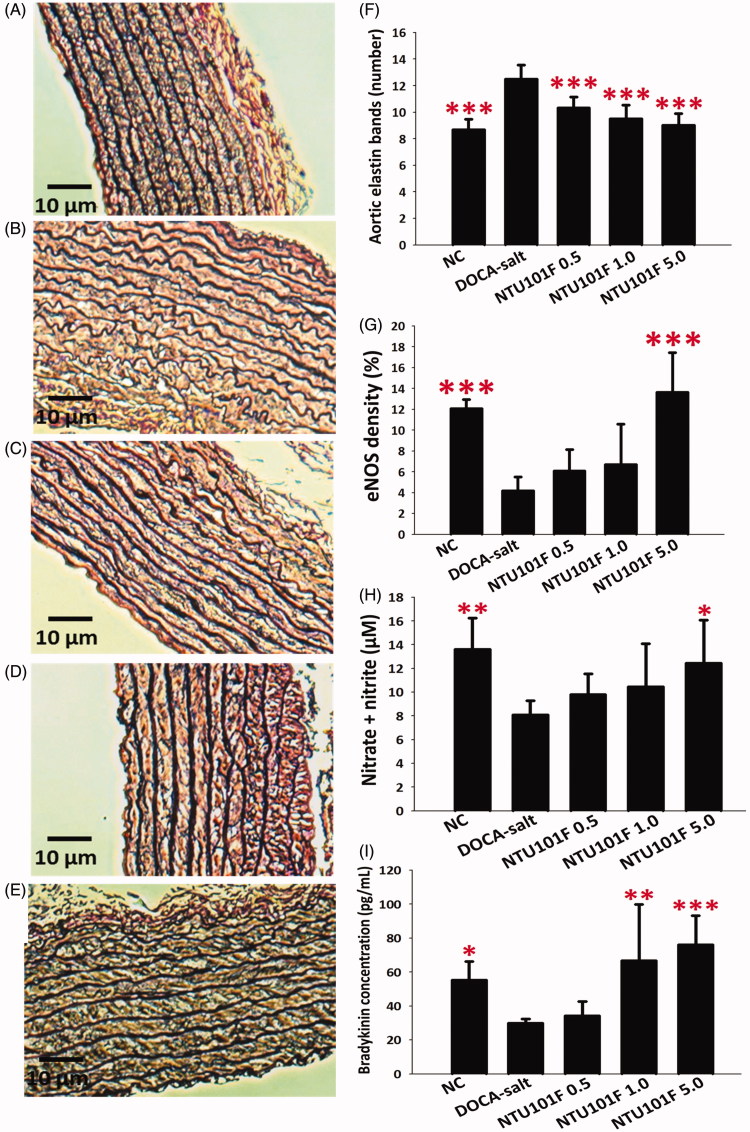
Effect of NTU101F on elastin arrangement and eNOS expression in the aorta
Elastin is a protein that maintains vascular elasticity and allows stretching of blood vessels. Figure 4(A–E) shows that DOCA-salt treatment caused disarrangement of aortic elastin compared to the control group, which was repaired dose-dependently by NTU101F administration. Moreover, NTU101F supplementation reduced the number of elastin bands in the arteries of hypertensive rats (p < 0.001; Figure 4(F)). In addition, immunohistochemistry analysis revealed that NTU101F in the high dose caused a significant (about 3-fold) increase in eNOS density within arcuate arteries compared to the DOCA-salt group (p < 0.001; Figure 4(G)). Moreover, DOCA-salt-treated rats had significantly decreased levels of NO, a vasodilators (NO and bradykinin) in the aorta compared to control rats (p < 0.05), while NTU101F reversed this effect (Figure 4(H,I)), which was consistent with the increase of eNOS density and reduction of hypertension.
Figure 4.
The effect of the ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented products on the aorta of rats with hypertension-induced vascular dementia. (A–E) Elastin expression in the aorta from rats treated with saline (A), DOCA-salt (B), NTU101F 0.5 (C), NTU101F 1.0 (D), and NTU101F 5.0 (E). The number of elastin bands (F), eNOS density (G), nitrate plus nitrite level (H), and bradykinin (I). Magnification, × 100. The meaning of abbreviations was shown in Figure 1. The data are presented as the mean ± SD (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 versus DOCA-salt according to Duncan’s multiple range test.
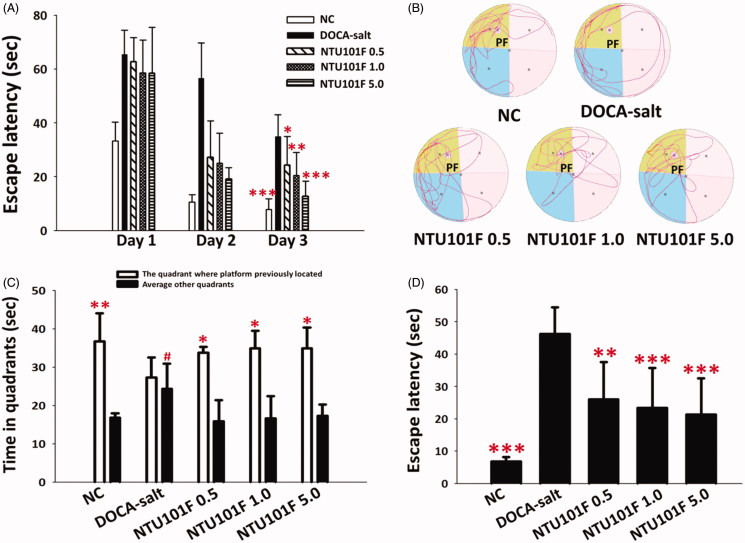
Effect of NTU101F on reference memory task
The rats showed a decrease in the escape latency time (ELT) at day 3 compared to day 1 (Figure 5(A)), which reflects the learning ability. However, a significant increase of the ELT at day 3 was observed for DOCA-salt-treated rats compared to control animals, indicating impairment of memory acquisition. However, NTU101F treatment repaired learning ability of the DOCA-salt rats, as evidenced by a significant reduction in the 3-day ELT (p < 0.05; Figure 5(A)); moreover, in rats treated with the highest NTU101F dose, the ELT was as short as that in the control group.
Figure 5.
The effect of ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented products on memory and learning ability of rats with hypertension-induced vascular dementia. (A) Reference memory task, (B) probe test locus (C) probe test, and (D) working memory task. The meaning of abbreviations was shown in Figure 1. The data are presented as the mean ± SD (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 versus DOCA-salt and #p < 0.05 versus NC according to Duncan’s multiple range test.
Effect of NTU101F on probe test
The escape platform was removed from the tank, and probe tests started after the reference memory tasks. A significant increase in time spent in the target quadrant (upper left) compared to that spent in other quadrants reflected normal retrieval (Figure 5B,C). Among the groups, DOCA-salt rats demonstrated the poorest test result, searching the target quadrant around the walls of the tank in a directionless manner (Figure 5(B)). On the contrary, all NTU101F-treated animals were more focused on the target quadrant in their escape pathway, which resulted in the increase of the times spent in the target quadrant, suggesting that NTU101F administration repaired the damage of the learning ability exerted by DOCA-salt-induced hypertension (Figure 5(C)).
Effect of NTU101F on working memory task
Short-term learning ability or working memory was evaluated based on the escape latency time. The results indicated that DOCA-salt treatment significantly weakened working memory, as evidenced by an increase in the escape latency time from 5 (control group) to 45 s (DOCA-salt group, p < 0.001). However, NTU101F-treated groups demonstrated a significantly shorter escape latency: about 25 s for the 0.5 NTU101F-treated group (p < 0.01), and about 20 s for the other NTU101F-treated groups (p < 0.001), indicating that NTU101F could ameliorate DOCA-salt-induced memory deficit (Figure 5(D)).
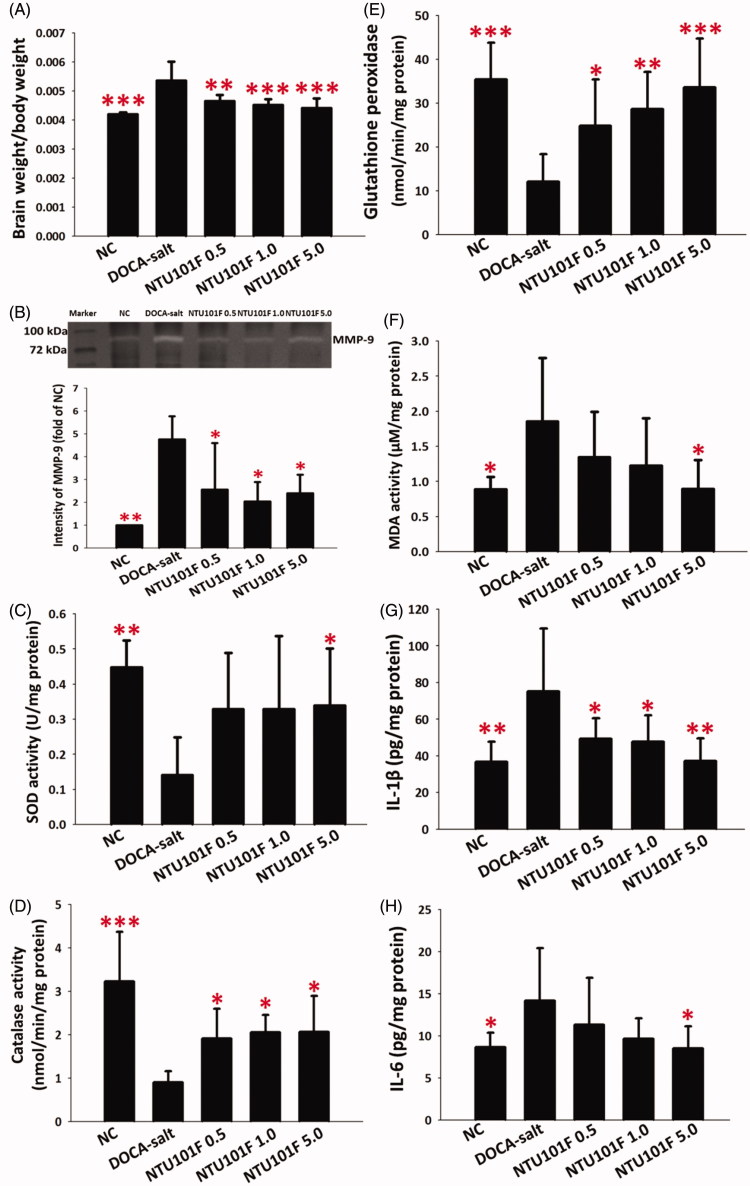
Effect of NTU101F on brain weight and MMP-9 activity
As shown in Figure 6(A), brain weight (normalized to body weight) in the DOCA-salt group was significantly increased compared to the control group (p < 0.001), but after NTU101F treatment it returned to the control level. Next, we measured the enzymatic activity of MMP-9 in the hippocampus by zymography and found that MMP-9 level in the DOCA-salt group was significantly higher than that in the control group (p < 0.01; Figure 6(B)). However, NTU101F feeding decreased MMP-9 activity in the DOCA-salt-treated animals (p < 0.05), although not to the level of the control group.
Figure 6.
The effect of ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented products on the brain body weight ratio (A), MMP-9 activity (B), and on the activity of antioxidant enzymes, lipid peroxidation, and the release of pro-inflammatory cytokines in the hippocampus of rats with hypertension-induced vascular dementia. (C–E) Enzymatic activity of superoxide dismutase (C), catalase (D), and glutathione peroxidase (E). The levels of malonyldialdehyde (F), IL-1β (G), and IL-6 (H). The meaning of abbreviations was shown in Figure 1. The data are presented as the mean ± SD (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 versus DOCA-salt according to Duncan’s multiple range test.
Effect of NTU101F on antioxidant enzymes and lipid peroxidation
The ability of NTU101F to reduce oxidative stress in the brain of hypertensive rats was assessed by measuring the activity of antioxidant enzymes (SOD, CAT and GPx) and lipid peroxidation level (malonyldialdehyde, MDA) in the hippocampus (Figure 6(C–F)). The results indicated that NTU101F administered to DOCA-salt-treated rats tended to increase the activity of SOD, CAT and GPx, and decrease MDA levels; the difference in SOD activity and MDA between rats treated with DOCA-salt and the highest NTU101F dose was statistically significant (p < 0.05).
Effect of NTU101F on release of pro-inflammatory cytokines in the hippocampus
Activated microglial cells secrete a variety of pro-inflammatory cytokines, including IL-1β and IL-6, which eventually results in neuronal injury. Our data indicated that DOCA-salt significantly induced the secretion of pro-inflammatory IL-1β and IL-6 in the hippocampus (p < 0.05; Figure 6(G,H)). However, the treatment with NTU101F 5.0 significantly lowered IL-1β and IL-6 levels compared to the DOCA-salt group (p < 0.05; Figure 6(G,H)).
Discussion
The brain is highly dependent on the adequate blood delivery of oxygen and glucose; accordingly, the disturbance of cerebral circulation impairs neuronal function and, in the long-term, induces brain damage (Iadecola 2004). Moreover, we found that ethanol extracts of Lactobacillus paracasei subsp. paracasei NTU 101-fermented products significantly reversed the oxygen-glucose deprivation and reperfusion (OGD/R)-induced neuroblastoma cell death in vitro; however, the water extracts of Lactobacillus paracasei subsp. paracasei NTU 101-fermented products was found to be less effective (data not shown). Hypertension causes structural remodelling of the vasculature, compromising oxygen and nutrient supply to the brain, and is, therefore, considered a leading risk factor for vascular cognitive impairment (Sharma & Singh 2012a,b, 2013). DOCA-salt rats, a salt- and volume-dependent but renin-independent (low plasma renin levels) model of hypertension has been widely used for the assessment of hypertension-induced changes and secondary complications, including vascular endothelial dysfunction (Morton et al. 1979; Bockman et al. 1992; Sharma & Singh 2012a,b, 2013). Here, we show that DOCA-salt treatment increased oxidative stress in the rat brain and impaired vascular endothelial and renal function, which was accompanied by VaD, as evidenced by the decline in cognitive abilities. However, these negative hypertension-related effects were significantly attenuated by oral administration of NTU101F, suggesting that fermentation products of L. paracasei subsp. paracasei NTU 101 possess considerable activity in preventing and alleviating in cognitive damages associated with hypertension. An angiotensin-converting-enzyme inhibitor (ACEI) has been shown to reduce the incidence of dementia or slow down the rate of cognitive decline in patients with hypertension in some clinical studies (Yasar et al. 2008; Sink et al. 2009). Milk fermented with L. helveticus strains can produce ACEI biogenic tripeptides as antihypertensive ingredients in spontaneously hypertensive rats (SHR) (Seppo et al. 2003). Our previously study found that milk fermented with NTU 101 has ACEI activity and decreased systolic and diastolic blood pressure in the SHR (Liu et al. 2011). These findings suggest that oral administration of NTU101F has antihypertensive effects, possibly via ACEI activity, in DOCA-salt rats.
Cerebral endothelial cells have powerful effects on vascular tone; they regulate cerebral blood flow by releasing vasodilators, such as NO, bradykinin, and prostacyclin (Andresen et al. 2006). The water extract of L. plantarum TWK10-fermented soy milk was shown to decrease blood pressure by promoting NO production, resulting in vasodilation and further improvement to hypertension (Liu et al. 2015). Reduced circulating levels of NO metabolites have been reported in patients with white matter lesions, indicating possible oxidative stress (Shibata et al. 2004). Another important aspect of endothelial cell function is the regulation of blood-brain barrier (BBB) permeability. The integrity of the BBB is vitally important for maintaining the homeostasis of the cerebral microenvironment, a prerequisite for normal brain function (Faraco & Iadecola 2013). The administration of NTU101F increased SOD activity, and decreased lipid peroxidation and oxidative stress, preventing NO from forming damaging free radicals; in addition, NTU101F induced eNOS expression, which should stimulate NO synthesis. These NTU101F activities can promote vasodilation, resulting in the improvement of hypertension.
Uncontrolled expression of MMPs can result in tissue injury and inflammation (Lukashev & Werb 1998), while their pharmacologic inhibition is shown to reduce tissue damage and oedema (Romanic et al. 1998). Aberrant MMP-9 proteolytic activity results in the degradation of tight junction proteins as well as basal membrane components, which has been shown to be associated with the increase in BBB permeability, brain infraction, edema (Lee et al. 2007; Rosenberg & Yang 2007), and stroke (Gasche et al. 1999; Castellanos et al. 2003). Our findings indicate that NTU101F can reduce MMP-9 activity, as well as cerebral secretion of pro-inflammatory cytokines and brain weight in hypertensive rats, suggesting that it can prevent the decline in brain function due to deregulation of BBB permeability and increase in cerebral inflammation.
As evidenced by the Morris water maze test, which is the most widely used model to examine cognition in rodents (Sharma & Singh 2012a, 2012b; Morris 1984), DOCA-salt treatment decreased spatial learning and memory in rats; however, NTU101F administration significantly improved cognitive abilities in these animals. NTU101F-induced neuroprotective activities can be related to the reduction of cellular reactive oxygen species (ROS) generation and prevention of oxygen–glucose deprivation and reperfusion (OGD/R)-induced cell death (data not shown). Neuronal apoptosis has been found to promote deficits in spatial learning and memory in rats subjected to chronic cerebral hypoperfusion (Pappas et al. 1996). In these conditions, oxidative stress leads to inflammation, which compromises endothelial wall integrity and blood supply, resulting in cell damage and loss (Ma et al. 2013). It has been reported that the DOCA-salt induced VaD may be due to increase in oxidative stress and the acetylcholinesterase activity in brain (Sharma & Singh 2013). Our results indicate that NTU101F exerted antioxidative and anti-inflammatory effects in the hippocampus, suggesting that it can effectively inhibit oxidative stress- and inflammation-related mechanisms in the brain, thus providing neuroprotection and preventing the loss of cognitive abilities associated with hypertension.
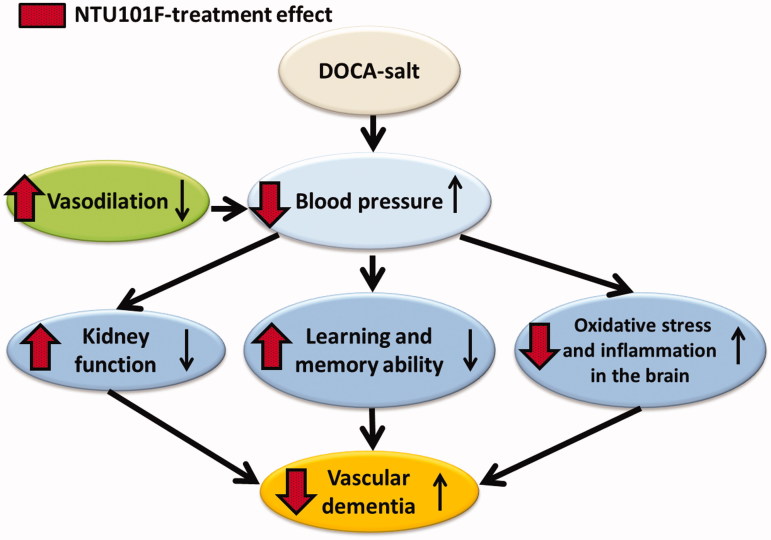
In summary, our findings indicate that the effects of experimental hypertension, such as endothelial and renal dysfunction, and subsequent VaD can be attenuated by the treatment with ethanol extract of L. paracasei subsp. paracasei NTU101-fermented products (Figure 7). This is the first report on the beneficiary effects of NTU101-fermented material on hypertension-associated VaD. We consider that these beneficiary effects depend on glyceryl 1,3-dipalmitate, the major neuroprotective compound which was characterized using ultra-performance liquid chromatography-mass spectrometry and nuclear magnetic resonance (Cheng et al. 2016).
Figure 7.
Proposed mechanism of ethanol extract of L. paracasei subsp. paracasei NTU 101-fermented products effect on rats with hypertension-induced vascular dementia.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Al-Qazzaz NK, Ali SH, Ahmad SA, Islam S, Mohamad K.. 2014. Cognitive impairment and memory dysfunction after a stroke diagnosis: a post-stroke memory assessment. Neuropsychiatr Dis Treat. 10:1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen J, Shafi NI, Bryan RM.. 2006. Endothelial influences on cerebrovascular tone. J Appl Physiol. 100:318–327. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. . 2011. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 23:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berecek KH, Barron KW, Webb RL, Brody MJ.. 1982. Vasopressin-central nervous system interactions in the development of DOCA hypertension. Hypertension. 4:131–137. [PubMed] [Google Scholar]
- Bockman CS, Jeffries WB, Pettinger WA, Abel PW.. 1992. Reduced contractile sensitivity and vasopressin receptor affinity in DOCA-salt hypertension. Am J Physiol. 262:1752–1758. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Davalos A.. 2003. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 34:40–46. [PubMed] [Google Scholar]
- Cheng MC, Tsai TY, Pan TM.. 2015. Anti-obesity activity of the water extract of Lactobacillus paracasei subsp. paracasei NTU 101 fermented soy milk products. Food Funct. 6:3522–3530. [DOI] [PubMed] [Google Scholar]
- Cheng MC, Leu YL, Tsai TY, Pan TM.. 2016. Screening and identification of neuroprotective compounds produced by Lactobacillus paracasei subsp. paracasei NTU 101. J Funct Foods. 26:238–248. [Google Scholar]
- Chiang SS, Liu CF, Tseng KC, Mau JL, Pan TM.. 2012. Immunomodulatory effects of dead Lactobacillus on murine splenocytes and macrophages. Food Agric Immunol. 23:183–202. [Google Scholar]
- Chiu CH, Lu TY, Tseng YY, Pan TM.. 2006. The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Appl Microbiol Biotechnol. 71:238–245. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Barber WJ, Lombard JH, Osborn JL, Liard JF.. 1986. Relationship between body fluid volumes and arterial pressure. Fed Proc. 45:2864–2870. [PubMed] [Google Scholar]
- Cryan JF, Dinan TG.. 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 13:701–712. [DOI] [PubMed] [Google Scholar]
- DiBona GF.1994. Neural control of renal function in health and disease. Clin Auton Res. 4:69–74. [DOI] [PubMed] [Google Scholar]
- Faraco G, Iadecola C.. 2013. Hypertension: a harbinger of stroke and dementia. Hypertension. 62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes S, Bruneval P, Hagege A, Heudes D, Ghostine S, Bouby N.. 2002. Chronic V2 vasopressin receptor stimulation increases basal blood pressure and exacerbates deoxycorticosterone acetate-salt hypertension. Endocrinology. 143:2759–2766. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH.. 1999. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 19:1020–1028. [DOI] [PubMed] [Google Scholar]
- Gentile MT, Poulet R, Di Pardo A, Cifelli G, Maffei A, Vecchione C, Passarelli F, Landolfi A, Carullo P, Lembo G.. 2009. beta-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 30:222–228. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M.. 2002. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2:289–300. [DOI] [PubMed] [Google Scholar]
- Iadecola C.2004. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 5:347–360. [DOI] [PubMed] [Google Scholar]
- Iyer A, Chan V, Brown L.. 2010. The DOCA-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev. 6:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB.1999. Hypertension mechanisms causing stroke. Clin Exp Pharmacol Physiol. 26:563–565. [DOI] [PubMed] [Google Scholar]
- Kotlyar E, Vita JA, Winter MR, Awtry EH, Siwik DA, Keaney JF, Sawyer DB, Cupples LA, Colucci WS, Sam F.. 2006. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J Card Fail. 12:122–127. [DOI] [PubMed] [Google Scholar]
- Krishnan PH, MacDonald T.. 2004. The current epidemic of primary aldosteronism: causes and consequences. J Hypertens. 22:2039–2040. [DOI] [PubMed] [Google Scholar]
- Lee BH, Ho BY, Wang CT, Pan TM.. 2009. Red mold rice promoted antioxidase activity against oxidative injury and improved the memory ability of zinc-deficient rats. J Agric Food Chem. 57:10600–10607. [DOI] [PubMed] [Google Scholar]
- Lee BH, Lo YH, Pan TM.. 2013. Anti-obesity activity of Lactobacillus fermented soy milk products. J Funct Foods. 5:905–913. [Google Scholar]
- Lee CL, Kuo TF, Wu CL, Wang JJ, Pan TM.. 2010. Red mold rice promotes neuroprotective sAPPalpha secretion instead of Alzheimer's risk factors and amyloid beta expression in hyperlipidemic Abeta40-infused rats. J Agric Food Chem. 58:2230–2238. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL.. 2007. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 38:2563–2568. [DOI] [PubMed] [Google Scholar]
- Lee TC, Zhao YD, Courtman DW, Stewart DJ.. 2000. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation. 101:2345–2348. [DOI] [PubMed] [Google Scholar]
- Lin FM, Chiu CH, Pan TM.. 2004. Fermentation of a milk-soymilk and Lycium chinense Miller mixture using a new isolate of Lactobacillus paracasei subsp. paracasei NTU101 and Bifidobacterium longum. J Ind Microbiol Biotechnol. 31:559–564. [DOI] [PubMed] [Google Scholar]
- Liu CF, Pan TM.. 2010. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J Food Drug Anal. 18:77–86. [Google Scholar]
- Liu CF, Tung YT, Wu CL, Lee BH, Hsu WH, Pan TM.. 2011. Antihypertensive effects of Lactobacillus-fermented milk orally administered to spontaneously hypertensive rats. J Agric Food Chem. 59:4537–4543. [DOI] [PubMed] [Google Scholar]
- Liu YY, Zeng SY, Leu YL, Tsai TY.. 2015. Antihypertensive effect of a combination of uracil and glycerol derived from Lactobacillus plantarum strain TWK10-fermented soy milk. J Agric Food Chem. 63:7333–7342. [DOI] [PubMed] [Google Scholar]
- Lukashev ME, Werb Z.. 1998. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 8:437–441. [DOI] [PubMed] [Google Scholar]
- Ma X, Sun Z, Liu Y, Jia Y, Zhang B, Zhang J.. 2013. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen Res. 8:2050–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 11:47–60. [DOI] [PubMed] [Google Scholar]
- Morton JJ, Casals-Stenzel J, Lever AF, Millar JA, Riegger AJ, Tree M.. 1979. Inhibitors of the renin-angiotensin system in experimental hypertension, with a note on the measurement of angiotensin I, II and III during infusion of converting-enzyme inhibitor. Br J Clin Pharmacol. 7:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S.. 2012. Host-gut microbiota metabolic interactions. Science. 336:1262–1267. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Torre JC, Davidson CM, Keyes MT, Fortin T.. 1996. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res. 708:50–58. [DOI] [PubMed] [Google Scholar]
- Puchtler H, Waldrop FS.. 1979. On the mechanism of Verhoeff's elastica stain: a convenient stain for myelin sheaths. Histochemistry. 62:233–247. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA.. 2009. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 6:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC.. 1998. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 29:1020–1030. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y.. 2007. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 22:1–9. [DOI] [PubMed] [Google Scholar]
- Seppo L, Jauhiainen T, Poussa T, Korpela R.. 2003. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 77:326–330. [DOI] [PubMed] [Google Scholar]
- Shackelford C, Long G, Wolf J, Okerberg C, Herbert R.. 2002. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 30:93–96. [DOI] [PubMed] [Google Scholar]
- Sharma B, Singh N.. 2012a. Defensive effect of natrium diethyldithiocarbamate trihydrate (NDDCT) and lisinopril in DOCA-salt hypertension-induced vascular dementia in rats. Psychopharmacology (Berl). 223:307–317. [DOI] [PubMed] [Google Scholar]
- Sharma B, Singh N.. 2012b. Experimental hypertension induced vascular dementia: pharmacological, biochemical and behavioral recuperation by angiotensin receptor blocker and acetylcholinesterase inhibitor. Pharmacol Biochem Behav. 102:101–108. [DOI] [PubMed] [Google Scholar]
- Sharma B, Singh N.. 2013. Pharmacological inhibition of inducible nitric oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, convalesce behavior and biochemistry of hypertension induced vascular dementia in rats. Pharmacol Biochem Behav. 103:821–830. [DOI] [PubMed] [Google Scholar]
- Shibata H, Nabika T, Moriyama H, Masuda J, Kobayashi S.. 2004. Correlation of NO metabolites and 8-iso-prostaglandin F2a with periventricular hyperintensity severity. Arterioscler Thromb Vasc Biol. 24:1659–1663. [DOI] [PubMed] [Google Scholar]
- Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Yasar S, Atkinson H, Robbins M, Psaty B, et al. . 2009. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the cardiovascular health study. Arch Intern Med. 169:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.2009. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 81:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, et al. . 2013. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 144:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaschitz A, Pilz S, Ritz E, Obermayer-Pietsch B, Pieber TR.. 2010. Aldosterone and arterial hypertension. Nat Rev Endocrinol. 6:83–93. [DOI] [PubMed] [Google Scholar]
- Tsai TY, Chu LH, Lee CL, Pan TM.. 2009. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk-soy milk supplemented with Momordica charantia. J Agric Food Chem. 57:2065–2071. [DOI] [PubMed] [Google Scholar]
- Tsai YT, Cheng PC, Pan TM.. 2014. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl Microbiol Biotechnol. 98:1–10. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, Oconnor FC, Wright JG, Lakatta LE, Yin FCP, Lakatta EG.. 1993. . Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 88:1456–1462. [DOI] [PubMed] [Google Scholar]
- Weber KT, Brilla CG, Cleland JG, Cohn JN, Hansson L, Heagerty AM, Laragh JH, Laurent S, Ollivier JP, Pauletto P.. 1993. Cardioreparation and the concept of modulating cardiovascular structure and function. Blood Press. 2:6–21. [DOI] [PubMed] [Google Scholar]
- Weber KT, Sun Y, Katwa LC.. 1996. Wound healing following myocardial infarction. Clin Cardiol. 19:447–455. [DOI] [PubMed] [Google Scholar]
- Yagi K.1976. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 15:212–216. [DOI] [PubMed] [Google Scholar]
- Yasar S, Zhou J, Varadhan R, Carlson MC.. 2008. The use of angiotensin-converting enzyme inhibitors and diuretics is associated with a reduced incidence of impairment on cognition in elderly women. Clin Pharmacol Ther. 84:119–126. [DOI] [PubMed] [Google Scholar]