Abstract
Sensory nerve fibers differ not only with respect to their sensory modalities and conduction velocities, but also in their relative roles for pain hypersensitivity. It is presently largely unknown which types of sensory afferents contribute to various forms of neuropathic and inflammatory pain hypersensitivity. Vesicular glutamate transporter 3-positive (VGluT3+) primary afferents, for example, have been implicated in mechanical hypersensitivity after inflammation, but their role in neuropathic pain remains under debate. Here, we investigated a possible etiology-dependent contribution of VGluT3+ fibers to mechanical and cold hypersensitivity in different models of inflammatory and neuropathic pain. In addition to VGluT3−/− mice, we used VGluT3-channelrhodopsin 2 mice to selectively stimulate VGluT3+ sensory afferents by blue light, and to assess light-evoked behavior in freely moving mice. We show that VGluT3−/− mice develop reduced mechanical hypersensitivity upon carrageenan injection. Both mechanical and cold hypersensitivity were reduced in VGluT3−/− mice in neuropathic pain evoked by the chemotherapeutic oxaliplatin, but not in the chronic constriction injury (CCI) model of the sciatic nerve. Further, we provide direct evidence that, despite not mediating painful stimuli in naive mice, activation of VGluT3+ sensory fibers by light elicits pain behavior in the oxaliplatin but not the CCI model. Immunohistochemical and electrophysiological data support a role of transient receptor potential melastatin 8-mediated facilitation of synaptic strength at the level of the dorsal horn as an underlying mechanism. Together, we demonstrate that VGluT3+ fibers contribute in an etiology-dependent manner to the development of mechano-cold hypersensitivity.
Keywords: cold hypersensitivity, mice, optogenetics, pain, primary afferents, VGluT3
Introduction
Various animal models for pain have been developed to mimic clinically relevant pain conditions and to understand the neuronal mechanisms underlying altered nociception; e.g., during an inflammation and in neuropathy (Sandkühler, 2009; Jaggi et al., 2011). Different animal models of chronic pain reproduce distinct sensory symptoms in pain patients manifested as lowered withdrawal thresholds or enhanced response magnitudes to innocuous or noxious mechanical, heat, or cold stimuli (Kim et al., 1997; Le Bars et al., 2001). It is presently a matter of debate whether changes in the perception of different sensory modalities; e.g., assessed in humans via quantitative sensory testing, or pain etiology better predict the treatment outcome (Attal et al., 2008; Maier et al., 2010).
In rodent pain models, inflammatory pain is typically characterized by a fast onset of mechanical and heat hypersensitivity, but leads to variable degrees of cold hypersensitivity (Allchorne et al., 2005). The chronic constriction injury (CCI) leads to enhanced mechanically-, heat-, and cold-evoked responses (Bennett and Xie, 1988). In contrast, the oxaliplatin model completely lacks a development of heat hypersensitivity (Ta et al., 2009). It has been speculated that in these diverse animal pain models different types of primary afferent fibers contribute to the observed pain symptoms (Minett et al., 2014).
Sensory nerve fibers express different types of vesicular glutamate transporters (VGluTs). For example, VGluT3+ sensory C-fibers are activated by low-threshold mechanoreceptive stimuli, as well as mild cooling (Seal et al., 2009), the latter in the temperature-detection range of the transient receptor potential melastatin 8 (TRPM8) channel, the main detector of environmental cooling (Bautista et al., 2007). In a recent study, VGluT3 was shown to be important for mechanical hypersensitivity during inflammation and after nerve injury (Seal et al., 2009). Whether VGluT3 also contributes to cold hypersensitivity upon tissue or nerve injury has not yet been investigated. It would be an intriguing possibility that mechanical and cold hypersensitivity, which co-occur in a range of chronic pain conditions (Bessou and Perl, 1969; Maier et al., 2010) share a common pathophysiological background possibly involving VGluT3+ primary afferents in an etiology-dependent manner. However, it is currently unknown whether selective activation of VGluT3-expressing sensory nerve fibers is at all capable of evoking aversive responses.
By using VGluT3−/− mice, we first studied the role of VGluT3 in basal nociception and the possible contribution of the transporter to augmented mechanical and cold responsiveness in different animal models of inflammatory and neuropathic pain (Winter et al., 1962; Bennett and Xie, 1988; Ta et al., 2009). We were able to directly assess the contribution of VGluT3+ sensory fibers to the behavior of naive and neuropathic mice by using an optogenetic approach. Crossing VGluT3-cre mice to floxed Channelrhodopsin-2 mice (VGluT3-ChR2 mice) enabled us to selectively activate VGluT3+ fibers by light and to evaluate the resulting behavior in freely moving mice.
Materials and Methods
Animals
We used 4- to 5-week-old male mice of the following genotypes: C57BL/6 wild-type (WT) mice; VGluT3−/− mice (Seal et al., 2008), and Ai32 mice (Madisen et al., 2012) crossed to VGluT3-Cre mice (Grimes et al., 2011), to create VGluT3-ChR2 mice. Genotyping was performed with the following primers: (1a) eYFP forward: AGC TGA CCC TGA AGT TCA TCT G; (1b) eYFP reverse: ACT CCA GCA GGA CCA TGT GAT; (2a) VGLUT3 Cre forward: CTC TGA CAG ATG CCA GGA C; (2b) VGLUT3 cre reverse: CTG CCCA GAG TCA TCC T. Animals were single-housed under standard conditions with ad libitum access to food and water under a standard 12 h light/dark regime. All procedures were conducted in accordance with guidelines set by the Ethical Committees for the use of laboratory animals at the Medical University of Vienna and the Austrian Ministry for Science and Research. These experiments conform to the standards as specified by the European Union and the International Association for the Study of Pain.
Behavior
Testing conditions.
On three consecutive days before testing, and on each testing day, mice were habituated for at least 1 h to each of the testing environments. The experimenter was blinded to the genotype and to the treatment of the animals until data analysis was completed. The testing room conditions were as follows: room temperature 21 ± 1°C, humidity 40–60%, luminance ∼500 lx. Experiments were performed during the light cycle.
Carrageenan-induced inflammatory pain.
Mice were anesthetized with 1.25–1.5% isoflurane, and 20 μl 2% λ-carrageenan (Sigma) dissolved in saline was injected unilaterally into the plantar hindpaw to model inflammatory pain. The sham control group received vehicle (saline) injections.
Chronic constriction injury.
CCI was performed as described previously (Leitner et al., 2013). Briefly, mice were deeply anesthetized with 1.25–1.5% isoflurane and the right sciatic nerve was exposed at the mid-thigh level. After removal of adherent tissue, three loose ligatures with chromic gut 6/0 (SMI) were tied 1 mm apart around the nerve, proximal to the trifurcation. Muscle and skin were closed with sutures (Silkam 6/0 and Safil 4/0, respectively; Ethicon) and wound clips.
Oxaliplatin-induced neuropathy.
Oxaliplatin was used as a model for chemotherapy-induced neuropathy (Ta et al., 2009). Mice received two treatment cycles, each consisting of 5 consecutive daily intraperitoneal injections of 3 mg/kg oxaliplatin (Tocris Bioscience; cumulative dose, 30 mg/kg) with 5 d of rest in between both treatment cycles. For injections, oxaliplatin was dissolved in a 5% glucose solution (B. Braun) at a concentration of 0.25 mg/ml. Sham-treated animals received glucose injections. The weight of the animals was monitored twice a week during the testing period.
von Frey test.
Mice were placed in acrylic glass cylinders on a wire mesh floor and allowed to habituate for at least 1 h before testing. Mechanical sensitivity was tested by applying calibrated von Frey filaments (Stoelting) to the plantar surface of the hindpaw. The “up and down paradigm” established by Chaplan et al. (1994) was used, starting with the 0.6 g filament.
Plantar heat test.
Animals were placed in acrylic glass cylinders on the heated base (30°C) of the plantar heat apparatus (Stoelting) and allowed to habituate to the test environment for at least 1 h before testing. Heat sensitivity was measured by application of radiant heat (60 mW/cm2) to the plantar surface of the hindpaw. To minimize tissue damage, the cutoff latency was set to 20 s. Measurements were taken alternatingly on both hindpaws with at least 5 min interstimulus interval.
Cold plantar assay.
As previously described (Brenner et al., 2012), mice were placed in acrylic glass cylinders on a 6.2-mm-thick elevated glass plate and allowed to habituate for at least 3 h. To test cold sensitivity, a dry-ice pellet (Ø: 1 cm) was applied to the lower glass surface underneath the hindpaw of the animal. The withdrawal latency in response to the dry ice-induced cooling of the glass surface was measured. Hindpaws were tested alternatingly with at least 7 min in between two consecutive stimulations. To exclude tissue damage, the cutoff latency was set to 20 s.
Acetone test.
Mice were placed in acrylic glass cylinders on an elevated wire mesh and allowed to habituate to the environment for at least 1 h before testing. Using a micropipette, 40 μl of acetone were applied to the plantar hindpaw of the animal. The first 10 s of the response were disregarded due to the mechanical stimulation of the paw. The behavior of the animals during the following 60 s was observed and scored (Caspani et al., 2009). In addition, the time the animal spent licking, brushing, and flinching the paw was measured.
Dynamic cold plate.
As previously described (Yalcin et al., 2009), a thermal plate apparatus (Bioseb) was set to a decreasing slope of 1°C/min (30–0°C). The behavior of the mice on the plate was videotaped and the number of jumps was counted and plotted versus the temperature.
Optogenetic stimulation.
VGluT3-ChR2 mice were placed in acrylic glass cylinders on a wire mesh floor and habituated for at least 1 h before testing. An optical fiber (Ø: 1000 μm, Thor Labs), coupled to a 470 nm LED (Thor Labs) was positioned closely to the plantar surface of the hindpaw and a 10 s light stimulus (60–80 mW/mm2 at the fiber tip) was applied. Each hindpaw was stimulated twice (oxaliplatin model), or the operated paw was stimulated three times (CCI model), with at least 15 min pause in between two stimulations. The behavior of the mice was videotaped to determine the reaction latency and to evaluate the behavior of the animals with the following score; 0: no behavior, 1: glancing at the paw, 2: paw withdrawal, 3: flinching, 4: paw shaking, 5: paw licking (Abbott et al., 1995; Caterina et al., 2000; Caspani et al., 2009).
Spinal cord slice and whole-mount preparation
Mice were deeply anesthetized and the lumbar spinal cord was quickly removed with attached dorsal roots and dorsal root ganglia (DRGs). Ventral roots and connective tissue were removed. For measuring miniature EPSCs (mEPSCs), transverse slices (700–1000 mm thick) from segments L2–L6 were cut using a vibrating microslicer (DTK-1000, Dosaka EM). Slices or lumbar whole-mount preparations with attached dorsal roots and DRGs were incubated in a solution kept at 33°C containing the following (in mm): NaCl 95, KCl 1.8, KH2PO4 1.2, CaCl2 0.5, MgSO4 7, NaHCO3 26, glucose 15, sucrose 50, pH 7.4, osmolarity 310–320 mosmol/L, and oxygenated with 95% O2 and 5% CO2.
A single slice or whole-mount was placed in the recording chamber and continuously superfused with oxygenated recording solution at a rate of 3–4 ml/min. The recording solution was identical to the incubation solution except for the following (in mm): NaCl 127, CaCl2 2.4, MgSO4 1.3, and sucrose 0. All experiments were performed at a bath temperature of 33.5°C.
Electrophysiological recordings
Transversal slice recordings.
Neurons of the dorsal horn were visualized with “Dodt” infrared optics (Dodt et al., 1998) using a 40× water-immersion objective on an Olympus BX50WI upright microscope equipped with a video camera. All recordings were made in the whole-cell patch-clamp configuration at a holding potential of −70 mV using an Axopatch 200B patch-clamp amplifier and the pCLAMP 10 software package (both Molecular Devices). No correction for the liquid junction potential was made. Signals were sampled at 10 kHz, and analyzed off-line using pCLAMP 10. Patch pipettes (2–5 MΩ) from borosilicate glass were pulled on a horizontal puller (P-87, Sutter Instruments) and filled with a solution composed of the following (in mm): K-MeSO3 120, KCl 20, MgCl2 2, HEPES 20, EGTA 0.5, and Na2ATP 2. mEPSCs were measured in the presence of tetrodotoxin (1 μm, Ascent Scientific). From minute 6 to 10 after establishing the whole-cell configuration, WS-3 (200 μm, Tocris Bioscience) was applied to the recording solution. Series resistance was controlled before and after mEPSC-recordings. Only cells with stable series resistances ≤30 MΩ were used for analysis.
Whole mount recordings.
Dorsal horn field potentials (DHFPs) were recorded via a borosilicate glass electrode (pipette-resistance: 2–5 MΩ) filled with recording solution. The electrode was inserted into the substantia gelatinosa, cranial to the dorsal root entry zone. Recordings were performed in current-clamp mode with a gain of 100. To selectively activate VGluT3+ primary afferents, DHFPs were evoked by stimulating the DRG with blue (5 ms, 470 nm) light pulses. To evoke DHFPs through unselective stimulation, a suction electrode was used to apply current (100 μs, 10 mA) to the dorsal root (Ikeda et al., 2003).
Test pulses were applied every 60 s to evoke DHFPs during the recording period. WS-3 (200 μm) was applied to the bath solution 20 min after the start of the recordings, and washed out after 10 min of application.
Immunohistochemistry and in situ hybridization
For immunohistochemistry deeply anesthetized VGluT3-ChR2 mice were transcardially perfused with 20 ml 0.9% NaCl, containing 10 U/ml heparin, followed by 60 ml of cold 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer (PB, pH 7.3). Tissue was quickly removed and postfixed in the same fixative overnight at 4°C. For TRPM8 immunohistochemistry lumbar DRGs were immediately removed after sacrificing the animals and postfixed in 4% PFA in PB for 2 h. Spinal cords with attached dorsal roots and DRGs were cryoprotected in 20% sucrose in 0.1 m PB at 4°C overnight and snap-frozen at −80°C. Ten micrometer thick sections were cut on a cryotome (Leica, CM350S), thaw mounted onto glass slides and allowed to air dry for 1 h. Slices were washed in PBS, blocked, and permeabilized with 5% normal donkey serum in 0.3% Triton X-100 in PBS (TRPM8: 0.15%). Primary antibodies were diluted in blocking solution and incubated overnight at 4°C: mouse anti-neuronal nuclear antigen (NeuN; 1:500, Millipore); rabbit anti-tyrosine hydroxylase (TH, 1:1000; Millipore), biotinylated isolectin B4 (IB-4; 1:500, Vector Laboratories), sheep anti-calcitonin gene-related peptide (CGRP; 1:1000, Biomol), mouse anti-neurofilament 200 (NF200, 1:1000; Millipore), and polyclonal rabbit anti-TRPM8 antibody (1:500; Dr M. Tominaga, Okazaki Institute for Integrative Bioscience; purchased from Hölzel Diagnostika). After rinsing with PBS solution, the slices were further incubated with the corresponding secondary antibodies for 2–3 h at room temperature: donkey anti-sheep IgG Cy3 (1:400, Jackson ImmunoResearch), donkey anti-mouse IgG Cy3 (1:400, Millipore Bioscience Research Reagents), streptavidin Cy3 (1:400, Jackson ImmunoResearch), donkey anti-rabbit IgG Cy5 (1:200, Millipore Bioscience Research Reagents), or donkey anti-rabbit IgG Cy3 (1:400, Millipore Bioscience Research Reagents). Slices were washed with PBS and then coverslipped in a glycerol-based medium containing n-propyl-gallate (Sigma-Aldrich). Images were viewed and recorded with an inverted fluorescence microscope (Olympus BX51) equipped with a CCD camera (Olympus, XM10), and analyzed using CellD Software (AnalySIS, Olympus). Only neurons with clearly visible nuclei on sections being at least 40 μm apart were included in the analysis (n = 3 animals, unless stated otherwise).
For nonradioactive in situ hybridization, full-length Trpm8 sense and antisense probes were amplified from a plasmid (McKemy et al., 2002). Tissue sections were prepared according to the immunohistochemistry-protocol. After the inhibition of endogenous peroxidase (15 min in 0.3% H2O2), permeabilization of the tissue (5 min in 0.2 m HCl; 5 min in 1 μg/ml proteinase K) and acetylation (10 min in 0.25% acetic anhydride) the slices were hybridized overnight at 65°C. Following 50% formamide washes at 55°C, slices were incubated for 2 h in mouse antidigoxigenin antibody conjugated with peroxidase (Roche). Trpm8-mRNA was detected by subsequent treatment with Tyramide Signal Amplification system coupled with Cy3 (PerkinElmer) for 8 min. Hybridization with the corresponding sense probe did not lead to a detectable signal. Immunohistochemistry for TH and eYFP (goat anti-GFP, 1:500, antikoerperonline.de; donkey anti-goat IgG Cy2, 1:400, Jackson ImmunoReseach) was performed subsequently, according to the above-mentioned protocol.
Statistical analyses
All data are expressed as mean ± SEM. Unless stated otherwise, all statistical analyses were conducted using GraphPad Prism 6. A two-way repeated-measures (RM) ANOVA with Bonferroni′s post hoc test was used to compare treatment and genotype groups in the behavior time course tests (groups and time as independent variables). To analyze single time-point group-differences, a two-tailed Student's t test was applied. For the analysis of the immunohistochemical data, a two-step cluster-analysis was performed with cross sectional area of eYFP+ cells as continuous variable (analysis performed in SPSS). Given numbers (n) refer to the numbers of mice used, unless stated otherwise.
Results
VGluT3−/− and wild-type mice do not differ in their responses to mechanical, heat, or cold stimuli
First, we asked whether VGluT3-expressing neurons contribute to the baseline withdrawal thresholds for mechanical and heat stimuli. Global VGluT3−/− mice did not differ from WT animals in their responses to mechanical forces (Fig. 1A; n = 9) or radiant heat stimuli (Fig. 1B; n = 9). We next set out to thoroughly investigate the responsiveness of VGluT3−/− mice to cold stimuli in a broad variety of behavioral tests. We assessed evaporative cooling-induced behavior upon acetone application (Caspani et al., 2009), the paw withdrawal latencies on the plantar cold assay (Brenner et al., 2012) and the number of jumps on the dynamic cold plate, to investigate the reaction pattern in a broad low temperature range (Yalcin et al., 2009). Both strains showed the same magnitude of acetone-evoked responses (Fig. 1C; n = 9), similar paw withdrawal latencies on the plantar cold assay (Fig. 1D; n = 9), and the same response pattern on the dynamic cold plate (Fig. 1E; n = 11). Together, these results confirm and extend previous findings in showing a similar responsiveness of VGluT3−/− and WT mice to mechanical, heat, and cold stimuli. Thus, VGluT3+ cells, including VGluT3+ primary afferents are not required for the innate behavioral response of naive mice to mechanical, heat, or cold stimuli.
Figure 1.
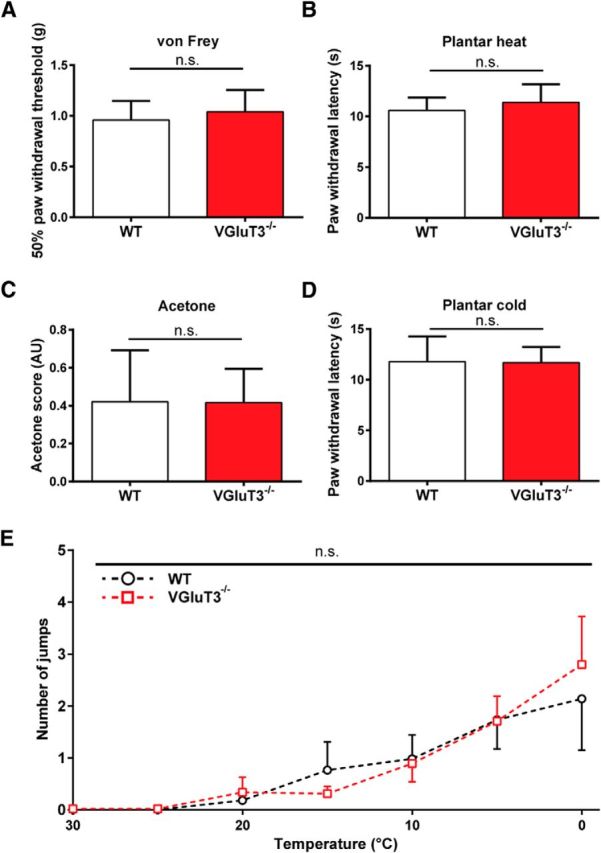
Behavioral responses to mechanical, heat, and cold stimuli do not differ between naive WT and VGluT3−/− mice. A, Mechanical paw withdrawal thresholds obtained upon von Frey monofilament stimulation in naive WT (white) and VGluT3−/− (red) mice (p = 0.41). B, Heat-evoked paw withdrawal thresholds obtained with the plantar heat test in naive WT and VGluT3−/− mice (p = 0.3). C, Response scores of acetone-induced evaporative cooling behavior in naive WT and VGluT3−/− mice (p = 0.96). D, Cold-evoked paw withdrawal thresholds obtained in the cold plantar test in naive WT and VGluT3−/− mice (p = 0.93). E, Number of jumps in WT and VGluT3−/− mice in the dynamic cold plate cooled from 30 to 0°C at a slope of 1°C per minute (p = 0.92). All values are mean values ± SEM obtained in 9 (A–D) and 11 (E) mice per genotype. Statistical significance was evaluated using an unpaired, two-tailed t test (A–D) and a one-way ANOVA with Bonferroni's correction, where the group was used as dependent variable (E). AU, Arbitrary units.
VGluT3−/− mice exhibit reduced mechanical and cold hypersensitivity dependent on the experimental model used.
Carrageenan-induced inflammation
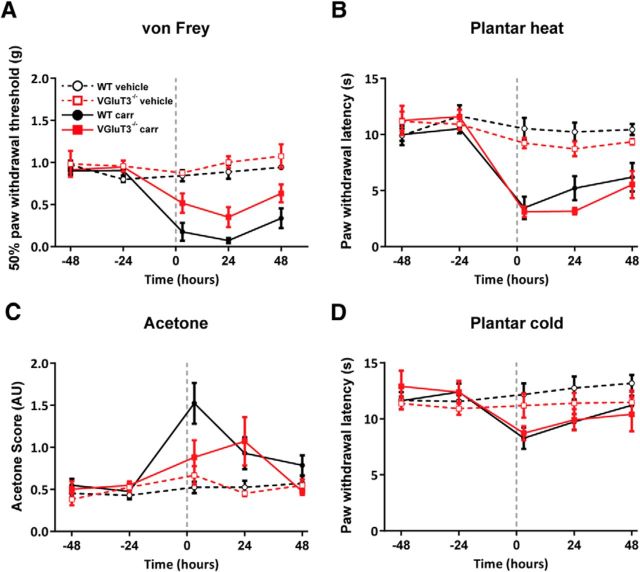
VGluT3−/− mice lack mechanical hypersensitivity in the carrageenan model of acute inflammation (Seal et al., 2009). In the next set of experiments, we therefore induced acute inflammation with a carrageenan injection into the plantar hindpaw in WT and VGluT3−/− mice and tested for mechanical, heat and cold sensitivity (Fig. 2; Table 1). Mechanical paw withdrawal thresholds decreased in both mouse strains, as compared with sham-treated mice of the respective genotype, after the intraplantar carrageenan injection. Reductions in mechanical paw withdrawal thresholds were, however significantly less pronounced in VGluT3−/− mice compared with WT mice (Fig. 2A; Table 1). In contrast, heat hypersensitivity developed to a similar degree in WT and VGluT3−/− mice after carrageenan injections (Fig. 2B; Table 1). Both strains developed a small and comparable increase in responsiveness to cooling by acetone and in the plantar cold test. WT and VGluT3−/− mice displayed small drops in response latencies, which however, were only significant in WT mice. In neither test did WT and VGluT3−/− mice differ in the degree of cold hypersensitivity after carrageenan injection (Fig. 2C,D; Table 1). These results confirm the reduced mechanical but not thermal hypersensitivity in VGluT3−/− compared with WT mice in this model.
Figure 2.
VGluT3−/− mice exhibit mechanical hypersensitivity upon carrageenan injection. Responses of carrageenan- (solid trace) and sham-treated (dashed trace) WT (black) and VGluT3−/− (red) mice to mechanical, heat, and cold stimuli on two baseline, and three time points after intraplantar injection (3, 24, and 48 h). The injection was performed at time point 0, as indicated with the gray dashed line. A, Carrageenan-injected, but not vehicle-injected, mice exhibited reductions in mechanical paw withdrawal thresholds (treatment effect: WT, p = 0.0001; VGluT3−/−, p = 0.0003; genotype effect: p = 0.021). B, Heat sensitivity measured with the plantar heat test was not affected in sham-treated WT and VGluT3−/− mice, but was enhanced to a similar degree in both genotypes after carrageenan injection (treatment effect: WT, p = 0.0001; VGluT3−/−, p = 0.0001; genotype effect: p = 0.812). C, The response scores in the evaporative cooling (acetone) test for cold hypersensitivity were similar in vehicle-treated WT and VGluT3−/− mice, and were likewise mildly enhanced in both genotypes after carrageenan injection (treatment effect: WT, p = 0.004; VGluT3−/−, p = 0.064; genotype effect: p = 0.239). D, Paw withdrawal latencies tested with the plantar cold assay did not change significantly in any of the tested groups (treatment effect: WT, p = 0.454, VGluT3−/−, p = 0.096; genotype effect: p = 0.801). Data are expressed as mean ± SEM and analyzed using a two-way ANOVA, followed by a Bonferroni's correction (time and groups as dependent variables, n = 7 per genotype and treatment). AU, Arbitrary units; carr, carrageenan treated.
Table 1.
Response values and statistics of naïve and treated WT and VGluT3−/− mice in the different behavioral settings
| Model | Modality/test | WT |
Treatment effect p | KO |
Treatment effect p | Genotype effect p | ||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||
| Carrageenan | Mechanical/von Frey (g) | 0.9 ± 0.045 | 0.19 ± 0.05 | 0.0001 | 0.93 ± 0.05 | 0.49 ± 0.06 | 0.0003 | 0.021 |
| Heat/Plantar heat (s) | 10.25 ± 0.45 | 4.95 ± 0.65 | 0.0001 | 11.43 ± 0.94 | 3.93 ± 0.47 | 0.0001 | 0.812 | |
| Cold/Acetone (score) | 0.51 ± 0.07 | 1.1 ± 0.12 | 0.004 | 0.52 ± 0.07 | 0.81 ± 0.12 | 0.064 | 0.239 | |
| Mild cooling/Cold plantar (s) | 12.0 ± 0.71 | 9.73 ± 0.52 | 0.454 | 12.6 ± 1.1 | 9.7 ± 0.6 | 0.304 | 0.801 | |
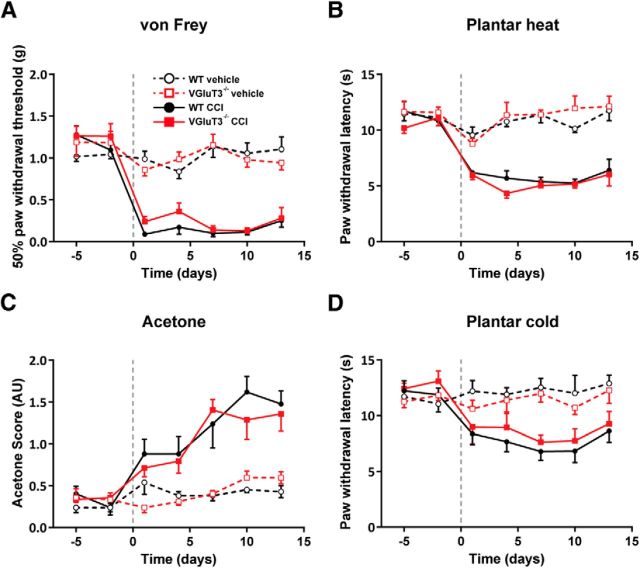
| CCI | Mechanical/von Frey (g) | 1.19 ± 0.1 | 0.14 ± 0.03 | 0.0001 | 1.26 ± 0.12 | 0.23 ± 0.04 | 0.0001 | 0.615 |
| Heat/Plantar heat (s) | 11.27 ± 0.67 | 5.79 ± 0.26 | 0.0001 | 10.64 ± 0.59 | 5.31 ± 0.28 | 0.0001 | 0.725 | |
| Cold/Acetone (score) | 0.32 ± 0.07 | 1.22 ± 0.1 | 0.0002 | 0.34 ± 0.09 | 1.11 ± 0.08 | 0.0002 | 0.619 | |
| Mild cooling/Cold plantar (s) | 12.05 ± 0.72 | 7.65 ± 0.41 | 0.0013 | 12.75 ± 0.7 | 8.51 ± 0.5 | 0.048 | 0.302 | |
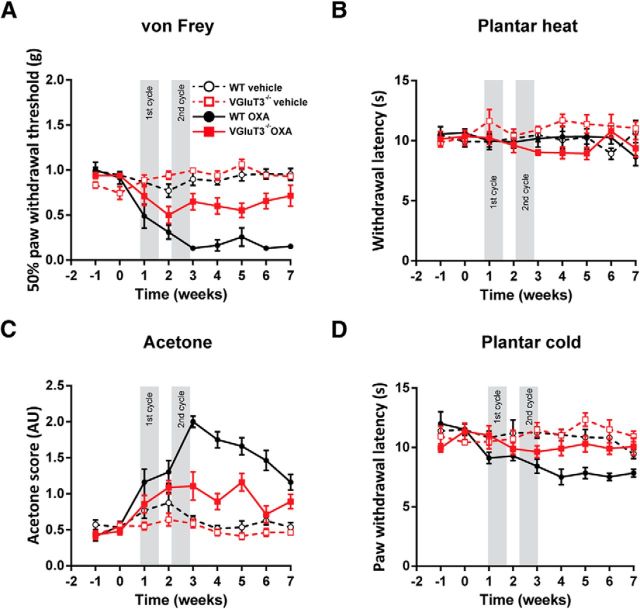
| Oxaliplatin | Mechanical/von Frey (g) | 0.96 ± 0.074 | 0.23 ± 0.03 | 0.0001 | 0.94 ± 0.03 | 0.63 ± 0.03 | 0.0003 | 0.0001 |
| Heat/Plantar heat (s) | 10.6 ± 0.45 | 9.95 ± 0.24 | 0.969 | 10.24 ± 0.52 | 9.56 ± 0.21 | 0.0003 | 0.223 | |
| Cold/Acetone (score) | 0.48 ± 0.06 | 1.5 ± 0.06 | 0.0001 | 0.45 ± 0.05 | 0.96 ± 0.05 | 0.001 | 0.0001 | |
| Mild cooling/Cold plantar (s) | 12.76 ± 0.72 | 8.21 ± 0.19 | 0.0002 | 10.65 ± 0.57 | 10.1 ± 0.22 | 0.003 | 0.0006 | |
Values are mean response ± SEM obtained in the different behavioral paradigms, as indicated, as well as p values obtained in statistical tests. Data were analyzed using a two-way ANOVA with time and group as dependent variables with Bonferroni's correction (n = 7 in all groups). Treatment effect was determined by comparison of the time-course data of naïve against treated mice of the same genotype. Genotype effect was calculated by the comparison of the time-course data of WT and VGluT3−/− mice that received the same treatment regime. Bold p values indicate a significant genotype effect.
Chronic constriction injury
Next, we studied the role of VGluT3+ neurons in the CCI model. This neuropathic pain model typically leads to prominent mechanical and cold hypersensitivity, as well as heat hypersensitivity (Kim et al., 1997). WT and VGluT3−/− mice developed similar reductions in paw withdrawal thresholds in the von Frey test postsurgery (Fig. 3A; Table 1). Both strains did not differ in their mechanical thresholds at any time-point of testing. Heat hypersensitivity, as determined with the plantar heat test, also developed with a similar time course and to a similar degree in WT and VGluT3−/− mice (Fig. 3B; Table 1). In both cold assays, CCI-treated WT and VGluT3−/− mice showed a comparable increase in cold-evoked nocifensive behavior over the entire time course (Fig. 2C,D; Table 1). Together, in the CCI model, there were no significant differences between WT and VGluT3−/− mice in any of the tests used. Thus, VGluT3+ primary afferents are not required for the development of mechanical, heat, or cold hypersensitivity in the CCI model of neuropathic pain.
Figure 3.
VGluT3−/− mice do not differ from WT mice in their behavioral responses to mechanical, heat, and cold stimuli after CCI injury. CCI- (solid trace) and sham-treated (dashed trace) WT (black) and VGluT3−/− mice (red) were tested on two baseline, and seven time points postsurgery for their responses to mechanical, heat, and cold stimuli. The CCI surgery was performed at time point 0, as indicated with the gray dashed line. A, After CCI- but not sham-treatment, WT and VGluT3−/− mice showed a comparable significant decrease in their mechanical paw withdrawal thresholds (treatment effect: WT, p = 0.0001; VGluT3−/−, p = 0.0001; genotype effect, p = 0.615). B, In the plantar heat test, CCI-treated WT and VGluT3−/− mice, but not sham-treated mice, showed a significant decrease in their reaction latencies to the heat stimulus (treatment effect: WT, p = 0.0001; VGluT3−/− p = 0.0001; genotype effect: p = 0.725). C, In the evaporative cooling test, CCI- but not sham-treated mice of both genotypes showed a significant, but comparable increase in their acetone scores (treatment effect: WT, p = 0.0002; VGluT3−/−, p = 0.0002; genotype effect: p = 0.619). D, In the plantar cold test, CCI- but not sham-treated mice exhibited a comparable significant decrease in their reaction latencies after the surgically induced peripheral nerve lesion (treatment effect: WT, p = 0.0013; VGluT3−/−, p = 0.048; genotype effect: p = 0.302). Data are expressed as mean ± SEM and analyzed using a two-way ANOVA, followed by Bonferroni's correction (time and groups as dependent variables; n = 7 per genotype and group). AU, Arbitrary units.
Oxaliplatin-induced neuropathy
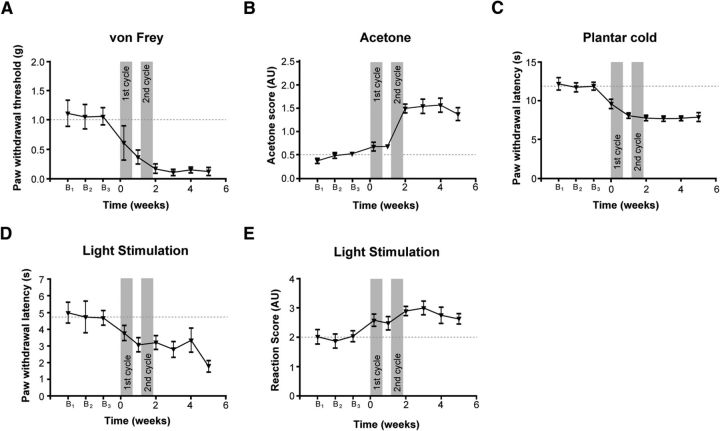
We then tested the responses of VGluT3−/− mice in the oxaliplatin model. To this end, we performed a cyclic oxaliplatin injection paradigm, closely mimicking clinical use of oxaliplatin for cancer treatment (André et al., 2004; Ta et al., 2009). Both, WT and VGluT3−/− mice developed a decrease in mechanical thresholds compared with sham-injected mice, starting after the first injection, and lasting until the end of the testing period 7 weeks after the first injection (Fig. 4A; Table 1). VGluT3−/− mice showed, however, a significantly smaller reduction of mechanical thresholds as compared with WT mice. Heat hypersensitivity developed in neither WT nor VGluT3−/− mice upon oxaliplatin treatment (Fig. 4B; Table 1). In the acetone evaporative cooling test, as well as the cold plantar assay, WT and VGluT3−/− mice developed an increased responsiveness upon oxaliplatin injection, indicating cold hypersensitivity (Fig. 4C,D; Table 1). Cold hypersensitivity was, however, significantly smaller in VGluT3−/− mice as compared with WT animals (Fig. 4C,D; Table 1). Together, these results indicate that VGluT3+ cells are necessary for the full expression of mechanical and cold hypersensitivity in oxaliplatin-induced neuropathy.
Figure 4.
VGluT3−/− mice exhibit reduced mechanical and cold hypersensitivity upon oxaliplatin treatment. Oxaliplatin- (solid trace) and sham-treated (dashed trace) WT (black) and VGluT3−/− mice (red) were tested on two baseline, and seven time points during and postcyclic oxaliplatin injections over a 9-week time course for heat, cold, and mechanical thresholds. The oxaliplatin injection cycles are indicated with gray boxes. A, Oxaliplatin- but not vehicle-injected mice developed a gradual and significant reduction in their mechanical paw withdrawal thresholds over the testing time course. Thresholds were reduced to a lesser degree in VGluT3−/− compared with WT mice (treatment effect: WT, p = 0.0001; VGluT3−/−, p = 0.0003; genotype effect, p = 0.0001). B, Heat hypersensitivity, as determined on the plantar apparatus, did not differ from baseline in any group, though a small difference was observed between sham and oxaliplatin-treated VGluT3−/− mice (treatment effect, WT p = 0,969, VGluT3−/−, p = 0.0003, genotype effect, p = 0.223,). C, Acetone evoked behavioral scores increased during the course of oxaliplatin treatment in WT and VGluT3−/− mice, but not in sham-treated mice. Acetone scores were increased to a lesser degree in VGluT3−/− mice compared with WT animals (treatment effect: WT, p = 0.0001; VGluT3−/−, p = 0.01; genotype effect: p = 0.0001). D, Cold response latencies were decreased in oxaliplatin-treated, but not vehicle-treated WT and VGluT3−/− mice. Latencies were reduced to a lesser degree in oxaliplatin-treated VGluT3−/− compared with WT mice (treatment effect: WT, p = 0.0002; VGluT3−/−, p = 0.003; genotype effect: p = 0.0006). Data are expressed as mean ± SEM and were analyzed using a two-way ANOVA with Bonferroni's correction (n = 7 per genotype and treatment; time and groups as dependent variables). AU, Arbitrary units; OXA, oxaliplatin treated.
Signaling by VGluT3+ primary afferents is enhanced in the oxaliplatin but not the CCI model of neuropathic pain
Next, we used an optogenetic approach to directly assess the contribution of VGluT3+ primary afferents to nociception in freely moving naive and neuropathic mice. To this end, VGluT3-Cre mice were crossed to Ai32 mice expressing light-activated ChR2 coupled to the marker protein eYFP in a Cre-dependent manner (VGluT3-ChR2 mice).
Characterization of VGluT3-ChR2 mice
To examine the distribution of ChR2-eYFP, we performed immunohistochemical staining on lumbar DRGs of VGluT3-ChR2 mice. Nineteen percent of all neurons identified with the NeuN expressed eYFP (279/1466 neurons). Enhanced YFP was not expressed in DRG neurons of Cre-negative littermates. Forty-four percent of eYFP+ neurons in VGluT3-ChR2 mice coexpressed NF200, a marker for myelinated fibers (Fig. 5A,D; 119/271 neurons). Conversely, 32% of NF200+ neurons expressed eYFP (Fig. 5E; 119/376 neurons). Fifty-two percent of eYFP+ DRG neurons coexpressed TH, identifying them as low-threshold mechanoreceptive C-fibers (C-LTMRs; Li et al., 2011; Lou et al., 2013; Fig. 5A–D; 567/1106 neurons), and 94% of TH+ C-LTMRs expressed eYFP (Fig. 5E; 567/603 neurons). Enhanced YFP+ neurons scarcely expressed markers for nociceptive neurons, such as IB-4 or CGRP (Fig. 5B–D; 11/356 neurons and 23/479 neurons, respectively); and eYFP expression was negligible in the IB-4+ or CGRP+ cell populations (Fig. 5E; 11/335 and 23/482 neurons, respectively). The mean cross sectional area of eYFP+/NF200+ neurons was 808 ± 36 μm2 (n = 119), whereas NF200−/TH+ or NF200−/TH− neurons were considerably smaller (295 ± 3 and 291 ± 6 μm2, respectively; n = 567 and 324). In summary, these results are in line with a previous report, identifying three populations of VGluT3+ primary afferents (Lou et al., 2013): (1) A-fibers, transiently expressing VGluT3, likely innervating Merkel cells; (2) TH+ C-LTMRs; and (3) TH− C-fibers.
Figure 5.
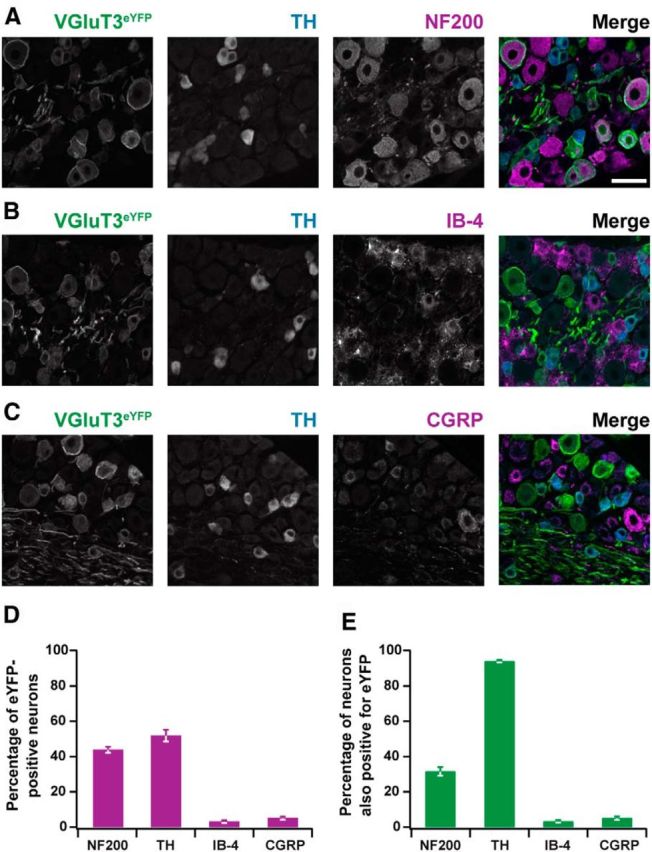
Immunohistochemical characterization of VGluT3-ChR2 mice. A–C, Sample immunohistochemical images of lumbar DRG depicting colocalization of eYFP, indicating VGluT3 expression (VGluT3eYFP) with TH and NF200, IB-4, or CGRP. Merged images are shown on the right (VGluT3eYFP, green; TH, blue; NF200, IB-4, or CGRP, magenta). Scale bar 50 μm. D, Percentage of eYFP+ neurons costaining for other markers, as indicated. E, Percentage of NF200+, TH+, IB-4+, or CGRP+ DRG neurons expressing eYFP (D, E; mean ± SEM, n = 3 mice each, except for NF200 stainings, where only 2 mice were used).
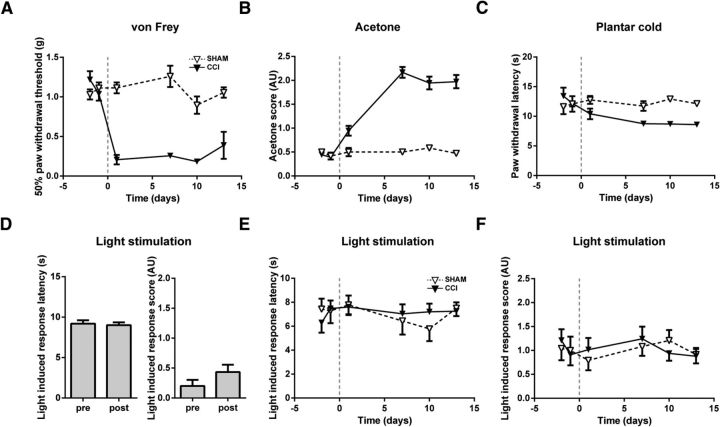
To activate VGluT3+ sensory fibers in freely moving mice, a flash of blue (470 nm) light was applied to the plantar hindpaw. Naive VGluT3-ChR2 mice showed only minor responses to the 470 nm light stimulation, such as glancing at the paw or weak withdrawal responses without further attention to the stimulated paw. Upon stimulation with 617 nm light, which does not activate ChR2, VGluT3-ChR2 mice randomly exhibited paw movements without apparent association with the light stimulus (Fig. 6D; n = 11).
Figure 6.
Light-evoked responses were not altered in VGluT3-ChR2 mice upon CCI treatment. Standard behavioral tests and light stimulations with 470 or 617 nm light in CCI- and sham-treated VGluT3-ChR2 mice. A, Mechanical paw withdrawal thresholds were reduced in CCI- but not sham-treated VGluT3-ChR2 mice over the CCI time course (treatment effect: p < 0.001; CCI, n = 11; sham, n = 8). B, Acetone-evoked responses increased in CCI- but not sham-treated VGluT3-ChR2 mice over the testing time course (treatment effect: p < 0.001; CCI, n = 6; sham, n = 6). C, Paw withdrawal latencies were reduced in CCI- but not sham-treated VGluT3-ChR2 mice in response to mild cold stimuli on the plantar cold assay (treatment effect: p < 0.001; CCI, n = 6; sham, n = 6). D, Paw withdrawal latency and response score of CCI-treated VGluT3-ChR2 mice in response to light stimuli of 617 nm wavelength were not significantly altered after CCI surgery, (treatment effect latency: p = 0.62; treatment effect score: p = 0.089; n = 11;). E, Light-induced (λ = 470 nm) paw withdrawal latencies of VGluT3-ChR2 mice were stable over the CCI time course testing period (treatment effect: p = 0.85; CCI, n = 11; sham: n = 8). F, Response scores upon light stimulation (λ = 470 nm) were not altered after CCI injury (treatment effect: p = 0.87; CCI, n = 11; sham, n = 8). Data are expressed as mean ± SEM and were analyzed using a two-way ANOVA with Bonferroni's correction. AU, Arbitrary units.
Chronic constriction injury
After CCI surgery, mechanically- and cold-evoked responses were consistently aggravated, confirming development of a neuropathy (Fig. 6A–C). Neither paw withdrawal latencies, nor response scores to either 470 or 617 nm light stimulation (Fig. 6D–F) were altered for up to 13 d after CCI surgery. These findings further support our hypothesis that VGluT3+ sensory fibers do not contribute to mechanical or cold hypersensitivity in the CCI model of neuropathic pain.
Oxaliplatin-induced neuropathy
To assess the role of VGluT3+ sensory fibers in chemotherapy-induced pain, we performed the light stimulation paradigm in VGluT3-ChR2 mice subjected to cyclic oxaliplatin treatment. Oxaliplatin treatment resulted in reduced mechanical and cold withdrawal thresholds in VGluT3-ChR2 mice (Fig. 7A,C; n = 12), and to increased cold-induced responses in the acetone test (Fig. 7B), confirming the development of mechanical and cold hypersensitivity, respectively. Upon stimulation with blue light, oxaliplatin-treated VGluT3-ChR2 mice displayed significantly shorter paw withdrawal latencies as compared with their baseline testing (Fig. 7D; n = 12). In addition, VGluT3-ChR2 mice showed pronounced flinching, paw shaking or paw licking behavior, which often persisted for several seconds after withdrawal from the light stimulus. Accordingly, the response score was significantly increased after oxaliplatin treatment (Fig. 7E; n = 12). These findings demonstrate that activation of VGluT3+ fibers leads to nocifensive behavior in the oxaliplatin model of neuropathic pain.
Figure 7.
Light-evoked responses were enhanced in VGluT3-ChR2 mice upon oxaliplatin treatment. Responses of oxaliplatin-treated VGluT3-ChR2 mice to mechanical and cold stimuli, and blue light. Oxaliplatin-treatment is indicated with gray boxes. A–C, Cyclic oxaliplatin injections induced a decrease in mechanical withdrawal thresholds (A; p < 0.001), an increase in cooling evoked nocifensive behavior in the acetone test (B; p < 0.001), and a reduction in paw withdrawal latencies to cold stimuli (C; p < 0.001). D, Oxaliplatin treatment led to a decrease in paw-withdrawal latencies upon stimulation with blue light (λ = 470 nm) at the plantar hindpaw (p = 0.003). E, Light induced nocifensive behavior was enhanced in oxaliplatin-treated VGluT3-ChR2 mice (p = 0.002). Data are presented as mean ± SEM and analyzed using a RM one-way ANOVA with Bonferroni's correction (n = 12; time was used as dependent variable). AU, Arbitrary units; B, baseline testing timepoint.
To assess the validity of the light stimulus task, we performed a correlation analysis between light-evoked behavior and conventional behavioral tests in the CCI and the oxaliplatin models of neuropathic pain. Upon oxaliplatin treatment, mechanical paw withdrawal thresholds closely correlated with cold-induced paw withdrawal latencies. This indicates a parallel development and simultaneous aggravation of mechanical and cold hypersensitivity over the oxaliplatin time course (Fig. 8A; r = 0.7). Light-induced paw withdrawal latencies correlated with mechanical withdrawal thresholds (Fig. 8B; r = 0.41), and with cold-induced paw withdrawal latencies (Fig. 8C; r = 0.301). The acetone score, a behavioral measurement used to describe the severity of cold hypersensitivity rather than a threshold value for paw withdrawal, correlated well with the response score obtained upon light stimulation (Fig. 8D; r = 0.388). In contrast, we did not find any correlation of light-evoked behavior and conventional test values in the CCI-model of neuropathic pain (Fig. 8B′–D′), despite a good correlation of the von Frey and the plantar cold test (Fig. 8A′). These results further support our hypothesis that VGluT3+ sensory fibers contribute to mechanical and cold hypersensitivity in the oxaliplatin-, but not the CCI model of neuropathic pain.
Figure 8.
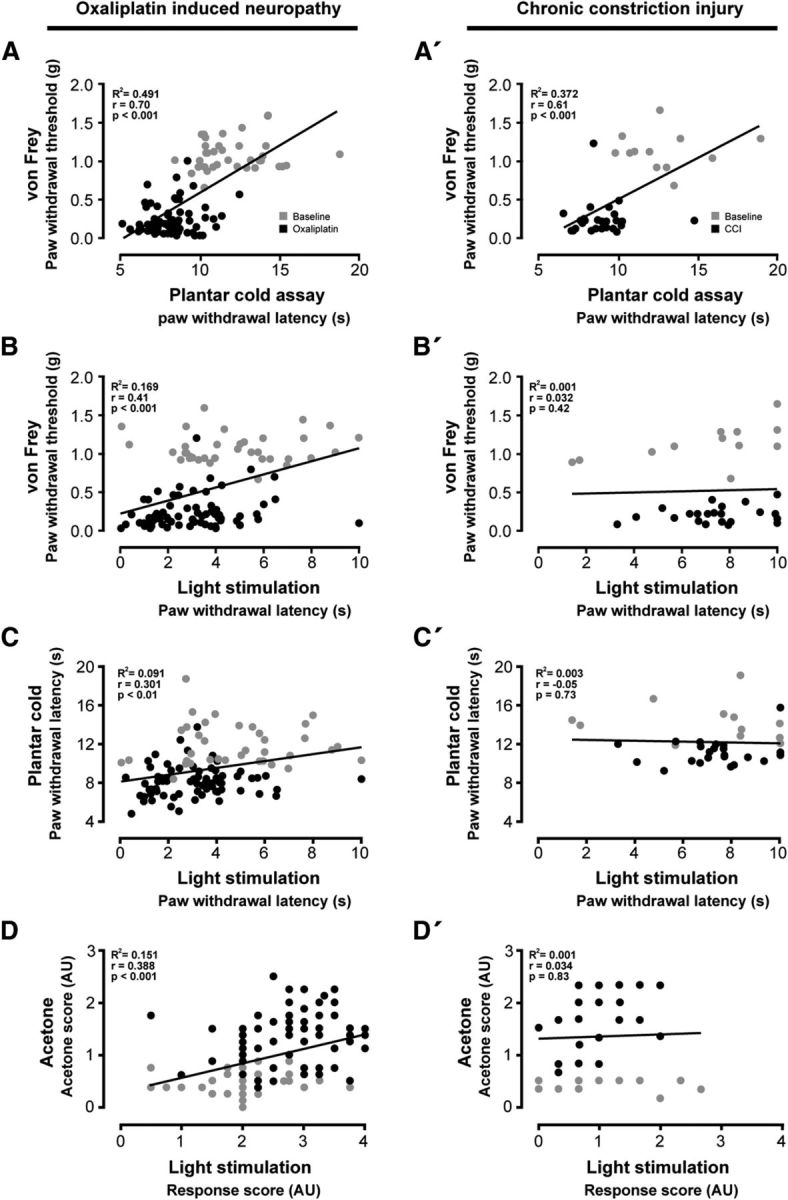
Light-evoked behavior correlates with mechanical and cold hypersensitivity in oxaliplatin-treated but not in CCI-treated VGluT3-ChR2 mice. Correlation of mechano-cold hypersensitivity and light-induced nocifensive behavior observed in the oxaliplatin (A–D) and the CCI (A′–D′) time course. The responses assessed in each behavioral test are compared with one another to validate their comparative power. Values obtained during baseline conditions are indicated in gray, those after treatment in black. A, A′, The observed mechanical thresholds in the von Frey test are compared against the cold-induced paw withdrawal latencies of the plantar cold test (oxaliplatin, r = 0.7; CCI, r = 0.61). B, B′, Mechanical thresholds of the von Frey test are compared with light-induced reaction latencies (oxaliplatin, r = 0.41; CCI, r = 0.032). C, C′, Cold-induced paw withdrawal latencies of the plantar cold test are plotted against the reaction latencies obtained with the light stimulation test (oxaliplatin, r = 0.3; CCI, r = −0.05). D, D′, The scoring obtained in the acetone test is compared with the light stimulation induced behavior scoring (oxaliplatin, r = 0.39; CCI, r = 0.034). Data were analyzed using a correlation-analysis to determine the Pearson-coefficient (r) and the coefficient of determination (R2; oxaliplatin, n = 108, 12 mice at 9 testing time-points; CCI n = 38, 6 mice at 6 testing time-points,). AU, Arbitrary units.
Signaling by VGluT3+ primary afferents is enhanced by spinal TRPM8 receptor activation
It has been suggested that TRPM8 receptors are involved in the expression of cold hypersensitivity in the oxaliplatin model of neuropathic pain (Gauchan et al., 2009; Kawashiri et al., 2012). We therefore tested the hypothesis that VGluT3+ DRG neurons coexpress TRPM8. To this end, we performed immunohistochemical stainings for TRPM8 in DRG tissue of VGluT3-ChR2 mice and quantified the number of eYFP+ cells coexpressing TRPM8 protein (Fig. 9A). A two-step cluster-analysis, based on the cross sectional area of eYFP+ cells, predicted two subpopulations of VGluT3+ neurons (n = 311, model-predictor = 0.8, cluster-centers determined at 195 and 776 μm2 for subpopulations 1 and 2, respectively; Fig. 9B). According to classical conduction velocity studies (Harper and Lawson, 1985), the small cells with a mean cross-sectional area of 195 μm2 likely corresponded to VGluT3+ C-fibers, whereas the larger cells with a mean area of 776 μm2 are consistent with being A-fibers. We found that 90% of VGluT3+ C-fibers and 10% of VGluT3+ A-fibers coexpressed TRPM8 (subpopulations 1 and 2, respectively; Fig. 9B) when assessed with immunohistochemical stainings. As the specificity of TRPM8 antibodies in mouse DRG is debated (Knowlton et al., 2013), we additionally performed fluorescence in situ hybridization (Fig. 9C). With the latter method, which tends to be less sensitive than radioactive labeling methods (Kobayashi et al., 2005), we detected Trpm8-mRNA in 16 ± 7% of small diameter eYFP+ neurons (568/3291 cells of three animals). Large diameter eYFP+ neurons virtually did not coexpress Trpm8-mRNA (4/954 cells positive). Interestingly, 90 ± 2% of Trpm8-mRNA+ neurons were eYFP+, whereas only 4 ± 3% coexpressed TH (Fig. 9C; 42/692 neurons). Together, these results clearly show TRPM8 receptor expression in a subpopulation of VGluT3+ fibers.
Figure 9.
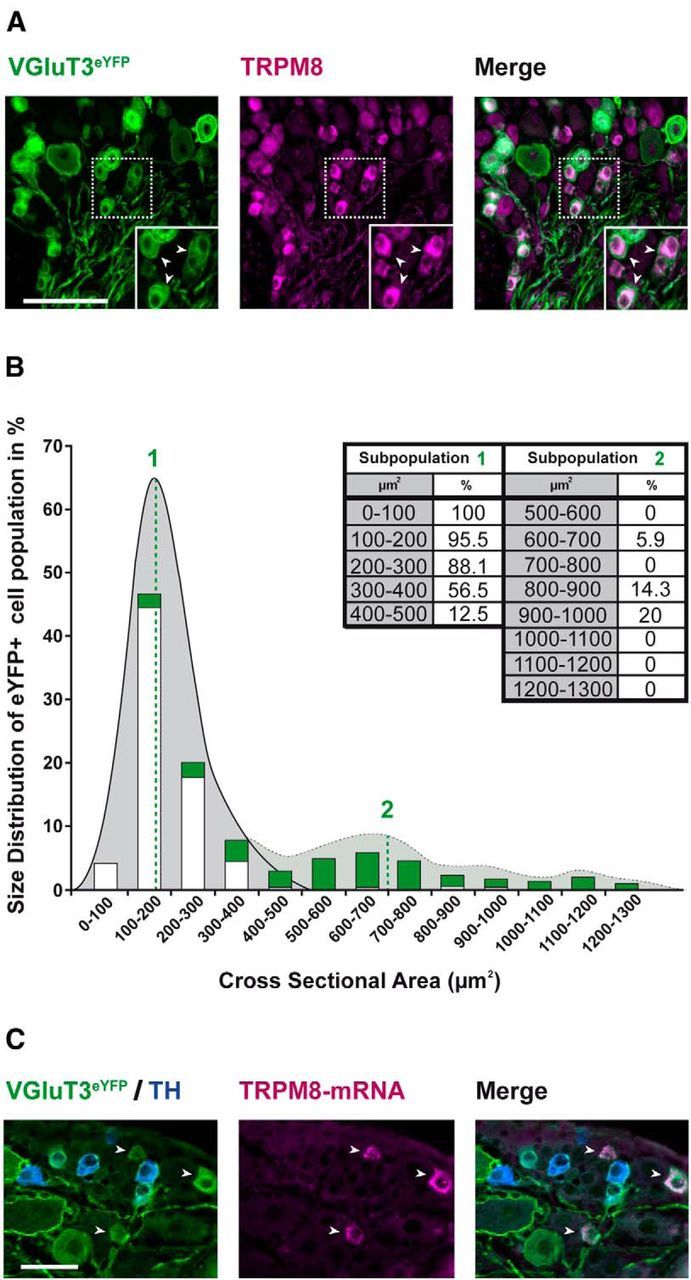
VGluT3+ DRG neurons coexpress TRPM8. Immunohistochemical stainings and in situ hybridization of lumbar DRG neurons from VGluT3-ChR2 mice. A, Sample images depicting eYFP-fluorescence (left, green), indicating VGluT3 expression (VGluT3eYFP), TRPM8 immunohistochemistry (middle, magenta), and colocalization of both fluorophores (right, white; Scale bar, 100 μm; white arrows indicate colocalization events in cells with small somata). Insets, A magnification of the area depicted with the broken line. B, Quantification of immunohistochemical data. A Gaussian distribution cluster analysis (gray area) reveals two clusters of cells in the eYFP+ cell population. Cluster centers are indicated with green numbers and lines (n = 311 cells, model-predictor = 0.8, cluster centers determined at 195 and 776.2 μm2; n = 3 mice, 10 sections per mouse). A size-distribution plot reveals that most eYFP+ cells have a small cross-sectional area. Green bars represent the percentage of eYFP+ cells within the indicated range of cross sectional areas. White bars represent the portion of TRPM8+ cells within the eYFP+ subpopulation (percentage values are displayed in the inset table). C, Sample images depicting eYFP fluorescence (left, green), indicating VGluT3 expression, overlaid with immunohistochemistry for TH (blue), Trpm8 in situ hybridization (middle, magenta), and a merge of all signals (right, white; Scale bar, 50 μm; white arrows indicate colocalization of Trpm8 and eYFP).
TRPM8 receptors are commonly expressed on central terminals of TRPM8+ primary afferent neurons (Proudfoot et al., 2006) where their activation can modulate synaptic transmission (Tsuzuki et al., 2004). To test whether VGluT3+ or unidentified sensory fibers express functional TRPM8 receptors, we next measured evoked field potentials in spinal cord whole-mount preparations from VGluT3-ChR2 mice with attached dorsal roots and DRGs. Field potentials, used as a measure for the strength of synaptic transmission, were either nonselectively elicited by electrical stimulation of the dorsal root at C-fiber intensity, or with a 470 nm light flash to stimulate VGluT3+ fibers selectively.
Topical application of the TRPM8 receptor agonist WS-3 (200 μm) increased the area of electrically-evoked field potentials in the spinal dorsal horn to 163 ± 13.8% of baseline (Fig. 10A; n = 9). However, when VGluT3+ primary afferents were selectively stimulated with blue light, application of WS-3 led to an increase of evoked field potentials to 430 ± 115% of baseline (Fig. 10A; n = 6). The much stronger facilitation of light-evoked field potentials (p = 0.043) indicates a preferential effect of TRPM8 receptor activation on VGluT3+ primary afferents. To investigate whether the WS-3-mediated facilitation is mediated pre- or postsynaptically, we measured mEPSCs in lamina I and II of the spinal dorsal horn via whole-cell patch-clamp recordings in transversal spinal cord slices (Fig. 10C). During the application of WS-3 mEPSC, amplitudes remained stable (Fig. 10D; control: 36 ± 9 pA, WS-3: 39 ± 9 pA, n = 10, p = 0.07), whereas the mEPSC rate increased (Fig. 10D; increase from 1.4 ± 0.6 events per second to 4.3 ± 1.9 events per second, n = 10, p = 0.03). Together, these results indicate a strong facilitation of VGluT3+ fiber-mediated field potentials in the spinal dorsal horn likely due to presynaptic TRPM8 receptor activation.
Figure 10.
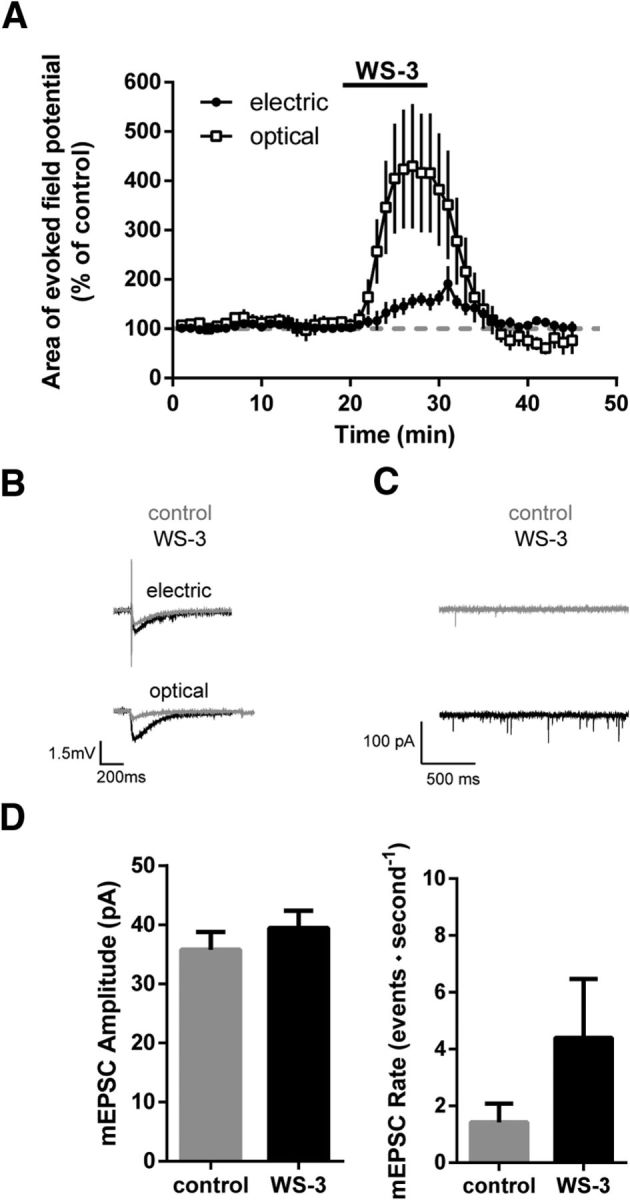
VGluT3+ fibers are facilitated by TRPM8 receptor activation. Electrophysiological investigation of VGluT3+ and unidentified fibers. A, In a whole-mount preparation, application of the TRPM8 agonist WS-3 (200 μm) facilitated the electrically evoked field potentials in WT mice to ∼163 ± 13.8% of control (n = 9). Light-evoked field potentials recorded in VGluT3-ChR2 mice were facilitated significantly stronger by WS-3 (facilitation to ∼430 ± 115% of control; stimulation effect, p = 0.043). B, Sample traces of optically- and electrically-evoked field potentials, before and during WS-3 application. C, Sample mEPSC recordings from spinal lamina I and II neurons before and during application of WS-3 (200 μm). D, Upon WS-3 application, the mEPSC amplitude did not change (p = 0.07), whereas the mEPSC rate increased (p = 0.03, n = 10). Data are expressed as mean ± SEM and were analyzed using a two-way ANOVA (A) with time and groups as dependent variables or a paired Student's t test (D).
Discussion
Here we show that VGluT3+ primary afferents express functional TRPM8 receptors and show altered signaling in the oxaliplatin-model of neuropathic pain. This likely contributes to cold hypersensitivity in inflammatory as well as neuropathic pain evoked by oxaliplatin, but not the CCI model.
Etiology-dependent role of VGluT3+ fibers for pain hypersensitivity
Pain models vary in the nature of the pain-trigger (Jaggi and Singh, 2010 for review). Even seemingly similar pain models differ mechanistically and in their magnitude of responses to external mechanical, heat, and cold stimuli (Kim et al., 1997; Dowdall et al., 2005). Furthermore, recent evidence indicates that different types of primary afferent fibers contribute differentially to the various models of pain (Minett et al., 2014). VGluT2+ fibers have been suggested to mediate acute pain, as well as heat pain after injury (Scherrer et al., 2010; Rogoz et al., 2012). VGluT3+ fibers, in contrast, do not contribute to heat hypersensitivity but might be involved in mechanical hypersensitivity upon inflammation or nerve injury (Seal et al., 2009). We now demonstrate that (1) VGluT3+ fibers play an important role for mechanical and cold hypersensitivity, and (2) that the role of VGluT3+ fibers strictly depends on pain etiology. Our data show that VGluT3+ neurons are involved in inflammatory pain and oxaliplatin-induced neuropathic pain but not in neuropathic pain in the CCI model, adding to the emerging awareness that similar pain symptoms are not necessarily mediated by the same underlying mechanisms (Minett et al., 2014).
Earlier conclusions that VGluT3+ fibers contribute to mechanical hypersensitivity in the spared nerve injury (SNI) model (Seal et al., 2009) were based on findings in global VGluT3−/− mice. However, in a mouse mutant lacking VGluT3+ C-fibers in a Runx1-dependent manner (Lou et al., 2013), mechanical hypersensitivity developed normally in the SNI model, suggesting that VGluT3+ primary afferent fibers are not necessary for neuropathic pain in this model (Lou et al., 2013). The reduced mechanical hypersensitivity in the global VGluT3−/− mouse (Seal et al., 2009) compared with the Runx1-dependent knock-out rather suggests that spinal or supraspinal brain areas, which also express VGluT3+ cells (Fremeau et al., 2004), might contribute to mechanical hypersensitivity in the SNI model. In contrast, in the CCI model used in the present study, we did not observe any differences between WT and the global VGluT3−/− mice which were also used by Seal et al. (2009). We therefore conclude that despite similar neuropathic symptoms and in contrast to the SNI model, neither central nor peripheral VGluT3+ neurons are necessary for the pain phenotype in the CCI model.
In global VGluT3−/− mice, oxaliplatin-induced mechanical and cold hypersensitivity were significantly less severe than in WT mice which is in striking contrast to the CCI model, strongly suggesting the involvement of different pathological mechanisms in CCI- and oxaliplatin-induced neuropathic pain. And indeed, in the CCI model, neuropathic pain results from pathological changes in the CNS, such as reduced GABAergic inhibition and spread of excitation from deep, to more superficial laminae of the spinal dorsal horn (Ibuki et al., 1997; Schoffnegger et al., 2008; Leitner et al., 2013). Oxaliplatin-induced neuropathy, in contrast, is thought to largely result from an increased excitability of primary afferent fibers (Adelsberger et al., 2000), possibly due to increased voltage gated sodium channel (Nav1.8) expression, or decreased expression of inhibitory potassium channels (Descoeur et al., 2011).
Selective activation of VGluT3+ fibers in behaving mice
Conditional knock-out mice can be very helpful in identifying elements which are necessary for a given response, but may inevitably fail when searching for elements which are sufficient for that response. In our case, global VGluT3−/− mice revealed that either peripheral or central VGluT3+ neurons are significantly involved in oxaliplatin-induced hypersensitivity. To test whether VGluT3+ primary afferents are sufficient to elicit nocifensive behavior, we used an optogenetic approach. Our data show that naive VGluT3-ChR2 mice do not exhibit nocifensive responses upon light stimulation, which suggests that activation of VGluT3+ fibers is probably not sufficient to elicit pain in naive mice. In contrast, when ChR2-expression is driven by the Nav1.8 promoter in the same conditional mouse line (Ai32; Daou et al., 2013), mice show clear nocifensive behavior including jumping and vocalization, as expected when activating nociceptive C-fibers.
In our model, ChR2 expression was restricted to VGluT3+ fibers, which consist of at least three distinct populations: TH+, and TH− VGluT3+ C-fibers, as well as a group of A-fibers, likely innervating Merkel cells (Lou et al., 2013). Interestingly, after oxaliplatin injections, VGluT3-ChR2 animals showed enhanced behavioral responses to stimulation with light, such as licking or biting of the paw, which are typically associated with nociception. This behavioral pattern suggests that VGluT3+ fibers are not classical nociceptors. They may rather detect low-threshold mechanical or cooling stimuli in naive mice, and are rendered to mediate hypersensitivity after lesions of the somatosensory system. In oxaliplatin-treated mice, activation of VGluT3+ fibers becomes sufficient to elicit nocifensive behavior as demonstrated by increased pain scores in the light stimulus test, in which behavioral responses were classified according to similar behavioral scores as used in classical pain tests (Abbott et al., 1995; Caterina et al., 2000; Caspani et al., 2009). The correlation of response magnitudes in the light-evoked behavior with conventional pain behavioral tests further supports that the light-evoked withdrawal latency and nocifensive scores are useful measures for assessing pain-related behavior. Our results from VGluT3−/− mice and the optogenetic activation of VGluT3+ sensory fibers provide convergent lines of evidence for the involvement of VGluT3+ neurons in the expression of painful neuropathy in the oxaliplatin-model. Our data do not support any contribution of VGluT3 or VGluT3+ fibers to pain in the CCI model or in naive animals. The data further demonstrate that optogenetic stimulation can be used as a robust and reliable tool to study the contribution of specific primary afferent nerve fiber subpopulations to pain.
TRPM8 expression of VGluT3+ fibers
In chronic neuropathic pain conditions resulting from lesions of the somatosensory system, nociceptive and non-nociceptive signals can be synaptically amplified at the first synaptic relay in the superficial dorsal horn (Ikeda et al., 2006; Drdla et al., 2009; Drdla-Schutting et al., 2012). TRPM8 receptors are expressed on the central terminals of primary afferent fibers and their activation leads to the modulation of synaptic strength (Tsuzuki et al., 2004; Proudfoot et al., 2006). We now show that TRPM8 receptors are expressed in a significant proportion of VGluT3+ C-fibers and TRPM8 receptor activation leads to a preferential enhancement of neurotransmitter release from VGluT3+ fibers, possibly by release of calcium from intracellular stores (Tsuzuki et al., 2004). It has been shown that TRPM8 expression is increased upon oxaliplatin injection, and that Trpm8−/− mice do not develop oxaliplatin-induced cold hypersensitivity (Gauchan et al., 2009; Descoeur et al., 2011). An increased expression of TRPM8 in VGluT3+ fibers would thus not only lower the detection threshold of cold stimuli in the periphery, but could also ease a TRPM8-dependent facilitation of synaptic transmission centrally in the spinal dorsal horn (Tang et al., 2013). Our results suggest that adverse effects of oxaliplatin treatment result in part from enhanced signaling by VGluT3+ fibers. It has been recently shown that the endogenous TRPM8 modulator membrane bound phosphoinositide-interacting protein (Pirt) enhances TRPM8-mediated cellular calcium influx (Tang et al., 2013). The role of Pirt in chronic pain conditions remains elusive, but it seems likely that an increased expression of Pirt could be a mechanism of increased cold sensitivity of TRPM8-expressing fibers after oxaliplatin treatment.
Clinical relevance
Mechanical and cold hypersensitivity affect up to 40–60% of patients treated with oxaliplatin (André et al., 2004; Kemeny et al., 2004). Our data now suggest that VGluT3+ fibers play a key role in both mechanical and cold hypersensitivity after oxaliplatin treatment. Distinct subtypes of VGluT3+ primary afferents may, however, be involved in mechanical and cold allodynia, as TH− small diameter neurons seem to preferentially express the TRPM8 receptor. Interestingly, oxaliplatin also causes auditory deficits in humans (Ding et al., 2012) and VGluT3-expressing cells are essential for normal hearing as VGluT3−/− mice are deaf (Seal et al., 2008). Oxaliplatin-induced painful neuropathy and auditory deficits may therefore have a common underlying mechanism, suggesting that VGluT3+ cell types are especially vulnerable to oxaliplatin treatment.
Similar to other types of neuropathic pain, oxaliplatin-induced neuropathy is difficult to treat. Patients respond poorly to pharmacological interventions and it has been suggested that modality rather than etiology may be the better predictor for treatment outcome in neuropathic pain conditions (Pradhan et al., 2010). Our data now imply differential mechanisms contributing to different etiology. This suggests that pain therapies may be differentially effective in patients with oxaliplatin-induced neuropathy, compared with patients with peripheral nerve injury. And indeed, the pharmacological intervention with gabapentin has been shown to be ineffective in oxaliplatin-induced neuropathy, whereas it has beneficial effects in patients with traumatic peripheral nerve injury (Rao et al., 2007; Gordh et al., 2008). Consequently, clinical studies on the effectiveness of a treatment in “neuropathic” pain patients may fail to reach a statistically significant outcome, if patients with different neuropathic pain etiology were included.
It seems that etiology, possibly in combination with modality, allows the development of more specific pharmacological tools, likely associated with reduced side effects. We conclude that the development of pharmaceuticals selectively acting on VGluT3+ primary afferents might be beneficial for the treatment of oxaliplatin-induced peripheral neuropathy.
Footnotes
This work was supported by a grant from the Austrian Science Fund (FWF, project W1205) to J.S. and a scholarship from the Deutsche Schmerzgesellschaft eV to P.D. We thank Rebecca Pauline Seal for the VGluT3-Cre and VGluT3−/− mice, and helpful discussions on an earlier version of the paper, Hongkui Zeng for the Ai32 mice, Babette Maleiner for expert technical assistance, the Julius laboratory at UCSF for the TRPM8 probe, and Ulrike Köck and Sonja Forss-Petter for help with the in situ hybridization.
The authors declare no competing financial interests.
References
- Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na+ channel kinetics on rat sensory neurons. Eur J Pharmacol. 2000;406:25–32. doi: 10.1016/S0014-2999(00)00667-1. [DOI] [PubMed] [Google Scholar]
- Allchorne AJ, Broom DC, Woolf CJ. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005;1:36. doi: 10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138:343–353. doi: 10.1016/j.pain.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Brenner DS, Golden JP, Gereau RW., 4th A novel behavioral assay for measuring cold sensation in mice. PLoS One. 2012;7:e39765. doi: 10.1371/journal.pone.0039765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4:e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiro-da-Silva A, Mogil JS, Séguéla P. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci. 2013;33:18631–18640. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, Lazdunski M, Eschalier A, Authier N, Bourinet E. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Allman BL, Salvi R. Review: ototoxic characteristics of platinum antitumor drugs. Anat Rec (Hoboken) 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Frick A, Kampe K, Zieglgänsberger W. NMDA and AMPA receptors on neocortical neurons are differentially distributed. Eur J Neurosci. 1998;10:3351–3357. doi: 10.1046/j.1460-9568.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- Dowdall T, Robinson I, Meert TF. Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav. 2005;80:93–108. doi: 10.1016/j.pbb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Drdla R, Gassner M, Gingl E, Sandkühler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- Drdla-Schutting R, Benrath J, Wunderbaldinger G, Sandkühler J. Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration. Science. 2012;335:235–238. doi: 10.1126/science.1211726. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gauchan P, Andoh T, Kato A, Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Gordh TE, Stubhaug A, Jensen TS, Arnèr S, Biber B, Boivie J, Mannheimer C, Kalliomäki J, Kalso E. Gabapentin in traumatic nerve injury pain: a randomized, double-blind, placebo-controlled, cross-over, multi-center study. Pain. 2008;138:255–266. doi: 10.1016/j.pain.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Seal RP, Oesch N, Edwards RH, Diamond JS. Genetic targeting and physiological features of VGLUT3+ amacrine cells. Vis Neurosci. 2011;28:381–392. doi: 10.1017/S0952523811000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/S0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jäger T, Sandkühler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Singh N. Differential effect of spironolactone in chronic constriction injury and vincristine-induced neuropathic pain in rats. Eur J Pharmacol. 2010;648:102–109. doi: 10.1016/j.ejphar.2010.08.050. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol. 2011;25:1–28. doi: 10.1111/j.1472-8206.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Kawashiri T, Egashira N, Kurobe K, Tsutsumi K, Yamashita Y, Ushio S, Yano T, Oishi R. L type Ca 2+ channel blockers prevent oxaliplatin-induced cold hyperalgesia and TRPM8 overexpression in rats. Mol Pain. 2012;8:7. doi: 10.1186/1744-8069-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny N, Garay CA, Gurtler J, Hochster H, Kennedy P, Benson A, Brandt DS, Polikoff J, Wertheim M, Shumaker G, Hallman D, Burger B, Gupta S. Randomized multicenter phase II trial of bolus plus infusional fluorouracil/leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J Clin Oncol. 2004;22:4753–4761. doi: 10.1200/JCO.2004.03.119. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδc-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Leitner J, Westerholz S, Heinke B, Forsthuber L, Wunderbaldinger G, Jäger T, Gruber-Schoffnegger D, Braun K, Sandkühler J. Impaired excitatory drive to spinal GABAergic neurons of neuropathic mice. PLoS One. 2013;8:e73370. doi: 10.1371/journal.pone.0073370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci. 2013;33:870–882. doi: 10.1523/JNEUROSCI.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Minett MS, Falk S, Santana-Varela S, Bogdanov YD, Nassar MA, Heegaard AM, Wood JN. Pain without nociceptors? Nav1.7-Independent Pain Mechanisms. Cell Rep. 2014;6:301–312. doi: 10.1016/j.celrep.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Yu XH, Laird JM. Modality of hyperalgesia tested, not type of nerve damage, predicts pharmacological sensitivity in rat models of neuropathic pain. Eur J Pain. 2010;14:503–509. doi: 10.1016/j.ejpain.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110:2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- Rogoz K, Lagerström MC, Dufour S, Kullander K. VGLUT2-dependent glutamatergic transmission in primary afferents is required for intact nociception in both acute and persistent pain modalities. Pain. 2012;153:1525–1536. doi: 10.1016/j.pain.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci U S A. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffnegger D, Ruscheweyh R, Sandkühler J. Spread of excitation across modality borders in spinal dorsal horn of neuropathic rats. Pain. 2008;135:300–310. doi: 10.1016/j.pain.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain. 2009;5:9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Kim A, Masuch T, Park K, Weng H, Wetzel C, Dong X. Pirt functions as an endogenous regulator of TRPM8. Nat Commun. 2013;4:2179. doi: 10.1038/ncomms3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki K, Xing H, Ling J, Gu JG. Menthol-induced Ca2+ release from presynaptic Ca2+ stores potentiates sensory synaptic transmission. J Neurosci. 2004;24:762–771. doi: 10.1523/JNEUROSCI.4658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hindpaw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain. 2009;10:767–773. doi: 10.1016/j.jpain.2009.01.325. [DOI] [PubMed] [Google Scholar]