Abstract
Cortinarius coalescens Kärcher & Seibt is a rare European species of the subgenus Phlegmacium, section Phlegmacioides, neglected in recent molecular studies. New primers (CortF and CortR) designed for species in the section Phlegmacioides allowed to obtain ITS rDNA sequence data from the holotype collection of C. coalescens; according to the results, this epithet has priority over C. crassorum Rob. Henry ex Rob. Henry, C. pardinus Reumaux, and C. parargutus Bidaud, Moënne-Locc. & Reumaux. Morphological and ecological observations on recent collections of C. coalescens from the Czech Republic in comparison with the co-occurring C. largus are discussed. Nomenclatural and taxonomic comments on C. tomentosus Rob. Henry, C. balteatotomentosus Rob. Henry, and C. subtomentosus Reumaux are also provided. So far, C. coalescens is known with certainty from Germany, France, and the Czech Republic, where it grows in deciduous forests on acid to neutral soils. Arsenic and its compounds were determined in C. coalescens and related species of the section Phlegmacioides: C. largus, C. pseudodaulnoyae, and C. variecolor. Total arsenic concentrations were in the range 3.6–30.2 mg kg−1 (dry matter) and arsenobetaine was the major arsenic compound.
Keywords: Cortinariaceae, Phlegmacioides clade, Bioaccumulation, Arsenic, Arsenobetaine, Soil
Introduction
In late summer seasons of 2005, 2009, and 2010, the first author of this paper collected a Cortinarius species of the subgenus Phlegmacium with a conspicuously tomentose cap. The collections were found in deciduous forest plantations at two distant sites in Central Bohemia, Czech Republic, on rather acid bedrock. The specific 30% KOH reaction with a yellow ring on flesh indicated their affinity to the section Phlegmacioides (Brandrud 1998; Moser 1961). According to their ITS rDNA sequences, both collections were identical but there was no positive match in the GenBank and UNITE databases. The use of keys to European Cortinarius species published in Horak (2005) and Knudsen and Vesterholt (2012) did not result in any satisfactory identification but further literature search led to C. pardinus Reumaux, of which the color drawing (Bidaud et al. 1995, Plate 166) agreed well with the appearance of the Czech collections. Later on, the ITS rDNA sequence matched perfectly with that of the holotype of C. pardinus published by Liimatainen et al. (2014). However, Brandrud (1998) considered this species synonymous with C. coalescens Kärcher & Seibt, which was covered by neither the type study of Liimatainen et al. (2014) nor the identification keys. The question of the correct name of this species hence remained unresolved. Chemical analyses of the Czech collections revealed elevated levels of several trace elements in fruit bodies, particularly arsenic, which as an element occurring in both inorganic and organic forms in mushrooms (Nearing et al. 2014). The accumulation and speciation of arsenic in macrofungi depends on particular fungal species/genus (Šlejkovec et al. 1997) and might be of chemotaxonomic importance, but no data on arsenicals in Cortinarius spp. have ever been reported. Information on the arsenic speciation in various organisms is crucial for understanding the elements geobiochemical cycling and its role in the environment. We have, therefore, attempted to obtain molecular data from the holotype of C. coalescens to clarify its affinity to C. pardinus and solve its taxonomy. Furthermore, we have investigated arsenic accumulation and speciation in fruit bodies of the Czech collections and also in several closely related Cortinarius species of the Phlegmacioides clade.
Materials and methods
Collections studied
For the list of studied collections, see Table 1, herbarium acronyms are given according to Thiers (2017). Czech collections of C. coalescens, C. largus, and C. variecolor were sampled and documented fresh in the field; herbarium specimens are deposited at CB, PRM, and FR. The rest of the collections was studied on loan from the herbaria FR, G, O, PRM, and TUB.
Table 1. Cortinarius coalescens and related Cortinarius collections under study.
| ID | Cortinarius | Site, date | Host trees on site | Herbarium voucher | EMBL-Bank |
|---|---|---|---|---|---|
| X-57 | coalescens | GER, Kronberg, 13.IX.1977 | Castanea sativa | FR 0070020, holotype | LT827038 |
| X-103 | coalescens | GER, Kronberg, 31.VIII.2014 | Castanea sativa | FR 0070047 | LT827041 |
| X-104 | coalescens | GER, Kronberg, 23.IX.1995 | Castanea sativa | FR 0070043 | LT827048 |
| X-105 | coalescens | GER, Kronberg, 22.VIII.2014 | Castanea sativa | FR 0070046 | LT827040 |
| X-167 | coalescens | GER, Kronberg, 13.IX.1977 | Castanea sativa | FR-0070041, isotype F300a | LT827042 |
| X-168 | coalescens | GER, Kronberg, 13.IX.1977 | Castanea sativa | FR-0247030, isotype F300b | LT827039 |
| X-11, B-839 | coalescens | CZ, Český Šternberk, 2.VIII.2009 | Quercus robur | PRM 923844 | LT827035 |
| X-218, B-840 | coalescens | CZ, Český Šternberk, 23.VIII.2009 | Quercus robur | PRM 923845 (FR-0070025a) | LT827037, LT827049, LT827050, LT827051 |
| X-43, B-841/b | coalescens | CZ, Běleč-Jinčov, 27.VIII.2010 | Carpinus, Tilia | PRM 923847 | LT827043 |
| X-219 | coalescens | CZ, Běleč-Jinčov, 27.VIII.2010 | Carpinus, Tilia | PRM 923847 (violet cap) | LT827036 |
| - | crassorum | FR | Not reported | PC (Henry 87.127, holotype) | LT855664 |
| X-107 | largus | GER, Söllingen (Pfinztal), 23.VIII.2010 | Fagus sylvatica | FR 0070030 | LT827044 |
| X-217, B-503b | largus | CZ, Český Šternberk, 6.IX.2009 | Quercus robur | PRM 923846 | LT827045 |
| X-260, ASP-70 | pseudodaulnoyae | CZ, Ranšpurk, 10.X.2013 | Carpinus, Tilia, Quercus | PRM 944023 | LT827047 |
| K-14, X-358 | subtomentosus | FR, French Ardennes, Mount Damion | Carpinus, Quercus, Fagus | Reumaux 1112, holotypeb | LT844659 |
| X-316, ASP-65 | variecolor | CZ, Černá v Pošumaví, 14.VIII.2016 | Picea abies | CB 21393 | LT827046 |
Duplicate of the original collection from PRM
Isotype: PRM 945504 (ex herb. Moënne-Loccoz n. 370)
Basidiospores of mature basidiomes (from lamellae in herbarium specimens) were measured; the statistical analysis is based on the measurement of 30–50 spores per collection. Minimum and maximum length/width values of spore size are given in brackets and represent the 5th and 95th percentiles, respectively. Spore length/width quotients (Q-values) are presented as the 5th percentile, median, and 95th percentile, respectively. Spore dimensions were measured on pictures taken with a Canon PowerShot A650 IS digital camera connected to a Zeiss Primo Star LED microscope (Full Köhler); a Zeiss Plan-Achromat 100×/1.25 oil immersion objective was used. Measurements on screen and estimations were carried out using the AxioVision 4.8.1 software. Scanning electron micrographs of the basidiospores were taken by scanning electron microscope TESCAN Vega3 XMU on lamellae sputter coated in gold.
Phylogenetic study
DNA was extracted according to Borovička et al. (2015a). ITS rDNA (ITS1–5.8S–ITS2) and LSU regions were amplified by the polymerase chain reaction (PCR) using the primer pairs ITS1F–ITS4 (or ITS4B) and NL1–NL4, respectively, under the PCR regime as described by Borovička et al. (2011). Additional molecular markers EF-1α (translation elongation factor 1α) and rpb2 (RNA polymerase II second largest subunit) were amplified and sequenced according to Borovička et al. (2012, 2015b). The obtained amplicons were purified with isopropanol and sequenced at Macrogen Europe. Sequences were edited in BioEdit (Hall 1999) and submitted to the EMBL Nucleotide Sequence Database (EMBL-Bank).
In both holotype and isotype collections of C. coalescens, ITS rDNA sequencing repeatedly failed. Moreover, both isotypes were heavily contaminated by conidial fungi (Fig. 1). Thus, we designed primers that selectively amplify the ITS rDNA region of Cortinarius spp. belonging to the section Phlegmacioides. These primers have been designed based on motifs present in sequences of C. coalescens (LT827035), C. patibilis (AY669543), C. eliae (AY669542, as C. largus), C. largus (AY669552 and AY174794, both as C. coalescens), C. balteatoalbus (AY669517), C. spadicellus (AY669539), C. variecolor (AY174796, UDB011729), C. vacciniophilus (AY669518), C. balteatocumatilis (AY174801), and C. balteatus (AY669526). Sequences of Penicillium daleae (JN198424), Neurospora crassa (M13906), and Xerocomellus rubellus (JX030209) were used as the outgroup. The resulting primers CortF (5′-CCTTTGTGCCTATA AACCTATAC-3′) and CortR (5′-GTATAGGTTTATAG GCACAAAGG-3′) were used in combination with universal primers ITS4 and ITS1F, respectively, and amplified 5.8S rRNA, ITS1, and ITS2 regions.
Fig. 1.
Sequenced isotype of Cortinarius coalescens (FR 0247030) contaminated by conidial fungi
Based on BLASTn searches and previous studies (Liimatainen et al. 2014; Garnica et al. 2016), ITS rDNA sequences of the Phlegmacioides clade were downloaded from the databases GenBank (Bidaud and Bellanger 2016; Garnica et al. 2003, 2005, 2016; Liimatainen et al. 2014; Shao et al. 2016) and UNITE (sequence UDB001081, direct submission without a reference). The partial sequence (ITS2) of the holotype of Cortinarius crassorum Rob. Henry ex Rob. Henry was added from an unpublished type study (Frøslev et al., in prep.). The final dataset including newly generated sequences and the additional downloaded ones were composed of 60 ingroup sequences (59 from Europe and one from North America) representing the Phlegmacioides clade. Cortinarius russus was selected for the outgroup, which, based on morphological concept, belonged to the section Phlegmacioides (Brandrud 1998) but phylogenetically seems to be more distantly related (Liimatainen et al. 2014; Garnica et al. 2016).
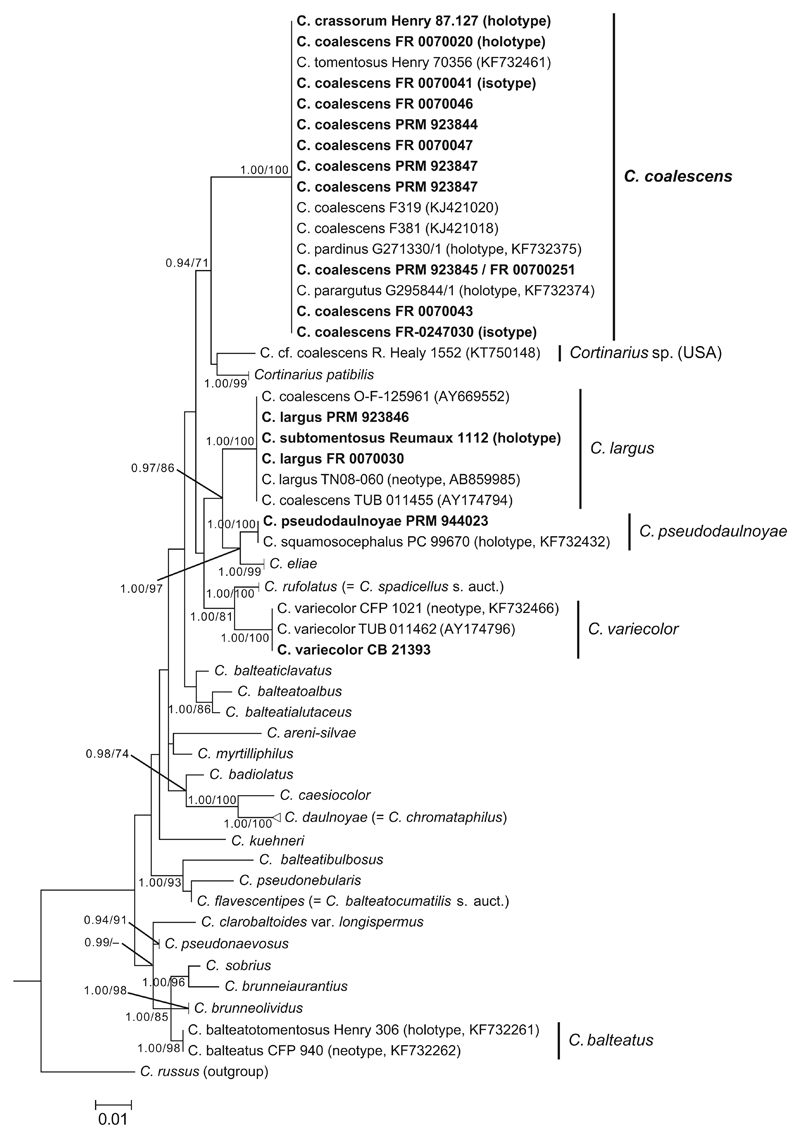
Sequences were aligned with MAFFT online version 7 (Katoh and Toh 2008) using the E-INS-i strategy (Katoh et al. 2005) with default settings. SeaView (Gouy et al. 2010) was used to adjust the alignment manually. Gaps were treated as missing data. Bayesian inference (BI) analysis was carried out with MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) applying the GTR + G substitution model for the dataset. Four incrementally heated simultaneous Markov chain Monte Carlo (MCMC) were run over 10 million generations with trees sampled every 1000 generations and an initial burn-in of 3000 trees (30%). For the remaining trees, a 50% majority rule consensus tree was computed. In addition, RAxML analysis (Stamatakis 2014) was performed with raxmlGUI (Silvestro and Michalak 2012) using rapid bootstrap analysis with 1000 replicates and the GTRGAMMA substitution model. Bayesian (BI) posterior probabilities (PP) >0.90 and maximum likelihood (ML) bootstrap values (BS) >70% were considered sufficient for support. For tree editing and visualization, MEGA6 (Tamura et al. 2013) was used. The topologies of phylogenetic trees from the Bayesian and ML analyses were congruent, and the ML tree is illustrated (Fig. 4).
Fig. 4.
Maximum likelihood phylogeny of the Phlegmacioides clade inferred from ITS rDNA sequence data. Branch support is indicated only when the Bayesian posterior probability and maximum likelihood bootstrap values are >0.90 or >70%, respectively. Sequences of species not being in the focus of this study are shown as compressed clades. Newly generated sequences are highlighted in bold. The bar indicates 0.01 expected change per site per branch
Soil survey and analyses
Soil types, soil properties, and arsenic distribution in soil profiles were investigated at both sites of C. coalescens in the Czech Republic: Český Šternberk and Běleč. Typical soil profiles were chosen on the basis of soil survey. Horizons designation followed the scheme of Jahn et al. (2006). Soils were classified according to the IUSS Working Group WRB (2015). Except O horizons, all samples were collected using a single gouge auger. The pH values were potentiometrically measured (van Reeuwijk 2002) in distilled water and in 1 M KCl with a SenTix electrode using a soil:solution ratio of 1:2.5. The pH values were assessed by the scale of Baize (1993). The soil profile of Český Šternberk was located at the altitude of 369 m with coordinates 49°49′05.2″N and 14°56′02.8″E. The soil sequence of Běleč was sampled at the altitude of 377 m, with coordinates 50°02′28.0″N and 13°59′02.1″E.
Total arsenic content and its mobility were investigated at both sites. Soil samples from particular soil horizons were dried at room temperature and sieved through a 2-mm stainless steel mesh. A representative part of each sample was milled in an agate mill (Fritsch, Germany) and aliquots of approximately 250 mg were used for the determination of total element contents by instrumental neutron activation analysis (INAA) with epithermal neutrons (Cejpková et al. 2016). Certified standard reference material SRM 2711a (Montana II Soil, NIST, USA) was used as the control.
In addition, we sequentially analyzed two arsenic soil fractions (“non-specifically sorbed” and “specifically adsorbed”), which are considered the most bioavailable (Wenzel et al. 2001). In 50-mL centrifuge tubes, 1.600 ± 0.005 g of milled soil with added 40 mL 0.05 M (NH4)2SO4 (Suprapur, Merck) was shaken for 4 h in an overhead shaker (Reax 2, Heidolph) at room temperature. After centrifugation, the supernatant liquid was carefully decanted, filtered (0.45-μm ProFill PTFE filters, Fisherbrand) and promptly analyzed by ICPSFMS (Element2, Thermo Scientific). Then, 40 mL of 0.05 M NH4H2PO4 (TraceSELECT, Fluka) was added to the residue and samples were shaken for 16 h and further processed and analyzed as described above. Procedural blank was processed in the same way.
The bioaccumulation factor (BAF, Falandysz and Borovička 2013) was calculated as the ratio of the chemical concentration in fruit bodies and sum of the most bioavailable arsenic fractions in topsoil horizons (Ah, Ahg). Arsenic concentrations in soils reported in this study are related to dry matter.
Chemical analysis of fungi
In total, seven Cortinarius samples (coalescens, largus, variecolor, and pseudodaulnoyae) were investigated for their concentrations of arsenic and 33 other elements, as well as for their arsenic speciation; element and arsenic species concentrations reported in this study are related to dry matter. Dried pulverized samples were digested in triplicate with nitric acid (p.a., ≥ 65%, Carl Roth, Karlsruhe, Germany) in a microwave-assisted autoclave, and then the total arsenic concentrations were determined with an inductively coupled plasma triple quadrupole mass spectrometer (ICPQQQMS, Agilent 8800, Agilent Technologies, Waldbronn, Germany). Sample preparation and measurement were carried out according to Ertl et al. (2016). Ultrapure water (Merck Millipore, Darmstadt, Germany) was used for the preparation of all solutions. Arsenic was measured in oxygen reaction mode at m/z 75 → 91, the other elements according to Ertl et al. (2016). Be, Ge, In, and Lu were used as internal standards. The certified standard reference material SRM 1573a (Tomato Leaves, NIST, USA) was used for quality control.
For arsenic speciation analysis, each sample was prepared in triplicate. About 50 mg (weighed to 0.1 mg) was extracted with 2 mL of ultrapure water, sonicated in an ultrasonic bath for 15 min, centrifuged at 5500×g, and then filtered through 0.2-μm Nylon® syringe filters (Marcherey-Nagel, Düren, Germany). Then, the extracts were analyzed with high performance liquid chromatography (HPLC, anion- and also cation-exchange chromatography, HPLC 1200, Agilent Technologies) coupled to an ICPQQQMS, according to Scheer et al. (2012). Solutions of arsenobetaine (AB), dimethylarsinic acid (DMA), methylarsonic acid (MA), arsenate [As (V)], trimethylarsine oxide (TMAO), arsenocholine (AC), and tetramethylarsonium ion (TETRA) were used for quantification (0.05–100 μg As/L). Again, the arsenic signal was recorded at m/z 75 → 91. Further on, hydrogen peroxide was added to the extracts to a final concentration of 10% v/v. The mixtures were shaken, put in an oven for one hour at 45 °C and then also analyzed with HPLC-ICPQQQMS, like the pure extracts before.
Results
Taxonomy
Cortinarius coalescens Kärcher & Seibt, Z. Mykol. 54 (1): 78 (April 1988), MB#124551.
= Cortinarius crassorum Rob. Henry ex Rob. Henry, Doc. Mycol. 19 (73): 66 (June 1988), MB#134934.
= Cortinarius pardinus Reumaux in Bidaud et al., Atlas des Cortinaires 7: 230 (1995), MB#447133.
= Cortinarius parargutus Bidaud, Moënne-Loccoz & Reumaux, Atlas des Cortinaires 9: 372 (1999), MB#820859.
Icones: Kärcher and Seibt (1988, Plate 1, a–c), Kärcher and Seibt (1991, Figs. 1 and 2), Bidaud et al. (1995, Plate 166, as C. pardinus), Bidaud et al. (1999, Plate 233, as C. parargutus). Color photographs of Czech collections of C. coalescens are presented in Fig. 2 and scanning electron micrographs of basidiospores in Fig. 3.
Fig. 2.
a Cortinarius coalescens found at Český Šternberk (21 August 2005, not documented by herbarium specimen). b Cortinarius coalescens collected at Běleč (PRM 923847). c, d Cortinarius coalescens collected at Český Šternberk (PRM 923845, duplicate FR 0070025). All photos by Jan Borovička
Fig. 3.
Basidiospores of Cortinarius coalescens and Cortinarius largus under a scanning electron microscope (scale bars = 5 μm). a Cortinarius coalescens (FR 0070020, holotype). b Cortinarius coalescens (PRM 923845). c Cortinarius largus (FR 0070030). d Cortinarius largus (PRM 923846)
With the aid of the newly designed Cortinarius primers, we were able to sequence the holotype and two isotype collections of C. coalescens (Table 1, Fig. 4). Altogether, 16 ITS rDNA sequences of C. coalescens and morphologically alike species were generated in this study (Table 1). Furthermore, sequences of rpb2 (LT827049) and EF-1α (LT827051) were obtained from C. coalescens (PRM 923845) and are available for future molecular studies.
In both BI and ML analyses, the sequences of C. coalescens as interpreted here (based on the study of the type specimen) forms a strongly supported (PP = 1.00/BS = 100%) terminal clade. The species clusters together with the North European C. patibilis and with a North American species originally identified as C. cf. coalescens (Shao et al. 2016); however, the relationship towards these two species was less supported (BI = 0.94, ML = 71%). The maximum intraspecific ITS variability of C. coalescens is low (0.3%), while the minimum interspecific ITS variability is 3% towards C. patibilis, which is the nearest known species, differing by 21 substitution and indel positions from C. coalescens. The relationship of C. coalescens with other species in the Phlegmacioides clade could not be reconstructed in the present study using a single locus (ITS) only.
Two sequences published earlier as C. coalescens (Garnica et al. 2003, 2005) belonged to the morphologically and ecologically very similar C. largus, which is, however, more distantly related based on the phylogenetic analyses. For the species we here refer to as C. coalescens, the binomial C. pardinus Reumaux (= C. parargutus) was applied in the type study of Liimatainen et al. (2014) as the oldest name having ITS rDNA sequence data from the type specimen at that time. Our extensive study, however, revealed that the holotypes of C. coalescens and C. crassorum (both were described in 1988) had identical ITS rDNA sequences to C. pardinus and C. parargutus types, thus leaving the two latter synonyms of C. coalescens. Cortinarius crassorum appeared to be synonymous as well, because its effective publication was dated 2 months later than that of C. coalescens (June vs. April).
Soil survey and analyses
Data on soils are summarized in Table 2. At Český Šternberk, the soil profile showed the following succession of horizons: O-Ah-ABw-Bw-Cr. Without the O horizon (2 cm thick), the soil thickness was 76 cm. This site is covered by Cambisol, which is characterized by weathering of the parent material and by the development of the Bw horizon. Cambisol from the Vrábov site has a weakly acid reaction and an acid one in the ABw horizon. The soil profile at Běleč showed the following succession of horizons: O-Ahg-ABg-Bg-Cg. Without the 2-cm-thick O horizon, the soil thickness was 70 cm. The soil cover was represented by Stagnosol, which shows periodically reducing conditions resulting in stagnic properties. Acid reaction was observed in the Ahg and ABg horizons and weakly acid in the Bg and Cg horizons.
Table 2. Soil characteristics and arsenic contents at Czech sites of Cortinarius coalescens.
| Locality | Depth (cm) | Horizon | pH (H2O) | pH (KCl) | As (total) (mg kg−1) | As (F1) (μg kg−1) | As (F2) (μg kg−1) | As (ΣF) (μg kg−1) |
|---|---|---|---|---|---|---|---|---|
| Běleč | 0–18 | Ahg | 4.67 | 3.84 | 16.2 | 34.3 | 550 | 584 |
| 18–36 | ABg | 4.64 | 3.74 | 14.2 | 16.1 | 447 | 463 | |
| 36–50 | Bg | 5.27 | 3.75 | 15.4 | 8.17 | 711 | 719 | |
| 50–70 | Cg | 5.78 | 4.26 | 17.0 | 5.40 | 1257 | 1263 | |
| Č. Šternberk | 0–11 | Ah | 6.01 | 4.92 | 5.75 | 29.0 | 331 | 360 |
| 11–21 | ABw | 4.88 | 3.62 | 5.05 | 14.5 | 217 | 231 | |
| 21–40 | Bw | 5.20 | 3.67 | 3.72 | 7.91 | 127 | 135 | |
| 40–76 | Cr | 6.00 | 3.84 | 2.72 | 3.17 | 109 | 112 |
F1: arsenic fraction extracted by ammonium sulfate; F2: arsenic fraction extracted by ammonium dihydrogen phosphate; ΣF: sum of fractions F1 and F2 Total arsenic determined for SRM 2711a was 104 ± 2 mg kg−1
At Český Šternberk, the total arsenic concentrations in soils decrease with depth from 5.75 mg kg−1 (Ah horizon) to 2.72 mg kg−1 (Cr horizon), and this trend is seen also in both mobile arsenic fractions. At Běleč, the total arsenic levels are considerably higher (14.2–17.0 mg kg−1) but rather uniformly distributed among horizons. In the Bnon-specifically sorbed arsenic fraction^, arsenic mobility decreases with depth. This is in contrast with what is seen in the “specifically adsorbed” arsenic fraction, which is the highest at the bottom of the soil profile.
Arsenic in fruit bodies
The total arsenic concentrations in the fruit bodies ranged from 3.6 to 30.2 mg kg−1, with a mean of 13 ± 8 mg kg−1. The results of all individual samples are given in Table 4. The results for the other 33 elements can be found in Supplementary Table S1. It is worth mentioning that the concentrations of silver, cadmium, and mercury were higher than in most non-accumulating macrofungi from pristine sites. For silver, between 10 and 41 mg kg−1 were found in the samples. The cadmium concentrations were only slightly lower, namely 2.3–20 mg kg−1, and 0.8–4.9 mg kg−1 of mercury was present in the samples.
Table 4. Total arsenic concentrations, extracted arsenic, and arsenic species in investigated Cortinarius spp. (mg kg−1 in dry matter).
| Sample ID | Cortinarius | Total As mg kg−1 | Extracted As mg kg−1 | DMA mg kg−1 | AB mg kg−1 | TMAO mg kg−1 | AC mg kg−1 | TETRA mg kg−1 | MA mg kg−1 | Inorganic As mg kg−1 | UNK 1 mg kg−1 | UNK 2 mg kg−1 | UNK 3 mg kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASP-65 | variecolor | 13.2 ± 0.8 | 11.4 ± 0.4 | 0.05 | 8.6 | 0.02 | 0.06 | 0.17 | nd | nd | 0.02 | 0.01 | 0.29 |
| ASP-70 | pseudodaulnoyae | 12.3 ± 0.8 | 10.7 ± 0.3 | 0.10 | 8.1 | 0.08 | 0.06 | 0.01 | nd | nd | 0.004 | 0.02 | 0.16 |
| B-503b | largus | 7.4 ± 0.4 | 5.2 ± 0.7 | 0.21 | 4.7 | 0.23 | 0.05 | 0.07 | nd | nd | 0.01 | 0.03 | nd |
| B-839 | coalescens | 11.7 ± 0.5 | 10.9 ± 0.2 | 0.05 | 8.6 | 0.21 | 0.07 | 0.19 | nd | nd | 0.02 | 0.04 | nd |
| B-840 | coalescens | 3.6 ± 0.4 | 2.6 ± 0.3 | 0.04 | 2.1 | 0.23 | 0.02 | 0.09 | nd | nd | 0.01 | 0.05 | nd |
| B-841 | coalescens | 30.2 ± 0.3 | 27.8 ± 0.6 | 0.05 | 22.1 | 0.21 | 0.24 | 0.43 | nd | nd | 0.03 | 0.04 | nd |
| B-841b | coalescens | 13.6 ± 0.5 | 6.6 ± 0.8 | 0.02 | 5.8 | 0.05 | 0.05 | 0.13 | nd | nd | 0.004 | 0.02 | nd |
UNK: unknown arsenic species; nd: not detectable; limit of detection = 0.002 mg kg−1
Concentrations of total and extracted arsenic are presented as mean and standard deviation from three replicates. Standard deviations of arsenic species within the three replicates were around 5% or less
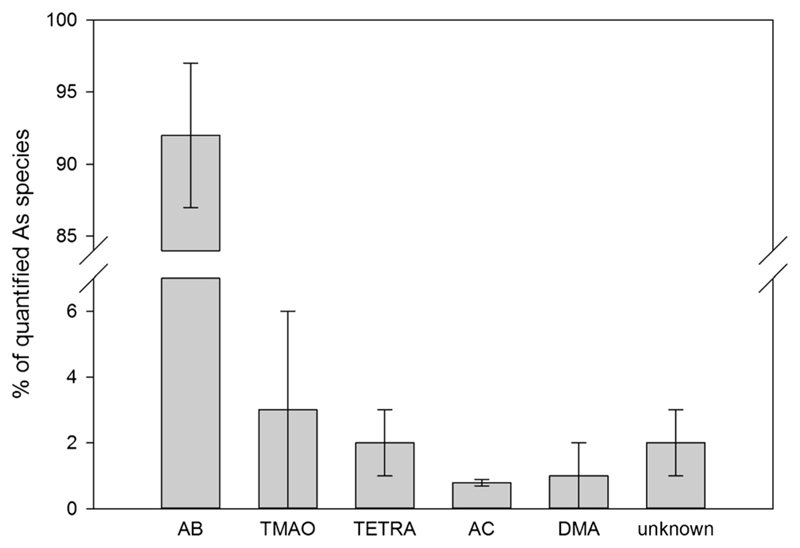
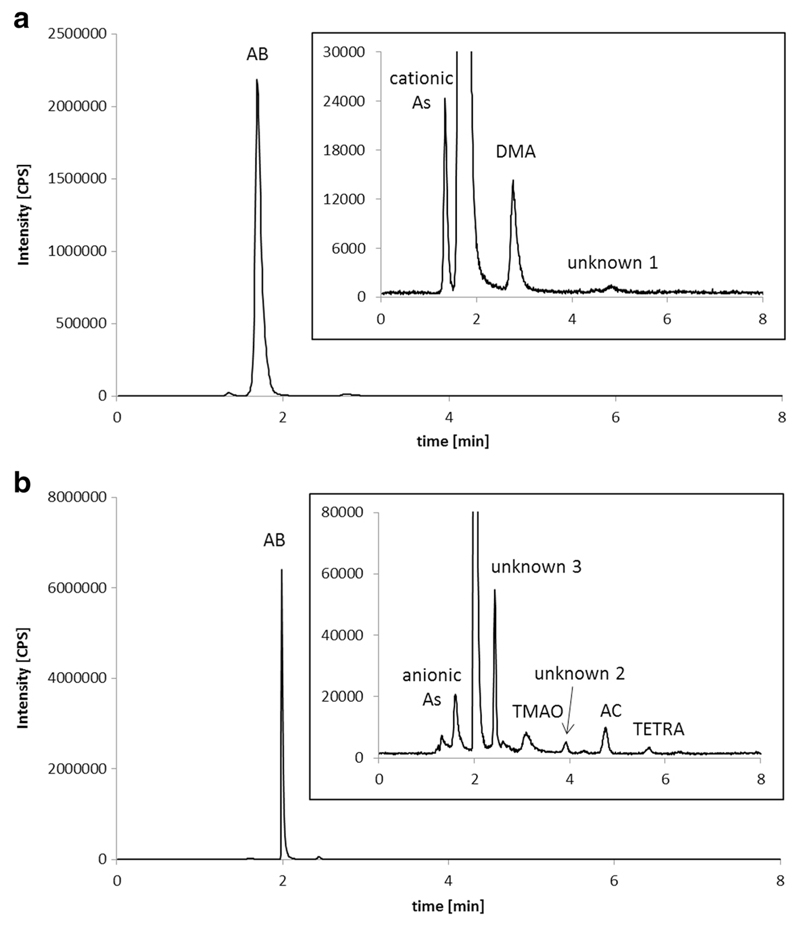
Overall, 80 ± 20% (range: 48–93%) of the arsenic was extractable with water. The column recoveries were between 80 and 100%. Most of the arsenic was present as AB. The investigated samples comprised 60 ± 10% of the total arsenic in this form, which is 92 ± 5% of all arsenic species that could be detected in the extracts with the chosen methods. Besides this, significant amounts of DMA, TMAO, AC, and TETRA were found as well (see Fig. 5 and Table 4). The limit of detection was 0.002 mg kg−1 for all arsenic species. MA and inorganic arsenic were not detected in any of the extracts. Traces of other arsenic species, which could not be identified until now, were also present (see chromatograms in Fig. 6). Two of them were found consistently in all of the samples. Unknown 1 was eluting from the anion-exchange column after 4.8 min, well distinguishable from MA (4.4 min). Unknown 2 was detected in the chromatograms of the cation-exchange method, with a retention time of 3.9 min, between TMAO and AC. Two samples (C. variecolor and C. pseudodaulnoyae) also contained significant concentrations of another arsenic compound (“unknown 3”), which eluted from the cation-exchange column after 2.5 min, between AB and TMAO. All of the unknown species were stable upon oxidation with hydrogen peroxide.
Fig. 5.
Arsenic species in extracts of investigated Cortinarius spp. (in % of all detected arsenic species). AB: arsenobetaine, TMAO: trimethylarsine oxide, TETRA: tetramethylarsonium ion, AC: arsenocholine, DMA: dimethylarsinic acid
Fig. 6.
Whole chromatograms and details of one extract of Cortinarius pseudodaulnoyae (ASP-70). a Anion-exchange conditions. b Cation-exchange conditions
Discussion
Taxonomy and ecology of C. coalescens
According to the phylogenetic analyses, C. coalescens is a sister species of C. patibilis, a similarly looking species which is, however, associated with conifers and of a North American species (found in Massachusetts, USA), which was identified as C. cf. coalescens. The color photograph of the latter undoubtedly showed a typical member of the section Phlegmacioides (Shao et al. 2016). At its type locality in Germany, C. coalescens grows under Castanea sativa on neutral soil (Kärcher and Seibt 1988). According to this study, it can also be associated with other deciduous trees (Quercus, Tilia/Carpinus) and topsoils with weakly acid to acid reaction. The current distribution of this species covers Germany, Czech Republic, and France. The Norwegian collection (O 125961) reported in Brandrud (1998) turned out to be C. largus after ITS rDNA sequencing (GenBank AY669552) as well as the Spanish collection (GenBank MF589755) reported by Kärcher and Seibt (1988).
With regard to morphological characters, it was observed that C. coalescens can have a violaceous cap margin (Fig. 2b, left specimen), which is probably a rare feature reported for neither type collections of C. coalescens nor synonymous taxa. However, the identity of this aberrant fruit body was confirmed by ITS rDNA sequencing (LT827036). The possible occurrence of violaceous tinges on cap further complicates field identification of C. coalescens, especially distinguishing it from C. largus. The latter species is common in Central Europe, but it also grows under deciduous trees and may be similarly colored. Unfortunately, there are no significant differences in either spore size (Table 3) or spore ornamentation (Fig. 3). At Český Šternberk, C. largus (Fig. 7) was found growing directly at the same site where C. coalescens was collected. The only apparent character separating both species in the field was the tomentose cap (at least near the margin) in C. coalescens, especially when dry (Fig. 2a, b). However, C. largus is a rather variable species and probably only molecular markers enable reliable separation from C. coalescens.
Table 3. Investigated collections of Cortinarius coalescens and related species with indicated spore size ranges and Q-values.
| Cortinarius | Collection | Spore size (μm) | Q-value |
|---|---|---|---|
| coalescens | FR 0070020 | 10.39) 11.09–11.43–11.76 (12.46) × (6.03) 6.23–6.39–6.64 (6.95) | (1.64) 1.71–1.76-1.84 (1.93) |
| coalescensa | G 3432 | 10.36) 10.86–11.35–12.08 (12.82) × (5.55) 5.89–6.24–6.41 (6.66) | (1.70) 1.76–1.80-1.95 (2.18) |
| coalescens | PRM 923844 | 9.63) 10.21–10.63–10.88 (11.9) × (5.53) 5.74–5.98–6.07 (6.30) | (1.60) 1.68–1.80-1.86 (2.01) |
| coalescens | PRM 923845 | 9.73) 10.25–10.37–10.95 (11.29) × (5.49) 5.70–5.85–6.05 (6.41) | (1.66) 1.69–1.80-1.89 (1.94) |
| coalescens | PRM 923847 | 9.84) 10.23–10.40–10.67 (10.98) × (5.32) 5.62–5.84–6.05 (6.26) | (1.66) 1.72–1.80-1.86 (1.98) |
| largusb | O 125961 | 10.74) 11.35–11.95–12.71 (13.30) × (5.88) 6.14–6.43–6.64 (7.11) | (1.69) 1.83–1.87-1.93 (2.01) |
| largusc | TUB 011455 | 10.50) 11.15–11.31–11.74 (12.34) × (5.94) 6.13–6.29–6.59 (6.95) | (1.66) 1.74–1.79-1.82 (1.93) |
| largusd | PRM 945504 | 9.77) 10.76–11.08–11.72 (12.25) × (5.29) 5.70–5.85–6.06 (6.57) | (1.73) 1.84–1.90-1.95 (2.08) |
| largus | PRM 923846 | 10.78) 11.08–11.56–12.16 (12.39) × (5.82) 6.02–6.17–6.35 (6.53) | (1.72) 1.79–1.89-1.94 (2.02) |
| largus | FR 0070030 | 9.91) 10.42–10.74–11.11 (11.62) × (5.51) 5.86–5.99–6.18 (6.40) | (1.64) 1.71–1.81-1.86 (1.95) |
| pseudodaulnoyae | PRM 944023 | 10.29) 10.87–11.25–11.54 (12.06) × (6.26) 6.54–6.70–6.92 (7.12) | (1.54) 1.62–1.67-1.72 (1.77) |
Fig. 7.
Cortinarius largus from Český Šternberk (PRM 923846, B-503b), where it shares the habitat with C. coalescens. Photo by Jan Borovička
The problem of C. tomentosus and C. subtomentosus
In the protologue of C. pardinus, Reumaux highlighted its close affinity to C. tomentosus Rob. Henry ex Rob. Henry (Bidaud et al. 1995). This would suggest that C. tomentosus might be the correct name for C. coalescens. However, C. tomentosus was not discussed in the study by Liimatainen et al. (2014) and has a highly problematical nomenclatural history.
The first description of C. tomentosus (Henry in Kühner and Romagnesi 1953) was invalid, as no Latin diagnosis was included. In 1958, the name was published again but with no indication of the type (Henry 1958). A later publication of C. tomentosus (Henry 1985a) was invalid again (no Latin diagnosis provided).
Notably, the sequence KF732461 (as C. pardinus) obtained from a specimen originally labeled as C. tomentosus from the herbarium of R. Henry in PC (also indicated as the paratype of C. tomentosus in Henry 1985a) matches that of C. coalescens. The valid description of C. tomentosus is dated to December 1985 (Henry 1985b), but C. tomentosus became an illegitimate name because of having the same holotype (Henry 306) as Cortinarius balteatotomentosus Rob. Henry ex Rob. Henry (1985a). According to Liimatainen et al. (2014), C. balteatotomentosus is a synonym of C. balteatus. Thus, C. tomentosus falls within the nomenclatural and taxonomic history of C. balteatus as follows:
Cortinarius balteatus (Fr.) Fr., Epicr. Syst. Mycol.: 257. 1838
≡ Agaricus balteatus Fr., Observ. Mycol. 2: 138. 1818. [basionym].
= Cortinarius subbalteatus Kühner, Bull. Mens. Soc. Linn. Lyon 24, 2: 40. 1955.
= Cortinarius balteatotomentosus Rob. Henry ex Rob. Henry, Bull. Soc. Mycol. France 101 (1): 4. April 1985.
= Cortinarius tomentosus Rob. Henry ex Rob. Henry, Doc. Mycol. 16 (61): 24. December 1985. [nom. illeg., Art. 52.1: holotype of Cortinarius balteatotomentosus included].
= Cortinarius subopimus Bidaud, Atlas des Cortinaires 7: 231. 1995.
–Cortinarius tomentosus Rob. Henry in Kühner & Romagnesi, Fl. Analyt. Champ. Supér. (Paris): 282. 1953. [nom. nud., Art. 39.1: no Latin description or diagnosis].
–Cortinarius balteatotomentosus Rob. Henry, Bull. Soc. Mycol. France 74: 303. 1958. [nom. inval., Art. 40.1: no indication of type].
–Cortinarius tomentosus Rob. Henry, Bull. Soc. Mycol. France 101 (1): 4. 1985. [nom. nud., Art. 39.1: no Latin description or diagnosis].
According to its description, Cortinarius subtomentosus Reumaux (Reumaux 1988) would also possibly represent the same species as C. coalescens. Curiously enough, both taxa were described in April 1988. However, the partial ITS rDNA sequence obtained from the holotype of C. subtomentosus is distinct and matches that of C. largus (Fig. 4).
Arsenic in Cortinarius species and underlying soils
High concentrations of toxic metals and metalloids in macrofungal fruit bodies are often found in samples from pristine areas. The biological importance of the accumulation process itself is unknown and element accumulation is often characteristic for particular fungal species or genera (Falandysz and Borovička 2013). Moreover, macrofungi are able to discriminate between the homologs arsenic and antimony (Borovička et al. 2006), and preferred metals are selectively accumulated regardless of their bioavailability in the underlying soil (Kubrová et al. 2014). In the case of arsenic, some macrofungi can take up even more than 1000 mg As kg−1(Falandysz and Rizal 2016).
Arsenic concentrations in soils found at both sites of C. coalescens fall within the range of natural levels (compare with Kabata-Pendias 2011). Arsenic contents of C. coalescens from Běleč (samples B-841 and B-841b; 13.6 and 30.2 mg kg−1) were higher than those detected in collections from Český Šternberk (samples B-839 and B-840; 3.6 and 11.7 mg kg−1), which might be caused by considerably lower arsenic soil content at the latter site. When compared with soils, arsenic is accumulated in fruit bodies, especially when related to the most bioavailable arsenic soil fractions; the BAF ranges between 11 and 51. Arsenic concentrations in additional Cortinarius species of the Phlegmacioides clade (Table 4) were in the range of 7.4–13.2 mg kg−1. Reported concentrations for common species of other ectomycorrhizal genera like Russula, Lactarius, Amanita, and Boletus s.l. are mostly below 5 mg kg−1 (Kalač 2010; Falandysz and Rizal 2016), and the contents found in Cortinarius species of the subgenus Phlegmacium can, thus, be considered elevated (see also Borovička et al. 2015a).
Besides arsenic, the concentrations of silver, cadmium, and mercury were interesting as well. With 30 ± 10 mg kg−1 of silver, the investigated samples are still far away from fungi like Amanita strobiliformis, which are able to accumulate more than 1000 mg Ag kg−1 (Borovička et al. 2007). Still, when compared to normal samples from pristine areas (Borovička et al. 2010), the concentrations reported here are clearly elevated. Also, in the case of cadmium, with 2.3–20 mg kg−1, the concentrations are not new record holders, but on the upper end of the range for common macrofungi (Kalač and Svoboda 2000). Finally, the mercury concentrations (0.8–4.9 mg kg−1) can be considered as slightly increased too (Kalač and Svoboda 2000).
Qualitatively, the arsenic speciation appeared to be very similar in all extracts, which suggests no chemotaxonomic importance of arsenicals within the Phlegmacioides clade. The major arsenic compound was AB, besides small quantities of TMAO, TETRA, AC, and DMA. Interestingly, all of the four latter species were present in quite similar amounts. This speciation profile has rarely been found in nature. In the article by Nearing, Koch and Reimer, where they investigated arsenic compounds of 73 mushroom samples, there are only three macrofungi where the arsenic speciation is comparable to our present findings, but in different proportions: Coprinopsis atramentaria, Hebeloma velutipes, and Boletus edulis (Nearing et al. 2014).
What is also astonishing is the absence of inorganic arsenic (typically the main arsenic species in soils) and MA, which is the alleged first product of the biomethylation pathway of inorganic arsenic in microbiota (Bentley and Chasteen 2002; Cullen 2014). The major compound AB accounted for 65–88% of the extracted arsenic. The origin of methylated arsenicals in macrofungi is still unclear, as methylation of arsenic has not been demonstrated in vitro in most of the tested macrofungal genera (Nearing et al. 2015). Therefore, it is unclear whether AB is formed during the vegetative or reproductive life stages of fungi, or by surrounding microbial community (Nearing et al. 2016). Investigation of this phenomenon and identification of the detected unknown arsenic species was beyond the scope of this study but will be attempted in the future.
Supplementary Material
Electronic supplementary material The online version of this article (doi:10.1007/s11557-017-1331-z) contains supplementary material, which is available to authorized users.
Acknowledgements
We thank Luis Alberto Parra-Sánchez for the invaluable comments on nomenclature, Jan Běťák for donating his collection of C. pseudodaulnoyae, and Tereza Tejklová for the kind assistance with the literature. Furthermore, we thank the curators of herbaria FR, G, O, PC, PRM, and TUB for providing their collections for microscopic and molecular study. André Bidaud and Patrick Reumaux are acknowledged for sending us the holotype of C. subtomentosus. This research was supported by the joint project GAČR GF16-34839 L (Czech Science Foundation) – FWF I 2352-B21 (Austrian Science Fund). The visit of Bálint Dima in PC was financially supported by SYNTHESYS, the European Union-funded Integrated Activities grant (application FR-TAF-4253). Institutional support for the institutes of the Czech Academy of Sciences was provided by the Long-term Development Projects RVO61388971, RVO67985831, and RVO61389005. INAA irradiations were carried out at the infrastructure of the NPI CAS Řež supported through the projects LM2011019 and LM2015074 (Ministry of Education, Youth and Sports of the Czech Republic).
References
- Baize D. Soil science analyses: a guide to current use. John Wiley & Sons Ltd; Chichester: 1993. [Google Scholar]
- Bentley R, Chasteen TG. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol Mol Biol Rev. 2002;66:250–271. doi: 10.1128/mmbr.66.2.250-271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaud A, Bellanger JM. À propos de Cortinarius daulnoyae Quél. J JEC. 2016;18:13–23. [Google Scholar]
- Bidaud A, Moënne-Loccoz P, Reumaux P, Henry R. Atlas des Cortinaires VII. Fédération Mycologique Dauphiné-Savoie; Annecy: 1995. [Google Scholar]
- Bidaud A, Moënne-Loccoz P, Reumaux P, Henry R. Atlas des Cortinaires IX. Fédération Mycologique Dauphiné-Savoie; Annecy: 1999. [Google Scholar]
- Borovička J, Řanda Z, Jelínek E. Antimony content of macrofungi from clean and polluted areas. Chemosphere. 2006;64:1837–1844. doi: 10.1016/j.chemosphere.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Borovička J, Řanda Z, Jelínek E, Kotrba P, Dunn CE. Hyperaccumulation of silver by Amanita strobiliformis and related species of the section Lepidella. Mycol Res. 2007;111:1339–1344. doi: 10.1016/j.mycres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Borovička J, Kotrba P, Gryndler M, Mihaljevič M, Řanda Z, Rohovec J, Cajthaml T, Stijve T, Dunn CE. Bioaccumulation of silver in ectomycorrhizal and saprobic macrofungi from pristine and polluted areas. Sci Total Env. 2010;408:2733–2744. doi: 10.1016/j.scitotenv.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Borovička J, Noordeloos ME, Gryndler M, Oborník M. Molecular phylogeny of Psilocybe cyanescens complex in Europe, with reference to the position of the secotioid Weraroa novae-zelandiae. Mycol Prog. 2011;10:149–155. doi: 10.1007/s11557-010-0684-3. [DOI] [Google Scholar]
- Borovička J, Rockefeller A, Werner PG. Psilocybe allenii—a new bluing species from the Pacific coast, USA. Czech Mycol. 2012;64:181–195. [Google Scholar]
- Borovička J, Bušek B, Mikšík M, Dvořák D, Jeppesen TS, Dima B, Albert L, Frøslev TG. Cortinarius prodigiosus—a new species of the subgenus Phlegmacium from Central Europe. Mycol Prog. 2015a;14:29. doi: 10.1007/s11557-015-1051-1. [DOI] [Google Scholar]
- Borovička J, Oborník M, Stříbrný J, Noordeloos ME, Parra Sánchez LA, Gryndler M. Phylogenetic and chemical studies in the potential psychotropic species complex of Psilocybe atrobrunnea with taxonomic and nomenclatural notes. Persoonia. 2015b;34:1–9. doi: 10.3767/003158515X685283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandrud TE. Cortinarius subgenus Phlegmacium section Phlegmacioides (= Variecolores) in Europe. Edinb J Bot. 1998;55:65–156. doi: 10.1017/S0960428600004364. [DOI] [Google Scholar]
- Cejpková J, Gryndler M, Hršelová H, Kotrba P, Řanda Z, Synková I, Borovička J. Bioaccumulation of heavy metals, metalloids, and chlorine in ectomycorrhizae from smelter-polluted area. Env Poll. 2016;218:176–185. doi: 10.1016/j.envpol.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Cullen WR. Chemical mechanism of arsenic biomethylation. Chem Res Toxicol. 2014;27:457–461. doi: 10.1021/tx400441h. [DOI] [PubMed] [Google Scholar]
- Ertl K, Kitzer R, Goessler W. Elemental composition of game meat from Austria. Food Addit Contam B. 2016;9:120–126. doi: 10.1080/19393210.2016.1151464. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Borovička J. Macro and trace mineral constituents and radionuclides in mushrooms: health benefits and risks. Appl Microbiol Biotechnol. 2013;97:477–501. doi: 10.1007/s00253-012-4552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falandysz J, Rizal LM. Arsenic and its compounds in mushrooms: a review. J Environ Sci Health C. 2016;34:217–232. doi: 10.1080/10590501.2016.1235935. [DOI] [PubMed] [Google Scholar]
- Garnica S, Weiß M, Oertel B, Oberwinkler F. Phylogenetic relationships of European Phlegmacium species (Cortinarius, Agaricales) Mycologia. 2003;95:1155–1170. doi: 10.2307/3761917. [DOI] [PubMed] [Google Scholar]
- Garnica S, Weiß M, Oertel B, Oberwinkler F. A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, Agaricales) derived from morphological and molecular data. Can J Bot. 2005;83:1457–1477. doi: 10.1139/b05-107. [DOI] [Google Scholar]
- Garnica S, Schön ME, Abarenkov K, Riess K, Liimatainen K, Niskanen T, Dima B, Soop K, Froeslev TG, Jeppesen TS, Peintner U, et al. Determining threshold values for barcoding fungi: lessons from Cortinarius (Basidiomycota), a highly diverse and widespread ectomycorrhizal genus. FEMS Microbiol Ecol. 2016;92:fiw045. doi: 10.1093/femsec/fiw045. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Henry R. Suite à l’étude des Cortinaires. Bull Soc Mycol Fr. 1958;74:249–361. [Google Scholar]
- Henry R. Nouvelle étude de Cortinaires. Bull Soc Mycol Fr. 1985a;101:1–13. [Google Scholar]
- Henry R. Validations—Diagnoses Latines. Doc Mycol. 1985b;16:47–54. [Google Scholar]
- Henry R. Nouvelles validations et typifications. Doc Mycol. 1988;19:63–68. [Google Scholar]
- Horak E. Röhrlinge und Blätterpilze in Europa. Elsevier; München: 2005. [Google Scholar]
- IUSS Working Group WRB. World Soil Resources Reports No. 106. FAO; Rome: 2015. World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps; p. 192. [Google Scholar]
- Jahn R, Blume HP, Asio VB, Spaargaren O, Schad P. Guidelines for soil description. 4th edn. FAO; Rome: 2006. [Google Scholar]
- Kabata-Pendias A. Trace elements in soils and plants. 4th edn. CRC Press; Boca Raton: 2011. [Google Scholar]
- Kalač P. Trace element contents in European species of wild growing edible mushrooms: a review for the period 2000–2009. Food Chem. 2010;122:2–15. doi: 10.1016/j.foodchem.2010.02.045. [DOI] [Google Scholar]
- Kalač P, Svoboda L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000;69:273–281. doi: 10.1016/S0308-8146(99)00264-2. [DOI] [Google Scholar]
- Kärcher R, Seibt D. Beitrag zur Kenntnis der Pilzflora des Rhein-Main-Gebietes. Teil 1 – Pilzgesellschaften im Kronberger Edelkastanienhain – Cortinarius subgenus Phlegmacium und Myxacium. Z Mykol. 1988;54:77–92. [Google Scholar]
- Kärcher R, Seibt D. Beitrag zur Kenntnis der Pilzflora des Rhein-Main-Gebietes. Teil 3 – Neues über Cortinarius (Phl.) coalescens Kärcher & Seibt (1988) sowie Diskussionen über nahestahende Taxa aus Laubwaldgesellschaften. Z Mykol. 1991;57:249–252. [Google Scholar]
- Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma KI, Toh H, Miyata T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H, Vesterholt J, editors. Funga Nordica. 2nd edn. Nordsvamp; Copenhagen: 2012. [Google Scholar]
- Kubrová J, Žigová A, Řanda Z, Rohovec J, Gryndler M, Krausová I, Dunn CE, Kotrba P, Borovička J. On the possible role of macrofungi in the biogeochemical fate of uranium in polluted forest soils. J Haz Mat. 2014;280:79–88. doi: 10.1016/j.jhazmat.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Kühner R, Romagnesi H. Flore Analytique des Champignons Supérieurs (agarics, bolets, chanterelles) Masson; Paris: p. 1953. [Google Scholar]
- Liimatainen K, Niskanen T, Dima B, Kytövuori I, Ammirati JF, Frøslev TG. The largest type study of Agaricales species to date: Bringing identification and nomenclature of Phlegmacium (Cortinarius) into the DNA era. Persoonia. 2014;33:98–140. doi: 10.3767/003158514X684681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MM. Die Gattung Phlegmacium. Julius Klinkhardt; Bad Heilbrunn Obb: 1961. [Google Scholar]
- Nearing MM, Koch I, Reimer KJ. Arsenic speciation in edible mushrooms. Environ Sci Technol. 2014;48:14203–14210. doi: 10.1021/es5038468. [DOI] [PubMed] [Google Scholar]
- Nearing MM, Koch I, Reimer KJ. Uptake and transformation of arsenic during the vegetative life stage of terrestrial fungi. Env Poll. 2015;197:108–115. doi: 10.1016/j.envpol.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Nearing MM, Koch I, Reimer KJ. Uptake and transformation of arsenic during the reproductive life stage of Agaricus bisporus and Agaricus campestris. J Environ Sci. 2016;49:140–149. doi: 10.1016/j.jes.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Reumaux P. En marge de l’Atlas des Cortinaires (validation d’espèces nouvelles) Bull Trim Féd Mycol Dauph-Sav. 1988;28:25–30. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, Pollak J, Tellez-Plaza M, Silbergeld EK, Guallar E, Navas-Acien A. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods. 2012;4:406–413. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Tang S, Healy RA, Imerman PM, Schrunk DE, Rumbeiha WK. A novel orellanine containing mushroom Cortinarius armillatus. Toxicon. 2016;114:65–74. doi: 10.1016/j.toxicon.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12:335–337. doi: 10.1007/s13127-011-0056-0. [DOI] [Google Scholar]
- Šlejkovec Z, Byrne AR, Stijve T, Goessler W, Irgolic KJ. Arsenic compounds in higher fungi. Appl Organomet Chem. 1997;11:673–682. doi: 10.1002/(SICI)1099-0739(199708)11:8<673::AID-AOC620>3.0.CO;2-1. [DOI] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers B. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium; 2017. [continuously updated], http://sweetgum.nybg.org/ih/ [Google Scholar]
- van Reeuwijk LP. Procedures for soil analysis. International Soil Reference and Information Centre (ISRIC); Wageningen: 2002. Technical paper 9. [Google Scholar]
- Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta. 2001;436:309–323. doi: 10.1016/S0003-2670(01)00924-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.