Abstract
During infections, TLR-mediated responses require tight regulation to allow for pathogen removal, while preventing overwhelming inflammation and immunopathology. The triggering receptor expressed on myeloid cells (TREM)-2 negatively regulates inflammation by macrophages and impacts on phagocytosis, but the function of endogenous TREM-2 during infections is poorly understood. We investigated TREM-2’s role in regulating TLR4-mediated inflammation by studying wild-type and TREM-2−/− mice challenged with LPS and found TREM-2 to dampen early inflammation. Augmented early inflammation in TREM-2−/− animals was followed by an accelerated resolution and ultimately improved survival, associated with the induction of the negative regulator A20. Upon infection with Escherichia coli, the otherwise beneficial effect of an exaggerated early immune response in TREM-2−/− animals was counteracted by a 50% reduction in bacterial phagocytosis. In line with this, TREM-2−/− peritoneal macrophages (PMs) exhibited augmented inflammation following TLR4 stimulation, demonstrating the presence and negative regulatory functionality of TREM-2 on primary PMs. Significantly, we identified a high turnover rate because TREM-2 RNA is 25-fold down-regulated and the protein proteasomally degraded upon LPS encounter, thus ensuring a tightly regulated and versatile system that modulates inflammation. Our results illustrate TREM-2’s effects on infection-triggered inflammation and identify TREM-2 as a potential target to prevent overwhelming inflammation while preserving antibacterial-effector functions.
Keywords: peritonitis, inflammation, macrophages, negative regulator
Peritonitis is an Infection of the otherwise sterile peritoneal cavity by intestinal bacteria like Escherichia coli and a frequent source of sepsis (1). Despite the availability of antibiotics, mortality rates remain high, mostly due to uncontrolled systemic inflammation and organ failure that cannot be treated as of today (2).
Pattern recognition receptors expressed on immune cells are the first sensors of invading pathogens and crucial in inducing protective inflammatory responses (3, 4). TLR2 and TLR4 are of particular importance because they detect bacterial lipoproteins and LPS from E. coli to then elicit immune responses including the production of cytokines and chemokines that in turn attract neutrophils to the site of infection (5, 6). Thus, impaired TLR signaling substantially compromises these protective immune responses against E. coli, leading to uncontrolled bacterial spread, systemic inflammation, and subsequent organ failure (5, 6). Systemic inflammation and subsequent organ failure are the hallmarks of sepsis (7, 8) and the most frequent cause of death from peritonitis in humans. The unfettered and overwhelming inflammatory response during sepsis either develops as a consequence of inefficient elimination of bacteria and persistent triggering of immune responses or is due to an impaired resolution of inflammatory responses in the absence of pathogens. The fatal effects of persistent inflammation are best illustrated in models of endotoxemia, in which high doses of LPS induce a deadly systemic inflammatory response from which TLR4-deficient mice are protected (9). The outcome from severe infections and sepsis is therefore determined by a tightly regulated inflammatory response that ensures efficient bacterial clearance without causing inflammation-induced immunopathology. In concurrence with this notion, a number of regulatory molecules that modulate and fine-tune TLR-mediated inflammation have been identified over the last years (8, 10).
The triggering receptor expressed on myeloid cell (TREM)-2 is as such a prototypic regulator of TLR-mediated inflammation (11). TREM-2 is expressed on a variety of innate immune cells, including macrophages, dendritic cells, and microglia (11–13). To this end, several in vitro studies showed that TREM-2 negatively regulates TLR-induced responses, by a yet unknown mechanism. Bone marrow-derived macrophages (BMMs), thioglycolate-recruited peritoneal macrophages (PMs), and dendritic cells of TREM-2 knockout (TREM-2−/−) mice produce more inflammatory cytokines compared to wild-type (WT) macrophages in response to LPS or CpG (12–16). Although, to this end, an endogenous ligand remains unknown, one report demonstrated that TREM-2 might bind bacterial cell wall components like LPS and lipoteichoic acid (17). Significantly, TREM-2 is involved in bacterial phagocytosis. As such, TREM-2-deficient BMMs were shown to less efficiently phagocytose E. coli, whereas TREM-2 transfection enabled nonphagocytic Chinese hamster ovary cells to internalize E. coli and Staphylococcus aureus (18), and i.v. injection of TREM-2-overexpressing myeloid cells improved bacterial clearance and outcome in a model of cecal ligation and puncture (19).
TREM-2 is a single-pass transmembrane protein of the Ig superfamily and contains a very small intracellular domain with no known motifs for signal transduction (11, 12, 20). TREM-2 signaling occurs via pairing with the immunoreceptor tyrosine-based activating motif (ITAM)–bearing adaptor DNAX-activation protein of 12 kDa (DAP12) (11, 12), which is a promiscuous protein that interacts with different members of the TREM family and other receptors, such as killer cell Ig-like receptor, Ly49, myeloid-associated Ig-like receptor-II, or Sialic acid-binding Ig-type lectin (14, 21). Upon ligation of its associated receptor, downstream signaling events are shaped by Src family kinase-mediated phosphorylation of DAP12’s ITAM domain, followed by recruitment of the Syk protein tyrosine kinase. Syk is importantly involved in the downstream engagement of diverse signaling pathways, including NF-κB, PI3K, and PLCγ (14, 21, 22). Considering TREM-2’s inhibitory effects on macrophage activation, the phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase was found to negatively regulate PI3K recruitment to the TREM-2/DAP12 signaling complex (23). Moreover, the requirement for DAP12-dependent phosphorylation of downstream kinase 3 was shown recently to be involved in the TREM-2-associated prevention of inflammatory responses to low-dose LPS (24).
Based on the finding that mutations in the TREM-2 or DAP12 gene in humans lead to a condition called Nasu-Hakola disease, which is characterized by bone cysts and presenile dementia (25–27), most previous studies focused on TREM-2’s role in bone formation and the maintenance of brain homeostasis. As such, TREM-2 was found to be important in the differentiation of osteoclasts (28–31) and as a receptor for apoptotic neurons (32). Very recent data illustrate that rare heterozygous variants of TREM-2 are associated with an increased risk for Alzheimer’s disease (33).
Despite TREM-2’s reported function in inflammation and phagocytosis in vitro and in contrast to the closely related receptor TREM-1, which augments TLR-mediated inflammation and whose involvement was found to be associated with progressive inflammation during human and murine sepsis (8, 34–37), the biologic role of TREM-2 during infections in vivo is poorly understood. To this end, one report found TREM-2 to be involved in the bacterial clearance and control of inflammation during Pseudomonas aeruginosa keratitis (38), and one publication showed TREM-2-overexpressing bone marrow macrophages to enhance phagocytosis of bacteria in vivo (19). We recently investigated the function of TREM-2 during pneumococcal pneumonia and discovered a detrimental and cell-type-specific role for TREM-2. We found TREM-2 on alveolar macrophages to selectively suppress the production of the complement component C1q, which in turn impaired bacterial phagocytosis and outcome from pneumonia (39). However, the role of endogenous TREM-2 during Gram-negative infection and sepsis and the individual contribution of TREM-2 to the function of different immune cells in the course of inflammation therein are unclear. Using TREM-2-deficient mice, we studied the biologic function of TREM-2 in regulating TLR4-induced responses in vivo during endotoxemia and Gram-negative sepsis, as well as on a cellular level by investigating TREM-2’s expression and function on different macrophage subsets, including resident PMs.
Materials and Methods
Animals
Age-matched 8- to 12-wk-old female TREM-2−/− mice and WT C57BL/6 controls were used. TREM-2−/− mice were kindly provided by Marco Colonna (Washington University, St. Louis, MO, USA) and backcrossed onto a >98% B6 C57BL/6 background facilitated by genome-wide simple sequence length polymorphism typing at 10 cM intervals (Speed Congenics Facility of the Rheumatic Diseases Core Center, Washington University, St. Louis, MO, USA) (13). All in vivo experiments were approved by the institutional review board of the Medical University of Vienna and the Austrian Ministry of Sciences (BMWF-66.009/065-II/3b/2011).
Mouse models of endotoxemia and E. coli peritonitis
Mice were intraperitoneally injected with 37 mg/kg LPS from E. coli or 1 × 104 colony-forming units (CFU) E. coli (O18.K1) as described previously (40–42) and killed at indicated times to collect peritoneal lavage fluid (PLF), blood, and organs (41). Body temperature was measured rectally. PLF supernatants were collected for ELISA measurements; pelleted cells were stained with Türk’s solution and counted using a hemocytometer. Differential cell counts were performed on cytospin preparations stained with Giemsa. Liver samples were homogenized using Precellys 24 (Peqlab Biotechnologie GmbH, Erlangen, Germany) and prepared for PCR and ELISA as described earlier (43). Cytokines and chemokines were quantified using commercially available ELISAs (R&D Systems, Minneapolis, MN, USA). Transaminases were measured as described earlier (40). Bacterial loads were determined by plating serial dilutions of PLF, blood, or organ homogenates on blood agar plates. Plates were incubated overnight at 37°C, and colonies were counted the next day. For survival studies, mice were treated with either 37 or 43 mg/kg LPS or infected with 1 × 104 CFU E. coli, and their health state was checked every 2 h.
Cell isolation, culture, and stimulation
PMs were isolated by peritoneal lavage, and BMMs were isolated and differentiated as described earlier (44). Cells were cultured in Roswell Park Memorial Institute 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (Gibco) and 1% penicillin/streptomycin. For in vitro stimulations, cells were plated at 2.5 × 105/ml in 96-well plates and let to adhere overnight. Subsequently, cells were washed with PBS and stimulated with LPS (100 ng/ml) from E. coli or heat-killed E. coli (4 × 107 CFU/ml) for indicated time points. The translation inhibitor cyclohexamide and the proteasome inhibitor MG132 were used at 100 μg/ml.
Generation of TREM-2-overexpressing RAW 264.7 cells
Expression plasmids were generated by Gateway cloning using pDONR 201 as a shuttle vector and pCMV-StrepIII-HA-GW as an expression vector. The following primers were used to obtain Gateway-compatible fragments (containing att sites) by PCR: sense-attB1 green fluorescent protein (GFP), 5′-ggggacaagtttgtacaaaaaagcaggctagactgccatggtgagcaagggc-3′; antisense-attB1 GFP, 5′-ggggaccactttgtacaagaaagctgggttcttgtacagctcgtccat-3′; sense-attB1 TREM-2, 5′-ggggacaagtttgtacaaaaaagcaggctagactgccatgggaccctctccac-3′; and antisense-attB1 TREM-2, 5′-ggggaccactttgtacaagaaagctgggttcgtacctccgggtcc-3′. Constructs were stably overexpressed in RAW 264.7 cells by retroviral transfection as previously described (45).
Western blotting
For preparation of whole-cell extracts, cells (1–5 × 106) were stimulated with LPS (100 ng/ml) and lysed in RIPA buffer [50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% DOC, complete protease inhibitor cocktail tablets (Roche), 10 mM NaF, and 1 mM orthovanadate]. After centrifugation, supernatants were frozen at −80°C. Protein concentrations were determined using the Pierce BCA assay (Thermo Fisher Scientific, Waltham, MA, USA), and Western blotting was conducted as described earlier (46) using a biotinylated sheep anti-mouse TREM-2 antibody (R&D Systems) and Streptavidin-horseradish peroxidase (R&D Systems).
Phagocytosis assays
In vitro fluorescent-activated cell sorting (FACS)–based phagocytosis assays using FITC-labeled E. coli were performed as previously described (43). In vivo phagocytosis was assessed by injecting FITC-labeled E. coli (1 × 107 CFU/mouse) i.p. Peritoneal exudate cells were harvested 1 h later and analyzed by FACS using antibodies against CD3 (eBioscience, San Diego, CA, USA), F4/80, and Ly6G (BioLegend, San Diego, CA, USA).
RT-PCR
mRNA was isolated from cells using TRIzol Reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA), and cDNA was generated using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Quantitative RT-PCR was performed using iTaq SYBR Green Supermix (Bio-Rad), the StepOnePlus cycler (Applied Biosystems, Foster City, CA, USA), and specific primers for TREM-2 (200 bp, forward 5′-ttgctggaaccgtcaccatc-3′ and reverse 5′-cacttgggcaccctcgaaac-3′), A20 (296 bp, forward 5′-catccacaaagcacttattgaca-3′ and reverse 5′-aacaggactttgctacgacactc-3′), IL-6 (187 bp, forward 5′-ccacggccttccctacttca-3′ and reverse 5′-tgcaagtgcatcgttgttc-3′), and TLR4 (365 bp, forward 5′-ctcctgcctgacaccaggaagcttga-3′ and reverse 5′-aggccaattttgtctccacagccacca-3′).
Statistical analysis
Statistical evaluation was performed using GraphPad Prism software (La Jolla, CA, USA). Data are represented as the mean ± sem and were analyzed using either Student’s t test, comparing 2 groups, or 1-way ANOVA analysis, followed by Tukey multiple comparison test, for more groups. Survival studies were analyzed using a log-rank (Mantel-Cox) test. Differences with a P value ≤0.05 were considered significant.
Results
TREM-2 delays LPS-induced inflammation and increases mortality from endotoxemia
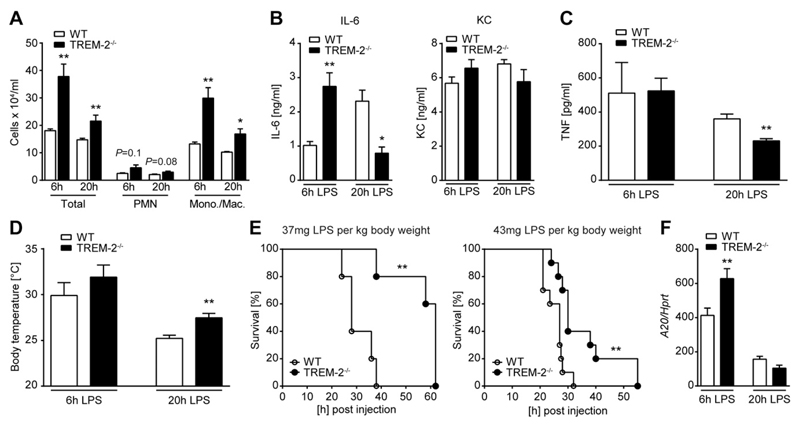
TREM-2 has been shown to regulate TLR4-mediated responses (15) and to bind LPS (17). To investigate the impact of TREM-2 on the in vivo inflammatory response to LPS, we challenged WT and TREM-2−/− mice intraperitonally with LPS from E. coli and harvested organs 6 h later, at a time point before mice show any clinical signs of disease. In line with published data showing that TREM-2 negatively impacts TLR4-mediated inflammation in vitro, the absence of endogenous TREM-2 resulted in a more pronounced early (6 h) inflammatory response. This was indicated by a substantially increased influx of cells (Fig. 1A), consisting of macrophages and neutrophils, and enhanced IL-6 and keratinocyte-derived chemokine (KC) levels (Fig. 1B) in the peritoneal cavity of TREM-2−/− as compared to WT animals. TNF levels and body temperature were similar between WT and knockout animals at this early time point (Fig. 1C, D). Interestingly, this augmented early inflammatory response in TREM-2−/− mice was followed by an accelerated resolution of inflammation, as indicated by a rapid decline of peritoneal cells (Fig. 1A) and IL-6 (Fig. 1B) 20 h after LPS administration. Lower plasma TNF-α levels (Fig. 1C) at this late time point correlated with less pronounced hypothermia (Fig. 1D) in TREM-2-deficient animals, whereas WT mice still displayed signs of ongoing inflammation (Fig. 1B, D). As a result of the rapidly resolving inflammatory response, TREM-2−/− mice showed a significantly improved survival from LPS-induced shock (Fig. 1E). The dynamic of an enhanced early inflammatory response, followed by an accelerated resolution in the absence of TREM-2, led us to hypothesize that TREM-2 might not only tune down inflammatory genes but might also affect the expression of negative regulators of TLR signaling that promote the resolution of inflammation. We therefore tested WT and TREM-2−/− livers for the induction of classic negative regulators of TLR4 signaling, such as IL-1R–associated kinase-M (IRAK-M), Tollip, and A20 (47). In line with this idea, we discovered robustly increased expression levels of the early NF-κB target gene A20 in TREM-2-deficient mice early (6 h) after administration of LPS compared to their WT counterparts (Fig. 1F), whereas TREM-2 did not impact on the other genes tested (data not shown). Together, TREM-2 deficiency was associated with an augmented immediate inflammatory response to LPS, an earlier induction of the negative regulator A20, and an accelerated resolution of inflammation that ultimately resulted in an improved survival.
Figure 1.
TREM-2 exerts detrimental effects during endotoxemia. WT and TREM-2−/− mice were intraperitonally injected with 37 mg/kg LPS. Peritoneal cell numbers (A), cytokines in PLF (B), and plasma (C) were assessed at 6 and 20 h postinjection. Body temperature was measured at 6 and 20 h postinjection (D). Survival (n = 5 per group) was monitored following i.p. LPS administration (37 or 43 mg/kg) over a period of 72 h (E). A20 was detected in liver samples 20 h after LPS administration by RT-PCR (F). Mono./Mac., monocyte/macrophage. PMN, polymorphonuclear leukocytes. Data presented in (A–D and F) are the mean ± sem of n = 6 per group and time; all data are representative of 2 independent experiments. *P ≤ 0.05; **P < 0.01.
Unaltered bacterial counts despite an enhanced inflammation in TREM-2-deficient mice
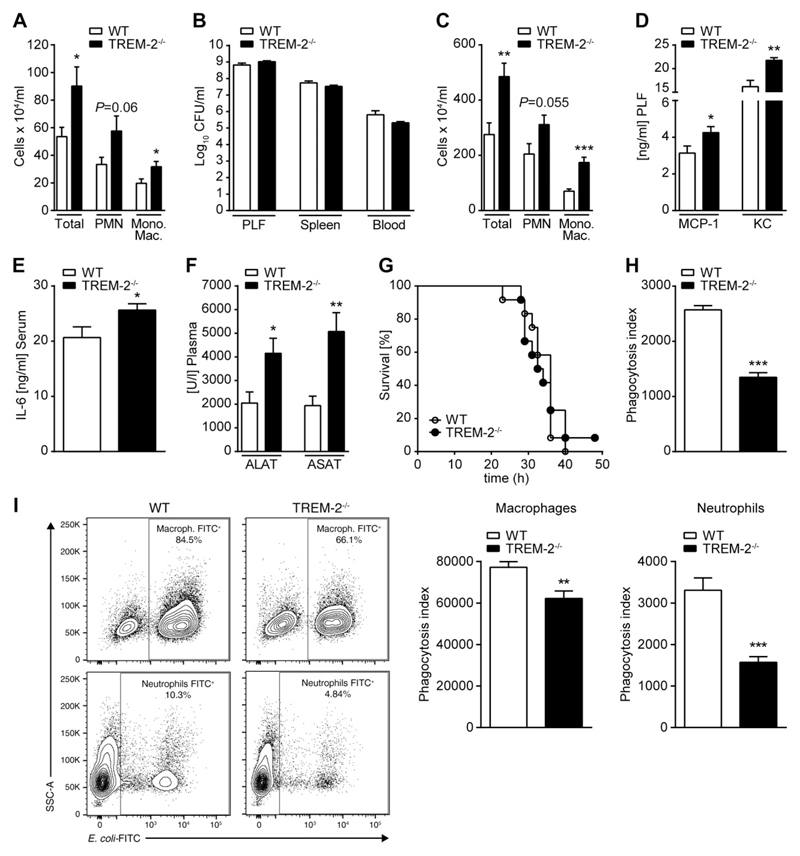
Based on these data, we wanted to study the potential role of endogenous TREM-2 in a clinically relevant model of Gram-negative peritonitis. WT and TREM-2−/− mice were infected intraperitoneally with E. coli and killed either 6 h postinfection, to evaluate the early inflammatory response, or 16 h postinfection, at a time when differences in bacterial clearance and organ damage can be assessed. Concurrent with our observations during endotoxemia, we discovered significantly higher numbers of macrophages and neutrophils in the peritoneal exudate of TREM-2-deficient mice 6 h postinfection, indicating a stronger early innate immune response to the bacteria (Fig. 2A). Based on the enhanced recruitment of phagocytes to the site of infection in TREM-2-deficient animals, we anticipated this response to positively contribute to bacterial clearance and containment of infection at later stages of the disease. However, the enhanced cell influx was not associated with an improved bacterial clearance at later stages because WT and TREM-2−/− animals had comparable bacterial numbers in the peritoneal cavity, blood, and distant organs (Fig. 2B), and TREM-2-deficient mice continued to exhibit more inflammatory cells in the PLF (Fig. 2C), indicating ongoing inflammation. This was accompanied by elevated peritoneal concentrations of monocyte chemotactic protein-1 (MCP-1) and KC (Fig. 2D), more pronounced signs of systemic inflammation, and sepsis-associated liver injury, as illustrated by elevated plasma levels of IL-6 (Fig. 2E) and of the transaminases aspartate transaminase (ASAT) and Alanine transaminase (ALAT) (Fig. 2F) in TREM-2−/− mice. Despite these differences in the inflammatory response, but in line with our finding of an unaltered bacterial clearance, the absence of TREM-2 did not impact on survival during E. coli peritonitis (Fig. 2G). The fact that TREM-2 deficiency neither improved bacterial clearance nor survival despite an enhanced early influx of inflammatory cells suggested that TREM-2 might in parallel affect crucial antibacterial effector mechanisms of phagocytes. Because TREM-2 was proposed to be a phagocytic receptor on BMMs in vitro (18), we next assayed the ability of WT and TREM-2−/− PMs to phagocytose E. coli and found TREM-2−/− primary PMs to be strongly impaired in their capability to ingest E. coli (Fig. 2H). In order to validate our in vitro finding, we then performed an in vivo phagocytosis assay and injected FITC-labeled bacteria i.p. into WT and TREM-2-deficient mice to then analyze the bacterial uptake by peritoneal exudate cells. Supporting and extending our in vitro data, we found TREM-2-deficient PMs and neutrophils to exhibit an impaired ability to phagocytose bacteria (Fig. 2I). Collectively, these results illustrate an ambiguous role of TREM-2 during bacterial infection because it negatively regulates early inflammation while at the same time being critical for the uptake of E. coli.
Figure 2.
TREM-2 dampens inflammation and promotes bacterial clearance in E. coli peritonitis. WT and TREM-2−/− mice (n = 8 per group) were intraperitonally infected with 1 × 104 CFU E. coli and killed either 6 or 16 h later. Peritoneal cell influx was accessed at 6 h postinfection (A). At 16 h postinfection, bacterial counts in PLF, spleen, and blood (B) and peritoneal cell numbers (C) were determined. MCP-1 and KC were measured in PLF (D) and IL-6 in plasma samples (E) by ELISA. Transaminase levels were detected in plasma (F). WT and TREM-2−/− mice (n = 12 per group) were infected intraperitonally with 1 × 104 CFU E. coli, and survival was monitored (G). Primary PMs of WT and TREM-2−/− mice were incubated for 1 h with FITC-labeled E. coli at 37°C, and the phagocytic uptake of bacteria was quantified by FACS (H). WT and TREM-2−/− mice were injected intraperitonally with FITC-labeled bacteria, and the bacterial uptake by peritoneal exudate cells was analyzed 1 h later by FACS (I). Data are representative of at least 2 independent experiments and depicted as the mean ± sem. PMN, polymorphonuclear leukocytes. *P ≤ 0.05; **P < 0.01; ***P < 0.001.
Glycosylated TREM-2 is present on resident PMs
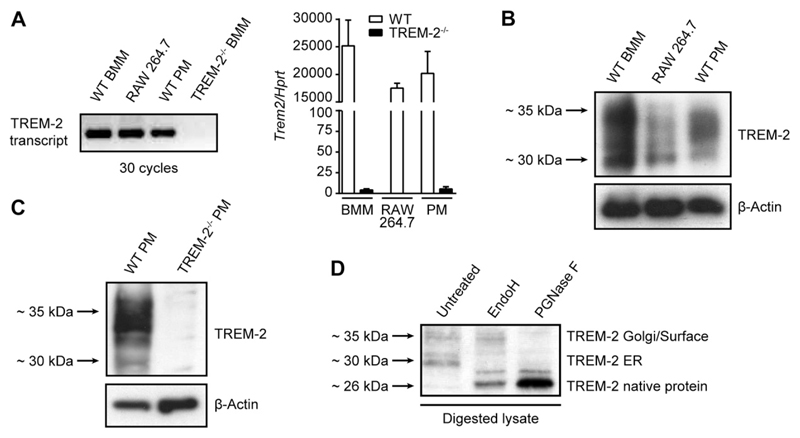
TREM-2−/− mice consistently exhibited an augmented early inflammatory response in the peritoneal cavity upon LPS or bacterial infection. PMs are strategically positioned immune cells in the peritoneal cavity and are crucial in eliciting early inflammation upon invasion of microbes (48, 49). Our data therefore suggest an important role for TREM-2 in regulating PM responses, although earlier reports pronounced TREM-2 to not be expressed on resident PMs (13). We therefore decided to challenge this notion and investigated the potential presence and regulation of TREM-2 on PMs. We first compared TREM-2 mRNA expression by primary PMs, BMMs, and RAW 264.7 cells, a PM-like cell line. We revealed substantial amounts of TREM-2 transcript in primary PMs (Fig. 3A), though slightly lower than transcript levels of BMMs, which were reported earlier to express TREM-2. Significantly, using Western blotting, we further confirmed the presence of TREM-2 protein in PMs, RAW 264.7 cells, and BMMs (Fig. 3B). TREM-2−/− PMs were used to verify the specificity of the antibody (Fig. 3C).
Figure 3.
TREM-2 is expressed in resident PMs and post-translationally modified. TREM-2 expression was assessed in BMMs, RAW 264.7 cells, and resident PMs on transcript level after 30 PCR cycles as well as by RT-PCR (A) or on a protein level by Western blotting (B, C). BMM lysates were digested with either Endo H or PGNase F, and TREM-2 was detected by Western blotting (D). Data are representative of 2 independent experiments.
Interestingly, we discovered at least 4 TREM-2-specific bands, a double band slightly >26 kDa, and another double band around 35 kDa (Fig. 3B). TREM-2 was earlier proposed to be post-translationally modified, with 2 predicted sites of N-linked glycosylation (50). To test this experimentally, we digested BMM lysate with the specific glycosidases endoglycosidase H (Endo H) and peptide-N-glycosidase F (PGNase F) and found the smaller size fragments to be Endo H sensitive, indicating core glycosylation, and the larger fragments around 35 kDa to be Endo H resistant, resembling the fully glycosylated forms of TREM-2 (Fig. 3D). These data suggest that TREM-2 is core glycosylated in the endoplasmic reticulum (ER) of macrophages and then further shuttled through the Golgi apparatus, where it gets further glycosylated.
TREM-2 has a short half-life and is rapidly down-regulated in response to LPS
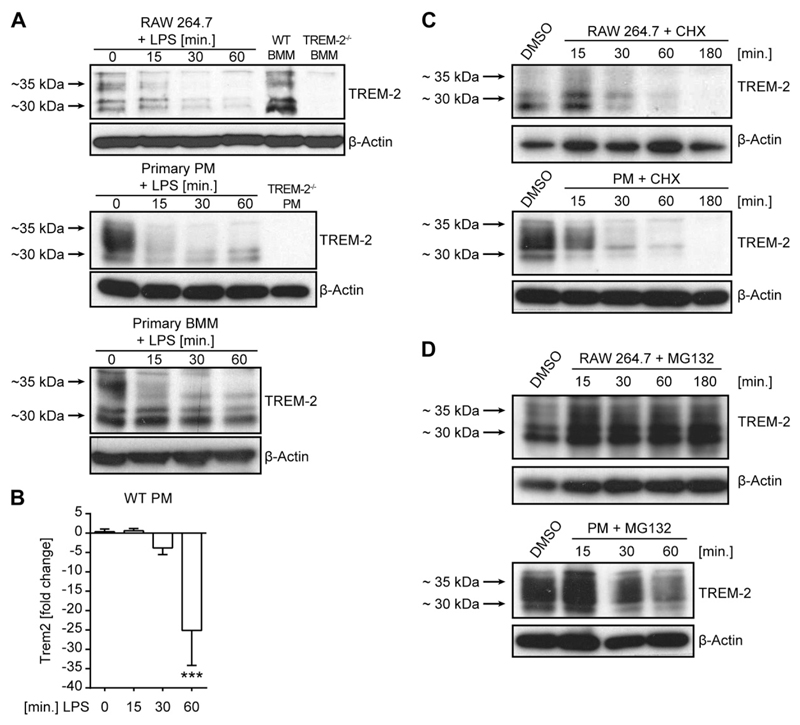
To finally understand why TREM-2’s effects on inflammation in vivo seemed restricted to early time points after microbial challenge we decided to examine the regulation and expression dynamic of TREM-2 in more detail. We thus assessed TREM-2 protein expression in selected macrophage populations upon LPS challenge and observed a rapid decline of TREM-2 protein levels upon LPS encounter, regardless of the macrophage subset tested (Fig. 4A). To investigate how LPS induced the prompt decline of TREM-2 protein, we first focused on TREM-2’s transcriptional regulation. Following 1 h of LPS treatment, TREM-2 transcript levels were strongly reduced in primary PMs (Fig. 4B). This supported a role for transcriptional regulation but also suggested that TREM-2 has a very short half-life and has to be continuously resynthesized, in order to maintain baseline expression. We tested this hypothesis using cyclohexamide, an established inhibitor of translation, and observed a rapid decline of TREM-2 expression (Fig. 4C), comparable to the decline we observed upon LPS treatment (Fig. 4A). To then assess the type of protein degradation, we studied the impact of proteasomal degradation of TREM-2 and added the proteasomal inhibitor MG132 to cells to discover a solid and immediate accumulation of TREM-2 protein (Fig. 4D). Collectively, our data suggest that TREM-2 has a very high turnover rate in macrophages and that TREM-2 expression is tightly controlled by continuous proteasomal degradation and resynthesis, thus ensuring a tightly regulated and very versatile system that regulates the inflammatory response.
Figure 4.
TREM-2 is rapidly down-regulated in response to LPS. TREM-2 protein levels in RAW 264.7 cells, primary PMs, and BMMs were detected upon LPS (100 ng/ml) stimulation over time (A). TREM-2−/− PMs were stimulated with LPS (100 ng/ml), and TREM-2 RNA levels, expressed as fold change compared to untreated WT, were determined at indicated time points (B). RAW 264.7 cells and primary PMs were cultured in the presence of either 100 μg/ml cyclohexamide (C) or 100 μg/ml MG132 (D), and TREM-2 protein levels were assessed. RNA measurements were performed in quadruplicates. Western blots are representative of at least 2 independent experiments. ***P < 0.001.
TREM-2 negatively regulates TLR4-mediated cytokine responses in primary PMs
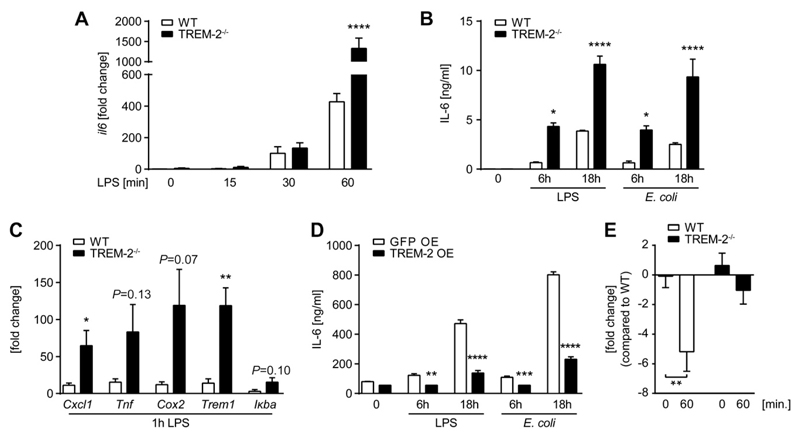
Having established that TREM-2 is expressed and tightly regulated on primary PMs, we wanted to finally address the potential effect of endogenous TREM-2 in regulating the inflammatory responses by PMs. We stimulated resident PMs from WT and TREM-2−/− mice with LPS or E. coli and discovered substantially higher IL-6 induction by TREM-2-deficient resident PMs as compared to WT PMs on mRNA (Fig. 5A) as well as on protein level (Fig. 5B). This was not only true for IL-6 but also for other genes, such as KC (Cxcl1), TNF-α, COX-2, TREM-1, and IκBα (Fig. 5C), which are typically induced by LPS, clearly suggesting TREM-2 to be present and functional as a negative regulator on these primary cells. As a control, we tested TREM-2-overexpressing RAW 264.7 macrophages upon stimulation with LPS or E. coli and discovered significantly less IL-6 in response to both stimuli (Fig. 5D). Based on a publication showing enhanced TLR4 expression in LPS-treated TREM-2-deficient alveolar macrophages (51), we compared TLR4 mRNA levels in quiescent and LPS-challenged primary PMs of WT and TREM-2−/− mice. Although we did not find any differences in baseline expression, TREM-2-deficient cells failed to down-regulate TLR4 mRNA in response to LPS (Fig. 5E), which might contribute to the augmented inflammatory response we observed in TREM-2-deficient PMs. Together with the boosted early inflammation we have observed in vivo, our data show that endogenous TREM-2 expression is strictly controlled on primary PMs and importantly contributes to the negative regulation of TLR4-triggered inflammation in vitro and in vivo.
Figure 5.
TREM-2 negatively regulates TLR4-mediated cytokine responses by PMs. Primary PMs from WT and TREM-2−/− mice were stimulated with LPS (100 ng/ml) or 4 × 107 CFU/ml E. coli for indicated times, and RNA levels, expressed as fold change compared to untreated WT, of different inflammatory genes (A, C) or protein levels of IL-6 (B) were quantified. RAW 264.7 cells overexpressing TREM-2 and GFP-control cells were stimulated with 100 ng/ml LPS or 4 × 107 CFU/ml E. coli, and IL-6 protein levels were assessed after 6 and 18 h in supernatants by ELISA (D). TREM-2−/− PMs were stimulated with LPS (100 ng/ml), and TLR4 mRNA levels, expressed as fold change compared to untreated WT, were assessed on resting and stimulated cells (E). Data are the mean ± sem of quadruplicates and representative of 2 independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P < 0.001; ****P < 0.0001.
Discussion
The fine-tuning of TLR-mediated signaling events is required to elicit a profound and powerful immune response while simultaneously triggering regulatory pathways that prevent overwhelming inflammation and ultimately restore tissue homeostasis (52). This beneficial balance is achieved through the production of inflammatory mediators upon infection and the simultaneous induction of genes that negatively feedback on inflammation (52, 53).
In the present study, we investigated the biologic function of TREM-2, which is considered a negative regulator of inflammation. We discovered that TREM-2 is essential in negatively regulating early inflammation during endotoxemia and Gram-negative sepsis in vivo and propose these effects to be attributed to TREM-2’s effects on resident PMs. The TREM-2-mediated delay of early inflammation proved detrimental during LPS-induced shock and was associated with a diminished induction of the negative regulator A20, ultimately resulting in prolonged inflammation and impaired survival of WT animals (Fig. 1). Upon infection with E. coli, TREM-2’s inhibitory impact on early inflammation was counteracted by its crucial role as a phagocytic receptor, which eventually resulted in an unaffected outcome when comparing WT and TREM-2−/− mice (Fig. 2). Given that the presence of TREM-2 consistently dampened early peritoneal inflammation in response to both, LPS and live bacteria, and taking into account the principal importance of resident PMs in sensing the presence of pathogens in the otherwise sterile peritoneal cavity (48, 49), we investigated expression and function of TREM-2 on resident PMs in more detail. We here show that primary, resting PMs express substantial amounts of TREM-2 (Fig. 3) and that this is functionally relevant both in vitro (Fig. 5) as well as during endotoxemia (Fig. 1) and bacterial infection (Fig. 2) in vivo.
Although it seems established that TREM-2 is expressed on inflammatory macrophages, this receptor was thus far considered absent on resident PMs, based on surface FACS staining (13). The current unavailability of commercial antibodies that reliably detect TREM-2 on cells together with the rapid down-regulation upon activation makes it challenging to conclusively demonstrate the presence of TREM-2 on primary PMs. However, functional data derived from our in vivo studies that exhibit changes in phagocytosis already 1 h after injection of bacteria (Fig. 2) as well as the altered inflammatory response early during peritonitis (Figs. 1 and 2), which was shown earlier to be induced by PMs (48, 49), strongly suggest the presence of TREM-2 on resident peritoneal phagocytes.
Our data illustrate that a large proportion of TREM-2 is stored intracellularly and that especially the surface band is highly sensitive to degradation (Fig. 4) and probably also shedding, as was shown by other groups (50). Based on the rapid down-regulation of (surface) TREM-2 upon activation of cells, we believe that Western blotting might be a more sensitive method to detect the presence of TREM-2 because this method does not require lengthy handling of cells and furthermore allows the simultaneous detection of intracellular and surface protein. In line with this, we believe that differences in TREM-2 protein levels between PMs and BMMs might result from PM activation and protein degradation as a result of time-consuming isolation procedures, whereas BMMs represent truly resting cells. TREM-2-deficient primary PMs exhibited an enhanced inflammatory reaction upon TLR4 activation in vitro. Based on publications showing TREM-2 to affect ERK signaling in human osteoclasts (30) and murine dendritic cells (54), we attempted to study ERK phosphorylation in primary PMs, but failed to obtain reproducible results. However, and in contrast to WT cells, we found TREM-2-deficient PMs to maintain steady levels of TLR4 mRNA following LPS stimulation, which was in line with previously published data in alveolar macrophages, where TREM-2 levels were reduced using small interfering RNA (51). Down-regulation of TLR4 in response to LPS is considered a negative regulatory mechanism that contributes to controlling the inflammatory response (47), and we suggest that the preservation of TLR4 expression might in part account for the hyperinflammatory phenotype of TREM-2-deficient PMs.
On a cellular level, we could show that processing of TREM-2 in macrophages involves glycosylation at 2 distinct sites and that all tested macrophage subsets, namely BMMs, RAW 276.4 cells, and resident PMs, store substantial amounts of TREM-2 in the ER (Fig. 3). We further observed that TREM-2 glycosylation seems to be cell-type and site specific because we discovered different glycosylation patterns between BMMs and PMs. This is not unusual because cell-type– and site-specific glycosylation patterns have been described for several proteins (55–57) and might regulate cell-type–specific binding affinities to respective ligands. A recent publication showed that TREM-2, when overexpressed in COS-7 or human embryonic kidney 293 cells, undergoes ectodomain shedding and that the remaining C-terminal fragment is further degraded by γ-secretase (50). However, we could not detect endogenous soluble TREM-2 in the serum of WT mice nor in the supernatant of PMs after trichloroacetic acid-precipitation in vitro (data not shown). This could either indicate cell-type-specific processing of TREM-2 or a lack of assay sensitivity. Looking closer into the regulation of TREM-2 expression in PMs, we found that TREM-2 protein levels are tightly regulated by continuous proteasomal degradation and resynthesis. LPS rapidly turns off TREM-2 transcription, and this immediately impacts on TREM-2 protein levels, due to its high turnover rate (Fig. 4). TREM-2 down-regulation was demonstrated earlier on both mRNA level in liver macrophages during endotoxemia (58) as well as on protein level upon overnight LPS challenge in vitro (13), but the celerity of TREM-2 down-regulation we discovered here upon LPS treatment was not known before.
Considering the fact that mortality from endotoxemia is attributed to overwhelming inflammation and that the absence of classic negative regulators leads to a higher susceptibility to septic shock, it seems counterintuitive at first that TREM-2 would exert detrimental effects in this model. TREM-2’s presence on resting cells and its rapid decline upon stimulation are in contrast to what is known about classic negative regulators of TLR signaling (47). A20 and IRAK-M are prototypic negative regulators, which are barely expressed or even absent in resting cells but strongly induced upon TLR ligation to then negatively feedback on the TLR signaling cascade (47, 59, 60). We therefore believe that tonic TREM-2 signaling fine-tunes the sensitivity of quiescent cells to a certain stimulus, and we propose that the mere presence of TREM-2 at the time of LPS administration was sufficient to delay the onset of inflammation. Thus, TREM-2 not only postponed the induction of the early immune response in WT mice but at the same time delayed the induction of the TLR-induced, early NF-κB target gene A20 (Fig. 1), a ubiquitin-editing protein that attenuates NF-κB signaling by promoting the degradation of RIPK1 and TRAF6, crucial mediators of TNF and TLR-induced NF-κB activation (61). As such, A20 negatively regulates cytokine responses and contributes to the termination of inflammation in mice and humans (52, 59, 62), and it was shown earlier that hepatic overexpression of A20 was sufficient to protect mice from LPS-induced shock (63).
We encountered a different situation when we challenged mice with live E. coli, i.e., a persistent stimulus, which is not as rapidly removed as LPS, and continuously replicates in vivo. Despite the fact that TREM-2 knockout mice displayed no difference in survival during E. coli–induced peritonitis, their immune response was severely altered (Fig. 2). Just like upon LPS administration, the absence of endogenous TREM-2 during E. coli infection led to accelerated phagocyte recruitment to the site of infection but did not affect the outcome from this disease. Because both a strong inflammatory response and an efficient elimination of bacteria via phagocytosis are required to clear bacterial peritonitis, we propose that the augmented inflammatory response was ultimately counteracted by impairment in bacterial phagocytosis, which, in line with previous studies (18, 19, 64), emphasizes the importance of TREM-2 as a phagocytic receptor. Our data demonstrate that in vivo, not only macrophages but also neutrophils were hampered in their ability to ingest E. coli, thus supporting our hypothesis that TREM-2’s impact on inflammation was counteracted by its effects on phagocytosis. We did not assess later time points after infection but expect also newly recruited macrophages, which were earlier shown to express TREM-2 (13), to phagocytose less bacteria in the absence of TREM-2. Regarding TREM-2’s effects on neutrophils, we can only speculate about TREM-2 presence on these cells because this has not been shown before. Alternatively, neutrophils might be affected by the altered inflammatory milieu, or soluble TREM-2, which has been shown to bind various bacteria (17), might be released in vivo to impact on the phagocytic capacity of neutrophils. Of interest, we recently observed that TREM-2 deficiency was beneficial during murine pneumococcal pneumonia, which was explained by an increased bacterial uptake by alveolar macrophages in the absence of TREM-2 (39). Mechanistically, we discovered a cell-type–specific effect because TREM-2−/− alveolar macrophages exhibited significantly higher levels of the complement component C1q that was sufficient to enhance the phagocytosis of bacteria. However, primary PMs from either WT or TREM-2−/− mice did not differ in C1q production (data not shown), and we therefore believe that the differences in the uptake of bacteria by PMs are directly caused by the absence of TREM-2 and its function as a phagocytic receptor. Taken together, we propose that TREM-2 plays a dual role during infection. TREM-2 crucially fine-tunes immediate TLR4-induced inflammation on resident PMs, resulting in delayed immediate inflammatory responses in vivo and in vitro, whereas at the same time acting as a phagocytic receptor for E. coli.
Acknowledgments
The authors thank L. Riki Cheever for her valuable comments and intellectual contributions to this paper. This work was funded by the Austrian Science Fund FWF (W1205). R.G. is a member of the Doctoral Program “Cell Communication in Health and Disease” [cofinanced by FWF and the Medical University of Vienna (to S.K.)]. The research leading to these results has received funding from the European Union Seventh Framework Program (FP7/2007-2013) under Grant Agreement 261382 (to M.S.).
Abbreviations
- ALAT
Alanine transaminase
- ASAT
aspartate transaminase
- BMM
bone marrow-derived macrophage
- CFU
colony-forming unit
- DAP12
DNAX-activation protein of 12 kDa
- Endo H
endoglycosidase H
- ER
endoplasmic reticulum
- FACS
fluorescent-activated cell sorting
- GFP
green fluorescent protein
- IRAK-M
IL-1R–associated kinase-M
- ITAM
immunoreceptor tyrosine-based activating motif
- KC
keratinocyte-derived chemokine
- MCP-1
monocyte chemotactic protein-1
- PGNase F
peptide-N-glycosidase F
- PLF
peritoneal lavage fluid
- PM
peritoneal macrophage
- TREM
triggering receptor expressed on myeloid cells
- WT
wild-type
Footnotes
The authors declare no conflicts of interest.
References
- 1.Holzheimer RG, Muhrer KH, L’Allemand N, Schmidt T, Henneking K. Intraabdominal infections: classification, mortality, scoring and pathophysiology. Infection. 1991;19:447–452. doi: 10.1007/BF01726463. [DOI] [PubMed] [Google Scholar]
- 2.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 5.Van ’t Veer C, van den Pangaart PS, Kruijswijk D, Florquin S, de Vos AF, van der Poll T. Delineation of the role of Toll-like receptor signaling during peritonitis by a gradually growing pathogenic Escherichia coli. J Biol Chem. 2011;286:36603–36618. doi: 10.1074/jbc.M110.189126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Westerloo DJ, Weijer S, Bruno MJ, de Vos AF, Van’t Veer C, van der Poll T. Toll-like receptor 4 deficiency and acute pancreatitis act similarly in reducing host defense during murine Escherichia coli peritonitis. Crit Care Med. 2005;33:1036–1043. doi: 10.1097/01.ccm.0000162684.11375.85. [DOI] [PubMed] [Google Scholar]
- 7.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93:329–342. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 10.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 11.Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 14.Hamerman JA, Lanier LL. Inhibition of immune responses by ITAM-bearing receptors. Sci STKE. 2006;2006:re1. doi: 10.1126/stke.3202006re1. [DOI] [PubMed] [Google Scholar]
- 15.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Hamerman JA. TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur J Immunol. 2012;42:176–185. doi: 10.1002/eji.201141679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171:594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 18.N’Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, Hamerman JA, Seaman WE. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184:215–223. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Zhang K, Jin Y, Zhu T, Cheng B, Shu Q, Fang X. Triggering receptor expressed on myeloid cells-2 protects against polymicrobial sepsis by enhancing bacterial clearance. Am J Respir Crit Care Med. 2013;188:201–212. doi: 10.1164/rccm.201211-1967OC. [DOI] [PubMed] [Google Scholar]
- 20.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 21.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrow AD, Trowsdale J. You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36:1646–1653. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 23.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Q, Long CL, Malhotra S, Humphrey MB. A physical interaction between the adaptor proteins DOK3 and DAP12 is required to inhibit lipopolysaccharide signaling in macrophages. Sci Signal. 2013;6:ra72. doi: 10.1126/scisignal.2003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasu T, Tsukahara Y, Terayama K. A lipid metabolic disease-“membranous lipodystrophy”-an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Acta Pathol Jpn. 1973;23:539–558. doi: 10.1111/j.1440-1827.1973.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 26.Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, Ganev GG, da Silva Júnior JP, Neto Evaldo S, Poffo MA, Walz R, Carlotti Júnior CG, et al. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy—PLOSL): a dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell Mol Neurobiol. 2004;24:1–24. doi: 10.1023/B:CEMN.0000012721.08168.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakola HP, Järvi OH, Sourander P. Osteodysplasia polycystica hereditaria combined with sclerosing leucoencephalopathy, a new entity of the dementia praesenilis group. Acta Neurol Scand. 1970;46(Suppl 43):79. [PubMed] [Google Scholar]
- 28.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colonna M, Turnbull I, Klesney-Tait J. The enigmatic function of TREM-2 in osteoclastogenesis. Adv Exp Med Biol. 2007;602:97–105. doi: 10.1007/978-0-387-72009-8_13. [DOI] [PubMed] [Google Scholar]
- 30.Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, Nakamura M, Ivashkiv LB. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arts RJ, Joosten LA, van der Meer JW, Netea MG. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93:209–215. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 35.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 36.Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173:7131–7134. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- 37.Gibot S, Kolopp-Sarda MN, Béné MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, Zhu M, Chen K, Nie X, Deng Q, Hazlett LD, Wu Y, Li M, Wu M, Huang X. TREM-2 promotes host resistance against Pseudomonas aeruginosa infection by suppressing corneal inflammation via a PI3K/Akt signaling pathway. Invest Ophthalmol Vis Sci. 2013;54:3451–3462. doi: 10.1167/iovs.12-10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharif O, Gawish R, Warszawska JM, Martins R, Lakovits K, Hladik A, Doninger B, Brunner J, Korosec A, Schwarzenbacher RE, Berg T, et al. The triggering receptor expressed on myeloid cells 2 inhibits complement component 1q effector mechanisms and exerts detrimental effects during pneumococcal pneumonia. PLoS Pathog. 2014;10:e1004167. doi: 10.1371/journal.ppat.1004167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knapp S, de Vos AF, Florquin S, Golenbock DT, van der Poll T. Lipopolysaccharide binding protein is an essential component of the innate immune response to Escherichia coli peritonitis in mice. Infect Immun. 2003;71:6747–6753. doi: 10.1128/IAI.71.12.6747-6753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knapp S, Matt U, Leitinger N, van der Poll T. Oxidized phospholipids inhibit phagocytosis and impair outcome in gram-negative sepsis in vivo. J Immunol. 2007;178:993–1001. doi: 10.4049/jimmunol.178.2.993. [DOI] [PubMed] [Google Scholar]
- 42.Matt U, Sharif O, Martins R, Furtner T, Langeberg L, Gawish R, Elbau I, Zivkovic A, Lakovits K, Oskolkova O, Doninger B, et al. WAVE1 mediates suppression of phagocytosis by phospholipid-derived DAMPs. J Clin Invest. 2013;123:3014–3024. doi: 10.1172/JCI60681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharif O, Matt U, Saluzzo S, Lakovits K, Haslinger I, Furtner T, Doninger B, Knapp S. The scavenger receptor CD36 downmodulates the early inflammatory response while enhancing bacterial phagocytosis during pneumococcal pneumonia. J Immunol. 2013;190:5640–5648. doi: 10.4049/jimmunol.1202270. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14:Unit 14.1. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bürckstümmer T, Bennett KL, Preradovic A, Schütze G, Hantschel O, Superti-Furga G, Bauch A. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat Methods. 2006;3:1013–1019. doi: 10.1038/nmeth968. [DOI] [PubMed] [Google Scholar]
- 46.Lagler H, Sharif O, Haslinger I, Matt U, Stich K, Furtner T, Doninger B, Schmid K, Gattringer R, de Vos AF, Knapp S. TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia. J Immunol. 2009;183:2027–2036. doi: 10.4049/jimmunol.0803862. [DOI] [PubMed] [Google Scholar]
- 47.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 48.Broche F, Tellado JM. Defense mechanisms of the peritoneal cavity. Curr Opin Crit Care. 2001;7:105–116. doi: 10.1097/00075198-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Holmes CJ. Peritoneal host defense mechanisms. Perit Dial Int. 1996;16(Suppl 1):S124–S125. [PubMed] [Google Scholar]
- 50.Wunderlich P, Glebov K, Kemmerling N, Tien NT, Neumann H, Walter J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J Biol Chem. 2013;288:33027–33036. doi: 10.1074/jbc.M113.517540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoogerwerf JJ, de Vos AF, van’t Veer C, Bresser P, de Boer A, Tanck MW, Draing C, van der Zee JS, van der Poll T. Priming of alveolar macrophages upon instillation of lipopolysaccharide in the human lung. Am J Respir Cell Mol Biol. 2010;42:349–356. doi: 10.1165/rcmb.2008-0362OC. [DOI] [PubMed] [Google Scholar]
- 52.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Anwar MA, Basith S, Choi S. Negative regulatory approaches to the attenuation of Toll-like receptor signaling. Exp Mol Med. 2013;45:e11. doi: 10.1038/emm.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouchon A, Hernández-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264:17615–17618. [PubMed] [Google Scholar]
- 56.Parekh RB, Dwek RA, Thomas JR, Opdenakker G, Rademacher TW, Wittwer AJ, Howard SC, Nelson R, Siegel NR, Jennings MG. Cell-type-specific and site-specific N-glycosylation of type I and type II human tissue plasminogen activator. Biochemistry. 1989;28:7644–7662. doi: 10.1021/bi00445a021. [DOI] [PubMed] [Google Scholar]
- 57.Zeck A, Pohlentz G, Schlothauer T, Peter-Katalinić J, Regula JT. Cell type-specific and site directed N-glycosylation pattern of FcγRIIIa. J Proteome Res. 2011;10:3031–3039. doi: 10.1021/pr1012653. [DOI] [PubMed] [Google Scholar]
- 58.Chen LC, Laskin JD, Gordon MK, Laskin DL. Regulation of TREM expression in hepatic macrophages and endothelial cells during acute endotoxemia. Exp Mol Pathol. 2008;84:145–155. doi: 10.1016/j.yexmp.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 61.Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Arvelo MB, Cooper JT, Longo C, Daniel S, Grey ST, Mahiou J, Czismadia E, Abu-Jawdeh G, Ferran C. A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology. 2002;35:535–543. doi: 10.1053/jhep.2002.31309. [DOI] [PubMed] [Google Scholar]
- 64.Correale C, Genua M, Vetrano S, Mazzini E, Martinoli C, Spinelli A, Arena V, Peyrin-Biroulet L, Caprioli F, Passini N, Panina-Bordignon P, et al. Bacterial sensor triggering receptor expressed on myeloid cells-2 regulates the mucosal inflammatory response. Gastroenterology. 2013;144:346–356. e343. doi: 10.1053/j.gastro.2012.10.040. [DOI] [PubMed] [Google Scholar]