Abstract
Context: Black tea has been reported to have significant antimutagenic and anticarcinogenic properties associated with its polyphenols theaflavins (TF) and thearubigins (TR). Similarly, Turkish black tea (TBT) also contains a considerable amount of TF and TR.
Objective: This study investigated the mutagenic, antimutagenic and anticlastogenic properties of TBT.
Materials and methods: The mutagenic and antimutagenic effects of TBT (10 to 40000 μg/plate) were investigated in vitro on Salmonella strains TA98 and TA100 with and without S9 fraction. Anticlastogenic effect was studied at concentrations of 300–1200 mg/kg TBT extract by chromosomal aberrations (CA) assay from bone marrow of mice.
Results: The results of this study did not reveal any mutagenic properties of TBT. On the contrary, TBT extract exhibited antimutagenic activity at >1000 μg/plate concentrations in TA98 strain with and without S9 activation (40% inhibition with S9 and 27% without S9). In TA100 strain, the antimutagenic activity was observed at >20,000 μg/plate TBT extracts without S9 activation (28% inhibition) and at >1000 μg/plate with S9 activation (59% inhibition). A significant decrease in the percentage of aberrant cells (12.33% ± 1.27) was observed in dimethylbenz(a)anthracene (DMBA) plus highest concentration (1200 mg/kg) of TBT extract-treated group when compared to only DMBA-treated group (17.00% ± 2.28).
Discussion and conclusion: Results indicated that TBT can be considered as genotoxically safe, because it did not exert any mutagenic and clastogenic effects. As a result, TBT exhibited antimutagenic effects more apparently after metabolic activation in bacterial test system and had an anticlastogenic effect in mice.
Keywords: Genotoxicity, chromosomal aberration assay, Ames test
Introduction
Tea is one of the most consumed beverages around the world. It is prepared by the infusion of Camellia sinensis (L.) Kuntze (Theaceae) leaf (Coimbra et al. 2006). There are more than 300 types of tea leaves commercially available and mainly categorized into three groups according to the process used for the preparation of the tea leaves, i.e. green, oolong and black tea. Among all these different types of tea, fermented black tea constitutes 75% of the worldwide tea production (Sang et al. 2011). Different types of biological activities and beneficial health effects of tea have been reported in previous studies. Its role against cancer, cardiovascular diseases, congenital abnormalities, neurodegenerative diseases, depression, and many other diseases have been discussed in literature (Hayat et al. 2015). Recent epidemiological studies have reported the protective effects of black tea consumption against some types of cancer including ovarian (Baker et al. 2007), lung (Wang et al. 2014) and prostate (Fei et al. 2014) as well as reducing the risk of cardiovascular diseases (Arab et al. 2009; Grassi et al. 2015).
Protective measures against carcinogenic development in the body are currently one of the leading research areas. Genotoxic effects such as mutations and chromosomal aberrations (CA) are one of main causes of cancer (Valdiglesias et al. 2009). Hence, mutagenicity, antimutagenicity and CA have been studied by many research groups. Black tea has been reported to have antimutagenic and anticlastogenic properties (Zengin et al. 2014; Hayat et al. 2015). Although the mechanism of antimutagenic and anticarcinogenic activities of black tea has not yet been fully understood, it has been reported that these activities are due to the presence of its polyphenols theaflavins (TF) and thearubigins (TR) (Gupta et al. 2002; Bhattacharya et al. 2014). Henning et al. (2003) studied polyphenol contents of 11 black tea samples from different blends. Their TF contents were in the range of 7.3–20 mg/100 mL after the preparation of standard infusion methods. TF content of Turkish black tea (TBT) was found as 18 mg/g dry weight (Üstündağ et al. 2016). TBT was the focus of our study since the difference in theaflavin content can change the protective effect of tea.
Although there are some studies regarding the TBT ingredients (Turkmen et al. 2007; Alasalvar et al. 2013; Üstündağ et al. 2016) no evidence was found related to its antimutagenic, anticlastogenic or anticarcinogenic activities. Considering the extent of worldwide studies on these kind of activities of green and black tea, this study investigates the antimutagenic and anticlastogenic effects of TBT using Salmonella mutagenicity assay in vitro and clastogenicity assay in vivo in mice.
Materials and methods
Chemicals
Dimethylbenz(a)anthracene (DMBA), sodium azide (SA), 4-nitro-o-phenylenediamine (NPD), biotin, nicotinamide adenine dinucleotide phosphate (NADP), glucose-6-phosphate, ampicillin trihydrate, benzo(a)pyrene (B(a)P), colchicine and agar were supplied from Sigma Chemical Company. Histidine was provided from Fluka and nutrient broth was supplied from Hi Media laboratories Ltd.
Animals
Male Balb C inbred mice, 12–14 weeks old, weighing 28–32 g, were used for the clastogenic and anticlastogenic assay and Sprague–Dawley male rats, weighing 150 to 200 g, were used for S9 preparation for this study. Animals were obtained from the Yeditepe University Medical School Experimental Research Center (Atasehir, Istanbul). They were housed in cages under standard condition and fed with standard rodent pellet diet and water ad libitum at 12 h light/dark cycle. Room temperature and relative humidity conditions were 28 ± 2 °C and 60% ± 5, respectively. The experimental protocol was approved by the Ethic Committee of Yeditepe University (No. 407).
Bacterial strains
Mutagenicity and antimutagenicity assays were carried out using the Salmonella strains of TA98 and TA100. The TA98 strain was provided by Gazi University, Faculty of Pharmacy, Department of Toxicology (Ankara, Turkey) and the TA100 strain was received from the Marmara Research Center (Marmara Research Center of the Scientific and Technological Research Council of Turkey, Gebze, Turkey). TA98 detects frame shift mutations and TA100 detects base pair mutations. These strains were primarily recommended by Maron and Ames (1983) for routine mutagenicity assays.
Preparation of S9 fraction
Rat liver homogenate was prepared according to the methods as explained by Ames et al. (1973), Garner et al. (1972) and Halder et al. (2005). Sprague–Dawley male rats, weighing 175–200 g, were fed with 0.1% phenobarbital in their drinking water for 7 days. On day 6, no food was supplied to the rats and they were euthanized on the following day. The rat liver homogenate (S9) was prepared by centrifugation at 9000 g, as explained by Maron and Ames (1983). Approximately 2 mL of S9 fractions were distributed in different small sterile cryovials, quickly frozen and stored at −80 °C for further use.
Preparation of lyophilized TBT extract
TBT infusion was prepared by submerging 20 g of grindered TBT leaves (CAYKUR, Filiz Cayi) in boiling distilled water (500 mL) for 5 min. After 5 min of brewing, tea infusion was filtered immediately, frozen at −20 °C and then lyophilized (Christ, Alpha 2-4 LD, Germany). Approximately 2 g of lyophilized TBT extract was obtained from TBT infusion and stored at +4 °C until further analysis.
Mutagenicity and antimutagenicity assays
For mutagenicity assay of TBT, standard plate incorporation test was carried as explained by Maron and Ames (1983). Both mutagenicity and antimutagenicity assays were performed with Salmonella tester TA98 and TA100 strains. TBT extract dissolved in hot distilled water and in different concentrations (0, 10, 100, 1000, 10000, 20000 and 40000 μg/plate) were used for both mutagenicity and antimutagenicity assay against the known mutagens and carcinogens. For testing mutagenicity, plates were co-incubated with the bacterial strains and prepared concentrations of TBT extract. Plates were inverted and placed at 37 °C for 48 h in dark, and revertant colonies were counted after incubation. To evaluate the impact of TBT metabolites, similar experiments were also carried out by incubating bacteria and extract with liver S9 fraction. Four plates were used for each concentration tested for experiments both with and without S9 fraction.
Antimutagenicity assays were carried out similarly using the same bacterial strains and the same TBT extract concentrations with and without S9 fraction. NPD was used as positive mutagen for TA98 strain without S9 and SA was used as positive mutagen for TA100 strain. B(a)P was used as positive mutagen for both TA98 and TA100 strains with S9 experiment. After incubation of inverted plates at 37 °C for 48 h in dark, revertant colonies were counted. The number of revertant colonies grown on plates containing the mutagen without TBT extract was defined as 100% which means 0% inhibition. The percentage of inhibition was calculated according to the formula: [(A−B)/(A−C)] × 100, where A = No. of histidine revertants in the absence of sample, B = No. of histidine revertants in the presence of sample, C = spontaneous revertants. The antimutagenic effect was considered moderate when the inhibitory effect was 25–40% and strong when it was more than 40%. Inhibitory effect less than 25% was considered as weak and was not recognized as a positive result (Zengin et al. 2014). Four plates were used for each TBT extract concentration for both with and without S9 experiments.
In vivo CA assay
Dose determination
Dose determination of TBT extract was based on the average daily tea consumption. It has been reported that approximately 5 cups (approximately 500–600 mL) of tea infusion per day is required to reduce the risk of some types of human cancer (Apostolides et al. 1996). Ground TBT leaves (20 g) brewed for 5 min in 500 mL of water equals to 5 cups of tea infusion per day for an adult. This means 2 g of TBT extract per day. In terms of body weight, this amount equals to 30 mg/kg daily for an adult weighing 65 kg. Considering this calculation, three dose levels at 300, 600 and 1200 mg/kg body weight were selected. These relatively higher doses were selected to justify the cumulative effects of tea consumption over a long period of time since our experimental protocol is based on only one application to animals.
Assay protocol
For CA assay, three different concentrations (300, 600 and 1200 mg/kg body weight) of TBT extract were administered by gavage to 3 sets of 4 animals in each (a total of 12 mice). Immediately after administration of TBT extract, DMBA prepared in corn oil was administered at 50 mg/kg dose by gavage to all the 12 TBT-treated mice. Four mice were also gavaged with distilled water, treated with DMBA and served as positive control. Similarly 4 mice were gavaged with distilled water, treated with corn oil, and served as negative control. Twenty-two h after gavage, all animals were injected with colchicine and euthanized by cervical dislocation after 2 h. Chromosomes from bone marrow were prepared as explained by Gupta et al. (2002). Bone marrow is the target tissue in this test since it is a highly vascularized tissue and it contains a population of rapidly cycling cells that can be readily isolated and processed. All the slides were coded and 75 well spread metaphase cells (40 ± 2 chromosomes) per animal were scored for CA. A total of 300 metaphase cells were scored for each dose of TBT plus DMBA and for both negative, positive controls and only TBT-treated groups. For analysis of Mitotic Index (MI), 1000 cells/animal were scored and expressed in percentages. CA was scored as explained in the WHO (World Health Organization 1985) and OECD guidelines (Organization for Economic Co-operation and Development 1997). Frequencies of chromatid and chromosome-type aberrations per cell were calculated. Gaps were also recorded as explained in the OECD guideline but not included as percentage of aberrant cells or as the frequency of aberrations per cell.
Statistical analysis
Experimental results were expressed as mean ± standard deviation. Dunnett and Crisafio (1995) multiple comparisons were carried out for mutagenicity and antimutagenicity data and for CA analysis data. p < 0.05 was considered as statistically significant.
Results
In this study, the mutagenic and antimutagenic activities of TBT extract (with doses of 40,000 μg/plate and lower) were investigated. The results showed that in the presence of the different concentrations of TBT extract, the mutation frequencies for the tested S. typhimurium strains TA98 and TA100 did not change significantly when compared to spontaneous mutation frequencies. Any of the tested concentrations did not induce a significant increase in the revertant number of TA98 and TA100 strains with or without S9 activation. Also, the results revealed that the different concentrations of TBT extract did not influence the viability of bacterial strains. The average revertant colony numbers in negative control were 30.50 ± 3.87 for TA98 and 175.25 ± 18.64 for TA100 with S9 and 28.25 ± 6.08 and 173.50 ± 8.81 without S9, respectively. The plates with the positive control mutagens showed significant increases relative to the spontaneous mutation rate in the two tested strains.
The revertant colony numbers observed in the antimutagenicity assay and inhibitory rate percentages of the TBT extract with and without S9 activation were given in Table 1. A significant decrease in the revertant colonies were observed from the positive mutagen (NPD) plus 1000 μg/plate TBT extract in TA98 without S9 activation when compared to only positive mutagen treated plates. TBT extract exhibited strong antimutagenic activities at doses of 10,000, 20,000 and 40,000 μg (70%, 78%, and 80% inhibition, respectively) and moderate antimutagenic activity (27% inhibition) at the dose level of 1000 μg against NPD in the absence of S9 mix in S. typhimurium TA98.
Table 1.
Antimutagenic activity of Turkish black tea (TBT) in Salmonella strains TA98 and TA100 with and without metabolic activation (S9) against known positive mutagens.
| TA98 |
TA100 |
||||||
|---|---|---|---|---|---|---|---|
| Without S9 | Number of revertant/plate | İnhibition (%) | Number of revertant/plate | İnhibition (%) | |||
| Negative control | 28.25 ± 6.08 | 173.50 ± 8.81 | |||||
| Positive control | 629.75 ± 54.84 | 667.75 ± 47.40 | |||||
| TBT Dose (μg/plate) + Positive mutagen (control) |
40,000 | 149.75 ± 11.59* | 80 | 433.75 ± 40.57* | 47 | ||
| 20,000 | 162.25 ± 12.82* | 78 | 527.25 ± 62.60* | 28 | |||
| 10,000 | 210.25 ± 59.81* | 70 | 606.25 ± 34.77 | 12 | |||
| 1000 | 467.25 ± 58.03* | 27 | 622.75 ± 39.45 | 9 | |||
| 100 | 619.50 ± 28.24 | 2 | 643.50 ± 49.59 | 5 | |||
| 10 | 613.75 ± 31.48 | 3 | 662.00 ± 50.65 | 1 | |||
| With S9 | |||||||
| Negative control | 30.50 ± 3.87 | 175.25 ± 18.64 | |||||
| Positive control | 84.25 ± 14.20 | 671.50 ± 34.89 | |||||
| TBT Dose (μg/plate) + Positive mutagen (control) |
40,000 | 29.25 ± 1.71* | 97 | 180.00 ± 7.12* | 99 | ||
| 20,000 | 38.00 ± 8.37* | 86 | 218.75 ± 38.80* | 91 | |||
| 10000 | 49.75 ± 12.50* | 64 | 277.50 ± 58.25* | 79 | |||
| 1000 | 59.50 ± 10.28 | 40 | 381.00 ± 50.15* | 59 | |||
| 100 | 82.25 ± 15.59 | 4 | 631.75 ± 65.56 | 8 | |||
| 10 | 84.00 ± 16.51 | 0.5 | 649.00 ± 53.81 | 5 | |||
p < 0.05; Positive mutagen vs. positive mutagen plus Turkish black tea treatment group. Dunnett multiple comparisons test. Sterile distilled water (100 ml/plate) was used as negative control. 4-Nitro-o-phenylenediamine (NPD) (20 μg/plate) was used as positive control (mutagen) for S. typhimurium TA98 strain and Sodiumazide (SA) (1 μg/plate) was used as positive control (mutagen) for S. typhimurium TA100 without S9 activation. Benzo(a)pyrene (B(a)P) (1.0 μg plate) was used as positive control (mutagen) for both strains with S9 activation.
TBT extract showed strong antimutagenicity at the dose of 10,000 (64% inhibition), 20,000 (86% inhibition) and 40,000 μg (97% inhibition) against B(a)P; 1000 μg dose of the extract exhibited moderate (40% inhibition) antimutagenic activity in the presence of S9 mix in TA98 strain. On the other hand, 100 and 10 μg doses of the extract were found to be weak antimutagenic with and without S9 activation in this strain.
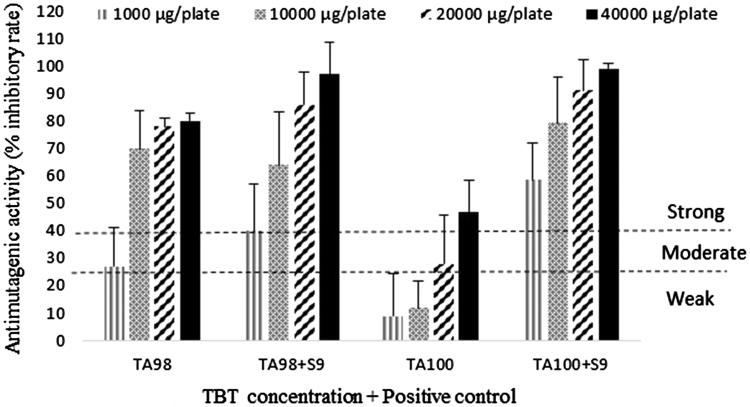
In TA100 strain, the antimutagenic effects were observed from the positive mutagen plus 20000 μg/plate TBT extract without S9 activation and from 1000 μg/plate with S9 activation. It was seen that TBT extract manifested moderate antimutagenicity (28% inhibition) at concentrations of 20000 μg and strong antimutagenic activity (47% inhibition) at dose of 40,000 μg against SA, while 10, 100, 1000, and 10,000 μg dose of extract were found to be weak antimutagenic in the absence of S9 mix in TA100 strain. On the other hand, except for 10 and 100 μg, all doses exhibited strong antimutagenic activity against B(a)P in the presence of metabolic activation system. The highest inhibition ratio (99%) was observed in 40,000 μg/plate dose group, followed by 20,000 μg (91%), 10000 μg (79%) and 1000 μg (59%) (Table 1, Figure 1).
Figure 1.
Antimutagenic activity (% inhibitory rate) of Turkish black tea extract against positive control (mutagen). 4-Nitro-o-phenylenediamine (NPD) (20 μg/plate) was used as positive control (mutagen) for S. typhimurium TA98 strain and sodium azide (SA) (1 μg/plate) was used as positive control (mutagen) for S. typhimurium TA100 without S9 activation. Benzo(a)pyrene (B(a)P) (1.0 μg plate) was used as positive control (mutagen) for both strains with S9 activation.
Table 2 shows the antigenotoxic effects of TBT extract in bone marrow cells of mice against the known carcinogen DMBA. A decrease in the CA was observed in the three higher concentrations (300, 600 and 1200 mg/kg body weight) of TBT extract plus DMBA treated series when compared with the group treated with only DMBA. A statistically significant decrease in CA was observed only in the highest dose of TBT extract plus DMBA treated group when compared to only DMBA treated group. No significant changes were observed when CA induced by only 1200 mg/kg of TBT extract compared to negative control series.
Table 2.
Chromosomal aberrations induced by DMBA and DMBA plus Turkish black tea (TBT) extract in vivo on bone marrow cells of mice.
| Treatment (mg/kg) | Metaphase cells scored | Gapsa | Aberrations/cellsb |
Aberrant cells % (Mean ± S.D.)c | Mitotic indices (Mean ± S.D.)c | |
|---|---|---|---|---|---|---|
| Chromatid type | Chromosome type | |||||
| DMBA (50mg/kg) | 300 | 31 | 0.127 | 0.073 | 17.00 ± 2.28 | 1.57 ± 0.24 |
| TBT 300 + DMBA | 300 | 28 | 0.117 | 0.037 | 14.67 ± 3.08 | 1.72 ± 0.29 |
| TBT 600 + DMBA | 300 | 25 | 0.127 | 0.047 | 15.67 ± 2.52 | 1.66 ± 0.16 |
| TBT 1200 + DMBA | 300 | 23 | 0.103 | 0.030 | 12.33 ± 1.27* | 1.62 ± 0.25 |
| Water control | 300 | 11 | 0.133 | 0.040 | 4.35 ± 1.68 | 2.18 ± 0.27 |
| Only TBT 1200 | 300 | 16 | 0.147 | 0.093 | 6.00 ± 1.68 | 1.93 ± 0.29 |
Total chromatid and chromosome gaps at each dose were recorded but not included as aberrations/cell.
Total number of aberrations (chromatid and chromosome type)/total number of cells scored per dose. Results are of 4 animals (75 metaphase cells/animal).
Results at each dose were compared to those of control using Dunnett’s multiple comparison with control.
p < 0.05 when compared with DMBA treated only. TBT 300 + DMBA:300 mg/kg TBT + DMBA; TBT 600 + DMBA:600 mg/kg TBT + DMBA; TBT 1200 + DMBA:1200 mg/kg TBT + DMBA; Only TBT 1200:1200 mg/kg TBT. DMBA: dimethylbenz(a)anthracene.
Discussion
Previous reports have already shown that Indian black tea has significant antimutagenic and anticarcinogenic activities (Gupta et al. 2002; Halder et al. 2005, 2008; Bhattacharya et al. 2014). These reports indicated that the antimutagenic and anticancer activities of black tea are mainly due to the presence of TF and TR content of black tea.
According to our results TBT extract was neither toxic nor mutagenic to the bacteria at the tested concentrations, and bacterial growth was normal.
In the antimutagenicity assay of our study, the protective effect of TBT against the known mutagens in both TA98 and TA100 strains increased after metabolic activation with S9 (Figure 1) indicating the apparent protective effect of TBT metabolites against mutation in these strains. The effect of metabolic activation on the antimutagenic activity of plant polyphenols was also stated by Edenharder et al. (1997). Buening et al. (1981) have also postulated that some flavonoids such as TF and TR are potent inhibitors of cytochrome P450 monooxygenases such as CYP1A1 and CYP1A2. This inhibition prevents mutagenic/carcinogenic metabolite formation from some procarcinogenic chemicals such as B(a)P and aflatoxin B1.
Overall, our findings of decreased number of revertant colonies in both TA98 and TA100 with or without S9 activation clearly indicate that TBT extract has significant antimutagenic effects against the known mutagens.
According to our clastogenicity and anticlastogenicity data, TBT extract did not exert clastogenicity at a dosage of up to 1200 mg/kg in vivo in mice when compared to distilled water control. In anticlastogenic study, CA was decreased in all the three concentrations of TBT plus DMBA-treated series when compared with only DMBA-treated series, but statistically significant decrease in CA was observed in the highest dose (1200 mg/kg) of TBT plus DMBA-treated group when compared with only DMBA-treated group.
Previous studies revealed that the antimutagenic and anticlastogenic properties of tea polyphenols are mostly due to their antioxidant activity which inactivates direct carcinogens and inhibits the activation of indirect carcinogens extracellularly. Polyphenols also induce cytochrome P450 resulting in detoxifying of the carcinogens intracellularly (Gupta et al. 2002). TF play an important role in the antimutagenic activity of black tea by scavenging free radicals and also inhibiting cytochrome P450-dependent bioactivation of the carcinogens (Shiraki et al. 1994; Catteral et al. 1998). In the present study the anticlastogenic effect of TBT against the known mutagen DMBA is in agreement with the previous reports on the anticlastogenic effects of Indian black tea and its polyphenols TF and TR against the known mutagens DMBA and cyclophosphamide (CP) in vivo in mice (Gupta et al. 2002; Halder et al. 2005). Halder et al. (2005) studied anticlastogenic effect of TF and TR by CA and micronuclei formation (MN) method and reported that TF was more potent than TR in their in vitro test system. The TF content of TBT, 18 mg/g dry weight, is higher than those of 11 different blends of black tea samples which are between 7.3 and 20 mg/100 mL (Üstündağ et al. 2016; Henning et al. 2003). Henning et al. (2003) obtained their results by preparing tea bag infusions containing 1.5–2.4 g black tea leaves per bag in 100 mL hot water. We can assume that 2 g of dry TBT leaves give 38 mg TF after infusion with 100 mL hot water. Therefore, our significant antimutagenic and anticlastogenic effects may be mainly due to the presence of high-TF content in TBT extract. This is the first report of the antimutagenic and anticlastogenic effects of TBT in vitro in Salmonella assay and in vivo in mice. Further studies will confirm the antimutagenic and anticlastogenic effect of TBT due to its high-TF content.
Funding Statement
The authors are grateful to the TÜBİTAK, Turkey for assigning Senior Visiting Scientist position to Dr. Ashok K. Giri to supervise and do this work at the Department of Toxicology, Faculty of Pharmacy, Yeditepe University, Istanbul, Turkey.
Acknowledgements
The authors are grateful to Kimberly Farber for reviewing our manuscript as a native speaker.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Alasalvar C, Pelvan E, Ozdemir KS, Kocadağlı T, Mogol BA, Paslı AA, Ozcan N, Ozçelik B, Gökmen V.. 2013. Compositional, nutritional, and functional characteristics of instant teas produced from low- and high-quality black teas. J Agric Food Chem. 61:7529–7536. [DOI] [PubMed] [Google Scholar]
- Ames BN, Durston WE, Yamasaki E, Lee FD.. 1973. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci USA. 70:2081–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides Z, Balentine DA, Harbowy ME, Weisburger JH.. 1996. Inhibition of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) mutagenicity by black and green tea extracts and polyphenols. Mutat Res. 359:159–163. [DOI] [PubMed] [Google Scholar]
- Arab L, Liu W, Elashoff D.. 2009. Green and black tea consumption and risk of stroke: a meta-analysis. Stroke. 40:1786–1792. [DOI] [PubMed] [Google Scholar]
- Baker JA, Boakye K, McCann SE, Beehler GP, Rodabaugh KJ, Villella JA, Moysich KB.. 2007. Consumption of black tea or coffee and risk of ovarian cancer. Int J Gynecol Cancer. 17:50–54. [DOI] [PubMed] [Google Scholar]
- Bhattacharya U, Adak S, Majumder NS, Bera B, Giri AK.. 2014. Antimutagenic and anticancer activity of Darjeeling tea in multiple test systems. BMC Complement Altern Med. 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buening MK, Chang RL, Huang MT, Fortner JG, Wood AW, Conney AH.. 1981. Activation and inhibition of benzo(a)pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally occurring flavonoids. Cancer Res. 41:67–72. [PubMed] [Google Scholar]
- Catteral F, Copeland E, Clifford MN, Ioannides C.. 1998. Contribution of theafulvins to the antimutagenicity of black tea: their mechanism of action. Mutagenesis. 13:631–636. [DOI] [PubMed] [Google Scholar]
- Coimbra S, Castro E, Rocha-Pereira P, Rebelo I, Rocha S, Santos-Silva A.. 2006. The effect of green tea in oxidative stress. Clin Nutr. 25:790–796. [DOI] [PubMed] [Google Scholar]
- Dunnett CW, Crisafio R.. 1995. The operating characteristics of some official weight variation tests for tablets. J Pharm Pharmacol. 7:314–327. [DOI] [PubMed] [Google Scholar]
- Edenharder R, Rauscher R, Platt KL.. 1997. The inhibition by flavonoids of 2-amino-3-methylimidazo[4,5-f]quinoline metabolic activation to a mutagen: a structure-activity relationship study. Mutat Res. 379:21–32. [DOI] [PubMed] [Google Scholar]
- Fei X, Shen Y, Li X, Guo H.. 2014. The association of tea consumption and the risk and progression of prostate cancer: a meta-analysis. Int J Clin Exp Med. 7:3881–3891. [PMC free article] [PubMed] [Google Scholar]
- Garner RC, Miller EC, Miller JR.. 1972. Liver microsomal metabolism of aflatoxin B1 to a reactive derivative toxic to Salmonella typhimurium TA 1530. Cancer Res. 32:2058–2066. [PubMed] [Google Scholar]
- Grassi D, Draijer R, Desideri G, Mulder T, Ferri C.. 2015. Black tea lowers blood pressure and wave reflections in fasted and postprandial conditions in hypertensive patients: a randomised study. Nutrients. 7:1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Chaudhuri T, Seth P, Ganguly DK, Giri AK.. 2002. Antimutagenic effects of black tea (World Blend) and its two active polyphenols theaflavins and thearubigins in Salmonella assays. Phytother Res. 16:655–661. [DOI] [PubMed] [Google Scholar]
- Halder B, Pramanick S, Mukhopadhyay S, Giri AK.. 2005. Inhibition of benzo[a]pyrene induced mutagenicity and genotoxicity by black tea polyphenols theaflavins and thearubigins in multiple test systems. Food Chem Toxicol. 43:591–597. [DOI] [PubMed] [Google Scholar]
- Halder B, Bhattacharya U, Mukhopadhyay S, Giri AK.. 2008. Molecular mechanism of black tea polyphenols induced apoptosis in human skin cancer cells: involvement of Bax translocation and mitochondria mediated death cascade. Carcinogenesis. 29:129–138. [DOI] [PubMed] [Google Scholar]
- Hayat K, Iqbal H, Malik U, Bilal U, Mushtag S.. 2015. Tea and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 55:939–954. [DOI] [PubMed] [Google Scholar]
- Henning SM, Fajardo-Lira C, Lee HW, Youssefian AA, Go VLW, Heber D.. 2003. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr Cancer. 45:226–235. [DOI] [PubMed] [Google Scholar]
- Maron DM, Ames BN.. 1983. Revised methods for the Salmonella mutagenicity test. Mutat Res. 113:173–215. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Co-operation and Development 1997. OECD guideline. 475. (adopted 21 July 1997). Mammalian bone marrow chromosome aberration test. Paris. [Google Scholar]
- Sang S, Lambert JD, Ho CT, Yang CS.. 2011. The chemistry and biotransformation of tea constituents. Pharmacol Res. 64:87–99. [DOI] [PubMed] [Google Scholar]
- Shiraki M, Hara Y, Osawa T, Kumon H, Nakayama T, Kawakishi S.. 1994. Antioxidative and antimutagenic effects of theaflavins from black tea. Mutat Res. 323:29–34. [DOI] [PubMed] [Google Scholar]
- Turkmen N, Velioglu YS, Sari F, Polat G.. 2007. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 12:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstündağ ÖG, Erşan S, Özcan E, Özan G, Kayra N, Ekinci FY.. 2016. Black tea processing waste as a source of antioxidant and antimicrobial phenolic compounds. Eur Food Res Technol. 242:1523–1532. [Google Scholar]
- Valdiglesias V, Méndez J, Pásaro E, Laffon B.. 2009. The importance of the in vitro genotoxicity evaluation of food components: the selenium In: Kocsis A and Molnar H, editors. Genotoxicity: evaluation, testing and prediction. New York: Nova Biomedical Books; p. 1–40 [Google Scholar]
- Wang L, Zhang X, Liu J, Li Z.. 2014. Tea consumption and lung cancer risk: a meta-analysis of case-control and cohort studies. Nutrition. 30:1122–1127. [DOI] [PubMed] [Google Scholar]
- World Health Organization 1985. Environmental health criteria 46, Guidelines for the study of genetic effects in human populations. Geneva: WHO; p. 29–44. [Google Scholar]
- Zengin G, Uysal A, Gunes E, Aktumsek A.. 2014. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): a potential source for functional food ingredients and drug formulations. PLoS One. 9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]