Abstract
Context: Baicalin (BL) and baicalein (B) as the major flavonoids of Scutellaria baicalensis Georgi (Lamiaceae) have been investigated intensively, and shown to possess a multitude of pharmacological activities.
Objective: This study systematically evaluates the stability of BL and B in monomer and total flavonoid fraction (FSR) form in vitro, and further studies whether the protective measures are effective to make B and BL stable enough to meet the requirement of quantitative analysis in various biological samples.
Materials and methods: The stability of BL and B was evaluated by investigating the influence factors such as pH (2.0, 3.0, 4.5, 6.8, 7.4 and 9.0), temperature (4, 25 and 40 °C), antioxidant (vitamin C and Na2SO3) and sunlight. After the protective measures were taken, stability of BL and B in plasma, urine and tissue homogenates was evaluated through post-preparative stability (stored at 4 °C for 24 h), three freeze–thaw cycles stability and long-term stability test (stored in refrigerator at −20 °C for 15 days). In addition, by comparing the degradation parameters of BL and B obtained from the monomer administration group with those of the FSR administration group, drug–drug interaction of coexistent components in FSR on the stability of BL and B was discussed.
Results: The degradation of BL and B was both pH- and temperature-dependent with their correlation coefficents for first-order kinetics equation larger than 0.99, and acidic environment (pH 2–4.5), lower temperature (<4 °C) and acidic antioxidant (e.g. vitamin C) were conducive to stabilize B and BL. Furthermore, coexistent components in FSR were proved to have function on inhibiting the degradation of BL and B in our study for the first time, which was characteristic of prolonging their biological half-life (t1/2) significantly, e.g., from 2.89 h to indefinite for BL (pH 6.8, 25 °C), from 2.63 h to 4.48 h for B (pH 6.8, 25 °C) and so on. Antioxidant of Na2SO3 could inhibit the degradation of BL with t1/2 increasing from 1.8 h to 3.5 h, but aggravate the bio-transformation of B with t1/2 decreasing from 0.92 h to 0.29 h. Our research proved that BL monomer, and BL and B in FSR form could be stabilized enough to meet the requirement of biological quantitative analysis under the protection of coexistent components in FSR.
Discussion and conclusion: The results obtained indicated that the stability of BL and B was affected not only by its environmental parameters, but also by the coexistent components in the effective total flavonoids fractions.
Keywords: Degradation, drug–drug interaction, HPLC
Introduction
Radix scutellariae, the dried roots of Scutellaria baicalensis Georgi (Lamiaceae), has been widely employed for the treatment of inflammation, pyrexia, jaundice, diarrhoea, hepatitis, etc. Flavonoids are considered to be the main active constituents of Radix scutellariae. Baicalin (BL) and baicalein (B), as the major flavonoids, have been investigated intensively, and shown to possess a multitude of pharmacological activities such as antibacterial, anti-inflammatory, antioxidant, antitumor and anti-allergic (Chen et al. 2015; Li et al. 2015; Choi et al. 2016; Yan et al. 2016; Zhou et al. 2016).
In pharmacokinetic and metabolism studies, drug levels are commonly monitored in plasma, urine or tissue homogenate. These studies often involve the duration of samples collection, storage and procession. As the degradation or biotransformation of target compounds during the sampling procedure can yield misleading results, the stability of labile compounds calls for attention during processing and storage. Due, in part, to the presence of 5,6,7-trihydroxyl and 6,7-dihydroxyl groups in the benzene ring, B and BL have demonstrated poor stability (Wang et al. 2008). Concern for accessing the stability of B and BL has kept growing for the past few years. Qiu et al. (2004) developed a RP-HPLC method to investigate the stability of BL in aqueous solution, and found that acid medium and relative lower temperature were conducive to its stability. Yu et al. (2002) studied the stability of BL in different pH aqueous solutions, organic solvents, rat plasma, bovine serum and some tissue homogenates, and found that BL was stable in acidic water and organic solutions, but unstable in basic water and biological media. Based on the above-mentioned research, Xing et al. (2005) developed a LC-MS/MS method to deeply investigate the stability of BL in buffered aqueous solutions at different pHs and in biological fluids in vitro. In addition to obtaining some conclusions in line with previous studies, they proved that the degradation of baicalin was pH- and temperature-dependent, and oxidation-reduction reaction was the major degradation process for BL in plasma and urine in vitro. The more valuable contribution of Xing’s study was that they advised some protective measures for processing and storage of biological samples containing BL, which could provide researchers a leisure time during analysis. About the stability studies on B, Zhu et al. (2006) discussed the influence of heat in airtight condition on thermos stability of B. Yao and Zhang (2006) studied the effects of light, temperature, pH and antioxidants on the stability of B by UV-Vis spectrometer. Wang et al. (2008) researched on the stability of BL and B in buffered aqueous solution at pH 7.4 and rat plasma by the HPLC-ECD technique. Some similar advice on improving the stability of samples were given by Xing et al. (2005), such as the of vitamin C (Vc) and HCl into the samples. However, whether B could be stabilized enough to meet the requirements of analysis method employed in pharmacokinetic or metabolism study was not evaluated in these reports. Although there were abundant papers on the in vivo analysis of B, the stability of B during the sampling process was not considered fully in these reports (Feng et al. 2010; Hou et al. 2010; Tong et al. 2012). In this study, we noticed that B was much more unstable than BL, and other compounds contained in total flavonoids fraction of Radix scutellariae (FSR) seemed to have the function of improving the stability of BL and B. So, whether the protective measure for BL stability recommended in the literature is also effective enough to ensure the quantification requirement of B in vivo and in vitro calls for further assessment. Meanwhile, drug–drug interaction on the stability of BL and B has not been reported.
In view of the above problems and previous research limitations, we comparatively investigated the influence of buffer aqueous pH, temperature, sunlight and antioxidant agents on the stability of BL and B in both monomer and FSR forms to search the most effective protection measure, which were further evaluated by post-preparative stability, three freeze–thaw cycles stability and long-term stability test in various biological samples, including plasma, urine and tissue homogenous. Some valuable suggestions on collecting, processing and storing biological samples are offered in our study.
Materials and methods
Chemicals and reagents
Baicalin, baicalein and daidzein were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (China). Wogonoside was obtained from Xi’an Rongsheng Biological Technology Co., Ltd (Xi’an, China). Maackiain (purity, >99%) was obtained in our laboratory (Figure 1). HPLC grade methanol and acetonitrile were obtained from Merk (Darmstadt, Germany). Deionized water used throughout the experiments was generated by a Millipore water purification system (Milford, MA). Other regents were of analytical grade. Scutellariae radix purchased from a local drug store in Guangzhou in 2015 was identified by Associate Professor Jinsong Zhou at Guangzhou University of Traditional Chinese Medicine. The voucher specimen was deposited at the Herbarium at Guangzhou University of Traditional Chinese Medicine.
Figure 1.
Molecular structures of baicalin (A), baicalein (B), wogonoside (IS-1, (C), daidzein (IS-2, D) and maackiain (IS-3, E).
Instrumentation and chromatographic conditions
The Shimadzu LC-20A HPLC system consisted of a Shimadzu SIL-20A pump, a Shimadzu LC-20A autosampler and a LC-20MAV photodiode array detector. Chromatographic elution was carried out on a Phenomenex Luna C18 column (particle size 5 μm, 4.6 × 250 mm ID) at ambient temperature. The mobile phase for the separation of BL and B samples was methanol:0.1% (v/v) aqueous formic acid (50:50) and methanol:0.1% (v/v) aqueous formic acid (65:35), respectively. The mobile phase for the separation of FSR consisted of a mixture of acetonitrile (A) and 0.1% (v/v) aqueous formic acid (B), by using a linear gradient elution program as follows: 32–36% A at 0–5 min, 35–50% A at 5–15 min and 50–50% B at 15–25 min. The flow rates were all 1.0 mL/min. The detection wavelength was set at 275 nm. An aliquot of 20 μL test solution was injected.
Preparation of the total FSR
Finely ground Radix scutellariae sample powder (40 mesh) was accurately weighed, and decocted twice by refluxing with water (1:10, g/mL) for 1 h. The extracted solution was concentrated to 0.5 g/mL calculated according to crude drug quantify. FSR was purified and enriched by acid precipitation method reported by literature (Wang et al. 2008), and the final effective part was obtained after spray drying. The content of total flavonoids in FSR was analyzed quantitatively by Mg–HCl colorimetric method (purity >60%), and the content of BL and B in FSR was accurately quantified by HPLC-DAD based on our previously developed method (Feng et al. 2012).
Animals and sample collection
Male Sprague–Dawley rats weighing 220 ± 50 g were supplied by the Center Animal Laboratory of Guangzhou University of Traditional Chinese Medicine. Animals were fasted for 18 h with free access to water prior to the experiments. Fresh plasma, urine and tissue samples (including liver, stomach, intestine and kidney) were collected below 4 °C prior to experiments. Tissue homogenates were obtained by homogenizing the tissues with cold methanol at a ratio of 1:2 (g/mL).
Sample pretreatment
Incubations in buffered aqueous solution of BL, B and FSR were terminated by adding 200 μL of cold methanol:0.5M hydrochloric acid (10:1, v/v, CMHC) containing internal standard (IS) of wogonoside (10 μg/mL), CMHC containing IS of daidzein (10 μg/mL) and CMHC containing IS of maackiain (10 μg/mL) to 20 μL measured incubated solution, respectively. The stability of BL and B in the termination medium obtained by refrigerated centrifugation at 13,000 rpm was evaluated.
Pretreatment for the analysis of biological samples was as follows: plasma, urine and tissue homogenate were acidified with hydrochloric acid (adding 20 μL HCl to 1 mL plasma and tissue homogenate, adding 200 μL HCl to 1 mL urine, pH 2.0) and placed on ice. After BL, B and FSR were added into the ice-cold biological matrix, spiked samples were processed either immediately, or after three freeze–-thaw cycles or after long-term freezing (−20 °C) procedure. To 100 μL of the above spiked plasma, urine and tissue homogenates, 50 μL of corresponding internal standard and 50 μL of 0.2% Vc were added. After protein precipitation with 400 μL ice-cold methanol, the supernatant for direct injection of biological samples was collected after thoroughly vortex mixing (3 min) and centrifuging at low temperature (4 °C, 13,000 g, 5 min). All the sampling procedures were operated on ice-bath, and the reagents and apparatus used were precooled to 4 °C in refrigerator.
Comparative stability of baicalin and baicalein in buffered aqueous solutions at different pHs
Baicalin (100 μg/mL), baicalein (100 μg/mL) and FSR (200 μg/mL) were incubated in six buffer systems at pH 2.0, 3.0, 4.5, 6.8, 7.4 and 9.0 for several hours, respectively. An aliquot of 20 μL incubated solution was measured at predetermined time of different intervals (pH 2.0, 3.0, 4.5: 0, 0.25, 0.5, 1.0, 1.5, 2.0, 4.0, 8.0, 12.0, 18.0 and 24.0 h; pH 6.8: 0, 0.25, 0.5, 1.0, 1.5, 2.0, 4.0, 6.0 and 8.0 h; pH7.4, 9.0: 0, 0.25, 0.5, 1.5 and 2.0 h), the supernatant obtained after sample pretreatment was injected into the LC system for analysis. Stability curves of BL and B were constructed by plotting the % value of the remaining drug versus time (the initial concentration was presumed to be 100 units). Each set of experiments was conducted in triplicate.
Comparative stability of baicalin and baicalein at different temperatures, in buffers containing different antioxidants and in sunniness condition
Baicalin (100 μg/mL), baicalein (100 μg/mL) and FSR (200 μg/mL) were incubated in phosphate buffer at pH 7.4 for 6 h. This experiment was performed at 4, 25 and 40 °C, respectively. The stability of BL and B in both monomer and FSR form in buffers (at pH 7.4, 25 °C) containing 0.2% Na2SO3 or Vc as antioxidant was evaluated. The influence of sunlight was also investigated. An aliquot of 20 μL incubated solution was measured according to the specific schedule at times of 0, 0.25, 0.5, 1.0, 1.5, 2.0, 4.0 and 6.0 h. The supernatant obtained after sample pretreatment was immediately injected into the LC system for analysis.
Comparative stability of baicalin and baicalein in biological fluids
Stability of BL and B in plasma, urine and tissue homogenates was evaluated after sample extraction process (samples were processed and stored under 4 °C for 24 h), three freeze–thaw cycles and long-term freezing at −20 °C (15 days) by samples in six replicates at terminal concentration level of 2–10 μg/mL. Stability was assessed by comparing the mean peak areas ratio of target compounds to internal standard obtained from biological samples after extraction with those from acid treated water and methanol spiked with equivalent amount of target references (RE). Samples were to be concluded stable if their bias were within ±15%.
Results
Comparative stability of baicalin and baicalein in buffered aqueous solutions at different pHs
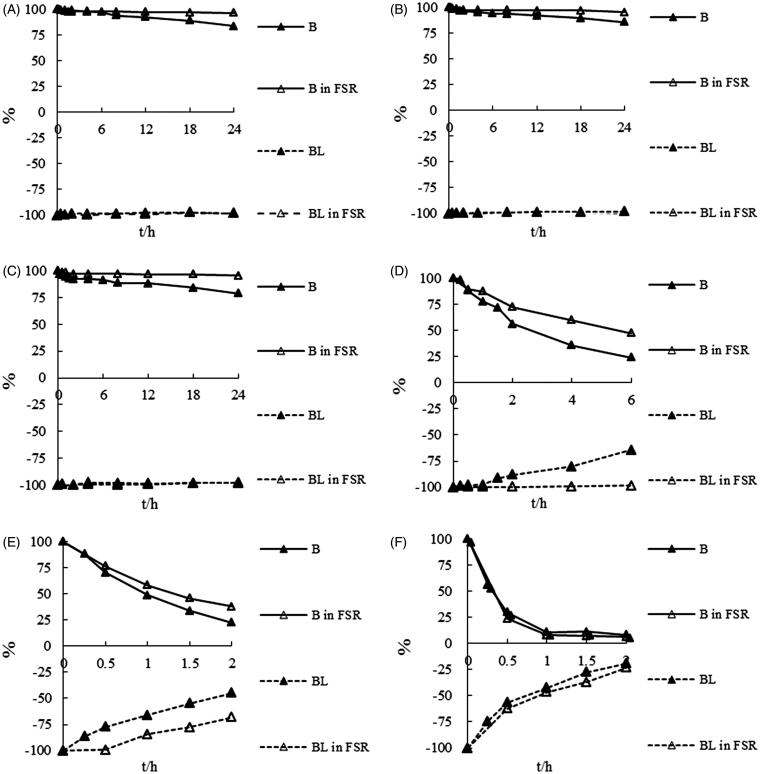
The stability profiles of BL and B in their monomer and FSR form in different pH buffered aqueous solutions at ambient temperature are shown in Figure 2. The profiles indicated that the stability of both BL and B were pH-dependent (R > 0.99) and their degradation would be aggravated greatly with the increasing aqueous solution pH values, For example, B in monomer administration group, t1/2: 130.8 h (pH 2.0)→88.5 (pH 3.0)→68.4 (pH 4.5)→2.63 (pH 6.8)→0.92 (pH 7.4)→0.31 (pH 9.0), was consistent with the literature (Xing et al. 2005). According to the degradation profiles of BL at pH 6.8 and pH 7.4 aqueous solutions, the protective effect of coexistent components in FSR could be observed.
Figure 2.
The degradation curves of baicalin and baicalein administrated in monomer and FSR form in aqueous buffers at different pH (A) pH 2.0; (B) 3.0; (C) 4.5 (D) 6.8; (E) 7.4; (F) 9.0.
Comparative stability of baicalin and baicalein at different temperature
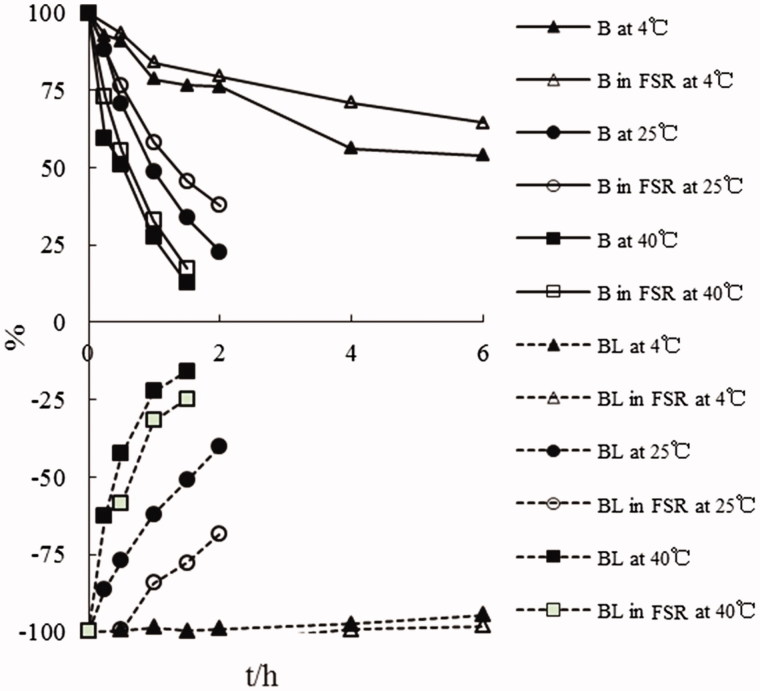
According to the stability results at different temperature shown in Figure 3, Tables 1 and 2, the degradation of BL and B in aqueous buffers at pH 7.4 was temperature-dependent. The protective effect of coexistent components in FSR against degradation of BL and B at different temperatures could be obviously observed. However, with the increase of temperature, this protective effect was found to be more insignificant.
Figure 3.
The degradation curves of baicalin and baicalein administrated in monomer and FSR form in aqueous buffers at pH 7.4 at different temperatures.
Table 1.
Degradation parameters of baicalin in buffered aqueous solutions (25 °C) administrated in monomer and FSR forms.
| Medium | Comp. | First-order kinetic equation | R | K | t1/2(h) |
|---|---|---|---|---|---|
| pH 2.0 (25 °C) | BL | Rather stable | ∝ | ||
| BL in FSR | Rather stable | ∝ | |||
| pH 3.0 (25 °C) | BL | Rather stable | ∝ | ||
| BL in FSR | Rather stable | ∝ | |||
| pH 4.5 (25 °C) | BL | Rather stable | ∝ | ||
| BL in FSR | Rather stable | ∝ | |||
| pH 6.8 (25 °C) | BL | LgC = −0.1043 t + 2.000 | 0.9865 | 0.24 | 2.89 |
| BL in FSR | Rather stable | ∝ | |||
| pH 7.4(25 °C) | BL | LgC = −0.1659 t + 1.9846 | 0.9968 | 0.382 | 1.81 |
| BL in FSR | LgC = −0.1049 t + 2.0431 | 0.9912 | 0.242 | 2.87 | |
| pH 9.0 (25 °C) | BL | LgC = −0.3467 t + 1.9654 | 0.9956 | 0.798 | 0.87 |
| BL in FSR | LgC = −0.2843 t + 1.9713 | 0.9925 | 0.69 | 1.01 | |
| pH 7.4 (4 °C) | BL | LgC = −0.0042 t + 2.0028 | 0.9361 | 0.0097 | 71.7 |
| BL in FSR | Rather stable | ∝ | |||
| pH 7.4 (40 °C) | BL | LgC = −0.4096 t + 1.9705 | 0.9878 | 0.943 | 0.73 |
| BL in FSR | LgC = −0.5682 t + 1.9738 | 0.9941 | 0.53 | 1.31 | |
| PH3.0 (40 °C) | BL in FSR | Rather stable | ∝ | ||
| VC | BL | Rather stable | ∝ | ||
| (25 °C, pH 7.4) | BL in FSR | Rather stable | ∝ | ||
| Na2SO3 | BL | LgC = −0.0871 t + 2.0056 | 0.9943 | 0.201 | 3.45 |
| (25 °C, pH 7.4) | BL in FSR | LgC = −0.0547 t + 2.0084 | 0.9906 | 0.126 | 5.50 |
Table 2.
Degradation parameters of baicalein in buffered aqueous solutions (25 °C) administrated in monomer and FSR forms.
| Medium | Comp. | First-order kinetic equation | R | K | t1/2 (h) |
|---|---|---|---|---|---|
| pH 2.0 (25 °C) | B | LgC = −0.0023 t + 1.988 | 0.9974 | 0.0053 | 130.8 |
| B in FSR | Rather stable | ∝ | |||
| pH 3.0 (25 °C) | B | LgC = −0.0034t + 2.0024 | 0.9953 | 0.0078 | 88.5 |
| B in FSR | Rather stable | ∝ | |||
| pH 4.5 (25 °C) | B | LgC = −0.0044 t + 1.977 | 0.9952 | 0.01 | 68.4 |
| B in FSR | Rather stable | ∝ | |||
| pH 6.8 (25 °C) | B | LgC = −0.1145 t + 2.007 | 0.9949 | 0.264 | 2.63 |
| B in FSR | LgC = −0.068 t + 1.997 | 0.9947 | 0.155 | 4.48 | |
| pH 7.4 (25 °C) | B | LgC = −0.3262 t + 2.013 | 0.9994 | 0.751 | 0.92 |
| B in FSR | LgC = −0.2115 t + 1.9908 | 0.9981 | 0.487 | 1.42 | |
| pH 9.0 (25 °C) | B | LgC = −0.9781 t + 1.991 | 0.9993 | 2.253 | 0.31 |
| B in FSR | LgC = −1.098 t + 1.974 | 0.9967 | 2.528 | 0.27 | |
| pH 7.4 (4 °C) | B | LgC = −0.0396 t + 1.9672 | 0.9846 | 0.091 | 7.6 |
| B in FSR | LgC = −0.0269 t + 1.9633 | 0.9869 | 0.062 | 11.19 | |
| pH 7.4 (40 °C) | B | LgC = −0.5682 t + 1.9738 | 0.9923 | 1.3086 | 0.53 |
| B in FSR | LgC = −0.5327 t + 1.9408 | 0.9851 | 1.2268 | 0.56 | |
| PH3.0 (40 °C) | B in FSR | Rather stable | ∝ | ||
| VC | B | LgC = −0.0126 t + 1.9905 | 0.9723 | 0.029 | 23.9 |
| (25 °C, pH 7.4) | B in FSR | LgC = −0.0087 t + 2.0014 | 0.9989 | 0.02 | 34.6 |
| Na2SO3 | B | LgC = −1.0272 t + 1.9569 | 0.9960 | 2.366 | 0.29 |
| (25 °C, pH 7.4) | B in FSR | 1/4 remain of original amount within 30 min, then slow declination | |||
Influence of antioxygen and sun light on stability of baicalin and baicalein aqueous solutions
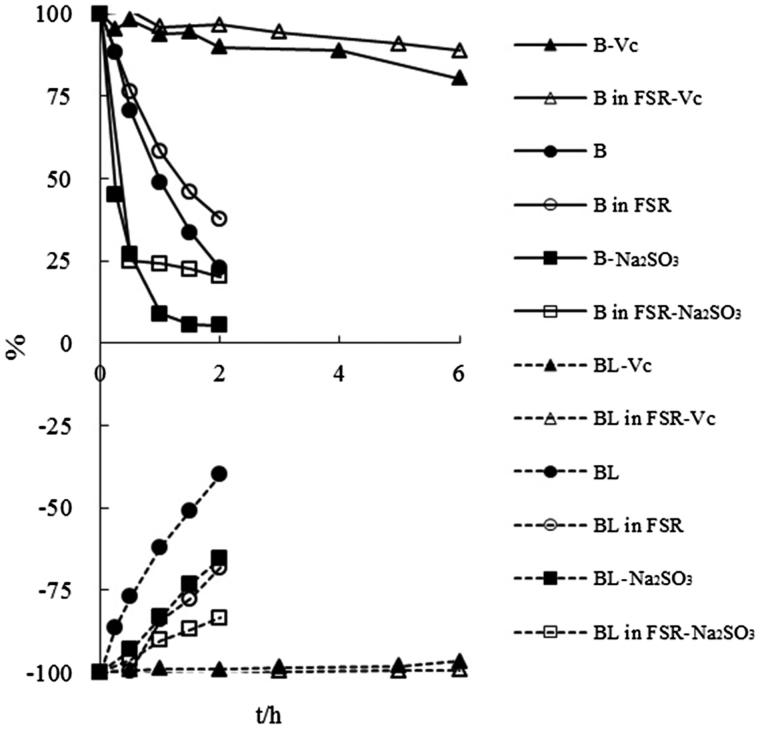
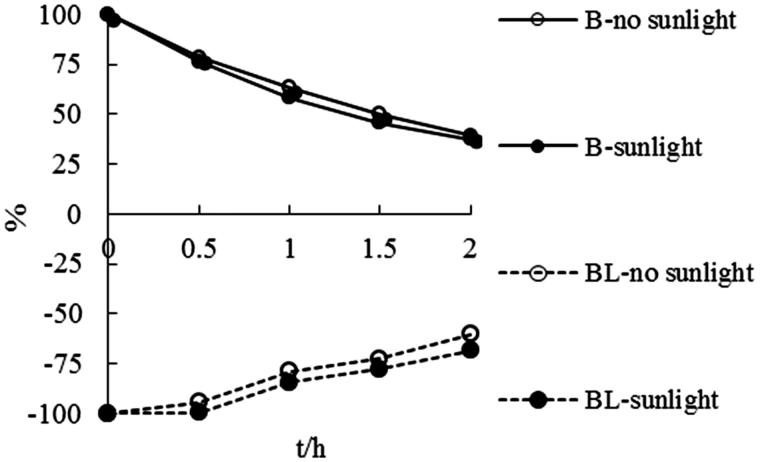
The stability of BL and B in buffer (pH 7.4, 25 °C), containing 0.1% Vc and 0.2% Na2SO3 as antioxidant was evaluated, As shown in Figure 4, the degradation of BL was suppressed after adding 0.1% Vc and 0.2% Na2SO3 as antioxidant, which was consistent with the literature. Judging from the trend line of ‘BL in SFR’ and ‘BL-Na2SO3’ presented in Figure 4, we found that the anti-degradation ability of coexistent components was equivalent to that of Na2SO3. The degradation of B in buffer (pH 7.4, 25 °C) could be effectively suppressed by adding Vc even though slight and constant degradation was still existent. But, contrary to the circumstances of addition Na2SO3 to BL samples, the degradation of B was found to be aggravated after the addition of Na2SO3 for the first time. In our study, the stability of BL and B in FSR form was also investigated under the condition of with or without sunlight illumination. The results (Figure 5) proved that sunlight had no effect on the stability of BL and B in FSR form, and blackout was unnecessary when processing the samples.
Figure 4.
The degradation curves of baicalin and baicalein administrated in monomer and FSR form in aqueous buffers (pH 7.4, 25 °C) with 0.1% Vc and 0.2% Na2SO3 as antioxidant.
Figure 5.
The degradation curves of baicalin and baicalein administrated in FSR form in aqueous buffers (pH 7.4, 25 °C) under sunlight or no sunlight condition.
Comparative stability of baicalin and baicalein in biological fluids
Post-preparative stability (PPS), three freeze–thaw cycles stability (TFTC) and long-term stability (−20 °C) experiments were designed for accessing whether BL (in FSR form) and B (in monomer and FSR form) could be effectively stabilized throughout the biological analysis process by taking the measures recommended above (Tables 3 and 4). Based on the stability experiments in vitro, we evaluated the effectiveness of these protective measures applied for pretreatment and storage of biological samples. In the case of BL in FSR form, as we had predicted that it showed the good stability in the acid treated urine, plasma and tested tissue homogenates (liver, stomach, small intestine and kidney), the relative error (RE) for post-preparative stability (stored at 4 °C for 24 h), three freeze–thaw cycles stability and long-term stability (stored in refrigerator at −20 °C for 15 days) of all biological samples were between the range of −12.8–8.0%, meeting the stability requirements of the biological quantitative analysis. The protective effect of coexistent components in FSR on stabilization of B, especially in plasma and kidney tissue homogenates matrix, could be obviously observed from the data summarized in Tables 3 and 4.
Table 3.
The stability of baicalin and baicalein in urine, plasma and the tissue homogenate of liver.
| Urine |
Plasma |
Liver |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BL-1 | B-1 | B-2 | BL-1 | B-1 | B-2 | BL-1 | B-1 | B-2 | |
| PPS | RE% | RE% | RE% | RE% | RE% | RE% | RE% | RE% | RE% |
| 0.5 h | 6.7 | 4.9 | −10 | −0.1 | −0.6 | −61 | −2.9 | −1.7 | −9.6 |
| 1h | −6.6 | −7.2 | −10.6 | −0.8 | −0.3 | −73.3 | −2.5 | −1.6 | −10.6 |
| 2h | 1.9 | −4.2 | −4.7 | −1.1 | −1.9 | −78.5 | −2.8 | −1.6 | −7.1 |
| 4h | −4.7 | −3.8 | −12.7 | 1.7 | −0.5 | 0.8 | 0.2 | −5.3 | |
| 6h | 7.2 | 6.5 | −9.8 | −1.3 | 5.6 | 0.8 | −3.2 | −6.1 | |
| 8h | −3.7 | −5.1 | −11.7 | −0.9 | 6 | −0.5 | −3.2 | −13 | |
| 12h | 3 | −1.2 | −3.9 | −1.4 | 6.8 | −3.3 | −5 | −10.2 | |
| 24h | 1.9 | −0.9 | −29.9 | −11.2 | −13.1 | −4.6 | −5.3 | −22.9 | |
| TFTC | −1.2 | −1.2 | 2.9 | −2.6 | −0.01 | −2.9 | −1.7 | −5.3 | 6.7 |
| −20 °C | |||||||||
| 1d | 6.8 | −0.5 | −4.7 | −0.3 | −6 | −13.2 | −8.9 | −11 | 6.7 |
| 5d | −7.8 | −6.9 | −6.3 | −10 | −9.3 | −27.6 | −4.6 | −7.9 | 7.4 |
| 10d | 4.8 | −11.8 | 0.3 | −1.6 | 3.4 | −52.7 | −12.8 | −9.5 | 1.6 |
| 15d | −5.1 | −13.4 | 4.8 | −2.1 | −4.6 | −63.8 | −10.5 | −11.8 | −6.7 |
1: in FSR form; 2: in monomer form.
Bold value refers to the deviation of a value greater than 15%.
Table 4.
The stability of baicalin and baicalein in the tissue homogenate of intestine, kidney and stomach.
| Small intestine |
Kidney |
Stomach |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BL-1 | B-1 | B-2 | BL-1 | B-1 | B-2 | BL-1 | B-1 | B-2 | |
| PPS | RE% | RE% | RE% | RE% | RE% | RE% | RE% | RE% | R% |
| 0.5 h | 0.9 | −1.9 | −3.4 | 1.4 | −1.4 | −3.9 | 1 | 7.2 | −1.5 |
| 1 h | −3.3 | −0.3 | −5.2 | −0.4 | −5.5 | −9 | −0.7 | 4.7 | 2.3 |
| 2 h | −3 | −2.3 | −5 | 1.5 | −7.6 | −16.6 | −0.3 | 4.5 | 5.5 |
| 4 h | −1.1 | −4.9 | −7.8 | −0.1 | −8.2 | −35.8 | 0.8 | 7.3 | −3.9 |
| 6 h | −4.5 | −0.9 | −8.1 | 0.5 | −27.4 | −57.3 | 1.8 | 8.3 | −6.4 |
| 8 h | −2.2 | −5.4 | −9.6 | 2.9 | −37.5 | −5.2 | −4.1 | 7.1 | |
| 12 h | −2.3 | −9.4 | −11.7 | 8 | −62 | 4.3 | −1 | −3.5 | |
| 24 h | 1.6 | −10.1 | −13.6 | 7.1 | −65.9 | −3.1 | 6.4 | 6 | |
| TFTC | −10.1 | 1.7 | −7.7 | −8.2 | 9.6 | −13.4 | −7.6 | −3.8 | −6.9 |
| −20 °C | |||||||||
| 1d | −5.1 | −3.5 | −7.2 | 6.7 | −4.2 | −11.6 | −6.2 | −1.3 | −3.5 |
| 5d | −8.9 | −5.6 | −5.1 | −3.3 | −6.5 | −14.8 | −4.6 | −0.7 | 6.8 |
| 10d | 0 | −4.9 | −8.3 | 5.2 | −6.1 | − | −9.2 | −8.3 | −7.2 |
| 15d | −2.1 | 5.3 | −9.7 | 0.9 | −0.5 | − | −2.1 | 3.9 | −3.5 |
1: in FSR form; 2: in monomer form.
Bold value refers to the deviation of a value greater than 15%.
Discussion
In buffered aqueous solutions at pH 2.0, 3.0 and 4.5, BL was rather stable for at least 24 h whether in its monomer form or in FSR form. In buffered aqueous solutions at pH 6.8, BL in FSR form could be stabilized for at least 6 h without any sign of degradation by coexistent components, and BL monomer degraded gradually with the increasing time. In buffered aqueous solutions at pH 7.4 and 9.0, the content of BL rapidly declined, especially at pH 9.0. In the case of B, it was of the worse stability as shown in Figure 2. Briefly speaking, unlike BL monomer, B monomer gradually degraded even at pH 2.0–4.5. According to the FDA guidance for the quantitative analysis of biological samples, taking ±15% deviation from original concentration as the acceptable maximum value, B monomer was stable within 18 h in buffered aqueous at pH 2.0–3.0, and within 12 h in buffered aqueous at pH 4.5. It is noticeable that B in FSR form could be so effectively protected by coexistent components at pH 2.0–4.5 that the degradation had been completely inhibited for at least 24 h. In buffered aqueous solutions at pH 6.8, 7.4 and 9.0, relatively rapid degradation of B in both monomer and FSR form was observed, but the degradation rate of B in FSR form was obviously slower than that in its monomer form if pH <7.4. The degradation rate constants (k) and half-lives (t1/2) of BL and B administrated in monomer and FSR forms in buffered aqueous solutions at different pHs are given in Tables 1 and 2 based on the first-order kinetics equation of lgC = lgC0 - Kt/2.303, where C and C0 are the concentrations at t and zero time, and K is the constant of reaction velocity.
Either in monomer form or in FSR form, B in buffered aqueous solution at pH 7.4 could not be stabilized enough for accurate quantification only by controlling the temperature. BL in two forms in buffered aqueous solution at pH 7.4 could be stabilized for at least 6 h by reducing the temperature to ≤4 °C. The degradation reaction of BL and B at 25 °C and 40 °C also fitted the first-order kinetics equation of lgC = lgC0 - Kt/2.303. It was worth mentioning that BL and B in FSR form would become rather stable without any sign of degradation for at least 12 h if the buffered aqueous solution was justified to pH <3.0 even if they were kept in 40 °C water bath temperature.
Meanwhile, we found that Vc was more effective than Na2SO3 to stabilize BL so that BL in buffer (pH 7.4) in its monomer and FSR form could be stabilized for at least 6 h without any sign of degradation even at room temperature. In case of Na2SO3, although it could slow down the degradation reaction rate of BL, the function was not effective enough to guarantee the requirements of quantification analysis in vivo and in vitro. The reason was probably the addition of Na2SO3, alkali weak acid salts, increased the pH value of the solution, and thereby improved the degradation. The pH influence on the stability of B was more significant than antioxidant protection of Na2SO3.
In summary, the stability of BL and B in aqueous would be greatly improved by adjusting the aqueous solution pH to acidic range with pH <4.5, adding acidic antioxidants and keeping at a low temperature. According to first-order kinetic equation and t1/2 listed in Tables 3 and 4, the anti-degradation ability order of these influence factors was follows: pH > Vc > temperature. The conclusion was deeply proved by our experiment results that when aqueous solution pH value was adjusted to pH =3 with hydrochloric acid (Tables 1 and 2), BL and B in FSR form was rather stable without any sign of degradation for at least 12 h even kept in 40 °C water bath. The stability of BL monomer was better than that of B. Accordingly, BL monomer in aqueous solution showed no degradation trend for hours under the condition of adjusting the aqueous solution to pH <4.5, adding Vc and storage at 4 °C. However, a visible continuous and slight degradation of B could still be observed even as the same protective measures were applied. Coexistent components in FSR presented excellent inhibiting function against the degradation of BL and B that B in FSR form in aqueous could be effectively stabilized without any sign of degradation if pH <3, under the cooperative protective function of coexistent components in FSR.
In previous studies, Xing et al. (2005) has systematically investigated the stability of BL monomer in biological fluids (plasma, urine and liver homogenate) in vitro, and found that the pH, but not the matrix of plasma or urine played an important role in the degradation of BL. They also deduced that other factors (besides pH) were also important in the reaction in tissues, such as gastric fluid or enzyme based on the results that the degradation rates of BL spiked in tissues were all higher than that in plasma. The main contribution of their study was the recommendation of the optimal conditions for collecting, processing and storing biological samples of BL. Our study proved that the stability of B in water aqueous could be improved by the same measures suitable for BL. But, due to its worse stability compared with BL, it was worth further studying whether the protective measures were also effective enough to meet the requirement of quantification analysis of B in various biological samples. BL and B were found relatively stable in organic solvents or acidic solutions, especially at pH 2–4, and their stability would be greatly improved by lowering environmental temperature and adding acid antioxidant. Based on the stability experiments in vitro, we evaluated the effectiveness of these protective measures for processing and storage of biological samples. In the case of BL in FSR form, as predicted it showed the good stability in the acid-treated urine, plasma and tissue homogenates (liver, stomach, small intestine and kidney). B in FSR form could be stabilized enough to meet the requirement of biological quantitative analysis in diverse biological matrix under the contribution of protective measures as well as the coexistent components. In the case of B monomer, its stability was obviously different in various biological samples. Specifically, the stability of B monomer in stomach and small intestine homogenates was relatively better than that in other biological samples, but the gradual degradation could still be observed in small intestine homogenate. In acid-treated urine and liver homogenate, data for three freeze–thaw cycles stability and long-term stability was satisfactory, but for post-preparative stability was acceptable only in the case of storage time less than 12 h. In acid-treated plasma and kidney homogenate, B monomer could not be stabilized effectively. Especially in plasma, B monomer has been degraded by 60% and 27.6% even stored at 4 °C after pretreatment, and the RE value just met the quantification requirement for merely 0.5 h (kept at 4 °C) and 5 days (in −20 °C refrigerator), respectively. So, matrix of plasma or kidney played an important role in the degradation of B, and the coexistent components in FSR would to some extent inhibit the matrix affection.
Conclusions
The results obtained in the study indicated that the stability of BL and B was affected not only by the environmental parameters such as pH, temperature, solvent, antioxidants and so on, but also by the coexistent components in the effective total flavonoids fractions (FSR). Their stability could be greatly improved by coexistent components in vivo and in vitro, which meant that the unique therapeutic effect of TCMs different from the single compound also owed much to drug–drug interaction on the stability of contributed components. Our research also illustrated that B monomer in plasma and kidney homogenate was extremely unstable; it could hardly be accurately quantified even if various protective measures mentioned above were taken, and the matrix of plasma or kidney played an important role in the degradation of B.
Funding Statement
This research work was financially supported by the Science and Technology Planning Project of Guangdong province [No. 2016A020226031] and the Program of Science and Technology of Guangzhou [No: 201607010334] of Guangdong Provincial Science and Technology Department.
Disclosure statement
The authors report that they have no conflicts in interest. The authors alone are responsible for the content and writing of this article.
References
- Chen H, Xu Y, Wang J, Zhao W, Ruan H.. 2015. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation and oxidative stress in rat. Int J Clin Exp Pathol. 8:10139–10147. [PMC free article] [PubMed] [Google Scholar]
- Choi EO, Jeong JW, Park C, Hong SH, Kim GY, Hwang HJ, Cho EJ, Choi YH.. 2016. Baicalein protects C6 glial cells against hydrogen peroxide-induced oxidative stress and apoptosis through regulation of the Nrf2 signaling pathway. Int J Mol Med. 37:798–806. [DOI] [PubMed] [Google Scholar]
- Feng J, Xu W, Xia T, Wei H, Cai F, Jiang B, Chen W.. 2010. Simultaneous determination of baicalin, baicalein, wogonin, berberine, palmatine and jatrorrhizine in rat plasma by liquid chromatography–tandem mass spectrometry and application in pharmacokinetic studies after oral administration of traditional Chinese. J Pharm Biomed Anal. 53:591–598. [DOI] [PubMed] [Google Scholar]
- Feng ZQ, Han J, Xie ZY, Liao QF, Zhang L.. 2012. Determination of plasma protein binding rate of multicomponent in Scutellaria baicalensis Georgi. Chin Pharmacol Bull. 28:286–289. [Google Scholar]
- Hou YC, Lin SP, Tsai SY, Ko MH, Chang YC, Chao PD.. 2010. Flavonoid pharmacokinetics and tissue distribution after repeated dosing of the roots of Scutellaria baicalensis in rats. Planta Med. 77:455–460. [DOI] [PubMed] [Google Scholar]
- Li K, Wang J, Shi M, Li J, Yan L, Zhang H, Lu C.. 2015. Prescription consisting of vitamin C and baicalin inhibits tumor growth by enhancing the antioxidant capacity in vivo. J Buon. 20:1368–1372. [PubMed] [Google Scholar]
- Qiu F, Tang X, He ZG, Li HZ.. 2004. Stability of baicalin aqueous solution by validated RP-HPLC. J China Pharma Sci. 13:134–137. [Google Scholar]
- Tong L, Wan M, Zhang L, Zhu YH, Sun H, Bi KS.. 2012. Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, oroxylin A and chrysin of Radix scutellariae extract in rat plasma by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 70:6–12. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang YJ, Li WF, Yang J, Jiang H, Sun WJ.. 2008. Study on stability of baicalein in mouse plasma by HPLC-ECD. China J Chin Mater Med. 33:2675–2678. [PubMed] [Google Scholar]
- Wang XL, Zhong FL, Li DM, Zhu B.. 2008. Study on extraction process of total flavonoids in Herba Desmodii Styracifolii. J Chin Med Mater. 31:900–902. [PubMed] [Google Scholar]
- Xing J, Chen XY, Zhong DF.. 2005. Stability of baicalin in biological fluids in vitro. J Pharm Biomed Anal. 39:593–600. [DOI] [PubMed] [Google Scholar]
- Yan WJ, Ma XC, Gao XY, Xue XH, Zhang SQ.. 2016. Latest research progress in the correlation between baicalein and breast cancer invasion and metastasis. Mol Clin Oncol. 4:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YH, Zhang LW.. 2006. Study on stability of baicalein. China J Spectro Lab. 23:346–348. [Google Scholar]
- Yu BT, Zhang ZR, Liu WS, Wang P, Yang T.. 2002. Studies on stability of baicalin. China Tradit Herb Drugs. 33:218–220. [Google Scholar]
- Zhou YJ, Wang H, Sui HH, Li L, Zhou CL, Huang JJ.. 2016. Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic rhinitis guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflamm Res. 65:603–612. [DOI] [PubMed] [Google Scholar]
- Zhu ZH, Yan HM, Wen YJ, Yang Y.. 2006. Influence of heat in airtight condition on thermostability of baicalin and baicalein. Henan Sci. 24:353–354. [Google Scholar]