Abstract
Context:Calophyllum inophyllum Linn. (Clusiaceae) (CI) is traditionally used to treat pain, inflammation, eye disorders and rheumatism.
Objective: The present study evaluates the antiarthritic activity of the ethanol extract of the stem bark (ESBCI) and seeds (ESCI) of Calophyllum inophyllum in Freund’s adjuvant induced arthritic Wistar albino rat model.
Materials and methods: ESBCI and ESCI were screened for in vitro anti-inflammatory activity by proteinase inhibition and membrane stabilization assays. Acute oral toxicity studies were conducted according to OECD-425 guidelines. Antiarthritic activity of ESBCI and ESCI at the dose of 250 mg/kg/p.o. was evaluated by Freund’s adjuvant induced arthritic rat model.
Results: ESBCI and ESCI have shown maximum inhibition at 250 μg/mL in proteinase inhibition and haemolysis assays. The LD50 of ESBCI and ESCI was found to be greater than 5000 and 2000 mg/kg/p.o., respectively. In Freund’s adjuvant induced arthritic rat model ESBCI, ESCI and Diclofenac treatment have shown 28.57, 36.36, and 43.51% as maximum reduction in rat paw oedema volume respectively when compared with the arthritic control rats. ESBCI and ESCI treatment at the dose level of 250 mg/kg/p.o. normalized the altered haematological and biochemical parameters of arthritic control rats. Histological and radiological evaluation confirmed the antiarthritic effect of ESBCI and ESCI.
Discussion: ESBCI and ESCI were found to show significant antiarthritic activity evidenced with clinical, biochemical, histological and radiological evaluations.
Conclusion: The present study indicates the antiarthritic activity of ESBCI and ESCI, however its mechanism of action has to be studied in the future.
Keywords: Rheumatoid arthritis, anti-inflammatory, Tamanu, histopathology of interphalangeal joints
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease which includes pathological changes such as hyperplasia of synovial membrane, infiltration of inflammatory cells, neovascularization, cartilage erosion and joint destruction (Chunxia et al. 2011). The prevalence of RA is about 1% of the world population and the epidemiology of arthritis in male to female ratio is 3:1 (Narendhirakannan et al. 2007). Cytokines play a major role in inflammation and damage the joint leading to tissue destruction during the development of RA which includes the tumor necrosis factor α (TNF-α), interleukin (IL) IL-1β and IL-6 (Yeom et al. 2006). Non-steroidal anti-inflammatory drugs (NSAID) and disease modifying antirheumatoid drugs (DMARD) have many applications in treating diseases but are associated with side effects like gastrointestinal tract complications, ulcers and cardiovascular problems (Emmanuel & Montgomery 1971; Singh et al. 1996; Gaffo et al. 2006). Major issues of the currently available medicines for RA include poor efficacy, potential side effects and high cost of biological agents. Thus, an efficient and safe alternative from herbs has drawn special attention from scientists worldwide.
Calophyllum inophyllum Linn Clusiaceae (CI) is commonly known as Kamani, Tamanu, Alexandrian laurel and Punnai. Calophyllum is an evergreen tree about 8–20 m tall with elliptical leaves, fragrant white flowers and large round nuts. The tree is used for its hard nature and its beauty as an ornamental tree. It is native to east Africa, India, South East Asia, Philippines, Taiwan, Melanesia, Australia, Southern and Eastern Polynesia. Oil from the nuts of CI is used for medicinal and cosmetic purposes (Kirtikar & Basu 1987). Traditionally this plant has been used to treat abrasions, pain, helminthiasis, bacterial and viral infections, inflammation, athlete's foot, bed sores, blisters, burns, cancer, cicatrizant, eczema, eye disorders, pain, psoriasis, rheumatism, ringworm, scabies, sedative, skin conditions, sore throat, stretch marks and wound care (Adeyeye 1991).
The methanol and chloroform extracts of the stem bark of CI possesses antibacterial and analgesic activity (Mishra et al. 2010). Calophyllolide isolated from the seeds reduces histamine inflammation and carrageenan induced tissue swelling in rats (Bhalla et al. 1980). Seeds contain inocalophyllins A and B (Shen et al. 2003). Two main activities in the seed oil were discovered by Professor Lederer and he has succeeded in isolating two essential components from the seed of CI. He identified totally a new fatty acid calophyllic acid and a lactone endowed with antibiotic properties to be at the origin of the oil’s amazing cicatrizing power (Lederer et al. 1953). The oil from the seeds is used externally for rheumatism and gout, and is specific for scabies (Jayaweera 1981). The traditional claim of CI oil for its therapeutic properties in the treatment of skin diseases and wound healing power was validated experimentally for its cytotoxic, wound healing and antibacterial properties (Léguillier et al. 2015). The inopyllums and (+)-calanolide isolated from the seeds of CI showed strong activity against human immunodeficiency virus type-1 (HIV-1) (Vlietinck et al. 1998). The extracts of CI were reported for antidepressant activity (Silpa et al. 2015) and neurobehavioral effects (Ibironke & Ugege 2015). Calophyllolide isolated from the nuts showed anti-inflammatory and antiarthritic activities in formaldehyde arthritis and adjuvant-induced arthritis in rats (Rastogi & Mehrotra 1998). Calophyllolide isolated from the seeds of CI has reduced histamine inflammation (Oliver-Bever 1986). Liu et al. (2015) studied the variation in calophyllolide content at different stages of maturity in CI and also found osteogenic activity in murine MC3T3-E1 cells. The stem bark of CI is the richest source of xanthones (Mah et al. 2011; Ee et al. 2011). The xanthones isolated from CI is well reported for its antiproliferative (Mah et al. 2014) and anti-inflammatory (Zakaria et al. 2014) activities. Traditionally the stem bark and seeds of CI are used to treat inflammations and rheumatism. To the best of our knowledge, the antiarthritic activity of the stem bark and seeds of CI were not experimentally validated, and hence the aim of present study is to experimentally validate the antiarthritic activity of the stem bark and seeds of CI in Freund’s adjuvant induced arthritic rat model.
Materials and methods
Plant material
The stem bark and seeds from CI were collected in the month of August from the river side of Vattakulam, Tirunelveli district, Tamil Nadu, India. The plant was authenticated by Dr. Soosai Raj, Department of Botany at St Joseph’s College, Trichy, Tamil Nadu, India. A voucher specimen representing the plant material has been deposited in the Department of Pharmaceutical Technology, Anna University BIT-Campus, Tiruchirappalli, India. The stem bark and seeds were made free from dust, cleaned, shade-dried and powdered by means of mechanical grinder and stored in an air-tight container for further use.
Drugs and chemicals
Solvents used for the extraction and analysis were of analytical grade and obtained from Merck Specialities Pvt. Ltd. (Mumbai, India). Diclofenac sodium was obtained from Kniss Pharma Laboratory Private Limited, Chennai, India. Freund’s complete adjuvant (FCA) used in the study was obtained from Sigma Aldrich (St. Louis, MO).
Preparation of the ethanol extract of stem bark and seeds of CI
The stem bark powder (280 g) was subjected to Soxhlet extraction using ethanol as the solvent in the ratio 1:5 for 24 h. In case of seed extraction, 200 g of the seed powder was weighed and extracted with 1750 mL of ethanol. Ethanol was recovered using a rotary vacuum evaporator. The extract was dried and the yield was calculated.
Preliminary phytochemical screening
Phytochemical analysis was performed using standard procedures to identify the phytoconstituents present in the leaves of CI as described by Kokate (1999).
In vitro anti-inflammatory assays
The proteinase inhibition assay was performed for ESBCI and ESCI according to the method followed by Oyedapo and Famurewa (1995). Diclofenac sodium was used as the standard drug. The percentage inhibition of the proteinase enzyme was calculated. Human Red Blood Cell (HRBC) membrane stabilization assay was performed for the ESBCI and ESCI according to the method followed by Sadique et al. (1989). Diclofenac sodium was used as the standard drug. Absorbance of the haemoglobin content in the suspension was measured spectrophotometrically at 560 nm and the percentage inhibition of haemolysis or membrane stabilization was calculated.
Experimental animals
Male Wistar albino rats weighing 120–150 g procured from Sri Venkateshwara Breeders, Bangalore, were used in the study. The study has been approved by Institutional Animal Ethical Committee of Bharathidasan Institute of Technology, Anna University, Tiruchirappalli – 620 024. IAEC Approval number is AUROT/IAEC/DEC 2014-001 DT 22.12.2014. Animals were housed in polypropylene cages (43 × 27 × 15 cm) lined with husk and not more than six animals per cage and maintained under standard environmental conditions (12 h light/dark cycles; temperature 22 ± 3 °C; 30–70% humidity, air ventilation) and were fed with standard pellet diet and fresh water ad libitum. The animals were acclimatized to the environment for two weeks prior to experimental use.
Acute oral toxicity study
Acute oral toxicity study for ESBCI and ESCI was conducted as per OECD guidelines 425. The test dose 5000 mg/kg of ESBCI and 2000 mg/kg of ESCI was administered orally to single rat each. If mortality was not observed additional two animals for ESBCI and four animals for ESCI was dosed. They were observed for the gross morphological changes and mortality up to 48 h.
Experimental setup
The animals were divided into seven groups of six animals in each group. The study period was conducted from 0–28 days in two different durations as 0–28 (developing phase) and 14–28 (developed phase) days (Sanmuga Priya et al. 2010). The standard drug Diclofenac sodium, ESBCI and ESCI were suspended in 1% sodium carboxy methyl cellulose (SCMC) and administered immediately.
Group I: Normal control
Group II: Negative control (arthritis induced rats without any treatment)
Group III: Positive control received 10 mg/kg per oral (p.o.) of Diclofenac sodium
Group IV: ESBCI received 250 mg/kg p.o. for 28 days from the day of induction of arthritis (developing phase)
Group V: ESCI received 250 mg/kg p.o. for 28 days from the day of induction of arthritis (developing phase)
Group VI: ESBCI received 250 mg/kg p.o for 14 days from the 14th day of induction of arthritis (developed phase)
Group VII: ESCI received 250 mg/kg p.o. for 14 days from the 14th day of induction of arthritis (developed phase)
Induction of arthritis
The rats were injected with 0.1 mL of the FCA by intraplantar injection in the left hind paw (Newbould 1963). Each mL of the adjuvant contains 1 mg of heat killed and dried Mycobacterium tuberculosis (strain H37Ra, ATTC 25177), 0.85 mL paraffin oil and 0.15 mL of mannide monooleate. After the injection of FCA, the paw volume of all the animals was measured using plethysmometer at 0, 7, 14, 21 and 28 days. The percentage inhibition of paw volume was calculated from the mean difference in the paw volume in the drug treated and control group using the following formula:
where Vc is the paw volume of the control group and Vt is the paw volume of the drug-treated group.
The hind legs of the experimental rats were photographed to view the morphology of the arthritic condition. At the end of the experiment, all the animals were sacrificed by administering high dose of chloroform as anaesthesia and blood was collected by cardiac puncture in plain and EDTA containing tubes, respectively, for serum separation. The serum samples were used for the estimation of marker enzymes like alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), C-reactive protein (CRP) and blood samples were used for analyzing the haematological parameters like red blood cell (RBC) count, white blood cell (WBC) count and haemoglobin (Hb) contents. The proximal interphalangeal joints from the adjuvant-induced arthritic rats were removed and fixed in 10% formalin and used for the histopathological studies. The hind legs were taken radiographs.
Analysis of haematological parameters
The haematological parameters like RBC and WBC counts were assessed by Chesbrough and McArthur (1972). The haemoglobin content was determined by the acid haematin method (Wintrobe 1975).
Analysis of biochemical parameters
The serum samples obtained were used for the analysis of biochemical parameters like ALP, AST, ALT and CRP using standard kits in the fully automatic biochemical analyzer.
Radiological studies
Radiographs were taken for the hind paw of all the experimental animals and examined for soft tissue swelling, bony erosions and narrowing of spaces between the joints (Harris 1990).
Histopathological studies
The interphalangeal joints were removed from the hind paw, washed with saline and fixed for 24 h in 10% formalin. After decalcification, the sections obtained were stained with eosin haematoxylin stain and viewed under 100× magnification.
Statistical analysis
The results were expressed as mean ± SEM. The statistical comparison was made between arthritic control and the treated groups. They were analyzed by one-way ANOVA followed by Dunnet’s multiple comparison test. The level of significance was set at p < .05.
Results
Preliminary phytochemical screening
The percentage yield of the ESBCI and ESCI obtained were 24.14% and 41.5% w/w, respectively. Preliminary phytochemical analysis revealed the presence of tannins, saponins, glycosides, flavonoids, steroids and terpenoids in ESBCI, whereas ESCI showed the presence of tannins, glycosides, saponins, flavonoids and terpenoids.
In vitro anti-inflammatory assays
The results of in vitro anti-inflammatory activity of ESBCI and ESCI are shown in Table 1. The results show that both standard and the test extracts showed equivalent activity at the concentration of 250 μg/mL. At 250 μg/mL, ESBCI showed 73.13% inhibition and ESCI showed 74.64% inhibition while the standard drug Diclofenac sodium produced 80.31% inhibition of proteinase enzyme. The percentage inhibition of test extracts is almost equal to the standard drug Diclofenac sodium. The results of the HRBC membrane stabilization assay showed significant anti-inflammatory activity in a concentration-dependent manner. At 250 μg/mL, ESBCI showed 71.10% inhibition of membrane haemolysis and the ESCI showed 72.31% inhibition that is nearly equal to the standard drug Diclofenac sodium which exhibited 72.55% protection of HRBC membrane in hypotonic solution.
Table 1.
In vitro anti-inflammatory assay of ESBCI and ESCI.
| Mean percentage of proteinase inhibition activity (%) |
Mean percentage inhibition of membrane haemolysis (%) |
||||||
|---|---|---|---|---|---|---|---|
| S.NO | Concentration (μg/ml) | Diclofenac sodium | ESBCI | ESCI | Diclofenac sodium | ESBCI | ESCI |
| 1. | 50 | 56.08 ± 0.243 | 31.38 ± 0.348 | 17.66 ± 0.143 | 30.23 ± 0.116 | 21.13 ± 0.153 | 12.74 ± 0.289 |
| 2. | 100 | 59.45 ± 0.124 | 46.15 ± 0.133 | 30.06 ± 0.510 | 43.58 ± 0.284 | 36.49 ± 0.271 | 20.23 ± 0.206 |
| 3. | 150 | 62.43 ± 0.065 | 58.92 ± 0.107 | 40.86 ± 0.309 | 52.43 ± 0.273 | 51.17 ± 0.314 | 46.89 ± 0.392 |
| 4. | 200 | 75.36 ± 0.092 | 68.95 ± 0.282 | 62.86 ± 0.302 | 68.61 ± 0.051 | 66.29 ± 0.069 | 53.83 ± 0.335 |
| 5. | 250 | 80.31 ± 0.109 | 73.13 ± 0.328 | 74.64 ± 0.095 | 72.55 ± 0.237 | 71.10 ± 0.181 | 72.31 ± 0.528 |
Values indicate Mean ± S.E., n = 3.
ESBCI: Ethanolic extract of the stem bark of Calophyllum inophyllum; ESCI: Ethanolic extract of the seeds of Calophyllum inophyllum.
Acute oral toxicity study
ESBCI and ESCI did not show any toxic or deleterious effects up to 5000 and 2000 mg/kg oral dose which indicates non-toxicity even at higher doses. The LD50 of ESBCI and ESCI were found to be greater than 5000 and 2000 mg/kg, respectively. As a result, 250 mg/kg was selected as the dose in order to evaluate in vivo antiarthritic activity in Freund’s adjuvant induced arthritic rat model.
Antiarthritic activity
Effect of ESBCI and ESCI on paw volume of Freund’s adjuvant induced arthritic rats
There was a significant increase in paw volume of the arthritic control group when compared to the normal group. Diclofenac sodium and ESCI treatment showed significant (p < .01) reduction (43.51 and 36.36% inhibition, respectively) in the paw volume of rats compared to the arthritic control group. ESBCI showed moderate effect (28.57% inhibition) on the prevention of paw oedema compared to the arthritic control group. The inhibition of ESCI obtained was nearly equal to that of the standard and was found to be effective (Table 2). The antiarthritic effect of the extracts in the developing phase of arthritis (Group VI) showed significant (p <.01) effect when compared to the developed phase (Group VII) of arthritis.
Table 2.
Effect of ESBCI and ESCI on paw volume of Freund’s adjuvant induced arthritic rats.
| Paw volume of the rats in ml Mean ± SEM (% inhibition) |
|||||
|---|---|---|---|---|---|
| Groups | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
| Group I | 0.92 ± 0.010 | 0.93 ± 0.006 | 0.95 ± 0.004 | 0.93 ± 0.006 | 0.94 ± 0.007 |
| Group II | 0.98 ± 0.004 | 1.44 ± 0.013 | 1.61 ± 0.006 | 1.56 ± 0.007 | 1.54 ± 0.006 |
| Group III | 0.90 ± 0.103 (8.16%) |
1.29 ± 0.009 b* (10.42%) | 1.21 ± 0.008 b* (25.31%) | 1.15 ± 0.013 b* (26.28%) |
0.87 ± 0.006 b* (43.51%) |
| Group IV | 0.97 ± 0.003 (1.02%) |
1.27 ± 0.008 b*c* (11.80%) |
1.26 ± 0.007 b*c* (21.73%) |
1.19 ± 0.003 b*c*(23.72%) | 1.10 ± 0.009 b*c* (28.57%) |
| Group V | 0.94 ± 0.009 (4.08%) |
1.32 ± 0.005 b*c* (8.82%) |
1.28 ± 0.007 b*c* (20.49%) |
1.22 ± 0.007 b*c* (21.15%) |
0.98 ± 0.007 b*c* (36.36%) |
| Group VI | 0.94 ± 0.004 (4.08%) |
1.27 ± 0.005 b*c* (11.80%) |
1.24 ± 0.004 b*c* (22.98%) |
1.21 ± 0.003 b*c* (22.24%) |
1.13 ± 0.003 b*c* (26.62%) |
| Group VII | 0.91 ± 0.003 (7.14%) |
1.25 ± 0.003 b*c* (13.19%) |
1.22 ± 0.004 b*c* (24.22%) |
1.19 ± 0.003 b*c* (23.72%) |
1.04 ± 0.042 b*c* (32.48%) |
Group I: Normal control; Group II: Negative control; Group III: Positive control; Group IV: ESBCI (28 days drug treatment); Group V: ESCI (28 days drug treatment); Group VI: ESBCI (14 days drug treatment); Group VII: ESCI (14 days drug treatment).
Values in parenthesis indicate the percentage inhibition of paw oedema for the respective groups. Values are expressed as Mean ± SEM, n = 6 animals in each group.
Comparisons were made between: b: Group II vs. groups III, IV, V, VI and VII; c: Group III vs. groups IV, V, VI and VII.
Represents the statistical significance at p < .01.
Effect of ESBCI and ESCI on haematological parameters of adjuvant induced arthritic rats
There was a significant (p < .05) decrease in RBC count and haemoglobin content, whereas increase in WBC count in the arthritic control group. ESBCI, ESCI and standard treated groups have shown significant (p < .05) increase in RBC and Hb content, whereas almost normal WBC count in the developing phases of arthritis (Table 3). ESBCI and ESCI in the developed phases of arthritis showed moderate effect when compared to the arthritic control group
Table 3.
Effect of ESBCI and ESCI on haematological parameters of control and adjuvant induced arthritic rats.
| Groups | RBC (millions/mm3) | WBC (thousands/mm3) | Hb (g/dL) |
|---|---|---|---|
| Group I | 7.19 ± 0.044 | 9.81 ± 0.018 | 14.11 ± 0.039 |
| Group II | 3.38 ± 0.038 | 19.88 ± 0.151 | 9.17 ± 0.037 |
| Group III | 5.05 ± 0.016 b* | 8.39 ± 0.009 b* | 13.16 ± 0.068 b* |
| Group IV | 5.52 ± 0.681 b*c* | 10.32 ± 0.062 b*c* | 10.61 ± 0.145 b*c* |
| Group V | 5.94 ± 0.021 b*c* | 9.57 ± 0.209 b*c* | 11.15 ± 0.047 b*c* |
| Group VI | 6.41 ± 0.006 b*c* | 9.94 ± 0.123 b*c* | 9.63 ± 0.024 b*c* |
| Group VII | 6.01 ± 0.005 b*c* | 8.58 ± 0.053 b*c* | 11.50 ± 0.014 b*c* |
RBC: Red blood cell; WBC: White blood cell; Hb: Haemoglobin.
Group I: Normal control; Group II: Negative control; Group III: Positive control; Group IV: ESBCI (28 days drug treatment); Group V: ESCI (28 days drug treatment); Group VI: ESBCI (14 days drug treatment); Group VII: ESCI (14 days drug treatment).
Values are expressed as mean ± SEM, n = 6 animals in each group.
Comparisons were made between: b: Group II vs. groups III, IV, V, VI and VII. c: Group III vs. groups IV, V, VI and VII.
Represents the statistical significance at p < .05.
Effect of ESBCI and ESCI on biochemical parameters of adjuvant induced arthritic rats
The arthritic control group showed a significant increase in the serum levels of ALP, ALT, AST and CRP, whereas the standard, ESBCI and ESCI-treated groups showed marked decrease in the serum levels of ALP, ALT, AST and CRP in the arthritic rats. ESCI in the developing phase of arthritis showed better effect compared to developed phase of arthritis. ESBCI in the developing and developed phases of arthritis showed moderate effect when compared to ESCI and standard. The values obtained were found to be statistically significant (p <.05) (Table 4).
Table 4.
Effect of ESBCI and ESCI on biochemical parameters of control and adjuvant induced arthritic rats.
| Groups | ALP | AST | ALT | CRP |
|---|---|---|---|---|
| Group I | 150.7 ± 0.013 | 59.41 ± 0.177 | 68.55 ± 0.245 | 105.5 ± 0.186 |
| Group II | 271.8 ± 1.379 | 115.2 ± 0.109 | 99.22 ± 0.074 | 135.6 ± 0.141 |
| Group III | 149.4 ± 0.471 b* | 70.42 ± 0.199 b* | 80.62 ± 0.158 b* | 110.3 ± 0.297 b* |
| Group IV | 199.9 ± 0.886b* | 94.44 ± 2.057b* | 88.22 ± 0.142b* | 122.3 ± 0.142 b* |
| Group V | 187.7 ± 0.409b* | 82.19 ± 0.193b* | 84.00 ± 0.258b* | 112.3 ± 0.209 b* |
| Group VI | 202.1 ± 0.828b* | 111.7 ± 0.557b* | 99.89 ± 0.432b* | 128.3 ± 0.329 b* |
| Group VII | 182.7 ± 0.330b* | 98.77 ± 0.290b* | 96.88 ± 0.209b* | 125.1 ± 0.188 b* |
ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; CRP: C reactive protein.
Group I: Normal control; Group II: Negative control; Group III: Positive control; Group IV: ESBCI (28 days drug treatment); Group V: ESCI (28 days drug treatment); Group VI: ESBCI (14 days drug treatment); Group VII: ESCI (14 days drug treatment).
Values are expressed as mean ± SEM, n = 6 animals in each group.
Comparisons were made between: b: Group II vs. groups III, IV, V, VI and VII. c: Group III vs. groups IV, V, VI and VII.
Represents the statistical significance at p < .05.
Histopathological study
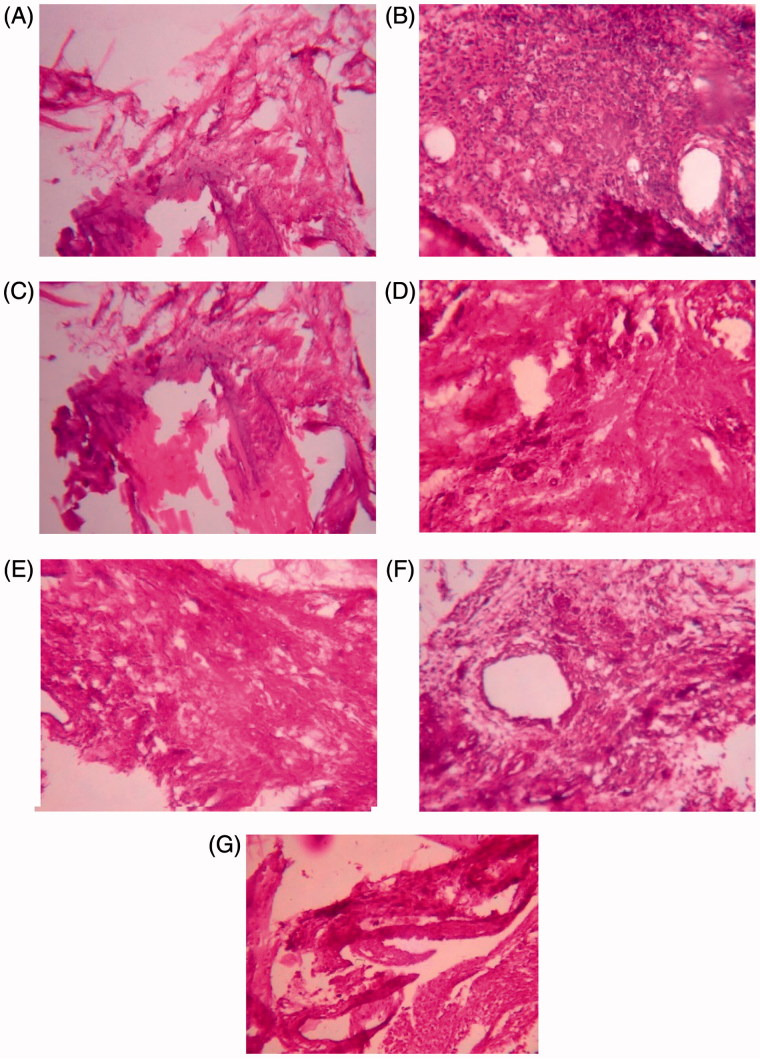
Histopathological study shows the differences in the normal ankle joint and adjuvant-induced arthritic rat joint. In this study, the histopathological studies of hind paw joints in the arthritic control group showed the prominent abnormalities like destruction of the bone marrow and extensive infiltration of the cells in the articular surface. Cellular infiltrations were abundant at synovial lining in the arthritic control rats. Infiltration of cells and destruction of bone marrow was not observed in ESCI and Diclofenac-treated group. Infiltration of cells was seen in the 14 days ESBCI treatment. ESCI in both the developing and developed phases of arthritis showed less infiltration of the cells and destruction of the bone marrow (Figure 1).
Figure 1.
Histopathology of proximal interphalangeal joints on adjuvant-induced arthritic rats. (A) Group I normal control; (B) group II negative control; (C) group III positive control; (D) group IV ESBCI (28 days treatment); (E) group V ESCI (28 days treatment); (F) group VI ESBCI (14 days treatment); (G) group VI ESCI (14 days treatment).
Radiological study
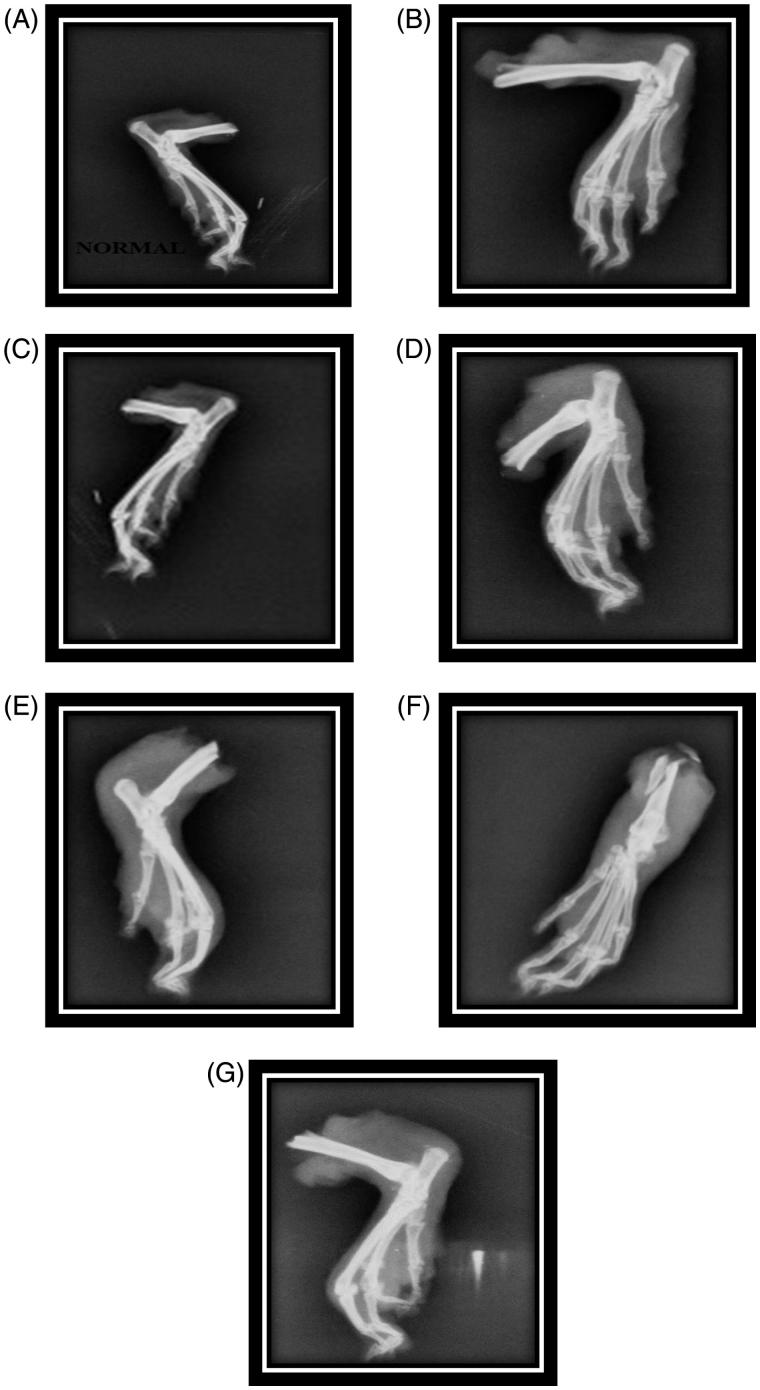
The radiographs of the rat joints in FCA-induced arthritic rat model shown in the Figure 2 depict the following observation. The arthritic control rats showed soft tissue swelling along with the narrowing of the joint spaces and bone destruction. ESCI-treated groups have prevented this bony destruction, by showing less soft tissue swelling and narrowing of joint spaces. Bone destruction was not observed in the Diclofenac-treated group. The ESBCI in the developing phase showed mild inflammation in the joint. Diclofenac and ESCI showed better effect compared to all other groups. Similar to the histopathological studies, the extract treatment for 28 days have shown significant prevention against bony destruction by showing less soft tissue swelling and narrowing of the joint spaces when compared with the developed phase (14 days) of extract treatment.
Figure 2.
Radiographs of hind legs in adjuvant-induced arthritic rats. (A) Group I normal control; (B) group II negative control; (C) group III positive control; (D) group IV ESBCI (28 days treatment); (E) group V ESCI (28 days treatment); (F) group VI ESBCI (14 days treatment); (G) group VI ESCI (14 days treatment).
Discussion
The percentage yields of the extracts were calculated and the extracts were subjected to preliminary phytochemical analysis to identify the phytoconstituents present. In vitro anti-inflammatory assays such as proteinase inhibitiory action and HRBC membrane stabilization assays were performed. Proteinase enzyme plays an important role in the development of tissue damage during inflammatory reactions (Das & Chatterjee 1995) and in this study the extracts of ESBCI and ESCI-treated groups showed maximum inhibition of proteinase enzyme compared to the standard drug Diclofenac sodium. The erythrocyte membrane is analogous to the lysosomal membrane and hence the HRBC membrane stabilization assay was performed to study the in vitro anti-inflammatory activity (Gandhidasan et al. 1991; Shenoy et al. 2010). Stabilization of lysosomal membrane is important in limiting the inflammatory response by preventing the release of constituents like activated neutrophils, bacterial enzymes and proteases, which causes further tissue inflammation and damage upon extracellular release. The NSAID’s act either by inhibiting the lysosomal enzyme or by stabilizing the lysosomal membrane (Vadivu & Lakshmi 2008). The results of the HRBC membrane stabilization assay showed significant anti-inflammatory activity in a concentration-dependent manner. It shows that ESCI was highly effective in stabilizing the lysosomal membrane, whereas ESBCI showed moderate effect when compared to the standard drug. Acute toxicity study was conducted as per OECD guideline 425. The ESBCI and ESCI did not show any toxic effects or lethality at the limit dose of 5000 and 2000 mg/kg p.o., respectively, which showed that the extracts are safe to use at higher concentrations.
Changes in the paw volume of the adjuvant-induced arthritic rats were measured using digital plethysmometer. From the results obtained, it was observed that ESCI was effective in equivalent to the standard drug Diclofenac sodium on reducing the increase in paw volume, whereas ESBCI showed moderate effect on prevention of paw oedema compared to the arthritic control group. Further, the effect of ESCI was also appreciable in developing phase rather than the developed phase of arthritis. In a previous study, the stem bark extract of CI at a dose of 400 mg/kg was studied in carageenan paw oedema and cotton pellet granuloma models, where the stem extract was found to show significant anti-inflammatory activity (Baig et al. 2014). The presence of calophyllolide (Bhalla et al. 1980) and inocalophyllins (Shen et al. 2003) were reported in the seeds of CI which may contribute to the higher antiarthritic activity of ESCI in reducing the paw volume. This study reports the anti-inflammatory effect of ESBCI and ESCI on FCA model confirming with the previous reports on other models.
The decrease in the RBC count and haemoglobin level in the arthritic control group shows the anemic condition in arthritic rats. Anemia is the most common extraarticular manifestation of rheumatoid arthritis, estimated to occur in 30% to 60% patients (Mowat 1971). The two most common reasons for anemia in arthritic patients are gastrointestinal blood loss from arthritic medication and bone marrow changes in patients with inflammatory arthritis which prevents the release of iron for incorporation into the RBC’s. ESBCI, ESCI and Diclofenac-treated groups showed significant recovery from anemia. The WBC count was reported to be increased in arthritic control rats, which may be due to the stimulation of the immune system against the invading antigens and the significant decrease in ESBCI and ESCI-treated groups showed its immunomodulation effect (Rajaram et al. 2015).
The assessment of the serum levels of ALP, ALT, AST and CRP is a tool to measure the antiarthritic activity of a particular drug. AST and ALT plays a vital role in the formation of biologically active chemical mediators such as bradykinins in the inflammatory process. The elevated levels of ALP in the adjuvant-induced arthritic rats may be due to the increase in the liver and bone fraction. This leads to localized bone loss in the form of bone erosion and periarticular osteopenia, as the enzyme is released into circulation in the course of bone formation and resorption (Borashan et al. 2009). CRP is a prototypic inflammatory biomarker of systemic inflammation which belongs to the class of acute phase proteins. The level of CRP increases during the inflammatory process that occurs in the body and the increase in the level of CRP is due to the rise in the plasma concentration of IL-6, which is produced by the macrophages (Pepys & Hierchfield 2003) and the adipocytes (Lau et al. 2005). In the present study, the adjuvant-induced arthritic rats in the control group showed elevated levels of ALP, ALT, AST and CRP when compared to Diclofenac, ESBCI and ESCI-treated groups. The alteration of the biochemical parameters was brought back to near normal in ESBCI and ESCI-treated groups in both the developing and developed phases of arthritis indicating that there might be decrease in bone loss and organ-protective mechanism, which may be due to the reduction in the release of chemical mediators of inflammatory process.
Histopathological study shows the differences in the normal ankle joint and adjuvant-induced arthritic rat joint. In general, histopathological studies on arthritic joint shows the prominent abnormalities from the normal joint like oedema formation, degeneration with partial erosion of the cartilage, destruction of bone marrow and extensive infiltration of inflammatory exudates in the articular surface. In the present study, the histopathological studies of hind paw joints in arthritic control rats showed the prominent abnormalities like destruction of the bone marrow and extensive infiltration of the cells in the articular surface. ESBCI and ESCI treatment have shown marked reduction in all the above-mentioned pathological conditions, indicating its effective antiarthritic activity by protecting the bone from degeneration.
Radiographic changes in RA conditions are useful diagnostic measures which indicate the severity of the disease. Soft tissue swelling is the earlier radiographic sign, whereas prominent radiographic changes like bony erosions and narrowing of joint spaces can be observed only in the developed stages of rheumatoid arthritis (Harris 1990). In the present study, ESBCI and ESCI was found to be effective in reducing the soft tissue swelling and narrowing of joint spaces, especially in developing phase of arthritis. The radiographic report confirms the effective antiarthritic activity of ESBCI and ESCI.
Conclusions
On evaluating the antiarthritic activity of ESBCI and ESCI, the effect of ESCI was found to be higher especially in the developing phase of arthritis. The better antiarthritic activity of the ESCI may be due to the presence of phytoconstituents such as calophyllolide, inophyllins and triterpenoids. Further, it validates the traditional use of CI seeds in the treatment of rheumatism. However, further studies are necessary to identify the active phytoconstituent responsible for the antiarthritic activity. The molecular mechanism involved in the antiarthritic activity of the plant extracts of CI, especially ESCI, can be studied in future to develop it as an alternate treatment for rheumatoid arthritis.
Disclosure statement
The authors declare that they have no competing interests.
References
- Adeyeye A.1991. Studies on seed oils of Garcinia kola and Calophyllum inophyllum. J Sci Food Agri. 57:441–442. [Google Scholar]
- Baig MD, Basheeruddin S, Silpa S, Venkateshwara Reddy A.. 2014. Antiinflammatory activity of ethanol extracts of leaf and stem bark of Calophyllum inophyllum on albino Wistar rats. Int J Pharm Sci Drug Res. 6:174–177. [Google Scholar]
- Bhalla TN, Saxena RC, Nigam SK, Misra G, Bhargava KP.. 1980. Calophyllolide: a new non-steroidal anti-inflammatory agent. Ind J Med Res. 72:762–765. [PubMed] [Google Scholar]
- Borashan FA, Ilkhanipoor M, Hashemi M, Farrokhi F.. 2009. Investigation the effects of Curcumin on serum hepatic enzymes activity in a rheumatoid arthritis model. Electr J Biol. 4:129–133. [Google Scholar]
- Chesbrough M, McArthur J.. 1972. Laboratory manual of rural tropical hospitals. London: The English Language Book Society and Churchill Livingstone. [Google Scholar]
- Chunxia C, Peng Z, Huifang P, Hanli R, Zehua H, Jizhou W.. 2011. Extracts of Arisaema rhizomatum C.E.C. Fischer attenuate inflammatory response on collagen-induced arthritis in BALB/c mice. J Ethnopharmacol. 133:573–582. [DOI] [PubMed] [Google Scholar]
- Das SN, Chatterjee S.. 1995. Long term toxicity study of ART‐400. Ind Indg Med. 16:117–123. [Google Scholar]
- Ee GCL, Mah SH, Rahmani M, Taufiq-Yap YH, Lim YM, Teh SS.. 2011. A new furanoxanthone from the stem bark of Calophyllum inophyllum. J Asian Nat Prod Res. 13:956–960. [DOI] [PubMed] [Google Scholar]
- Emmanuel JH, Montgomery RD.. 1971. Gastric ulcer and the anti-arthritic drugs. Postgrad Med J. 47:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffo A, Saag KG, Curtis JR.. 2006. Treatment of rheumatoid arthritis. Am J Health Syst Pharm. 63:2451–2465. [DOI] [PubMed] [Google Scholar]
- Gandhidasan R, Thamaraichelvan A, Baburaj S.. 1991. Anti-inflammatory action of Lannea coromandelica by HRBC membrane stabilization. Fitoterapia. 12:81–83. [Google Scholar]
- Harris ED.1990. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Eng J Med. 322:1277–1289. [DOI] [PubMed] [Google Scholar]
- Ibironke GF, Ugege OG.. 2015. Effects of the Extract of Calophyllum inophyllum on behavioral indices in rodents. Neurophysiology. 47:40–45. [Google Scholar]
- Jayaweera DMA.1981. Medicinal plants used in Ceylon, part 3, Colombo: National Science Council of Sri Lanka. [Google Scholar]
- Kirtikar KR, Basu BD.. 1987. Indian medicinal plants. Dehradun, India: International Book Distributors. [Google Scholar]
- Kokate CK.1999. Practical pharmacognosy. New Delhi, India: Vallabh Prakashan. [Google Scholar]
- Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S.. 2005. CRP increment due to rise in IL-6 produced predominantly by adipocytes. Am J Physiol Heart Circ Physiol. 288:H2031–H2041. [DOI] [PubMed] [Google Scholar]
- Lederer E, Dietrich P, Polonsky J.. 1953. On the chemical constitution of calophylloide and calophyllic acid from the nuts of Calophyllum inophyllum. Bull French Chem Soc. 5:546–549. [Google Scholar]
- Léguillier T, Lecsö-Bornet M, Lémus C, Rousseau-Ralliard D, Lebouvier N, Hnawia E, et al. 2015. The wound healing and antibacterial activity of five ethnomedical Calophyllum inophyllum oils: an alternative therapeutic strategy to treat infected wounds. PLoS One. 10:e0138602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu Y, Chen Z, Chiou W, Tsai Y, Chen C.. 2015. Calophyllolide content in Calophyllum inophyllum at different stages of maturity and its osteogenic activity. Molecules. 20:12314–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah SH, Ee GCL, Rahmani M, Taufiq-Yap YH, Go R, Teh SS.. 2011. A new pyranoxanthone from the stem bark of Calophyllum inophyllum. Lett Org Chem. 8:447–449. [Google Scholar]
- Mah SH, Ee GCL, Teh SS.. 2014. Antiproliferative properties of xanthones from Calophyllum inophyllum and Calophyllum soulattri towards human cancer cell lines. Planta Med. 80:P1L101. [Google Scholar]
- Mishra US, Narasimha Murthy P, Prashanta Kumar C, Panigrahi G, Mohapatra S, Pradhan D.. 2010. Antibacterial and analgesic effects of the stem barks of Calophyllum inophyllum. Int J Chem Tech Res. 2:973–979. [Google Scholar]
- Mowat AG.1971. Hematologic abnormalities in rheumatoid arthritis . Semin. Arthritis Rheum. 1:195–219. [DOI] [PubMed] [Google Scholar]
- Narendhirakannan RT, Subramanian S, Kandaswamy M.. 2007. Anti-inflammatory and lysosomal stability actions of Cleome gynandra L. studied in adjuvant induced arthritic rats. Food Chem Toxicol. 45:1001–1012. [DOI] [PubMed] [Google Scholar]
- Newbould BB.1963. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol Chemother. 21:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Bever B.1986. Medicinal plants in tropical West Africa. Cambridge: University Press. [Google Scholar]
- Oyedapo OO, Famurewa AJ.. 1995. Antiprotease and membrane stabilizing activities of extracts of Fagra zanthoxiloides, Olax subscorpioides and Tetrapleura tetraptera. Int J Pharmacog. 33:65–69. [Google Scholar]
- Pepys MB, Hierchfield GM.. 2003. C-Reactive protein: a critical update. J Clin Invest. 111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram C, Ravindra Reddy K, Bonnth Chandra Sekhar K.. 2015. Evaluation of antiarthritic activity of Caesalpinia pulcherimma in Freund’s complete adjuvant induced arthritic rat model. J Young Pharmacists. 7:128–132. [Google Scholar]
- Rastogi RP, Mehrotra XX. (1998). Compendium of Indian medicinal plants vol. 5. New Delhi: Central Drug Research Institute, Lucknow and Publications and Information Directorate; p. 14–14. [Google Scholar]
- Sadique J, Al‐Rqobahs WA, Bughaith MF, El-Gindy AR.. 1989. The bioactivity of certain medicinal plants on the stabilization of RBC membrane system. Fitoterapia. 60:525–532. [Google Scholar]
- Sanmuga Priya E, Senthamil Selvan P, Venkataraman S.. 2010. Evaluation of antiarthritic activity of Strychnos potatorum Linn seeds in Freund’s adjuvant induced arthritic rat model. BMC Complement Altern Med. 10:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YC, Hung MC, Wang LT, Chen CY.. 2003. Inocalophyllins A, B and their methyl esters from the seeds of Calophyllum inophyllum. Chem Pharm Bull. 51:802–806. [DOI] [PubMed] [Google Scholar]
- Shenoy S, Shwetha K, Prabhu K, Maradi R, Bairy KL, Shanbhag T.. 2010. Evaluation of anti-inflammatory activity of Tephrosia purpurea in rats. Asian Pac J Trop Med. 3:193–195. [Google Scholar]
- Silpa S, Inayat A, Venkateshwar Reddy A, Satyavathi D.. 2015. Evaluation of anti depressant and nootropic activity of Calophyllum inophyllum. Indo Amer J Pharm Res. 5:2136–2142. [Google Scholar]
- Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF.. 1996. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med. 156:1530–1536. [PubMed] [Google Scholar]
- Vadivu R, Lakshmi KS.. 2008. In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchinensis (Lour) Moore ssp. laurina. Bangladesh J Pharmacol. 3:121–124. [Google Scholar]
- Vlietinck AJ, De Bruyne T, Apers S, Pieters LA.. 1998. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med. 64:97–109. [DOI] [PubMed] [Google Scholar]
- Wintrobe MM.1975. Clinical hematology. 7th ed Philadelphia: Lea and Febiger; p. 114–115. [Google Scholar]
- Yeom MJ, Lee HC, Kim GH, Lee HJ, Shim I, Oh SK, Kang SK, Hahm DH.. 2006. Anti-arthritic effects of Ephedra sinica STAPF herb-acupuncture: inhibition of lipopolysaccharide-induced inflammation and adjuvant induced polyarthritis. J Pharmacol Sci. 100:41–50. [DOI] [PubMed] [Google Scholar]
- Zakaria MB, Vijayasekarana ZI, Muhammad NA.. 2014. Anti-inflammatory activity of Calophyllum inophyllum fruits extracts. Procedia Chem. 13:218–220. [Google Scholar]