Abstract
Context: The essential oil (EO) from Thymus capitatus Hoff. et Link. (Lamiaceae) has been traditionally used for its medicinal properties, such as anti-inflammatory, analgesic, antioxidant and antimicrobial properties.
Objective: Characterize the constituents from T. capitatus EO and further evaluate the antinociceptive activity by in vivo and in vitro procedures.
Materials and methods: Gas chromatography–mass spectrometry was used to identify and quantify the constituents of the T. capitatus EO. The antinociceptive activity was evaluated in vivo by the glutamate-induced nociception model in male Swiss mice (25 g), at doses of 3, 6 and 12 mg/kg, 1 h before evaluation of the licking time response (0–15 min). The mechanism of T. capitatus EO (1–500 μg/mL) on the isolated nerve excitability of Wistar rat (300 g) was assessed by the single sucrose technique.
Results and discussion: The EO of T. capitatus presented 33 components, mainly monoterpenes and sesquiterpenes, carvacrol (ca. 80%) was its major constituent. T. capitatus EO induced antinociception in orally treated mice (3, 6, and 12 mg/kg) reducing the licking time from control (100.3 ± 11.9 s) to 84.8 ± 12.2, 62.7.6 ± 9.9, and 41.5 ± 12.7 s, respectively (n = 8; p < 0.05). Additionally, we have demonstrated that T. capitatus EO (500 μg/mL) decreased the compound action potential amplitude (VCAP) of about 80.0 ± 4.3% from control recordings (n = 4; p < 0.05). Such activity was presumably mediated through a voltage-gated Na+ channels.
Conclusions: The present study demonstrated the antinociceptive activity of Thymus capitatus essential oil, which acts via peripheral nervous excitability blockade.
Keywords: Pain, glutamate, action potential
Introduction
The essential oils (EO) are volatile molecules generated by the secondary metabolism of higher plants and are produced by specialized secretory structures, such as glandular hairs and differentiated parenchyma cells (Lane et al. 2010). EO constituents belong mainly to two chemical groups: terpenoids (monoterpenes and sesquiterpenes of low molecular weight) and, to a lesser extent, phenylpropanoids (Regnault-Roger et al. 2012). These EO also have shown many biological activities, such as analgesic, anticonvulsant, anxiolytic, antioxidant, and antimicrobial properties (Faturi et al. 2010; Pergolizzi et al. 2010; Almeida et al. 2011).
Thymus (Lamiaceae) comprises over 300 species of hardy perennial herbaceous plants and subshrubs, which are native to southern Europe, Asia and North Africa (Tepe et al. 2004). Thyme is a distinguished condiment that is used in traditional medicine for several purposes, for example, as an anti-inflammatory and analgesic agent (Alamger et al. 2015; Amirghofran et al. 2016). The pharmacological activity of Thymus capitatus Hoff. et Link. EO have been demonstrated, including antiseptic, antioxidant, and antimicrobial properties (Consentino et al. 1999; Al-Mustafa & Al-Thunibat 2008; Mkaddem et al. 2010; Russo et al. 2013; Džamić et al. 2015), however, the antinociceptive and analgesic properties have not yet been evaluated. Therefore, the present study was designed to determine the major constituents from the T. capitatus EO and to evaluate its antinociceptive effects by in vivo and in vitro procedures.
Materials and methods
Chemicals
Pure standards for GC-MS analysis were purchased from Sigma-Aldrich (St. Louis, MO) and Extrasynthese (Lyon, France). Glutamate, morphine, Tween 80 (polyoxyethylene sorbitan monooleate), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). All salts used in physiological solution, sucrose, glucose and NaOH, were purchased from Vetec (Rio de Janeiro, Brazil). HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] was acquired from Amresco (Solon, OH).
Plant processing
Wild Thyme sample was collected in the middle of Sicily, in 2006, at bloom stage and dried at room temperature until no constant weight loss was observed at the collection point, Calascibetta lands (Enna province, Italy), 37°59′83″(N), 14°25′26″(E). The plant collection and identification was made by the Regional Unit UOT Enna, as described by Napoli et al. (2010), in which herbarium voucher specimen was deposited. Dried aerial part of the sample (100 g) was subjected to hydrodistillation in accordance with current European Pharmacopoeia 6.0 (2008) until there was no significant increase in the volume of oil collected (3 h). The essential oil obtained was dried over anhydrous sodium sulfate and stored under N2 in a sealed vial until required.
Oil analysis
Gas chromatographic (GC) analyses were run on a Shimadzu gas chromatograph, Model 17-A, equipped with a flame ionization detector (FID) and with an operating software Class VP Chromatography Data System, version 4.3 (Shimadzu Corporation, Kyoto, Japan). Analytical conditions were SPB-5 capillary column (15 m × 0.10 mm × 0.15 μm) and helium as the carrier gas (1 mL/min). Injection was done in split mode (1:200), injected volume was 1 μL (4% essential oil/CH2Cl2 v/v), and the injector and detector temperatures were 250 °C and 280 °C, respectively. Linear velocity in column 19 was cm/s. The oven temperature was held at 60 °C for 1 min, then programed from 60 to 280 °C at 10 °C/min then 280 °C for 1 min. Percentages of compounds were determined from their peak areas in the GC-FID profiles.
Gas chromatography-mass spectrometry (GC-MS) analysis was carried out in the fast mode on a Shimadzu GC-MS model GCMS-QP5050A, with the same column and the same operative conditions used for the analytical GC and the operating software GCMS solution version 1.02 (Shimadzu Corporation, Kyoto, Japan). We adjusted the ionization voltage at 70 eV, the electron multiplier at 900 V and the ion source temperature at 180 °C. Mass spectra data were acquired in the scan mode in an m/z range of 40–400. The same oil solutions (1 μL) were injected with the split mode (1:96).
The compounds were identified based on their GC retention index (relative to C9-C22 n-alkanes on the SPB-5 column), computer matching of spectral MS data with those from NIST MS 107 and NIST 21 libraries (National Institute of Standard and Technology 1998), comparison of the fragmentation patterns with those reported in the literature (Adams 2001) and, whenever possible, co-injections with authentic samples.
Animals
Adult male Swiss mice and Wistar rats, weighing around 25 and 300 g, respectively, were randomly housed in appropriate cages at 24 ± 2 °C on a 12 h light cycle with free access to food (Purina, São Paulo, Brazil). All procedures in this study were carried out in accordance with Institutional Animal Care and Use Committee (CEEA-UFPI and CEPA-UFPB, Brazil).
Glutamate-induced nociception
The procedure used was similar to that previously described by Beirith et al. (2002). In brief, a volume of 20 μL of glutamate (10 μM/paw) was injected intraplantarly in the ventral surface of the mice’s right hind paw. Animals were divided into five groups (n = 8), orally treated with vehicle (Tween 80 0.1% in distilled water) in the control group, T. capitatus EO (3, 6, and 12 mg/kg) and morphine (5 mg/kg), 1 h before experimentation. After drug treatment, the licking time (0–15 min) of the glutamate-injected paw was recorded and considered as indicative of nociception (Donato et al. 2015).
Electrophysiological experiments
The single sucrose gap technique was used as previously described by Gonçalves et al. (2010). Briefly, rat sciatic nerves (n = 4) were carefully removed and immediately positioned in an experimental chamber composed of five compartments (I–V), electrically isolated by solid vaseline. All compartments were filled with a physiological solution, composed of (in mM): NaCl 150; KCl 4; CaCl2 2; MgCl2 1; glucose 10 and HEPES 10. The pH was adjusted to 7.4 with NaOH. Compartments I and II of the chamber were used to apply supramaximal stimulation (6–10 V/100 ms), manually triggered at 5 min intervals (CF Palmer, London, UK). The compartment III was superfused by isotonic sucrose (290 mM; 1 mL/min) to electrically isolate the neighbouring recording compartments. Compartments IV and V were used for drug perfusion (1 mL/min) and data recording, acquired by a microcomputer-based 12-bit A/D converter (10.5 kHz) and later analyzed by proper software (Lynx, São Paulo, Brazil)).
T. capitatus EO was diluted into a vehicle (0.1% Tween 80 in physiological solution) at concentrations of 1, 100 and 500 μg/mL. Drug incubation was performed for 30 min followed by nerve washout with physiological solution for additional 30 min. To quantify drug effects, we analyzed the compound action potential (CAP) amplitude (VCAP, in mV) by measuring the difference between the baseline and the maximal voltage achieved. The CAP depolarization velocity (DVCAP, in V/s) was the rate between VCAP and the time required to reach the CAP peak. The time constant of repolarization (τrep, in ms) was calculated by the equation: V = V0*exp(−t/τ), as described by Alves et al. (2010).
Statistical analysis
Data are presented as the mean ± S.E.M. of independent experiments. To assess the significance level, analysis of variance (ANOVA) followed by Dunnet’s test for in vivo data (n = 8) and the two-tailed Student’s t-test for in vitro data (n = 4) were used. Differences between experimental and control groups were considered significant when p < 0.05.
Results
Table 1 lists the composition of the T. capitatus essential oil. In total, 33 components were fully identified, covering more than 96% of the total composition. Monoterpenes, both hydrocarbons and oxygenated, were the most highly represented classes, while sesquiterpenes and other classes were the least represented.
Table 1.
Chemical composition of T. capitatus essential oil (EO).
|
T. capitatus EO |
|||
|---|---|---|---|
| # | KIa | Compound | Areab (%) |
| 1 | 778 | 2-Methyl-butanoic acid methyl ester | 0.1 |
| 2 | 932 | α-Thujene | 0.7 |
| 3 | 939 | α-Pinene | 0.6 |
| 4 | 954 | Camphene | 0.2 |
| 5 | 982 | β-Pinene | 0.4 |
| 6 | 993 | β-Myrcene | 1.7 |
| 7 | 1008 | α-Phellandrene | 0.2 |
| 8 | 1015 | p-Menth-1-(7),8-diene | 0.1 |
| 9 | 1021 | α-Terpinene | 1.2 |
| 10 | 1033 | p-Cymene | 4.4 |
| 11 | 1035 | Limonene | 0.4 |
| 12 | 1052 | trans-β-Ocimene | 0.1 |
| 13 | 1063 | γ-Terpinene | 3.7 |
| 14 | 1072 | Sabinene Hydrate | 0.2 |
| 15 | 1091 | α-Terpinolene | 0.2 |
| 16 | 1100 | Linalool | 0.9 |
| 17 | 1126 | α-Campholenal | 0.1 |
| 18 | 1172 | Borneol | 0.4 |
| 19 | 1181 | Terpinen-4-ol | 0.8 |
| 20 | 1193 | α-Terpineol | 0.1 |
| 21 | 1248 | Nerol | 0.1 |
| 22 | 1255 | Neral | 0.1 |
| 23 | 1264 | Carvone | 0.1 |
| 24 | 1273 | Geranial | 0.1 |
| 25 | 1290 | Thymol | 0.3 |
| 26 | 1313 | Carvacrol | 79.9 |
| 27 | 1362 | Thymol acetate | 0.1 |
| 28 | 1425 | Caryophyllene | 2.3 |
| 29 | 1446 | Aromadendrene | 0.1 |
| 30 | 1460 | α-Humulene | 0.1 |
| 31 | 1511 | β-Bisabol | 0.2 |
| 32 | 1545 | α-Cadinene | 0.2 |
| 33 | 1589 | Caryophyllene Oxide | 0.2 |
| Monoterpene hydrocarbons | 13.7 | ||
| Oxygenated monoterpenes | 83.0 | ||
| Sesquiterpenes | 3.1 | ||
| Others | 0.1 | ||
KI: Kovat’s indices; RT: retention time.
Retention index (KI) relative to standard mixture of n-alkanes on SPB-5 column.
The numbering refers to elution order, and values (relative peak area percent) represent averages of 3 determinations.
We stated that the oxygenated monoterpene carvacrol (ca. 80%) strongly characterizes the T. capitatus EO being its major constituent. The two monoterpene hydrocarbons, namely p-cymene (ca. 4.4%) and γ-terpinene (ca. 3.7%) biosynthetically correlated to carvacrol, were the other two main components, although in lower concentrations (Table 1). Finally, among the other components, only the sesquiterpene caryophyllene reaches an appreciable amount (ca. 2.3%).
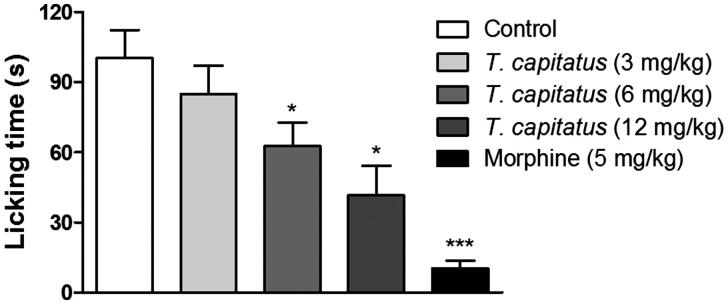
During the test of glutamate nociception, we observe that animals orally treated with T. capitatus EO at doses of 3, 6, and 12 mg/kg (Figure 1), reduced the licking time from control (100.3 ± 11.9 s) to 84.8 ± 12.2, 62.7.6 ± 9.9 and 41.5 ± 12.7 s (p < 0.05), respectively. As expected, the standard drug morphine (5 mg/kg) decreased the nociceptive response to 10.3 ± 3.4 s from control (p < 0.05).
Figure 1.
Antinociceptive effect of T. capitatus EO in mice. The T. capitatus EO was administered in mice orally (3–12 mg/kg) 1 h before initiating the glutamate-induced nociception method. Control animals were treated with vehicle alone. Morphine (5 mg/kg) was used as a positive control. Values are expressed as mean ± S.E.M, n = 8. *p < 0.05, ***p < 0.001 vs. control (ANOVA followed by Dunnet’s test).
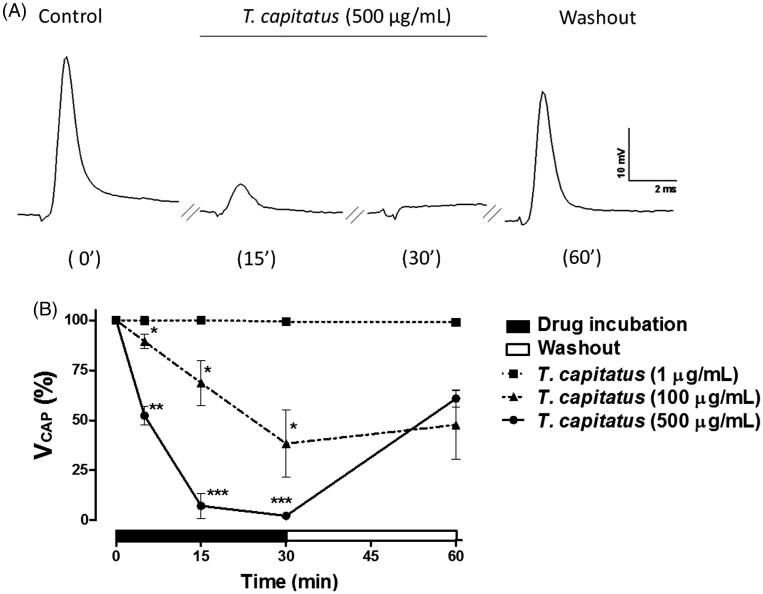
Testing the T. capitatus EO in the peripheral nerve excitability, we showed that 500 μg/mL was sufficient to completely block VCAP after 30 min of incubation and this effect was latter reverted by 30 min of drug removal (Figure 2(A)). In fact, 500 μg/mL of T. capitatus EO practically reached is maximum effect with only 15 min of incubation (Figure 2(B)). At 100 μg/mL of T. capitatus EO, we observed that VCAP was reduced to 10.6 ± 3.6, 31.4 ± 11.3, and 58.1 ± 16.8% from control, after 5, 15, and 30 min of incubation, respectively (Figure 2(B)). Vehicle alone did not induce any significant change in the CAP characteristics (data not shown).
Figure 2.
T. capitatus EO reversibly blocks the peripheral nerve excitability. (A) Representative CAP recordings obtained by the single sucrose gap technique after 5, 15 and 30 min of T. capitatus EO (500 μg/mL) incubation. After drug incubation, the nerve was washed out with the physiological solution for 30 min. Control records were obtained when sciatic nerves were submitted to the physiological solution only. Stimulation parameters were 6–10 V/0.1 ms. (B) Time- and concentration-dependent effects of T. capitatus EO (1–500 μg/mL) on CAP amplitude (VCAP) during 30 min incubation followed by nerve washout (30 min). Values are expressed as mean ± S.E.M, n = 4. *p < 0.05; **p < 0.01; ***p < 0.001 vs. control (Student’s t-test).
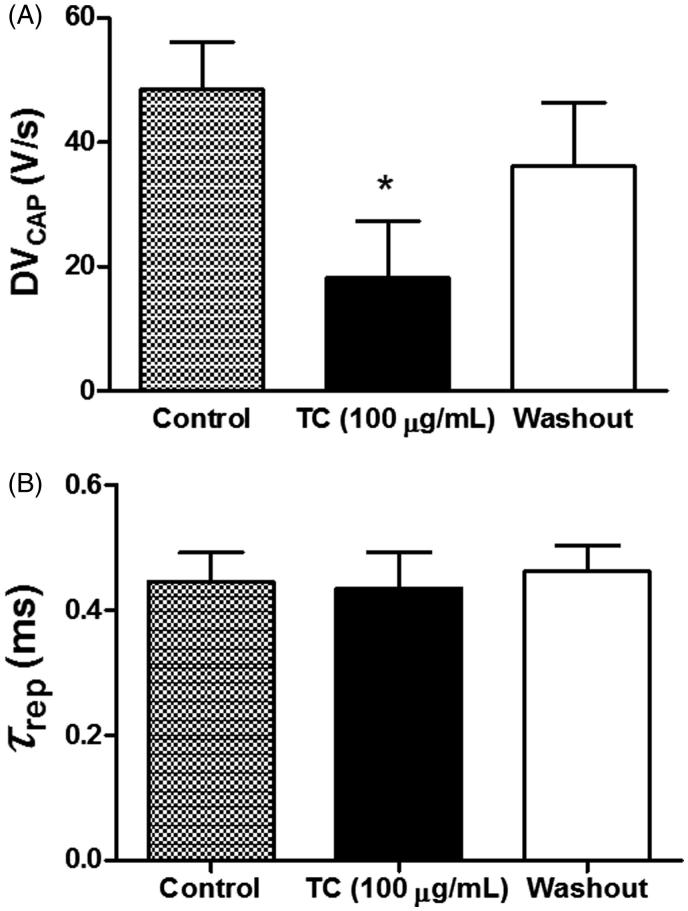
We also evaluated the effect of T. capitatus EO on DVCAP and τrep parameters in order to assess for alterations in the depolarization and/or repolarization phases of CAP recordings. Taken together, our results showed that T. capitatus EO (100 μg/mL) decreased DVCAP from 48.4 ± 7.6 V/s (control) to 18.1 ± 9.1 V/s (p < 0.05) after 30 min of incubation. Such effect was reversed to 26.1 ± 10.2 V/s after drug washout (Figure 3(A)). Meanwhile, no differences were observed on the CAP repolarization phase, measured by τrep (Figure 3(B)).
Figure 3.
Effects of T. capitatus on the depolarization and repolarization phases of the CAP. T. capitatus EO (TC, 100 μg/mL) was incubated in the rat sciatic nerve for 30 min followed by drug washout out with the physiological solution. The CAP parameters like (A) the depolarization velocity (DVPAC), and (B) the time constant of repolarization (τrep), were quantified and compared to control. Values are expressed as mean ± S.E.M, n = 4. *p < 0.05 vs. control (Student’s t-test).
Discussion
The aforementioned aromatic profile is similar to Sicilian Thyme, as recently reported in a study on 30 samples collected throughout the Sicilian island, suggesting that Thymus capitatus [syn. Coridothymus capitatus (L.) Rchb.f., Satureja capitata L., Thymbra capitata (L.) Cav.] is the most widespread wild species in the Sicilian area (Napoli et al. 2010). In the present study, we analyzed the EO composition of T. capitatus, demonstrating that it is composed of 33 different molecules, including monoterpenes and sesquiterpenes, and other compounds like phenols, alcohols, organic acids, aldehydes and ketones. The monoterpene carvacrol was found as the main component, corresponding to ∼80% of the whole oil (Table 1).
Recently, several studies have reported the antinociceptive activity of Thymus sp. extracts and their major constituents (Mahmoudi et al. 2011; Taherian et al. 2009; Cavalcante Melo et al. 2012). In this way, we evaluated whether T. capitatus EO also induces analgesy in vivo. For that, the glutamate-induced nociception model was performed in mice pretreated with T. capitatus EO by the oral tract, in order to imply that the drug was absorbed in vivo. Our data demonstrated significant analgesic activity and dose-dependency of T. capitatus EO (Figure 1).
At the peripheral level, the glutamate is involved in nociceptive transmission through primary afferent fibres, as well as in the development and maintenance of the pain response (Osikowicz et al. 2013; Bardoni 2013). This amino acid acts on ionotropic receptors such as N-methyl-d-aspartate (NMDA), increasing cation influx, like Na+ or Ca2+, through plasma membrane, activating post-synaptic transmission and deflagrating action potentials in nociceptive fibres, leading to pain sensation (Beirith et al. 2002; Srebro et al. 2015). Therefore, it is plausible to suggest that the constituents of T. capitatus EO might also interfere with the excitability of peripheral nerves.
To assess the effects of T. capitatus EO on the isolated nerve excitability, we performed the single sucrose technique. This method enables the study of bioactive compounds on the peripheral nerve by measuring the CAP evoked by external electrodes (Alves et al. 2010; Gonçalves et al. 2010). According to the presented data, we have demonstrated that T. capitatus EO impede nervous transmission by blocking the CAP amplitude, in a concentration- and time-dependent manner (Figure 2(A) and (B)). In addition, such effect was reverted by washing the drug out. These data also suggest that T. capitatus EO did not damage the nerve fibres functionality during the experimentation period.
The CAP recording is considered the algebraic sum of the action potential from all individual fibres of the nerve. Their shapes and properties indicate a single-action potential and therefore it can be a useful tool for an initial searching of neuroactive drug candidates. It has also been shown that inhibition of neuronal excitability, observed as a decrease in the CAP amplitude, might be associated with the blockade of the voltage-gated Na+ channels (Nav), which are responsible for the rising phase of the action potential in neurons (Araújo et al. 2011). The present study demonstrated that 100 μg/mL of T. capitatus EO delayed the CAP depolarization phase (DVCAP) after 30 min-incubation in rat sciatic nerves. Thus, we can suggest a Nav blocking-like effect promoted by T. capitatus EO in a reversible manner, since this effect was recovered by drug washout (Figure 3(A)). In fact, recent findings have demonstrated the ability of carvacrol, the major constituent of T. capitatus EO, to induce Nav blockade in DRG neurons (Joca et al. 2012, 2015). Those findings corroborate our results and strongly suggest that carvacrol is the main active molecule behind the antinociceptive effects of T. capitatus.
On the other hand, the voltage-gated K+ channels are believed to be an important factor in the repolarization phase of peripheral nerves, especially the delayed rectifier potassium channel (Kv). Some studies have demonstrated that the time constant of depolarization (τrep) of the CAP can be used as a valuable tool for the investigation of potential Kv blockers (Alves et al. 2010). Hence, it is unlikely that T. capitatus EO is related to this channel variety since no change in τrep was observed (Figure 3(B)).
In conclusion, our study reported that the carvacrol-rich essential oil from T. capitatus exerts its antinoceptive activity through peripheral nervous excitability blockade.
Funding Statement
The authors are grateful to CNPq and CAPES for providing financial support.
Acknowledgements
The authors are grateful to CNPq and CAPES for providing financial support.
Disclosure statement
The authors report no declarations of interest.
References
- Adams RP.2001. Identification of essential oil components by gas chromatographic/quadrupole mass spectrometry. Carol Stream (IL): Allured Publishing Corporation. [Google Scholar]
- Alamger, Mazhar U, Mushtaq MN, Khan HU, Maheen S, Malik MN, Ahmad T, Latif F, Tabassum N, Khan AQ. 2015. Evaluation of anti-inflammatory, analgesic and antipyretic activities of Thymus serphyllum Linn. in mice. Acta Pol Pharm. 72:113–118. [PubMed] [Google Scholar]
- Almeida RN, Agra MF, Maior FN, Sousa DP. 2011. Essential oils and their constituents: anticonvulsant activity. Molecules. 23:2726–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mustafa AH, Al-Thunibat OY.. 2008. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak J Biol Sci. 11:351–358. [DOI] [PubMed] [Google Scholar]
- Alves AMH, Gonçalves JCR, Cruz JS, Araújo DAM. 2010. Evaluation of the sesquiterpene (−)-alpha-bisabolol as a novel peripheral nervous blocker. Neurosci Lett. 472:11–15. [DOI] [PubMed] [Google Scholar]
- Amirghofran Z, Ahmadi H, Karimi MH, Kalantar F, Gholijani N, Malek-Hosseini Z. 2016. In vitro inhibitory effects of thymol and carvacrol on dendritic cell activation and function. Pharm Biol. 54:1125–1132. [DOI] [PubMed] [Google Scholar]
- Araújo DAM, Freitas C, Cruz JS.. 2011. Essential oils components as a new path to understand ion channel molecular pharmacology. Life Sci. 89:540–544. [DOI] [PubMed] [Google Scholar]
- Bardoni R.2013. Role of presynaptic glutamate receptors in pain transmission at the spinal cord level. Curr Neuropharmacol 11:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirith A, Santos ARS, Calixto JB.. 2002. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 924:219–228. [DOI] [PubMed] [Google Scholar]
- Cavalcante Melo FH, Rios ER, Rocha NF, Citó Mdo C, Fernandes ML, de Sousa DP, de Vasconcelos SM, de Sousa FC. 2012. Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J Pharm Pharmacol. 64:1722–1729. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Tuberoso CI, Pisano B, Satta M, Mascia V, Arzedi E, Palmas F. 1999. Palmas, in-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett Appl Microbiol. 29:130–135. [DOI] [PubMed] [Google Scholar]
- Donato F, Pavin NF, Goes AT, Souza LC, Soares LC, Rodrigues OE, Jesse CR, Savegnago L. 2015. Antinociceptive and anti-hyperalgesic effects of bis(4-methylbenzoyl) diselenide in mice: evidence for the mechanism of action. Pharm Biol. 53:395–403. [DOI] [PubMed] [Google Scholar]
- Džamić AM, Nikolić BJ, Giweli AA, Mitić-ćulafić DS, Soković MD, Ristić MS, Knežević-Vukčević JB, Marin PD. 2015. Libyan Thymus capitatus essential oil: antioxidant, antimicrobial, cytotoxic and colon pathogen adhesion-inhibition properties. J Appl Microbiol. 119:389–399. [DOI] [PubMed] [Google Scholar]
- European Pharmacopoeia 6.0 (2008). Determination of essential oils in herbal drugs. Strasbourg (France): Council of Europe. [Google Scholar]
- Faturi CB, Leite JR, Alves PB, Canton AC, Teixeira-Silva F. 2010. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 34:605–609. [DOI] [PubMed] [Google Scholar]
- Gonçalves JC, Alves Ade M, de Araújo AE, Cruz JS, Araújo DA. 2010. Distinct effects of carvone analogues on the isolated nerve of rats. Eur J Pharmacol. 645:108–112. [DOI] [PubMed] [Google Scholar]
- Joca HC, Cruz-Mendes Y, Oliveira-Abreu K, Maia-Joca RP, Barbosa R, Lemos TL, Lacerda Beirão PS, Leal-Cardoso JH. 2012. Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J Nat Prod. 75:1511–1517. [DOI] [PubMed] [Google Scholar]
- Joca HC, Vieira DC, Vasconcelos AP, Araújo DA, Cruz JS. 2015. Carvacrol modulates voltage-gated sodium channels kinetics in dorsal root ganglia. Eur J Pharmacol. 756:22–29. [DOI] [PubMed] [Google Scholar]
- Lane A, Boecklemann A, Woronuk GN, Sarker L, Mahmoud SS. 2010. A genomics resource for investigating regulation of essential oil production in Lavandula angustifolia. Planta. 231:835–845. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Morteza-Semnani K, Mojra E.. 2011. Anti-inflammatory and antinociceptive activity of Thymus pubescens extract. Fitoterapia. 79:361–365. [DOI] [PubMed] [Google Scholar]
- Mkaddem MG, Romdhane M, Ibrahim H, Ennajar M, Lebrihi A, Mathieu F, Bouajila J. 2010. Essential oil of Thymus capitatus Hoff. et Link. from Matmata, Tunisia: gas chromatography-mass spectrometry analysis and antimicrobial and antioxidant activities. J Med Food. 13:1500–1504. [DOI] [PubMed] [Google Scholar]
- Napoli EM, Curcuruto G, Ruberto G.. 2010. Screening the essential oil composition of wild Sicilian thyme. Biochem Syst Ecol. 38:816–822. [Google Scholar]
- National Institute of Standard and Technology (NIST). 1988. Mass Spectral Library. Technology Administration (MD): Department of Commerce. [Google Scholar]
- Osikowicz M, Mika J, Przewlocka B.. 2013. The glutamatergic system as a target for neuropathic pain relief. Exp Physiol. 98:372–384. [DOI] [PubMed] [Google Scholar]
- Pergolizzi JV, Pappagallo M, Raffa RB, Gharibo C, Phillips RB, Desjonquères S, Tabor A. 2010. Antinociceptive effect of Zanthoxylum rhoifolium Lam. (Rutaceae) in models of acute pain in rodents. J Ethnopharmacol. 129:227–231. [DOI] [PubMed] [Google Scholar]
- Regnault-Roger C, Vincent C, Arnason JT.. 2012. Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol. 57:405–424. [DOI] [PubMed] [Google Scholar]
- Russo M, Suraci F, Postorino S, Serra D, Roccotelli A, Agosteo GE. 2013. Essential oil chemical composition and antifungal effects on Sclerotium cepivorum of Thymus capitatus wild populations from Calabria, southern Italy. Braz J Pharmacog. 23:239–248. [Google Scholar]
- Srebro DP, Vučković SM, Savić Vujović KR, Prostran MS. 2015. TRPA1, NMDA receptors and nitric oxide mediate mechanical hyperalgesia induced by local injection of magnesium sulfate into the rat hind paw. Physiol Behav. 139:267–273. [DOI] [PubMed] [Google Scholar]
- Taherian AA, Babaei M, Vafaei AA, Jarrahi M, Jadidi M, Sadeghi H. 2009. Antinociceptive effects of hydroalcoholic extract of Thymus vulgaris. Pak J Pharm Sci. 22:83–89. [PubMed] [Google Scholar]
- Tepe B, Daferera D, Sökmen M, Polissiou M, Sökmen A. 2004. In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii M. Zohary et P.H. Davis. J Agric Food Chem. 52:1132–1137. [DOI] [PubMed] [Google Scholar]