Abstract
The tumor accumulation of nanomedicines relies on the enhanced permeability and retention (EPR) effect. In the last 5-10 years, it has been increasingly recognized that there is a large inter- and intra-individual heterogeneity in EPR-mediated tumor targeting, explaining the heterogeneous outcomes of clinical trials in which nanomedicine formulations have been evaluated. To address this heterogeneity, as in other areas of oncology drug development, we have to move away from a one-size-fits-all tumor targeting approach, towards methods that can be employed to individualize and improve nanomedicine treatments. To this end, efforts have to be invested in better understanding the nature, the complexity and the heterogeneity of the EPR effect, and in establishing systems and strategies to enhance, combine, bypass and image EPR-based tumor targeting. In the present manuscript, we summarize key studies in which these strategies are explored, and we discuss how these approaches can be employed to enhance patient responses.
Keywords: Cancer, Nanomedicine, Drug delivery, Tumor targeting, EPR
1. Introduction
Cancer is one of the major causes of death worldwide and its treatment remains to be very challenging [1]. First-line therapy of solid tumors is based on surgery, radiotherapy and/or chemotherapy. For metastasized tumors, or for lesions, which cannot be removed surgically, chemotherapy is among the very few treatment options available. Unfortunately, however, the therapeutic potential of classical chemotherapeutic drugs is limited, and they generally cause severe side effects [2].
Advances in nanotechnology and in chemical/pharmaceutical engineering have led to the development of many different drug delivery systems. These systems aim to improve the biodistribution and target site accumulation of chemotherapeutic drugs. Examples of drug delivery systems are polymer conjugates, micelles and liposomes, which typically have sizes ranging from 5 to 200 nm. These so called nanomedicine formulations have shown promising results in preclinical trials, and some of them are already routinely used in clinical practice [3].
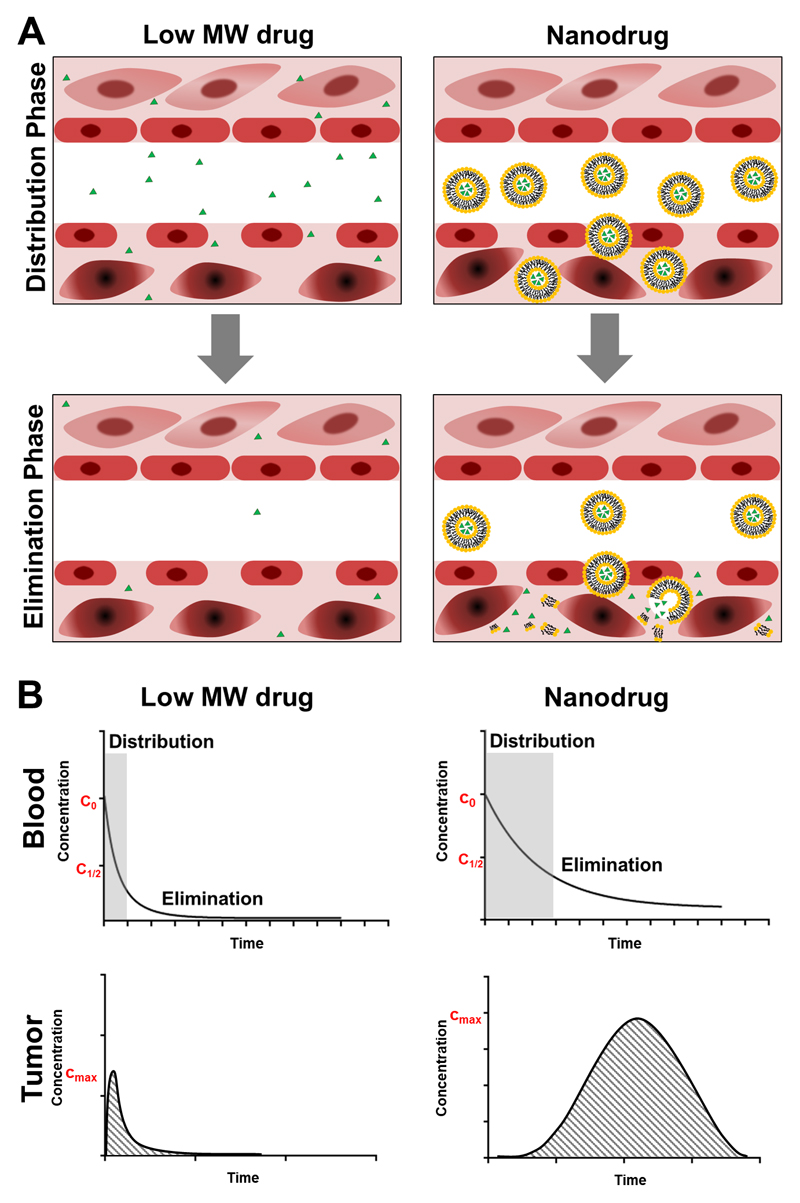
Conventional chemotherapy is based on low molecular weight drugs (generally less than 1000 Da) [4]. Due to their small size, chemotherapeutic agents, such as doxorubicin, cisplatin or gemcitabine, have unfavorable pharmacokinetics and a suboptimal biodistribution, as exemplified by a short blood half-life and prominent off-target accumulation in multiple healthy organs (Figure 1A). This, together with the unspecific mechanism of action of chemotherapeutic drugs and their large volume of distribution, causes severe side effects, such as myelosuppression, mucositis, neurotoxicity, nausea, vomiting and alopecia [5]. By increasing the size of systemically administered anticancer agents to at least 5-10 nanometers in diameter (i.e. exceeding the renal clearance threshold of ~40000 Da), kidney excretion can be reduced, blood half-lifes prolonged, and target site accumulation improved (Figure 1B). As an example, the encapsulation of doxorubicin into liposomes (Caelyx®/Doxil®) results in an increase in plasma half-life from 5-10 minutes for the free drug, to 2-3 days for the liposome-encapsulated drug [6]. In this specific case, as in many other liposomal and micellar nanomedicine formulations, surface modification with the stealthy polymer polyethyleneglycol (PEG) decreases aggregation and opsonization with plasma proteins, contributing to the prolonged circulation half-life [7,8].
Figure 1. Conventional low-molecular-weight chemotherapy versus EPR-based nanomedicine therapy.
A: Conventional small molecule chemotherapeutic drugs show high levels of off-target accumulation in healthy tissues during the distribution and elimination phase (upper parts of the panels on the left) and low levels of tumor accumulation (lower parts of the panels on the left). Conversely, nanodrugs prevent chemotherapy accumulation in healthy tissues (upper parts of the panels on the right), and promote accumulate at pathological sites (lower parts of the panels on the right). B: Typical pharmacokinetic profiles of small molecule drugs (left) and nanodrugs (right) in blood and tumors, exemplifying prolonged circulation properties and enhanced tumor accumulation over time.
By means of improved circulation times, nanomedicines can accumulate in tumors via the so called Enhanced Permeability and Retention (EPR) effect, which was first described by Matsumura and Maeda in 1986 [9]. EPR relies on specific pathophysiological characteristics of tumors vs. healthy tissues. In healthy tissues, low-molecular-weight drugs easily extravasate out of blood vessels, while nanomedicines are unable to do so, because of their size (Figure 1A). Conversely, in tumors, the abnormally wide fenestrations in the blood vessels allow for the extravasation of materials with sizes up to several hundreds of nanometers. This, together with the absence of lymphatic drainage, leads to a relatively effective and selective accumulation of nanomedicines in tumors [10–13].
Within the last couple of years, scientists have increasingly realized that the EPR effect is highly heterogeneous, changing over time during tumor development and possibly also being transient. This pathophysiological phenomenon does not only vary between mouse models and patients, but also among tumor types of the same origin, and among tumors and metastases within the same patient [14,15]. As a consequence, the clinical outcome of nanomedicine treatments is also highly heterogeneous, and not as good as anticipated on the basis of preclinical results [16]. The notion that the EPR effect strongly varies between individuals is of high importance, and may lead to misunderstandings and to a too pessimistic view on EPR-mediated passive tumor targeting (see e.g. [17], claiming that EPR is absent in patients, which is not the case and cannot be generalized [14,18]). In line with this reasoning, based on more than 100 preclinical studies, which were published during the last 10 years, Wilhelm and colleagues claim that nanoparticles often fail because of an overall median accumulation in tumors of only 0.7% ID. However, the authors do not discuss the heterogeneity of EPR, and they also do not take into account that for effective antitumor therapy and patient benefit, 0.7% ID may be sufficient [19], as it is much higher than what standard cytostatic compounds can typically achieve [20–22]. In this context, it has to be mentioned that multiple passively tumor-targeted nanomedicines have been successfully translated to the clinic and do clearly create patient benefit, in spite of the fact that their tumor accumulation may be “as low as 0.7% ID” [23].
To facilitate the translation of nanomedicines to the clinic, and to allow for individualized and improved anticancer nanomedicine therapies, it is crucial to get a better grip on the heterogeneity of the EPR effect in patients. Therefore, EPR-potentiating combination treatments, as well as diagnostic protocols which are able to visualize and quantify the extent of the EPR-mediated tumor targeting in individual patients, are urgently needed [24].
2. Principles of EPR
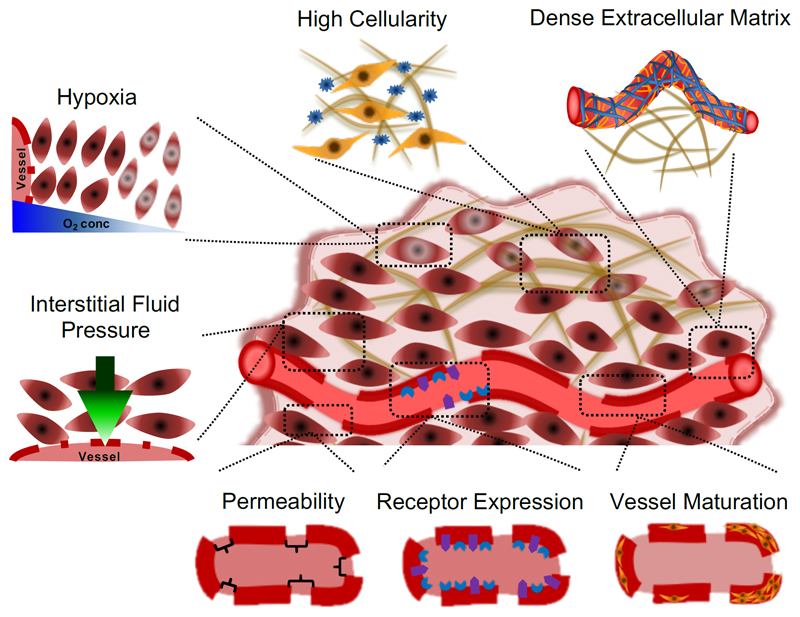
The tumor accumulation of nanomedicines is mainly based on the EPR effect, enabling the extravasation and retention of macromolecules and nanocarriers at pathological sites. The majority of solid tumors have a chaotic vasculature and microenvironment, which is associated with the production of an abnormal amount of vascular growth factors and vascular permeability enhancing factors (such as bradykinin, nitric oxide and prostaglandins), with the lack of functional lymphatic drainage, with an elevated interstitial fluid pressure, and/or with a dense and deregulated stromal compartment consisting of fibroblasts, smooth muscle cells and macrophages [25]. All these factors and features play a role in determining the extent of the EPR effect (Figure 2).
Figure 2. Biological barriers contributing to heterogeneity in EPR-mediated tumor targeting.
Multiple different vascular and microenvironmental parameters contribute to heterogeneity in EPR-based nanomedicine accumulation. At the vessel level, these include vascular permeability, endothelial cell receptor expression and vascular maturation. Stromal parameters which contribute to heterogeneity in EPR-based nano-tumor targeting are the extracellular matrix, tumor cell density, hypoxia and the interstitial fluid pressure. All of these pathophysiological parameters have to be considered when aiming to developed individualized and improved nanomedicine treatments.
One of the most critical features of tumors is their invasive and rapid growth. This excessive growth leads to solid stress, caused by the proliferation of a large number of cells within a spatially confined volume. To maintain tumor growth, the establishment of an own blood supply is mandatory for tumors larger than ~1-2 mm in diameter [26]. However, many of these angiogenic blood vessels are compressed as a result of solid stress, which together with the high levels of cell growth and metabolism in tumors leads to hypoxia, resulting in the production of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF).
The notion that tumors produce angiogenesis-enhancing factors was first described by Judah Folkman and colleagues in 1971 [27,28]. One of these factors was later identified as vascular permeability factor (VPF; which is VEGF) [29]. Research by Folkman’s group extended these findings, showing that increased levels of VPF/VEGF result in an upregulation of the corresponding receptors for this molecule (i.e. VEGFR2) on endothelial cells [30]. VEGF is responsible for endothelial cell survival, sprouting and vascular leakiness [31], thereby providing the basis for EPR-mediated tumor targeting.
Newly formed blood vessels typically occur in higher densities in tumor tissue [32,33], they often lack a smooth muscle layer and pericytes [34], they have a larger lumen and wider fenestrations (with sizes of up to 4.7 µm; note, however, that the majority of these fenestrations are in the order of 1-100 nm [13]), and they typically contain malfunctioning endothelial cells [35]. Additionally, vascular perfusion tends to be impaired, at least to some extent, and blood flow is sluggish [36,37].
Due to the lack of a properly functioning lymphatic drainage system, solid tumors furthermore tend to develop a high interstitial fluid pressure (IFP), which attenuates nanomedicine accumulation and penetration, especially in the core of tumors [38]. This high pressure also contributes to the compression of blood and lymphatic vessels, further adding to the high IFP, causing blood vessel collapse and inefficient tumor perfusion [39,40]. Decreasing the IFP and/or solid stress can decompress blood and lymphatic vessels, and it may help to increase perfusion and nanomedicine accumulation [41].
Another important factor contributing to the EPR effect is the stromal compartment, which can be subdivided into the extracellular matrix (ECM) and stromal cells. The latter include endothelial cells, pericytes, (myo)fibroblasts, smooth muscle cells, dendritic cells, macrophages and other immune cells. The density of ECM components, such as collagen and hyaluronic acid, strongly influences nanomedicine accumulation, as it forms a barrier which prevents the penetration of nanomedicines from the vessels deep into the tumor interstitium, further contributing to inhomogeneous distribution of drugs and drug delivery systems [42]. In this context, especially the collagen content and the collagen distribution seem to play a crucial role. The hypothesis that nanomedicine accumulation is compromised in collagen-rich tumors has been confirmed in several studies, showing that dense fibrillar collagen prevents large molecules as well as standard chemotherapeutic agents from penetrating deep into tumorous tissue [43–45]. However, ECM reduction has several limitations and systemic targeting of the ECM, which also affects healthy tissues, can induce adverse effects like thromboembolism [46,47].
In exemplary efforts to enhance tumor penetration, a dorsal skinfold chamber approach was used in immunocompromised mice bearing HSTS26T soft tissue sarcomas. The use of the hormone relaxin resulted in the up-regulation of the expression of matrix-degrading enzymes, like matrix-metalloproteinases, and in the degradation of the tumor ECM. After 12 days of relaxin treatment, it could be shown that the diffusion rate of extravasated IgG (150 kDa; ~10 nm) and dextran-2M (2000 kDa; ~54 nm) increased significantly, as a result of a more porous collagen matrix [43]. There are several tumors and tumor models with a very dense ECM and with very limited EPR-based accumulation, such as pancreatic ductal carcinomas [48,49]. Especially in such tumor types, ECM-degrading co-treatments like the reduction of hyaluronan, can help to promote nanomedicine accumulation and efficacy [50,51]. In this context, a special antibody-drug conjugate, which exploits the dense ECM as a scaffold for cancer stromal targeting (CAST) therapy has to be mentioned. CAST is directed against certain ECM components such as collagen 4 [52] and fibrin [53] and can be used to improve drug delivery and sustained release [54].
The cells in the tumor stroma play a crucial role in determining the efficiency of EPR-mediated tumor accumulation. Macrophages, for instance, strongly influence the retention of nanomedicines [55–57]. As an example, polymer-bound and fluorophore-labeled platinum(IV) prodrugs have been shown to strongly accumulate in tumor-associated macrophages (TAM) [58]. The polymeric prodrugs were injected via the tail vein and the accumulation in subcutaneously implanted HT1080 fibrosarcoma tumors was imaged in real-time using a dorsal window chamber. In this setup, the TAM acted as a nanoparticle depot and gradually released the payload to neighboring tumor cells. The depletion of TAM with clodronate liposomes prior to platinum prodrug treatment resulted in a reduction of prodrug concentrations in tumors, and it also reduced prodrug-induced tumor growth inhibition, indicating that nanomedicine accumulation and efficacy depend on macrophage content [58].
Besides considering the physiological characteristics of tumors, it is important to note that also the size of nanomedicine formulations affects nanomedicine targeting to different tumor compartments and cells [58]. As shown by Tsvetkova et al., riboflavin-mediated active targeting of differently sized star-PEGs (10 kDa and 40 kDa; i.e. approximately 7 and 13 nm in diameter) resulted in preferential uptake by tumor cells in case of 10 kDa nanocarriers and in increased uptake by tumor-associated macrophages in case of larger 40 kDa nanocarriers. While active targeting improves cell uptake and retention of a given formulation in tumor cells, the extravasation and accumulation is predominantly driven by the size of the compound. Even though the prolonged circulation of the 40 kDa riboflavin-PEG resulted in a higher overall tumor accumulation, cellular uptake was significantly higher for the 10 kDa riboflavin-PEG formulation, in spite of its shorter blood half-life times and less EPR-mediated accumulation [59].
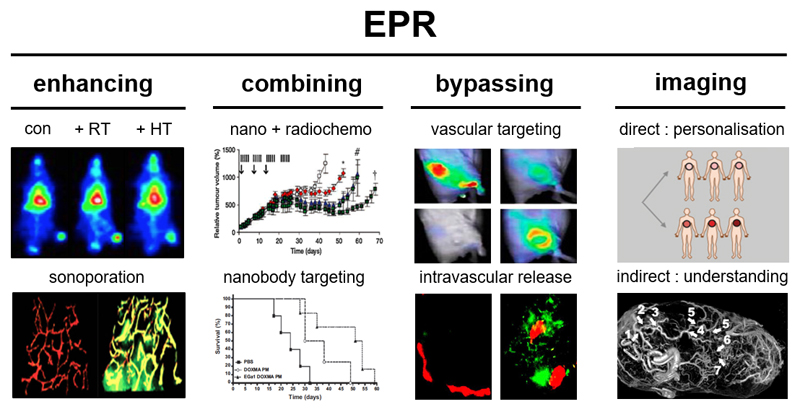
Considering all above notions, it appears that, while EPR-based nanomedicines may in principle hold promise for improving the efficacy of systemic anticancer drug therapy, there are still multiple biological and pathophysiological barriers that are withholding them from unlocking their full potential [24] (Figure 2). These relate to the high heterogeneity of tumors, and they call for companion diagnostics and nanotheranostics to monitor nanomedicine-based tumor targeting, as well as for combination treatments to enhance the EPR effect. We here summarize strategies to address these challenges, based on enhancing, combining, bypassing and imaging EPR-based tumor targeting (Figure 3).
Figure 3. Strategies to overcome heterogeneity in EPR-based tumor targeting.
Several strategies can be employed to improve nanomedicine-based anticancer therapy. From left: Enhancing: Pharmacological and physical means, such as radiotherapy (RT), hyperthermia (HT) (adapted from [101]) and sonoporation (adapted from [133]) can be used to enhance the EPR effect in tumors. Combining: Synergism between nanomedicine-based chemotherapy and clinically relevant fractionated radiotherapy leads to increased nanomedicine accumulation and enhanced efficacy (adapted from [99]). Active targeting with pharmacologically active ligands (e.g. anti-EGFR nanobodies) synergizes with the drug molecules entrapped within a given nanomedicine formulation (adapted from [173]). Bypassing: In case of tumors with low or no EPR, vascular targeting (e.g. via RGD-targeted nanocarriers; adapted from [188]) or the use of triggerable nanocarriers that release their payload intravascularly (e.g. from drug-loaded microbubbles; adapted from [125]) can be used to improve drug delivery in spite of low/no EPR effect. Imaging: The heterogeneity in EPR-based tumor targeting can be addressed via direct or indirect imaging approaches, employing either nanotheranostics and companion nanodiagnostics to monitor the biodistribution and target site accumulation of nanomedicines, or employing the use of established images probes and protocols to visualize tumor blood vessels and the microenvironment. Imaging tumor blood vessels and EPR-based tumor targeting can help to pre-select patients for more personalized nanomedicine treatments (adapted from [210] and [205]).
3. Enhancing EPR-mediated tumor-targeting
Several pharmacological and physical means can be employed to enhance the tumor accumulation and efficacy of EPR-based nanomedicines. Among pharmacological strategies, the most prominent are treatments with drugs which modulate VEGF signaling, with angiotensin agonists and antagonists, with tumor necrosis factor-alpha (TNF-α), with vessel promoting treatments and with nitric oxide-producing agents [60]. Physical means can include hyperthermia, radiotherapy and ultrasound. Several prominent examples of studies in which pharmacological and physical strategies are employed to enhance the accumulation and efficacy of EPR-based nanomedicine are described below.
3.1. Anti-angiogenic therapy
Anti-angiogenic drugs are traditionally used to deprive tumors from oxygen and nutrients [61]. When given at intermediate doses, anti-angiogenic agents can be employed to normalize the disorganized tumor vasculature of highly vascularized tumors to improve nanomedicine delivery [62]. This can e.g. be done using bevacizumab (Avastin®; a VEGF-blocking antibody) or sorafenib (Nexavar®; a small molecule VEGF receptor tyrosine kinase inhibitor) [63,64]. Intermediate dosing is necessary, as high doses can lead to a closing of the fenestrations between the endothelial cells, as well as to vessel pruning, which shuts down the perfusion of the tumor also limiting the delivery of anticancer agents. Consequently, pre-treatment with anti-angiogenic agents to enhance EPR-mediated tumor accumulation only works if the vasculature is normalized to a level at which vessels are still perfused. While the blockage of VEGF can lead to an unwanted reduction of vessel leakiness, other components such as bradykinin, a potent vascular permeability factor, are still released, maintaining vessel permeability and thus allowing for nanomedicine extravasation [65].
In a pioneering proof-of-concept preclinical study, the anti-VEGFR2 antibody DC101 was used to block the interaction between VEGF and its receptor, normalizing the vessels to the point that the perfusion of the tumors increased, necrotic areas disappeared and EPR-based nanomedicine accumulation enhanced. DC101 was injected in combination with either Doxil® (100 nm size) or Abraxane® (125 nm original size, 10 nm size after disassembly in blood, with paclitaxel bound to endogenous albumin). The study was performed in mice bearing either E0771 or 4T1 mouse mammary tumors. The use of intermediately dosed anti-angiogenic therapy enhanced the accumulation of paclitaxel (albumin-bound; 10 nm) in the tumor mass through restoration of convective drug delivery and through a reduction of the IFP [66], but it did not affect the concentrations of doxorubicin (Doxil®; 100 nm) in tumors, suggesting that vascular normalization affects EPR-mediated tumor targeting in a size-dependent manner [63]. This indicates that vascular normalization - besides resulting in an enhancement and/or homogenization of tumor blood flow - also results in a reduction of the pore cut-off size. In spite of these findings, in a clinical setting, bevacizumab-induced vascular normalization strongly enhances antitumor responses to Doxil®-based chemotherapy in patients with ovarian cancer, from 3.7 months for Doxil® alone, to 7.8 months for Doxil® plus bevacizumab [67]. Overall survival increased from 10 months to 33 months, respectively. On top of this, the concentration of liposomal doxorubicin per cycle could be lowered significantly (from 50 to 30 mg/m2), which may result in improved patient compliance and an increased quality of life. These findings indicate that vascular normalization can be employed to enhance the efficacy of EPR-based nanomedicine formulations.
3.2. TNF-α
Tumor necrosis factor-alpha (TNF-α) is a potent inflammatory mediator [68]. It enhances vascular leakiness to allow for leucocyte extravasation in case of inflammation, via the disruption of the endothelial cell adherence junction protein VE-cadherin [69], and it may thus also be useful to enhance the extravasation and accumulation of nanomedicines. In this context, it has been shown that the injection of TNF-α leads to a significantly 10-fold higher EPR-mediated accumulation (Figure 4A) of radiolabeled liposomes in mice bearing subcutaneous CT26 tumors compared to non-TNF-α-treated animals (Figure 4B) [70]. Clinical trials with various TNF-α formulations are ongoing, e.g. with Fibromun® (from Philogen), which is an antibody fused to TNF-α for melanoma treatment [71], and which is also used in combination with doxorubicin for soft tissue sarcoma treatment [72]. In case of certain soft tissue sarcomas, TNF-α is also used on its own, in combination with the low-molecular-weight anticancer agent melphalan, for isolated limb perfusion (ILP), to avoid amputation of the cancerous limb [73]. Patients receive ILP for 90 min, starting with a bolus injection of TNF-α directly into closed loop circulatory system in the isolated limb. Melphalan was administered 30 min later. When employing the combined ILP setup, 82% of affected limbs could be protected against amputation, as compared to 41% for melphalan-based ILP alone [73]. Although the results of this clinical trial are promising, the clinical use of TNF-α is limited to the treatment of local cancer sites, such as melanoma or soft tissue sarcoma (in combination with ILP), due to its systemic toxicity.
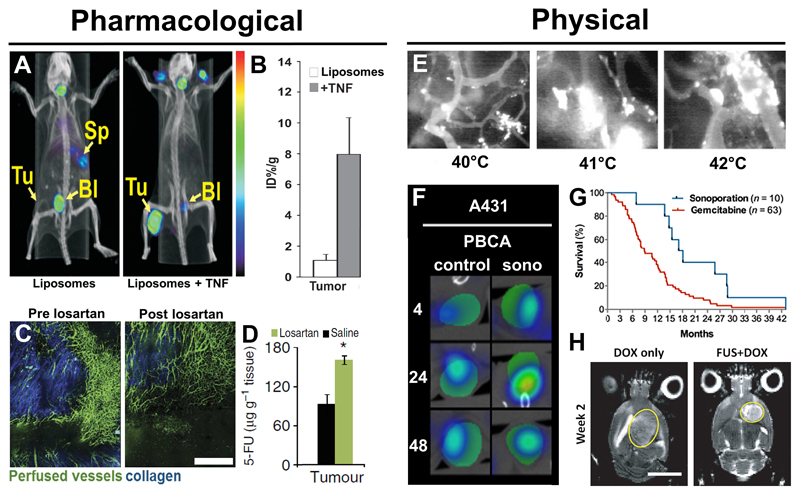
Figure 4. Pharmacological and physical means to enhance tumor accumulation.
Heterogeneity in EPR-based tumor targeting can be overcome by using different pharmacological and physical means. A-B: Accumulation of radiolabeled liposomes in tumors was increased after TNF-α application, which enhances vascular permeability and tumor penetration. The concentration of liposomes was substantially higher in TNF-α-treated tumors than in control tumors (adapted from [70]). C-D: Losartan, an angiotensin II receptor blocker, decompresses tumor blood vessels and leads to improved vessel perfusion. This results in enhanced accumulation of 5-fluorouracil (5-FU; adapted from [77]). E: Extravasation of liposomes from tumor blood vessels upon applying hyperthermia at different temperatures (adapted from [120]). F: CT-FMT images showing enhanced accumulation of fluorophore-labeled liposomes in tumors after sonoporation (adapted from [123]). G: Sonoporation in combination with gemcitabine has a positive impact on the survival of patients suffering from inoperable pancreatic cancer (adapted from [131]). H: Site-specific sonoporation in combination with liposomal doxorubicin inhibits the growth of rat glioma (FUS+DOX; indicated by yellow circles) more efficiently compared to treatment with liposomal doxorubicin alone (DOX only; adapted from [136]).
3.3. Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) can be used to enhance EPR-based accumulation because they amplify the effect of substances like bradykinin, which promote vessel permeability and dilation through the loosening of the fasciae adherens, i.e. the endothelial cadherin-mediated intercellular connections [74]. ARBs also modulate the expression of ECM components (e.g. reduction in collagen expression), which leads to vessel decompression and to enhanced EPR [12, 56]. Various ARBs can be used for this purpose [76], e.g. losartan, which is clinically used to treat chronic kidney diseases and hypertension, but also showed promising preclinical results in cancer treatments. Jain and colleagues used losartan to decompress tumor blood vessels, increase vascular perfusion and enhance tumor-targeted drug delivery [77]. Solid stress was measured via an ex vivo technique in which the extent of tumor tissue relaxation was measured with a surgical incision. After losartan treatment, the solid stress in four different tumor models (E0771 and 4T1 breast carcinoma as well as AK4.4 and Pan-02 pancreatic carcinoma) was found to be significantly decreased, and the perfused vessel fraction increased (Figure 4C), overall leading to a higher accumulation of 5-fluorouracil (5-FU) (Figure 4D). This effect was accompanied by a decrease in the expression of collagen 1, hyaluronic acid and cancer associated-fibroblasts, indicating that the increase in vessel perfusion is caused by vascular decompression resulting from the reduction of ECM components. Additionally, it has been reported that the distribution and efficacy of nanotherapeutics (e.g. Doxil) was increased upon losartan co-treatment through the suppression of collagen I synthesis [78]. Based on these results, losartan was selected for a clinical trial in pancreatic cancer in combination with 5-fluorouracil, leucovorin and oxaliplatin, as well as with proton beam radiation therapy. This phase II study is currently ongoing and final results are not available yet. However, initial results indicate that the losartan-based combination therapy led to a decrease in tumor size and in some cases even enabled surgical resection (i.e. making it possible to remove tumors which were not operable prior to combination treatment). The 2-year overall survival exceeded 60%, and the number of patients where a resection of the tumor was possible after combination therapy exceeded 50%, resulting in 2-year survival in the resected patient population of close to 80% [79–81]. A potential disadvantages of treatment with ARBs is that resistance may develop during long-term therapy [78,82].
3.4. Angiotensin-II
In contrast to the increase in vessel permeability and dilation of vessels through angiotensin II- antagonists, also vasoconstriction can be employed to enhance the EPR effect. Angiotensin-II (AT-II) injections induce hypertension through systemic vasoconstriction [83–85], which exclusively takes place in ‘healthy’ blood vessels resulting in clinical limitations for the treatment of patients with hypertension or brain tumors such as glioblastoma. Since tumor vessels are mostly immature and lack a properly differentiated and structured smooth muscle cell layer, they are not able to contract in response to AT-II. Still, there can be an effect on tumors, resulting from an increased blood flow caused by systemic hypertension and by vasoconstriction in tumor-feeding vessels, leading to the opening/enlargement of endothelial gaps in the tumor vasculature and increasing the blood pressure in tumor blood vessels, thereby enhancing convection. The fact that AT-II injections can lead to a better perfusion of tumorous tissues, to an improved EPR-mediated drug delivery, and to an enhanced nanomedicine efficacy has been shown by Maeda and colleagues in rodent xenograft models as well as in several patients with advanced solid tumors treated with the polymer-based nanoformulation SMANCS, which is a 16 kDa-sized conjugate of neocarzinostatin and poly(styrene-co-maleic acid) [86,87].
3.5. Vessel promotion
Instead of inhibiting angiogenesis, Wong et al. developed a strategy named vessel promotion, which focusses on increasing angiogenesis resulting in more vessels and eventually a higher delivery of chemotherapeutics [88]. Cilengitide, which binds to αvβ3 integrins and is usually associated with anti-angiogenesis [89], showed the opposite proangiogenic effect if applied at low doses [88]. Furthermore, this vessel promoting treatment was complemented with verapamil, a calcium channel blocking agent leading to higher blood flow, resulting in a significant increase of blood vessel perfusion of 10%. The combination of cilengitide, verapamil and gemcitabine, showed a significantly increased mean survival time (approximately doubled compared to gemcitabine only) in a mutagenic mouse model of pancreatic cancer (KPC mice) due to a lower tumor burden. Histological analysis of the triple-treated group (cilengitide, verapamil and gemcitabine) presented with significantly increased vessel density and significantly decreased hypoxia values compared to the placebo or gemcitabine only treated groups, showing the beneficial effects of vessel promotion in combination with standard chemotherapy. Similar results were reported for the use of recombinant human erythropoietin (Epo) in non-small cell lung cancer (NSCLC) tumor models, where the promotion of vessels induced an increase of 50% in vessel density and doubled the relative blood volume facilitating the delivery of carboplatin to tumor sites, which resulted in up to a 100% increase in delivered carboplatin [90]. Vessel promotion is one of several vessel modulating strategies to improve the delivery of chemotherapeutic agents and it might be a valuable tool to enhance nanomedicine accumulation in barely perfused tumors [91].
3.6. Radiotherapy
Ionizing irradiation can increase vascular leakiness via the up-regulation of VEGF and fibroblast growth factor (FGF) expression [92–94]. It furthermore leads to a decrease in cell density within tumors, and as a consequence of that, also to a reduced IFP [95], via the generation of radicals which damage the DNA and lead to tumor and endothelial cell apoptosis [96,97]. Taken together, these phenomena contribute to a better accumulation of both low-molecular-weight drugs and nanomedicine formulations in tumors. In addition, also the efficacy of nanomedicine-based chemotherapy can be increased upon combination with radiotherapy. This holds true both for classical external beam radiotherapy, as well as for internal peptide receptor radiotherapy [98]. In such setups, radiotherapy and nanomedicines can act synergistically, with radiotherapy enhancing the tumor accumulation of nanocarriers, and with nanocarriers enhancing the antitumor efficacy of radio-chemotherapy [99,100]. In this context, it has for instance been shown that radiotherapy has a positive effect on the accumulation of polymeric drug carriers in three different tumor types, all based on the Dunning R-3327 prostate carcinoma model [101]. Radiotherapy treatment significantly increased the accumulation of 31 and 65 kDa sized polymers (i.e. approximately 5 and 10 nm, respectively) in all tumor models, and most prominently in those with low levels of baseline leakiness. Conversely, polymeric drug delivery systems carrying either doxorubicin or gemcitabine both strongly enhanced the efficacy of clinically relevant regimens of fractionated radiotherapy [71]. These notions are confirmed by results reported by De Davies and colleagues, who combined liposomal doxorubicin (Doxil®) with radiotherapy in mice with osteosarcoma xenografts. Mice treated with both radiotherapy and Doxil® showed delayed tumor growth compared to the control group treated with Doxil® alone. Importantly, histological investigations of the tumor tissue revealed a deeper liposome penetration into tumor tissue for animals co-treated with radiotherapy [102]. These findings were further validated in a study in which mice with human fibrosarcoma xenografts (HT1080) were treated with Onivyde® (liposomal irinotecan) in combination with radiotherapy, showing complete eradication of tumors upon combined nano-chemo-radiotherapy, while in mice solely treated with Onivyde®, tumor growth was only delayed [103]. The combination of nano-chemotherapy with external beam radiotherapy has also already been evaluated in multiple clinical trials, showing not only improvements in efficacy, but also in tolerability. In one of the first exemplary trials performed in this context, seven patients with locally advanced sarcomas received radiolabeled liposomal doxorubicin plus radiotherapy. The response rate was found to be >70% without observation of severe toxicities [104], whereas severe side effects occurred when un-encapsulated chemotherapeutics were combined with radiotherapy [105,106]. Ionizing radiation has an effect on a variety of different cell types within the TME, and besides increasing vascular leakiness, it can also induce therapy resistance and metastasis [107,108]. This indicates that its implementation in multimodal combination therapies needs to be carefully considered and planned.
3.7. Hyperthermia
Over the years, hyperthermia has been extensively used for antitumor treatment, and it is generally combined with chemo- [109] and/or with radiotherapy [110–112]. Hyperthermia can be applied via several approaches, such as radiofrequency [113], microwaves [114], focused ultrasound [115], or intracavitary perfusion (i.e. with heated chemotherapy-containing solutions) [116], but is limited to locally well-defined, solid tumors. Hyperthermia generally leads to an increase in tumor blood flow and to an enhanced vascular permeability, thus promoting drug and oxygen supply to tumors [117,118]. In non-ablative settings, the applied temperatures typically range between 39 and 42 °C. Hyperthermia can be used to increase the EPR effect especially in non-leaky tumors in which the baseline levels of nanomedicine accumulation are low [101,119,120]. Employing a dorsal skin flap window chamber with human ovarian carcinoma (SKOV-3) tumors in athymic nude mice, Dewhirst and colleagues demonstrated enhanced extravasation of 100 nm liposomes out of tumor blood vessels into the interstitium upon gradually increasing the temperature to either 39, 40, 41 or 42 °C for 1 h, followed by i.v. injection of rhodamine-labeled liposomes (Figure 4E). In previous studies, it was found that this tumor model is rather impermeable for 100 nm-sized liposomes under normothermic temperatures, and that upon increasing the temperature, the extravasation of liposomes was enhanced significantly [120]. Based on these promising initial findings, as well as on combinations of hyperthermia with temperature-sensitive nanocarriers (see below, chapter 5.2), we anticipate that hyperthermia will gradually evolve to become a powerful clinical tool to enhance (nano-) drug accumulation and performance.
3.8. Sonoporation
Microbubbles are routinely used as contrast agents for ultrasound imaging. They can, however, also be employed to temporarily increase vessel perfusion and permeability [121,122], thereby improving drug delivery to tumors [123], upon application of ultrasonic waves to induce microbubble oscillation, cavitation or implosion [124]. There are several options for microbubble use to promote drug targeting to pathological sites: either via direct drug delivery (i.e. through the encapsulation of drug molecules in the microbubble core or shell, or binding of the drug or drug-containing nanoparticles to the shell of the microbubbles), or via indirect drug delivery (i.e. by co-injection of free drugs or drug delivery systems together with microbubbles) [125]. Direct drug loading of microbubbles was reported for lipid- as well as polymer-based microbubbles, generally showing higher loading efficiencies when drugs are loaded into polymer-based microbubbles [126]. However, the majority of studies focusing on microbubble loading with chemotherapeutic agents such as doxorubicin (Doxil®, where the cytotoxic agent is attached to the shell), bleomycin and docetaxel (the latter two entrapped into oils inside the microbubble core) employ lipid-based microbubbles [122,127,128]. It is expected that polymer-based microbubbles will be increasingly used for direct drug delivery in the future, because they can be more easily loaded with a variety of different drugs, and with a much higher loading capacity, and they can be additionally tailored with regard to e.g. shell thickness and mechanical properties [129]. Besides different loading capabilities, the microbubble type might also have an impact on the induced vessel leakiness which would be also important for both direct and indirect drug delivery. Lipid-based microbubbles are able to oscillate better, and the shell decomposes into fragments at elevating pressures whereas polymer-based microbubbles will remain largely intact and release the containing gas as a bubble through a shell defect [130]. Therefore, the effect of lipid- as well as polymer-based microbubbles on liposome accumulation upon ultrasound treatment was evaluated in two tumor models (A431 and BxPC-3) [123]. Both tumor models are known to have a poor EPR effect. While liposome accumulation was increased by up to 100% upon sonoporation in these models, and liposome penetration facilitated, no significant differences between lipid and PBCA microbubbles were observed (Figure 4F).
Recently, sonoporation in combination with gemcitabine-based standard chemotherapy showed a positive impact on the treatment of patients suffering from inoperable pancreatic cancer [131]. Sonoporation with gemcitabine almost doubled the mean overall survival of patients, from 8.9 months for gemcitabine alone (historical control cohort), to 17.6 months for gemcitabine plus ultrasound and (lipid-based) microbubbles (Figure 4G). Part of this substantial prolongation resulted from the fact that two patients with initially inoperable tumors could be subjected to surgical resection of the tumor [131]. Furthermore, an interventional clinical trial investigating the effect of contrast-enhanced US and sonoporation on the achieved tumor size reduction, applied during neoadjuvant chemotherapy administration in breast cancer, has recently started in our own laboratories [132]. Taking the above together, these efforts indicate that sonoporation may be a powerful non-invasive tool to increase the accumulation of drugs even in hardly treatable tumors such as pancreatic tumors.
Another promising application of sonoporation is the treatment of central nervous system (CNS)-related diseases such as neurodegenerative diseases or brain tumors. CNS drug therapies tend to be ineffective because of the presence of the blood-brain barrier (BBB), which is still intact in many tumors or neurodegenerative diseases such as Alzheimer, prohibiting drug delivery to pathological sites. One approach to circumvent this delivery problem is sonoporation, which induces a spatially and temporally controlled BBB opening, creating a window for drug delivery [133–135]. Hynynen, McDannold and colleagues are pioneers in the field of ultrasound-mediated brain vasculature opening and have shown that MRI-guided focused ultrasound is able to permeate the BBB, leading to an improved accumulation of liposomal doxorubicin in a rat 9L gliosarcoma model [136]. Rats treated with focused ultrasound and microbubbles in combination with liposomal doxorubicin showed prolonged tumor volume doubling times and had a 24% longer median survival compared to rats treated with liposomal doxorubicin alone (Figure 4H). Extending these results, a clinical trial was initiated in which patients with brain tumors, including glioblastoma multiforme, received MRI-guided focused ultrasound treatment together with lipid microbubbles and doxorubicin [137]. The added value of the sonoporation on the accumulation and efficacy of doxorubicin is currently under investigation [138], and the outcome of this pioneering study is eagerly awaited. Clinical sonoporation trials have also recently begun in patients with Alzheimer, to evaluate the minimal required ultrasound settings for a safe BBB opening [139].
3.9. Photodynamic therapy
Photodynamic therapy (PDT) refers to the treatment of tissues, typically tumors, with a photosensitizing agent, followed by activation via locally applied laser light [140]. It is based on the formation of reactive oxygen species (ROS), such as singlet oxygen (1O2), which damages nucleic acids and proteins, and leads to cell death. Clinical limitations of PDT are the penetration depth of the applied laser light (max. 1-2 cm), as well as the short migration distance of the produced oxygen radicals [141], which is typically less than 0.02 µm. These issues render the treatment of e.g. wide-spread tumors or metastases located deep in the body nearly impossible. For optimal efficacy, photodynamic therapy therefore has to be directed to specific (sub-) cellular targets, such as mitochondria (porphycene monomer [142]), lysosomes (chlorin e6 [143]) or the cell membrane (monocationic porphyrins [144]). Together, these effects lead to a reduction of the cell density in tumors, which decreases the IFP and the solid pressure, and which alleviates vessel compression, leading to a better perfusion of the vessels and to a higher accumulation of drugs and drug delivery systems. In an exemplary preclinical study, a monoclonal antibody-photosensitizer (i.e. panitumumab fused with the photosensitizer IR700; directed against the human epidermal growth factor receptor (EGFR; HER1)) was combined with laser light and with liposomal daunorubicin, to treat mixed tumors [145]. The subcutaneously inoculated tumors were composed predominantly of EGFR-positive A431 epidermoid carcinoma cells, mixed with a smaller fraction of EGFR-negative Balb-3T3 embryonic fibroblasts. The EGFR-targeted photosensitizer specifically accumulated in the regions of A431 epidermoid carcinoma cells and thus these areas showed massive necrosis after near-infrared laser light exposure. Treatment of the tumor with the EGFR-targeted photosensitizer prior to liposomal daunorubicin treatment led to a substantial increase in tumor permeability (an effect which the authors coined super-enhanced permeability and retention (SUPR) [146]), to a 5-fold increase in the tumor accumulation of liposomal daunorubicin, and to significantly enhanced therapeutic efficacy as compared to all relevant control groups. Follow-up studies aimed at identifying the mechanism of action behind PDT-induced super-enhanced permeability and retention, and showed that the increase in vascular permeability after photoimmunotherapy resulted from depolymerization of endothelial cell microtubules, giving rise to the formation of larger endothelial intercellular gaps in the endothelium, thereby promoting EPR [147].
4. Integrating EPR-based nanomedicines in combination therapies
Several of the above mentioned EPR-enhancing approaches have already alluded to the potential of combination regimens in which nanomedicines are joined with other treatment modalities, such as radiotherapy or hyperthermia. In addition to this, nanomedicines are also highly useful to improve the efficacy of different types of combination chemotherapy.
4.1. Multi-drug nanomedicines
Rationally designed chemotherapy combinations hold significant promise for the improvement of the outcome of systemic anticancer therapy [148]. Merging two different drugs within one nanomedicine formulation ensures the availability of both agents within the same cell, enhances the impact of each single agent, helps to avoid multidrug-resistance and likely also increases the tolerability of the two agents when given together, resulting in a clear improvement in therapeutic index [149,150]. Nanocarriers such as liposomes, micelles and polymers can be relatively easily co-loaded with two different anticancer agents to enable multi-drug treatment. Nanomedicines can actually also be efficiently combined with conventional chemotherapeutic drugs, generally improving both efficacy and tolerability [23,99,151]. Several of such combination nano-chemotherapy approaches are currently being evaluated in clinical trials [152], and they are likely to be extended in the near future to the use of nanomedicines together with antibody-drug conjugates (ADC) and/or with immunomodulating antibodies [153].
The combination of two different drugs within one nanomedicine formulation can be very beneficial. In the clinic, the standard procedure for the application of chemotherapy combinations is typically first establishing the maximum tolerated dose of one drug, and then start adding in the second drug [154], neglecting the notion that the most efficient therapeutic activity of those two drugs together may as well be at doses below the maximum tolerated dose(s). In this context, several studies reported a drug-ratio-dependent synergy showing that “ratiometric” co-encapsulation of two different drugs in liposomal nanocarriers improves anticancer efficacy [155,156]. A prototypic example for such a ratiometrically combined drug delivery approach is the liposomal formulation Vyxeos™ (CPX-351, Jazz Pharmaceuticals®), in which cytarabine and daunorubicin are combined within a single multilamellar liposome for the treatment of acute myeloid leukemia (AML). In a phase I dose-escalation clinical trial, it was proven that co-encapsulation into liposomal nanocarriers substantially increased the circulation times of the drugs while reducing the side effect profile. The traditional 7+3 regimen for AML consists of a continuous infusion of cytarabine (100 mg/m2 per day) from days 1-7, combined with a daily bolus of daunorubicin (60 mg/m2) on days 1-3. The ratiometric liposome formulation (i.e. cytarabine plus daunorubicin co-encapsulated in a 5:1 molar ratio) was only infused at days 1, 3 and 5, and showed a stable 5:1 molar ratio in both the plasma and the bone marrow (target organ) for up to 24 hours for all dose levels tested. The treatment showed increased efficacy, presenting with complete remissions in a significant portion of refractory AML patients (23%) and with very acceptable side effects (<10% grade 3 adverse events) [157]. In a phase III randomized trial, the efficacy of the conventional 7+3 treatment compared to Vyxeos™ was evaluated in more than 300 elderly patients with newly diagnosed secondary AML, showing a clear benefit for patients treated with the double-drug formulation: 47.4% of Vyxeos™-treated patients showed complete remission, compared to only 33.3% of patients receiving the conventional treatment, and the median overall survival time was almost doubled (6 vs. 10 months) [158]. Based on these findings, the authors proposed that the conventional 7+3 therapy should be replaced with Vyxeos™, an advice which the FDA partially followed by granting a breakthrough therapy designation for Vyxeos™. The FDA submission was completed for the treatment of AML in April 2017, with a request for priority review, and was approved in August 2017 [159].
The ratiometric combination of two drugs within one liposome has also been tested for several other chemotherapeutic treatments, including e.g. doxorubicin plus topotecan, and irinotecan plus floxuridine [152]. Thus far, besides CPX-351/Vyxeos™, only CPX-1 has been translated into clinical trials. CPX-1 is a liposome containing irinotecan and floxuridine in a fixed molar ratio of 1:1. In a phase II clinical trial, the effectiveness of the formulation for colorectal cancer treatments was evaluated and showed a disease control rate of 65% in irinotecan-naive patients and 38% in the irinotecan-refractory group. The improved response rate of the CPX-1 treated group can most likely be attributed to an EPR-based accumulation of CPX-1 in the colorectal cancer lesions [160]. Due to the nano-size of the liposomes, a rather selective accumulation in the lesions may be possible without strong enrichment in healthy tissues (other than liver and spleen), enabling the delivery of higher drug doses as compared to the standard therapy, in which a combination of conventional small molecule drugs is administered. The administered dose in the CPX-1 clinical trial was 210 u/m2, with one unit consisting of 1 mg irinotecan and 0.36 mg floxuridine, which is equal to 210 mg/m2 of irinotecan and 75.6 mg/m2 floxuridine. Compared to the conventional combination (180 mg/m2 irinotecan followed by 2400-3000 mg/m2 floxuridine), the nanomedicine-based ratiometric drug delivery approach allows for an overall lower drug dose, with constant drug release, which upon EPR-mediated accumulation results in higher drug concentrations in tumors for prolonged periods of time [161].
4.2. Combination of nanomedicines with standard chemotherapy
The combination of nanomedicines with standard chemotherapy treatments has also already shown promising results in the clinic. Abraxane®, for instance, is clinically applied together with gemcitabine for the first-line treatment of metastatic adenocarcinomas of the pancreas, and together with carboplatin for the treatment of locally advanced or metastatic non-small cell lung cancer [162]. In a phase III clinical trial, pancreatic cancer patients were infused with either Abraxane® (125 mg/m2) in combination with gemcitabine (1000 mg/m2) at days 1, 8, 29, 36 and 43, or with gemcitabine alone, weekly for 7-8 weeks. The combination treatment was found to be beneficial to the patients, as exemplified by a one-year survival rate of 35% compared to 22% in the gemcitabine alone group. The median progression-free survival time increased from 3.7 to 5.5 months. However, the combination treatment also presented with more side effects [163], but with additional adjustments in the treatment regimen (i.e. bi-weekly administration of both formulations on days 1 and 15 of a 28-day cycle), the side effect profile could be refined, and the treatment became better tolerable without a loss of efficacy [164].
4.3. Actively targeted nanomedicines for combination therapy
Combining antibody-based therapy with standard chemotherapy can be beneficial, as exemplified by the use of trastuzumab (Herceptin®) together with multiple different chemotherapeutic drugs for the treatment of patients with human epidermal growth factor receptor 2 (HER2) -positive metastatic breast cancer [165]. The antibody blocks the HER2 receptor, which is overexpressed in around 30% of breast cancer patients [166], and also around 20% of gastric cancer patients [167]. Blocking of HER2 signaling limits proliferation and induces apoptosis in tumor cells, thus decreasing the cell density in tumors [168,169]. This decrease leads to a reduced solid pressure, which decompresses blood vessels, increases tumor perfusion and enhances drug accumulation [170]. Antibodies such as trastuzumab can be directly coupled to chemotherapeutic agents, forming so called antibody-drug conjugates (ADCs), e.g. Kadcyla®. This formulation is a conjugate of trastuzumab and DM1 (T-DM1; i.e. emtansine or mertansine, which is a highly potent cytotoxic agent inhibiting the assembly of microtubules). Kadcyla® enters cells through receptor-mediated endocytosis and DM1 is activated through proteolytic lysosomal degradation, eventually inhibiting microtubule assembly and leading to cell death. The combination of trastuzumab and DM1 has no influence on the binding affinity to HER2, therefore the anti-tumor effects of DM1 and trastuzumab are preserved, rendering this combination construct even more effective [171]. Several clinical trials evaluated the effect of T-DM1 in HER2-positive breast cancer patients, showing a clear benefit compared to standard treatment, likely via combining anti-HER2 antibody effects with DM1-based chemotherapy effects. In the EMILIA trial, breast cancer patients received either T-DM1 or lapatinib plus capecitabine (control group). Patients receiving T-DM1 treatment presented with a longer progression-free survival (10 months for T-DM1 vs. 6 months for the control group) and increased overall survival (31 months for T-DM1 vs. 25 months for the control group). In 2013, these results led to the approval of T-DM1 by the FDA [171,172].
Compared to antibodies, nanobodies are easier to controllably conjugate to nanocarriers, and they are thus increasingly implemented in targeted nanomedicine studies. For example, an EGFR-targeted nanobody was linked to core-crosslinked polymeric micelles (PM) with covalently entrapped doxorubicin (DOX-PM) [173]. In vitro, nanobody-modified DOX-PM were significantly more effective in killing cancer cells than untargeted DOX-PM. In vivo, the nanobody-modified DOX-PM inhibited tumor growth, even in the absence of a chemotherapeutic drug, due to intrinsic activity of the anti-EGFR nanobodies. Based on this intrinsic anticancer activity, nanobody-targeted DOX-PM were more effective than untargeted DOX-PM, not only in inhibiting tumor growth, but also in prolonging animal survival (see Figure 3). Therefore, it can be concluded that the combination of receptor blockage via nanobodies (and also antibodies) and the simultaneous co-delivery of a chemotherapeutic agent within the same carrier is a highly promising strategy to improve the treatment of advanced solid malignancies.
4.4. Nano-immunotherapy
Despite recent successes of cancer immunotherapy, off-target effects as well as low immunogenicity and low response rates for most tumor entities remain major issues for this strategy. Recently, anticancer therapy focusing on combining immunotherapy with nanomedicines are investigated to tackle the challenges associated with the conventional approach, by decreasing immune-related adverse events while enhanced efficacy [174,175]. Nano-immunotherapy or nanotherapeutic cancer vaccines can advance the delivery of immunogenic cell death promotors, combine therapy with immune checkpoint inhibitors and/or deliver antigens and stimulate (via adjuvants) antigen-presenting cells (APCs) [176–178]. In such setups, besides its delivery purpose, the nanoformulation not only protects its cargo from early degradation, but also enhances cross-penetration as already described by Harding et al. in 1991 for ovalbumin encapsulated in acid-sensitive liposomes [179]. Most exogenous antigens are taken up via endocytosis and are thus degraded in lysosomes, resulting in major histocompatibility complex II (MHC-II) presentation, while endogenous antigens are degraded by proteases inside the cytosol, presenting to MHC-I and to CD8+ T cells. As most tumor antigens are exogenous, they need to be delivered to the cytosol to accomplish MHC-I antigen presentation, which is mandatory for efficient vaccination. Keller et al. showed cytosolic delivery of conjugated antigenic cargo in vitro via a pH-responsive polymeric micelle carrier with increased antigen uptake by APCs in draining lymph nodes, yielding a considerably greater T-cell activation through enhanced MHC-I presentation in vivo [180].
Cross-penetration and associated increased antigen surface presentation was also reported by Luo and coworkers for PC7A-nanoparticles (29 nm in diameter) upon accumulation in lymph nodes with the production of type I interferon. The latter was shown to be solely dependent on binding of the PC7A-nanoparticles to the stimulator of interferon genes (STING) and STING pathway activation. Combination with an immune checkpoint inhibitor for programmed cell death (PD-1) resulted in synergistically improved anti-tumor response and survival rate in tumor bearing mice [181]. The success of such combination therapies was also reported by Duan et al. by combining an immunogenic cell death-inducing nanoscale coordination polymer (NCP) nanocarrier, that was loaded with oxaliplatin, with the immune checkpoint inhibitor anti-PD-L1 as well as with a photosensitizer for photodynamic therapy (PDT) [182]. This combination of chemotherapy, antitumor immunity and PDT that synergized with immune checkpoint blockade showed the highest response rate compared to all controls in a bilateral colon carcinoma tumor model, with even an induction of abscopal effects and with induced cell death in distant tumors that were not irradiated [183]. The abscopal and synergistic effects of the combination therapy were further validated by the same group in a triple-negative breast cancer model with a modified nanoformulation suitable for combined PDT and immune checkpoint blockade. Only the combination therapy resulted in complete tumor regression, and it even prevented metastasis [182].
A phase I dose-escalation trial is currently recruiting patients with advanced malignant melanoma for cancer vaccination. Within this study, dendritic cells are targeted in vivo via intravenously injected tetravalent RNA-lipoplexes that trigger a dose-dependent release of interferon-α. The liposome formulation protects the RNA from early degradation and facilitates uptake by APCs, where the RNA is translated into four antigen encoding proteins, thus the nano-vaccine targets four different tumor-associated antigens [184].
In the clinic, tumor-infiltrating lymphocytes (TILs) can be employed to predict anti-tumor immune responses, where a low number of tumor-infiltrating lymphocytes indicates low or no therapy response. Targeting and/or modulating the tumor microenvironment might solve that problem by promoting tissue infiltration of immune cells converting tumors with a lack of TILs (“non-inflamed”) into those that are likely to respond to a certain treatment (“inflamed”) [185]. In this context, next to any defined target, Jiang et al. stressed that for the stimulation of the immune system, which enables the recognition and attack of malignant cells, the targeting concept of nanomedicine (when combined with immunotherapy) needs rethinking and does not solely rely on tumor accumulation and might instead also be achieved or at least enhanced by addressing immune cells in the immune cascade in e.g. liver or spleen. Thus, avoiding the recognition by the immune system and the mononuclear phagocyte system, as typically preferred for most nanomedicine formulations, might not be that desirable in nano-immunotherapy [186,187].
Overall the combination of several drugs within one nanocarrier and the application of antibody-drug conjugates, which first and foremost accumulate passively in tumors by virtue of the EPR effect, ensures efficient delivery of all drugs – ideally with different mechanisms of action to prevent cross-resistance – to the same cell, it enhances the impact of each single agent, it enables synergistic effects and it reduces side effects through the encapsulation of the drug into a nanocarrier, together resulting in a considerable enhancement in the outcome of combination anticancer (immuno-) therapy [186].
5. Bypassing the EPR effect
Patients suffering from tumors with a non-leaky vasculature, which are not amenable to EPR-based tumor targeting, only profit from nanomedicine therapy if the EPR effect can be bypassed. There are two major strategies to use nanomedicines as drug delivery agents to increase the tumor drug accumulation in spite of low EPR, i.e. active targeting to tumor blood vessels or triggered drug release within the tumor vasculature.
5.1. Vascular targeting
The first approach is the functionalization of nanomedicines with targeting ligands, such as antibodies or peptides, to enable specific binding to receptors (over-) expressed by the vasculature of tumors, and ideally not in healthy tissues. The comparison of the active formulation with its non-targeted counterpart shows two distinct advantages of the targeted formulation. Active targeting approaches lead – at least at early time points – to a higher local intravascular concentration of nanomedicines, compared to the non-targeted form, and increase the retention time in non-leaky tumor tissue. However, in a study by Kunjachan et al., active targeting could not outperform passive EPR-based targeting for 10-20 nm polymeric carriers [188]. It even decreased overall polymer accumulation because the conjugation of targeting moieties caused opsonization of the drug delivery system by macrophages in liver and spleen, thus reducing circulation time and the resulting EPR-mediated tumor accumulation. While MPS uptake of nanomedicine formulations is considered to be a drawback for the therapy of most tumors, it might be beneficial for the treatment certain specific tumors, such as hepatocellular carcinoma, in which TAM may act as local drug reservoirs [58].
A prototypic strategy for active vascular targeting is to functionalize nanocarriers with the Arg-Gly-Asp (RGD) peptide [189]. This peptide sequence is well known to bind to αvβ3-integrins, which are overexpressed on activated endothelial cells in tumors. Due to the ligand’s nature, this targeting approach has been claimed to enable the transport of the bound nanomedicine formulation through the vessel wall into the tumor interstitium, via integrin-mediated transcytosis [190]. In a study performed in mice bearing hepatoma (H22) tumors, RGD-targeted and fluorescently-labeled colloidal core-shell nanocapsules (lipid core and thin polymer membrane; 180-195 nm) loaded with paclitaxel were injected via the tail vein. The RGD-targeted nanocapsules caused an enhanced tumor growth inhibition compared to free paclitaxel and saline-treated animals (i.e. 3.5 vs. 12.0 vs 22.4-fold of tumor volume change, respectively) [191]. The RDG-targeted nanocapsules outperformed untargeted nanocapsules as well as PEG-nanocapsules, verifying the antitumoral effect of RGD-targeting, which is in this case thought to be based on increased endothelial cell targeting of these relatively large RGD-nanocapsules.
Another commonly used peptide-based vascular targeting ligand, i.e. Asn-Gly-Arg (NGR), binds to aminopeptidases (like CD13), which are expressed on endothelial cells of angiogenic blood vessels. NGR-targeted liposomes loaded with doxorubicin were used to treat orthotopic neuroblastoma xenografts in mice. The increased therapeutic effect of the liposomes was manifested through the damage of the tumor vasculature, which most likely led to an enhanced EPR effect. This led to a higher accumulation of doxorubicin in the interstitial space of the tumor and thus to a tumor mass reduction compared to naive or vehicle control mice injected with HEPES buffer or non-targeted liposomes (four of six mice showed a complete tumor reduction, the two others presented with >80% of tumor mass reduction compared to controls) [192,193]. A related study investigated the impact of vascular targeting ligand density on the surface of liposomes. Liposomes loaded with Omnipaque® (i.e. an iodine-based CT contrast agent) and surface-functionalized with different amounts of NGR peptides were injected into mice bearing squamous cell carcinoma xenografts (H520), and the tumor accumulation was visualized and quantified via CT imaging. Although both targeted formulations showed the same pharmacokinetic behavior in the blood, the formulation with the lowest amount of NGR (0.64 mol% vs. 2.56 mol%) presented with the highest tumor accumulation, compared to the one with a higher NGR concentration and the non-targeted controls (28% ID/g tumor vs. ~18% and 13% at 48 h after injection). The authors assumed that decreased stability, binding affinity and/or slower convection through the tumor may have caused these finding [194].
5.2. Intravascular release
A second important strategy to bypass EPR-based tumor accumulation is via triggering intravascular drug release within tumors by applying local external stimuli. The triggered release causes a high local concentration of free drug molecules, which can penetrate into the tumor via passive diffusion, almost independent of enhanced vascular leakiness and EPR. Different physical stimuli, especially hyperthermia and ultrasound [125,126,195–198], have been employed for the triggered intravascular release of drugs from carrier materials. The high local drug concentrations available within the vasculature upon triggered drug release and the physiological effects induced locally within tumors as a result of the applied physical stimuli (i.e. inducing/enhancing EPR; see Chapter 3) can act additively or even synergistically to improve therapeutic efficacy.
Temperature-sensitive liposomes are prototypic nanomedicine formulations for stimuli-responsive intravascular drug release and are typically used in combination with mild hyperthermia (39-42 °C). Hyperthermia, which can be induced via radiofrequency ablation or via focused ultrasound application, leads to drug release via a phase transition of the lipid layer of the liposomes. This approach has been extensively investigated in mice, rats and rabbits [199,200] and it has shown clear benefits as compared to the administration of free drugs or standard liposomes in multiple different tumor models, including cervical, lung and breast cancer [201]. As a result, hyperthermia combined with temperature-sensitive liposomes has been successfully translated to the clinic.
In a phase I clinical trial with lysolipid-based thermosensitive liposomal doxorubicin (LTLD; ThermoDox®) in combination with mild hyperthermia through radiofrequency ablation, patients with chest wall recurrence of advanced breast cancer received six cycles of ThermoDox® followed by mild hyperthermia. The treatment was well tolerated and no dose-limiting cardiac toxicity was observed. The overall local response rate was 48%, with 17% of the patients achieving a complete local response [202,203]. The effect of ThermoDox® combined with RFA treatment was further evaluated in hepatocellular patients in a phase III clinical trial, named HEAT study, which could not show an improved therapeutic outcome compared to standard therapy [204]. The reasons for this rather unexpected finding might be a lack of standardization and the inclusion of too many study sites. In an adapted clinical trial called OPTIMA, which is an advanced phase III trial, researchers are now aiming to overcome the hurdles of the previous HEAT study to prove the effectiveness of ThermoDox®. The potential use of therapies with intravascular drug release in combination with local hyperthermia allows bypassing the high heterogeneity in EPR in patients which are not amenable for sole EPR-based therapies.
6. Imaging EPR-based tumor targeting
The high heterogeneity in EPR between individual patients is more and more being considered as one of the major bottlenecks for nanomedicine formulations during their translation into the clinic. It is clear that the inclusion of patients with high vs. low levels of EPR in a clinical trial will lead to very different therapeutic outcomes, and may only show superiority in comparison to gold-standard treatments if patients showing sufficiently high levels of EPR-mediated accumulation can be pre-selected. Therefore, analogous to the development of patient pre-selection tools, like those used in case of e.g. trastuzumab (immunohistochemical staining of the HER2 receptor; using Herceptest®), probes and protocols are required to perform patient pre-selection for nanomedicine formulations, considering factors, such as vascular leakiness and perfusion, macrophage content and ECM density. In such an imaging based EPR assessment, a high tumor accumulation of a given nanomedicine is assumed to correlate with an increased antitumor response. Vice versa, if patients do not show sufficiently high levels of EPR, they are unlikely to show a good response. To visualize and quantify EPR-mediated tumor targeting and the evolvement of EPR during therapy via non-invasive imaging techniques, two major directions can be taken, i.e. indirect and direct imaging.
6.1. Indirect EPR imaging
The idea behind indirect EPR imaging is to non-invasively visualize and quantify tumor characteristics, which correlate with the accumulation of nanomedicines. Several preclinical studies have been published which look at key EPR-determining parameters of the tumor vasculature [205], and which correlate them with the accumulation and/or efficacy of nanomedicines. In an exemplary preclinical study performed in our own lab, the relative blood volume (rBV) in tumors was assessed using contrast-enhanced ultrasound imaging, and rBV values were correlated with the tumor accumulation of HPMA-based polymeric drug carriers. A decent positive correlation was observed, supporting the fact that imaging vascular parameters such as the rBV may be useful to predict EPR-mediated tumor targeting [123]. Another interesting recent study in this regard was published by Coll and collagues, who acquired MRI scans to characterize several tumor models and correlated parameters such as rBV and vessel permeability with the accumulation of fluorophore-labeled nanocarriers to detect suitable biomarkers for EPR-based nanomedicine accumulation [206]. Applying multi-modal imaging (MRI, µCT, US, microscopy), Sulheim and colleagues correlated the accumulation of polystyrene nanoparticles in different tumor models e.g. with the functionality of tumor vessels measured via the inflow of microbubbles using ultrasound [207]. However, using such indirect imaging biomarkers implies that we have to rely on one additional correlation and one additional source for variability, i.e. (1) tumor vascularization which correlates with nanomedicine accumulation, and (2) nanomedicine accumulation which correlates with treatment response. Direct EPR imaging, in which the tumor accumulation of companion nanodiagnostics or nanotheranostics (i.e. nanocarriers co-loaded with both a drug and an imaging agent) is directly visualized and quantified, therefore seems to be preferred [208–210].
6.2. Companion nanodiagnostics
An interesting intermediate option, i.e. between indirect vascular imaging and direct nanotheranostic imaging, relies on the use of companion diagnostics. In a recent preclinical study, Mulder and colleagues presented a so-called PET nanoreporter, which can serve as a companion diagnostic for PEGylated liposomes containing chemotherapeutics, such as Doxil®. Liposomes with highly similar physicochemical properties as compared to Doxil® were synthesized, and loaded with chelators allowing for 89Zr-labeling and PET imaging (Figure 5A). The tumor concentration of the companion nanodiagnostic and the nanotherapeutic correlated very well, especially also in tumors with delayed growth kinetics. Thus the 89Zr-labeled nanodiagnostic was proposed to be able to foresee the therapeutic outcome in individual tumors by predicting accumulation of the nanomedicine formulation (Figure 5B). As nanoreporter and doxorubicin concentrations in tumors also correlated relatively well with therapeutic efficacy, tumors likely to show a good therapeutic response may thus be pre-identified using this PET nanoreporter. Importantly, it could also be shown that the target site accumulation of the liposomal companion diagnostic correlated with the tumor localization of other nanomedicine formulations, such as PEG-PLGA nanoparticles, arguing for the development of broadly applicable companion nanodiagnostics [211].
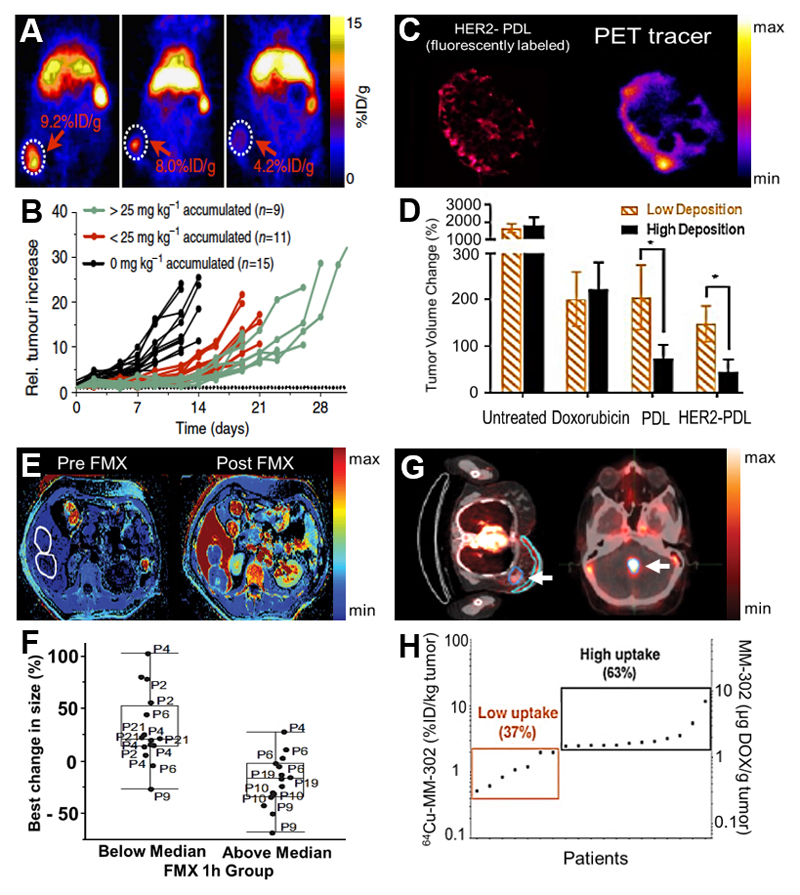
Figure 5. Imaging EPR to predict nanomedicine response.
A: Mouse study with a Zirconium-89-labeled liposomal PET nanoreporter showing highly heterogeneous tumor accumulation in individual animals. B: Relative tumor increase in different 4T1 tumor-bearing mice showing that the extent of tumor accumulation correlates with antitumor efficacy (A-B: adapted from [211]). C: PEGylated liposomes were labeled with a fluorophore and with a 64Cu PET-tracer to follow their tumor accumulation. Left image shows HER2-targeted doxorubicin liposomes in fluorescence microscopy, right image shows liposomes labeled with the PET-tracer. D: The accumulation of the companion diagnostic liposomes correlates with antitumor reponse, showing the smallest tumor volume changes for tumor with the highest levels of liposome accumulation (C-D: adapted from [212]). E: Color-coded MR images of patients before and after administration of the companion diagnostic ferumoxytol (FMX), allowing for quantification of nanoparticle tumor (encircled) accumulation. F: Clinical outcomes show that a high degree of FMX accumulation in tumors (i.e. above median; high EPR) corresponds to better therapeutic outcome, as exemplified by an overall decrease in average tumor size (E-F: adapted from [214]). G: PET-CT images exemplifying the accumulation of 64Cu-labeled HER2-targeted PEGylated liposomes loaded with doxorubicin in breast (left) and brain (right) tumor lesions. H: Correlation between liposome accumulation at the pathological site(s) and progression-free survival, showing that patients with higher uptake tend to present with better outcomes (adapted from [215]).
In a similar preclinical setup, Lee et al. labeled diagnostic PEGylated liposomes with 64Cu to predict the tumor accumulation of drug-containing liposomes in multiple different solid tumor models in mice (Figure 5C) [212]. It was found that the accumulation of the 64Cu-containing companion diagnostic liposomes corresponded well with the target site deposition of three different therapeutic liposomes. The macrodistribution and target site accumulation of the liposomal formulations, regardless of whether they were actively targeted or not, correlated with the accumulation of the companion nanodiagnostic. Without further investigation of the intratumoral microdistribution of the liposomes, the classification of tumors into high vs. low levels of accumulation was found to be sufficient to predict whether or not the tumor would respond to nanomedicine therapy (Figure 5D) [212].
A very pragmatic companion nanodiagnostic approach has been tested by Weissleder and colleagues, who employed the clinically approved iron-replacement agent ferumoxytol (Feraheme®; a ~30 nm-sized semi-long-circulating iron oxide nanoparticle which generates MRI contrast), to predict the accumulation of polymeric nanoparticles encapsulating docetaxel. Even though the companion nanodiagnostic and the therapeutic nanoparticle were different in terms of size and composition, a >85 % accuracy of co-localization in the tumor microenvironment was reported. Based on MRI measurements, tumors with high, medium and low ferumoxytol accumulation could be differentiated. In line with the accumulation of ferumoxytol, the highest docetaxel concentrations and the best tumor response were observed in the group of high ferumoxytol accumulating tumors [213].
Extending these efforts, in a first of its kind clinical trial, Merrimack Pharmaceuticals employed ferumoxytol as companion nanodiagnostic in patients, to evaluate if its tumor accumulation correlates with antitumor responses observed in mixed solid tumor patients treated with the recently approved liposomal irinotecan formulation Onivyde® [214]. MRI measurements were acquired at several different time points (i.e. pre, 1 h, 24 h and 72 h after the i.v. injection of ferumoxytol; Figure 5E) and they were correlated with irinotecan concentrations in biopsies (which were taken 72 h after Onivyde® injection). It was found that tumors with an above-median ferumoxytol accumulation showed a better therapeutic response upon Onivyde® treatment compared to patients with a below-average accumulation of ferumoxytol (Figure 5F). It is interesting to note in this regard that ferumoxytol concentrations in tumors at 1 h after i.v. injection gave the best association with lesion size reduction. If this finding is confirmed in other patients (and in other cancer types and study setups), this companion diagnostic approach, which relies on the repurposing of an already approved iron replacement nano-agent, would facilitate the clinical implementation of such imaging-based screening procedures, as the nano-diagnostic is available off the shelf, and as imaging can be performed almost immediately upon contrast agent administration, which is very pragmatic from a translational point of view. Further studies based on the same rationale and on a similar study-setup therefore seem to be strongly warranted.
6.3. Nanotheranostics for direct EPR imaging
The final and arguably most accurate approach to image the EPR effect and correlate imaging information with therapeutic outcome relies on the use of nanotheranostics, i.e. the combination of diagnostic and therapeutic agents within a single nanomedicine formulation. In this case, the quantification of imaging information enables the direct assessment of the amount of nanoparticles (and drug molecules) delivered to tumors. Merrimack Pharmaceuticals recently reported a study in which 64Cu-labeled HER2-targeted PEGylated liposomes containing doxorubicin were used to evaluate the EPR effect in patients with primary and metastatic breast cancer tumors (Figure 5G) [215]. They analyzed liposome accumulation in multiple lesions via quantitative PET imaging, and they also took biopsies, to determine doxorubicin concentrations in tumors and metastases, and then correlated these findings with therapeutic outcome (Figure 5H). Despite the fact that they showed an on average higher chance of progression-free survival when at least 1.2 µg doxorubicin per gram target tissue was present in all tumor lesions in a patient, they did not manage to find a strong correlation between the lowest lesion uptake value and progression-free survival. Based on these results, it seems that the treatment response is not only influenced by the mere amount of drug accumulating at the target site (which is highly heterogeneous in between patients and also in different lesions within the same patient), but also on other aspects, like intratumoral distribution, cellular uptake, drug release and sensitivity to drug. This is in line with the finding that the overall tumor accumulation of a nanomedicine formulation generally does not improve upon active targeting, while its intratumoral distribution may benefit, as a result of more target cell uptake and less macrophage uptake [216,217]. Although the authors state that even one not responding lesion can drive disease progression and therefore chose the lowest lesion uptake as the deciding parameter [215], it may be more convincing to additionally include the averaged accumulation over all lesions in a single patient. Furthermore, parameters which take the intratumoral micro-distribution into account, e.g. penetration depth or homogenous distribution, may improve the accuracy of the prediction. It will be interesting to see, which set of parameters will be established in the clinic to differentiate responding patients from non-responders. Another option could be the identification of the key reason for low accumulation in a tumor lesion (e.g. vessel- or microenvironment-related; via indirect imaging) followed by a suitable co-treatment (e.g. one of the strategies discussed above; see Chapter 2: enhancing EPR), to improve nanomedicine accumulation, penetration, intratumoral distribution and retention.
The above-mentioned examples show that several imaging tools and technologies are available to capture the heterogeneity in EPR-based tumor targeting, and that imaging biomarkers hold potential to guide clinical trials that evaluate the efficacy of nanomedicine formulations. The different indirect and direct imaging approaches each have their own advantages and limitations, in terms of their predictive power, versatility and clinical translatability. Whereas indirect imaging strategies might be the least accurate, they can be quite versatile and their clinical translation is rather straightforward. Analogously, repurposing of clinically approved and imageable nanoparticles like ferumoxytol can speed up the establishment of companion nanodiagnostics for patient pre-selection. Vice versa, nanotheranostic agents may be the best option to really accurately predict the performance of a given nanomedicine formulation. In that case, however, a new chemical entity (i.e. a chelator) has to be introduced into each nanotherapeutic formulation, to allow for radiolabeling, implying that each of these formulations again has to go through the complete set of preclinical and clinical toxicology experiments, to ensure the safety of this new chemical. This, of course, is more cumbersome as compared to off-the-shelf companion diagnostic approaches such as those based on e.g. ferumoxytol. If we manage to extend and expand some of the above mentioned efforts, future studies will teach us which levels of accuracy and specificity are required to assess EPR-mediated drug targeting to tumors, and which parameters are useful to predict if patients are likely to respond to EPR-based nanomedicine therapies.
7. Summarizing discussion
The highly variable nature of cancer, which is a result of various genetic mutations and its localization at different tissues throughout the body, leads to an enormously high heterogeneity in the composition of tumors, in the EPR effect of these tumors, and in the antitumor responses which are achieved. Therefore, when aiming to develop nanomedicines for clinical use, the heterogeneity of the EPR effect has to be taken into account, and strategies have to be developed to overcome this obstacle.
As described in the sections above, part of this can be accomplished by enhancing, combining or bypassing the EPR effect. Enhancement can be achieved using pharmacological or physical means, which can e.g. increase vessel perfusion or permeability, thereby allowing for increased nanomedicine accumulation at pathological sites. Approaches to bypass EPR include vascular targeting as well as triggered intravascular drug release. These techniques combine the beneficial pharmacokinetic and biodistributional properties of nanomedicines with the penetration ability of drugs locally released within the tumor vascular bed upon applying external stimuli, which can enable EPR-independent drug extravasation and penetration. Furthermore, imaging of the EPR effect in (different lesions in) individual patients can be employed to personalize nanomedicine treatments. Indirect imaging biomarkers, such as tumor perfusion or the relative blood volume (rBV), are one way to predict EPR-based nanomedicine accumulation. Direct imaging biomarkers are based on the use of companion nanodiagnostics or nanotheranostics. Patients with tumors having low levels of EPR could be identified prior to therapy and measures could be taken to modulate the EPR effect. Such a patient pre-selection would facilitate the clinical translation of nanomedicine formulations, employing the observed level of tumor accumulation as a biomarker to decide if patients should be included in clinical trials. Properly pre-selected patient populations will lead to improved response rates, fostering progression through the different phases of clinical evaluation, resulting in a higher number of approved nanomedicine products reaching the market [218].
It is of high importance to consider the variable nature of cancer not only in the clinic but also already in preclinical studies. The typically used models in preclinical research are based on relatively simple (and overly homogenous) cell line-based xenografts, which rely on the inoculation of human tumor cells in immunocompromised mice. These models are well established for preclinical research, with numerous different cell lines available. However, these tumor models are rather homogenous compared to tumors in patients, also because the inoculated tumor xenografts are all part of the same sub-clone, which leads to a lower degree of intratumoral heterogeneity. Furthermore, tumors in mice have a smaller absolute size as compared to most patient tumors when they are detected, but they are relatively larger, i.e. in relation to total body-size. Murine tumors also often lack the human microenvironment and stromal composition, which might be due to the very different growth kinetics, i.e. usually days to weeks in mice versus months to years in humans. Additionally, metastasis is often neglected in tumor xenografts.
The use of immuno-deficient mice is another shortcoming. It has been shown that the immuno-status affects the EPR effect, with nanomedicine accumulation being lower in mice lacking a proper immune system [219,220]. This may be due to altered macrophage density and activity in immunocompromised animals, but the exact reasons for this are not known. While the meta-analysis addressing this comparison presents with fairly high variability, it can be clearly observed that nanomedicine accumulation is lower in immunodeficient mice as compared to immunocompetent mice [219] (Figure 6A). Additionally, a second meta-analysis reported in the same paper shows the accumulation of liposomes and micelles in tumors induced at different locations (i.e. subcutaneous, orthotopic and metastases) indicating that also tumor location plays a key role, with a tendency for higher accumulation in orthotopic tumors (Figure 6B). It has to be mentioned, however, that also in this case, variability was very high [219]. Further differences between mice and patients which may be affecting the extent of the EPR effect (and therapeutic response) are listed in Figure 6C, stressing e.g. that tumors are typically induced in mice at very young age and develop rather fast, while in humans, tumors generally develop at old(er) age and progress over years. Therefore, new and advanced animal models are in need to overcome at least some of the limitations of the rodent models we routinely work with. These new and superior models should ideally display a more heterogeneous and thus more realistic version of the clinical situation [221]. Highly advanced and more realistic animal models can be obtained via chemically induced tumors or transgenic mice and have led to important findings regarding the development of tumors, but they are difficult to implement in drug targeting and therapeutic efficacy experiments, for multiple reasons, including spontaneous tumor formation (which requires extensive monitoring using e.g. imaging) and highly different kinetics of tumors [222].
Figure 6. Limited clinical translation: the role of (s.c.) cancer xenograft models.
A-B: EPR-based tumor accumulation of different nanomedicine formulations in immunocompetent versus immunocompromised mice. As compared to immunocompromised animals, immunocompetent mice tend to show increased accumulation. The location of xenograft tumors also impacts nanomedicine accumulation (adapted from [219]). C: Schematic overview of discrepancies between typically used preclinical tumor xenograft models and the real-life clinical situation. D: Comparing histology for a human primary tumor, its PDX model and the traditionally used cell line-based xenograft tumor model illustrates the fairly high similarity between the primary tumor and the PDX model, and the fairly low similarity between the primary tumor and the cell line-based xenograft tumor model (adapted from [232]).
To tackle the abovementioned issues, model systems have to be established and implemented which can deliver more representative results in a pragmatic manner. Among these systems, organoids and patient-derived xenografts (PDX) are the most attractive alternatives for preclinical research, as they allow for the regrowth of human tumors in vitro and in vivo. The tumor cells are harvested via biopsy or surgery, and upon growth in organoids or PDX models, most of the tumor stroma features and structures are still intact [223,224].
Organoids, which are becoming increasingly popular in basic and translational cancer research, require a scaffold mimicking the ECM to enable the three-dimensional growth of patient-derived tumor cells and allow for an extensive genetic characterization of tumor cells gained from patient probes [225,226]. They are highly useful for drug pre-screening [227] and thus for a potential selection of a beneficial combination treatment for certain tumor types. Promising results were reported especially for colorectal cancer, where a biobank - with organoids developed out of 20 patients with characterized RNA expression profiles and in which over 40 drugs were tested - could be established [228,229]. Upon sequencing, it was found that this biobank included the majority of known subtypes of colorectal cancer and that it thus mimicked the heterogeneity which is typically observed in the majority of patients, presenting a considerable advantage over drug screenings which are performed only in several selected cell lines. In recent clinical trials, researchers have started to implement liver and pancreatic cancer organoids in their work flow, allowing to compare healthy and tumor cells regarding genetic and cellular features and to screen drugs using in vitro models [230]. Organoids are also being employed for treatment prediction of e.g. esophageal cancer on neoadjuvant chemoradiotherapy to identify likely responding patients and to switch to other treatment options for likely non-responders [231].
Analogously, recent studies have underlined the potential of PDX models by showing that histological characteristics, such as collagen I patterns of the primary human tumor, are well displayed in a regrown PDX model in mice, especially if this approach is compared to a simplified cancer cell line-derived xenograft model (Figure 6D) [232]. Using pancreatic cancer PDX models, the impact of secreted protein acidic and rich in cysteine (SPARC) on the accumulation of nab-paclitaxel (Abraxane®) was studied in SPARC-positive versus SPARC-negative mice, and surprisingly it was found that nab-paclitaxel delivery is not relying on SPARC [233]. To verify the predictive value of PDX models in mice, ongoing clinical trials are focusing on drug screening or the collection of samples from triple negative breast cancer patients before and after neoadjuvant chemotherapy, to evaluate a potential correlation of treatment response in patients and in the corresponding PDX mouse models [234,235]. No results are published yet, but it is clear that the outcome of these studies is eagerly awaited.
While the use of PDX models appears to be beneficial, better mimicking the targeting and therapeutic situation in patients, there are also drawbacks, as PDX models require immunocompromised mice to be able to grow. Tumor nanomedicine accumulation patterns differ in immunocompromised versus immunocompetent mice, a mixed tumor stroma composed of human and murine cells will be developed, and also potential interactions between tumor cells (nanomedicine formulations) and -T cells are neglected [236]. In this context, it would be of great interest to increase the availability of immunocompetent mice with a humanized immune system, to narrow the gap between patients and PDX models, and to allow for more detailed and more informative (nano-) immunooncology studies [237].
Within the framework of this review, we propose to start thinking of setups where organoids and PDX models are more extensively integrated in nanomedicine research. We suggest to implement organoids and PDX models to characterize a patient’s tumor and to investigate which kind of treatment would be most promising (Figure 7). Organoids can serve to identify the most effective drug or the most effective combination of drugs and might also give first insights into the pathophysiology of tumors. The use of PDX models not only allows to study parameters such as the microenvironment and the vascularization of the tumor, but also the accumulation of drugs and nanomedicines leading to a therapy suggestion and indication on the extent of the EPR effect. The impact of EPR-enhancing treatments could be evaluated in tumors presenting a low EPR effect and the efficiency of the treatment can be analyzed using therapy experiments. However, there are major drawbacks translating organoids and PDX models into the clinic, mainly the time- and labor-intensive workflow and the low engraftment rates. The period between the biopsy and the final results may be too long to be beneficial for the individual patient. Therefore, a set-up of PDX libraries where scientists share information regarding the tumor type of the patient and the outcome of their experiments might be more convincing to facilitate an appropriate choice of therapy in the next generations of patients.
Figure 7. Use of organoids and PDX models to promote translational (nanomedicine) research.
Tumor cells harvested via biopsies can be used for the development of organoids as well as for PDX models. Organoids enable drug screening and cytotoxicity studies, while PDX models allow for in vivo drug accumulation and treatment response studies. When performed together, these setups may help to perform more efficient and more predictive preclinical research, and they may assist in identifying the the right (nano-) drug treatment for the right patient.
Last but not least, a key obstacle which has to be addressed when aiming to rapidly and efficiently translate a promising (nano-) therapeutic from the bench to the bedside relates to the way how we are conducting time-, labor- and cost-intensive clinical trials. However, the planning should start not with the clinical trial but already with the design of a new nanomedicine formulation, which should aim at a clear application, including a specific disease, with a high medical need, with a clear commercial potential, and with the proper screening tools to select the right patients (e.g. EPR-imaging, organoids, PDX models) [238]. Academic groups can unfortunately only play a minor role in the translation into the clinic, as most of them will – if at all – be only able to sponsor early phase exploratory studies which look for safety and clues of efficacy in small numbers of (often heavily pre-treated) patients. The pharmaceutical industry is needed for larger and later-stage trials, as well as for commercialization. To lower the costs of clinical trials, and to make them more efficient, the implementation of clinical trials with a lower, but carefully preselected number of patients to fulfill the needed requirements for FDA approval would be highly desirable, as is thoughtfully discussed by Workman and colleagues [239]. Pre-selection increases the chance for positive treatment responses; in the case of nanomedicine e.g. via use of imaging biomarkers to assess EPR-based accumulation in tumors and metastases. The downside of careful and critical patient pre-selection is that it can have a substantial impact on the duration of the clinical trials. Conversely, however, it may also lead to reduction in the trial arm size, resulting in overall cheaper trials, with higher predictive value. This would altogether lead to less variability, since the patient population will be better defined and in a way more homogenous. However, while this conduct will increase the success rates of clinical trials, it will also decrease the market size thus, raising the risk of making the drug less attractive for pharmaceutical industries from a commercial point of view.
Taken together, the high inter- and intra-individual heterogeneity of the EPR effect may be a key reason for explaining the relatively moderate clinical performance of nanomedicine formulations to date. As a consequence, the outcome of clinical nanomedicine trials is often worse than anticipated on the basis of preclinical experiments, which are typically performed in relatively homogenous mouse models. It is therefore crucial to better understand the pathophysiological characteristics which contribute to the EPR effect and which affect the accumulation, penetration, distribution, retention and efficacy of nanomedicine formulations. Additionally, nanomedicine formulations have to be developed while keeping clinical translatability firmly in mind. This not only entails optimization of size, drug loading and drug release, but also biocompatibility, pharmaceutical upscaling and batch-to-batch reproducibility, as well as imageability. A more rational and realistic design of nanomedicines, and an optimization of clinical trial design in which nanomedicines are being tested (e.g. via integration in combination regimens or via a pre-selection of the right patient subpopulation) will lead to more efficient clinical translation, to more approved nanomedicine drugs and to enhanced patient responses.
Acknowledgments
The authors gratefully acknowledge financial support by the European Research Council (ERC: Starting Grant 309495 (NeoNaNo) and Proof-of-Concept Grants 680882 (CONQUEST) and 813086 (PIcelles)), by the German research Foundation (DFG: La2937/1-2, SFB/TRR57, SFB1066, GRK2375) and by the European Union (EU-EFRE: European Fund for Regional Development: I3-STM 0800387 and EuroNanoMed III: NSC4DIPG).
References
- [1].WHO. Cancer. WHO; [accessed 07/06/2018]. (n.d.). http://www.who.int/mediacentre/factsheets/fs297/en/ [Google Scholar]
- [2].Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, Tattersall MH. On the receiving end--patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- [3].Greish K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: are we there yet? Drug Discov Today Technol. 2012;9:e161–e166. doi: 10.1016/j.ddtec.2011.11.010. [DOI] [PubMed] [Google Scholar]
- [4].de Jonge MJA, Verweij J. Renal Toxicities of Chemotherapy. Semin Oncol. 2006;33:68–73. doi: 10.1053/j.seminoncol.2005.11.011. [DOI] [PubMed] [Google Scholar]
- [5].Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E, TLC D-99 Study Group Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- [6].Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- [7].Park K. To PEGylate or not to PEGylate, that is not the question. J Controlled Release. 2010;142:147–148. doi: 10.1016/j.jconrel.2010.01.025. [DOI] [PubMed] [Google Scholar]
- [8].Gabizon AA, Barenholz Y, Bialer M. Prolongation of the Circulation Time of Doxorubicin Encapsulated in Liposomes Containing a Polyethylene Glycol-Derivatized Phospholipid: Pharmacokinetic Studies in Rodents and Dogs. Pharm Res. 10:703–708. doi: 10.1023/A:1018907715905. (n.d.) [DOI] [PubMed] [Google Scholar]
- [9].Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- [10].Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- [11].Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- [13].Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JSW. Effective Targeting of Solid Tumors in Patients With Locally Advanced Cancers by Radiolabeled Pegylated Liposomes. Clin Cancer Res. 2001;7:243–254. [PubMed] [Google Scholar]
- [15].Tanaka N, Kanatani S, Tomer R, Sahlgren C, Kronqvist P, Kaczynska D, Louhivuori L, Kis L, Lindh C, Mitura P, Stepulak A, et al. Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nat Biomed Eng. 2017;1 doi: 10.1038/s41551-017-0139-0. [DOI] [PubMed] [Google Scholar]
- [16].Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 2018;7:11. doi: 10.1186/s40169-018-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release Off J Control Release Soc. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- [18].Natfji AA, Ravishankar D, Osborn HMI, Greco F. Parameters Affecting the Enhanced Permeability and Retention Effect: The Need for Patient Selection. J Pharm Sci. 2017;106:3179–3187. doi: 10.1016/j.xphs.2017.06.019. [DOI] [PubMed] [Google Scholar]
- [19].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1 doi: 10.1038/natrevmats.2016.14. natrevmats201614. [DOI] [Google Scholar]
- [20].Laginha KM, Verwoert S, Charrois GJR, Allen TM. Determination of Doxorubicin Levels in Whole Tumor and Tumor Nuclei in Murine Breast Cancer Tumors. Clin Cancer Res. 2005;11:6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- [21].van Vlerken LE, Duan Z, Little SR, Seiden MV, Amiji MM. Biodistribution and pharmacokinetic analysis of Paclitaxel and ceramide administered in multifunctional polymer-blend nanoparticles in drug resistant breast cancer model. Mol Pharm. 2008;5:516–526. doi: 10.1021/mp800030k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cui Y, Zhang M, Zeng F, Jin H, Xu Q, Huang Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl Mater Interfaces. 2016;8:32159–32169. doi: 10.1021/acsami.6b10175. [DOI] [PubMed] [Google Scholar]
- [23].Lammers T, Kiessling F, Ashford M, Hennink W, Crommelin D, Storm G. Erratum: Cancer nanomedicine: is targeting our target? Nat Rev Mater. 2016;1 doi: 10.1038/natrevmats.2016.76. 16076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Greish K, Mathur A, Bakhiet M, Taurin S. Nanomedicine: is it lost in translation? Ther Deliv. 2018;9:269–285. doi: 10.4155/tde-2017-0118. [DOI] [PubMed] [Google Scholar]
- [25].Zhou Y, Kopeček J. Biological rationale for the design of polymeric anti-cancer nanomedicines. J Drug Target. 2013;21:1–26. doi: 10.3109/1061186X.2012.723213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- [27].Folkman J, Merler E, Abernathy C, Williams G. Isolation of a Tumor Factor Responsible for Angiogenesis. J Exp Med. 1971;133:275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Folkman J. What Is the Evidence That Tumors Are Angiogenesis Dependent? J Natl Cancer Inst. 1990;82:4–7. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- [29].Dvorak HF. Discovery of vascular permeability factor (VPF) Exp Cell Res. 2006;312:522–526. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- [30].Potente M, Gerhardt H, Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- [31].Verheul HM, Pinedo HM. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin Breast Cancer. 2000;1(Suppl 1):S80–84. doi: 10.3816/cbc.2000.s.015. [DOI] [PubMed] [Google Scholar]
- [32].Seki T, Fang J, Maeda H. Tumor-Targeted Macromolecular Drug Delivery Based on the Enhanced Permeability and Retention Effect in Solid Tumor. In: Lu Y, Mahato RI, editors. Pharm Perspect Cancer Ther. Springer; US: 2009. pp. 93–120. [DOI] [Google Scholar]
- [33].Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15:1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nagy JA, Chang S-H, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100:865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dudley AC. Tumor Endothelial Cells. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hori K, Saito S, Takahashi H, Sato H, Maeda H, Sato Y. Tumor-selective Blood Flow Decrease Induced by an Angiotensin Converting Enzyme Inhibitor, Temocapril Hydrochloride. Jpn J Cancer Res. 2000;91:261–269. doi: 10.1111/j.1349-7006.2000.tb00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hori K, Suzuki M, Tanda S, Saito S, Shinozaki M, Zhang Q-H. Fluctuations in Tumor Blood Flow under Normotension and the Effect of Angiotensin II-induced Hypertension. Jpn J Cancer Res. 1991;82:1309–1316. doi: 10.1111/j.1349-7006.1991.tb01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boucher Y, Baxter LT, Jain RK. Interstitial Pressure Gradients in Tissue-isolated and Subcutaneous Tumors: Implications for Therapy. Cancer Res. 1990;50:4478–4484. [PubMed] [Google Scholar]
- [39].Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- [40].Roose T, Netti PA, Munn LL, Boucher Y, Jain RK. Solid stress generated by spheroid growth estimated using a linear poroelasticity model. Microvasc Res. 2003;66:204–212. doi: 10.1016/S0026-2862(03)00057-8. [DOI] [PubMed] [Google Scholar]
- [41].Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced Apoptosis Decompresses Blood Vessels and Lowers Interstitial Fluid Pressure in Solid Tumors. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- [42].Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- [43].Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- [44].Yokoi K, Kojic M, Milosevic M, Tanei T, Ferrari M, Ziemys A. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. 2014;74:4239–4246. doi: 10.1158/0008-5472.CAN-13-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cathcart J, Pulkoski-Gross A, Cao J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015;2:26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Venning FA, Wullkopf L, Erler JT. Targeting ECM Disrupts Cancer Progression. Front Oncol. 2015;5 doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berger DP, Herbstritt L, Dengler WA, Marmé D, Mertelsmann R, Fiebig HH. Vascular endothelial growth factor (VEGF) mRNA expression in human tumor models of different histologies. Ann Oncol. 1995;6:817–825. doi: 10.1093/oxfordjournals.annonc.a059322. [DOI] [PubMed] [Google Scholar]
- [49].Jain RK. Transport of Molecules in the Tumor Interstitium: A Review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- [50].Kudo D, Suto A, Hakamada K. The Development of a Novel Therapeutic Strategy to Target Hyaluronan in the Extracellular Matrix of Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thompson CB, Shepard HM, O’Connor PM, Kadhim S, Jiang P, Osgood RJ, Bookbinder LH, Li X, Sugarman BJ, Connor RJ, Nadjsombati S, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- [52].Yasunaga M, Manabe S, Tarin D, Matsumura Y. Cancer-stroma targeting therapy by cytotoxic immunoconjugate bound to the collagen 4 network in the tumor tissue. Bioconjug Chem. 2011;22:1776–1783. doi: 10.1021/bc200158j. [DOI] [PubMed] [Google Scholar]
- [53].Yasunaga M, Manabe S, Matsumura Y. New concept of cytotoxic immunoconjugate therapy targeting cancer-induced fibrin clots. Cancer Sci. 2011;102:1396–1402. doi: 10.1111/j.1349-7006.2011.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Matsumura Y. Cancer stromal targeting (CAST) therapy. Adv Drug Deliv Rev. 2012;64:710–719. doi: 10.1016/j.addr.2011.12.010. [DOI] [PubMed] [Google Scholar]
- [55].Lee S, Kivimäe S, Dolor A, Szoka FC. Macrophage-based cell therapies: The long and winding road. J Control Release Off J Control Release Soc. 2016;240:527–540. doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Binnemars-Postma KA, Ten Hoopen HW, Storm G, Prakash J. Differential uptake of nanoparticles by human M1 and M2 polarized macrophages: protein corona as a critical determinant. Nanomed. 2016;11:2889–2902. doi: 10.2217/nnm-2016-0233. [DOI] [PubMed] [Google Scholar]
- [57].MacParland SA, Tsoi KM, Ouyang B, Ma X-Z, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WCW, McGilvray ID. Phenotype Determines Nanoparticle Uptake by Human Macrophages from Liver and Blood. ACS Nano. 2017;11:2428–2443. doi: 10.1021/acsnano.6b06245. [DOI] [PubMed] [Google Scholar]
- [58].Miller MA, Zheng Y-R, Gadde S, Pfirschke C, Zope H, Engblom C, Kohler RH, Iwamoto Y, Yang KS, Askevold B, Kolishetti N, et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun. 2015;6:8692. doi: 10.1038/ncomms9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tsvetkova Y, Beztsinna N, Baues M, Klein D, Rix A, Golombek SK, Al Rawashdeh W, Gremse F, Barz M, Koynov K, Banala S, et al. Balancing Passive and Active Targeting to Different Tumor Compartments Using Riboflavin-Functionalized Polymeric Nanocarriers. Nano Lett. 2017;17:4665–4674. doi: 10.1021/acs.nanolett.7b01171. [DOI] [PubMed] [Google Scholar]
- [60].Nel A, Ruoslahti E, Meng H. New Insights into “Permeability” as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics. ACS Nano. 2017;11:9567–9569. doi: 10.1021/acsnano.7b07214. [DOI] [PubMed] [Google Scholar]
- [61].Jain RK. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annu Rev Chem Biomol Eng. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- [63].Chauhan VP, Stylianopoulos T, Martin JD, Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Crawford Y, Ferrara N. VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res. 2009;335:261–269. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- [65].Matsumura Y, Kimura M, Yamamoto T, Maeda H. Involvement of the kinin-generating cascade in enhanced vascular permeability in tumor tissue. Jpn J Cancer Res Gann. 1988;79:1327–1334. doi: 10.1111/j.1349-7006.1988.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- [67].Verschraegen CF, Czok S, Muller CY, Boyd L, Lee SJ, Rutledge T, Blank S, Pothuri B, Eberhardt S, Muggia F. Phase II study of bevacizumab with liposomal doxorubicin for patients with platinum- and taxane-resistant ovarian cancer. Ann Oncol Off J Eur Soc Med Oncol. 2012;23:3104–3110. doi: 10.1093/annonc/mds172. [DOI] [PubMed] [Google Scholar]
- [68].van Horssen R, ten Hagen TLM, Eggermont AMM. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. The Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- [69].Friedl J, Puhlmann M, Bartlett DL, Libutti SK, Turner EN, Gnant MFX, Alexander HR. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood. 2002;100:1334–1339. [PubMed] [Google Scholar]
- [70].Qiao Y, Huang X, Nimmagadda S, Bai R, Staedtke V, Foss CA, Cheong I, Holdhoff M, Kato Y, Pomper MG, Riggins GJ, et al. A Robust Approach to Enhance Tumor-selective Accumulation of Nanoparticles. Oncotarget. 2011;2:59–68. doi: 10.18632/oncotarget.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Philogen SpA. Intratumoral Administration of L19IL2/L19TNF. Clin Internet Bethesda MD Natl Libr Med US. 2016 https://clinicaltrials.gov/ct2/show/NCT02076633. [Google Scholar]
- [72].Philogen SpA. L19TNFα in Patients With Advanced Solid Tumors. Clin Internet Bethesda MD Natl Libr Med US. 2016 https://clinicaltrials.gov/ct2/show/NCT01253837. [Google Scholar]
- [73].Eggermont AM, Koops HS, Klausner JM, Kroon BB, Schlag PM, Liénard D, van Geel AN, Hoekstra HJ, Meller I, Nieweg OE, Kettelhack C, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg. 1996;224:756. doi: 10.1097/00000658-199612000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J Controlled Release. 2012;164:138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- [75].Claesson-Welsh L. Vascular permeability—the essentials. Ups J Med Sci. 2015;120:135–143. doi: 10.3109/03009734.2015.1064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popovic Z, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4 doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Proton w/FOLFIRINOX-Losartan for Pancreatic Cancer - Full Text View - ClinicalTrials.gov. [accessed May 9, 2017]; (n.d.). https://clinicaltrials.gov/ct2/show/NCT01821729.
- [80].Promising Results for Folfirinox with Losartan in Locally Advanced Pancreatic Cancer. [accessed July 12, 2017];OncologyTube. (n.d.). http://www.oncologytube.com/v/10000718/promising-results-for-folfirinox-with-losartan-in-locally-advanced-pancreatic-cancer. [Google Scholar]
- [81].Fernández-del Castillo C. Confronts The Challenge of Pancreatic Cancer. [accessed July 12, 2017];PT Community. 2014 https://www.ptcommunity.com/journal/article/full/2014/4/281/carlos-fern-ndez-del-castillo-confronts-challenge-pancreatic-cancer. [PMC free article] [PubMed] [Google Scholar]
- [82].Sica DA, Gehr TWB, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44:797–814. doi: 10.2165/00003088-200544080-00003. [DOI] [PubMed] [Google Scholar]
- [83].Zlotecki RA, Boucher Y, Lee I, Baxter LT, Jain RK. Effect of angiotensin II induced hypertension on tumor blood flow and interstitial fluid pressure. Cancer Res. 1993;53:2466–2468. [PubMed] [Google Scholar]
- [84].Fujii Y, Hongo T, Masui H, Chiba T, Furukawa N, Nakajima H, Nasuda K, Yajima S, Horikoshi Y, Igarashi Y. Angiotensin-induced hypertension chemotherapy in children with advanced solid tumors. Acta Paediatr Jpn Overseas Ed. 1991;33:381–383. doi: 10.1111/j.1442-200x.1991.tb01570.x. [DOI] [PubMed] [Google Scholar]
- [85].Noguchi S, Miyauchi K, Nishizawa Y, Sasaki Y, Imaoka S, Iwanaga T, Koyama H, Terasawa T. Augmentation of anticancer effect with angiotensin II in intraarterial infusion chemotherapy for breast carcinoma. Cancer. 1988;62:467–473. doi: 10.1002/1097-0142(19880801)62:3<467::aid-cncr2820620304>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- [86].Li CJ, Miyamoto Y, Kojima Y, Maeda H. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. Br J Cancer. 1993;67:975–980. doi: 10.1038/bjc.1993.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Nagamitsu A, Greish K, Maeda H. Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: cases of advanced solid tumors. Jpn J Clin Oncol. 2009;39:756–766. doi: 10.1093/jjco/hyp074. [DOI] [PubMed] [Google Scholar]
- [88].Wong P-P, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MRL, Scudamore CL, Cereser B, Crnogorac-Jurcevic T, McDonald S, Elia G, Hagemann T, et al. Dual-Action Combination Therapy Enhances Angiogenesis while Reducing Tumor Growth and Spread. Cancer Cell. 2015;27:123–137. doi: 10.1016/j.ccell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- [89].Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10:753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Doleschel D, Rix A, Arns S, Palmowski K, Gremse F, Merkle R, Salopiata F, Klingmüller U, Jarsch M, Kiessling F, Lederle W. Erythropoietin improves the accumulation and therapeutic effects of carboplatin by enhancing tumor vascularization and perfusion. Theranostics. 2015;5:905–918. doi: 10.7150/thno.11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CTW, Storm G, Kiessling F, Lammers T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017;119:44–60. doi: 10.1016/j.addr.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, Yacoub A, Duigou GJ, Young CS, Grant S, Hagan MP, et al. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene. 2001;20:3266–3280. doi: 10.1038/sj.onc.1204258. [DOI] [PubMed] [Google Scholar]
- [93].Lee YJ, Galoforo SS, Berns CM, Erdos G, Gupta AK, Ways DK, Corry PM. Effect of ionizing radiation on AP-1 binding activity and basic fibroblast growth factor gene expression in drug-sensitive human breast carcinoma MCF-7 and multidrug-resistant MCF-7/ADR cells. J Biol Chem. 1995;270:28790–28796. doi: 10.1074/jbc.270.48.28790. [DOI] [PubMed] [Google Scholar]
- [94].Higgins GS, O’Cathail SM, Muschel RJ, McKenna WG. Drug radiotherapy combinations: review of previous failures and reasons for future optimism. Cancer Treat Rev. 2015;41:105–113. doi: 10.1016/j.ctrv.2014.12.012. [DOI] [PubMed] [Google Scholar]
- [95].Znati CA, Rosenstein M, Boucher Y, Epperly MW, Bloomer WD, Jain RK. Effect of Radiation on Interstitial Fluid Pressure and Oxygenation in a Human Tumor Xenograft. Cancer Res. 1996;56:964–968. [PubMed] [Google Scholar]
- [96].Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor Response to Radiotherapy Regulated by Endothelial Cell Apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- [97].Peschke P, Klein V, Wolber G, Friedrich E, Hahn EW. Morphometric analysis of bromodeoxyuridine distribution and cell density in the rat Dunning prostate tumor R3327-AT1 following treatment with radiation and/or hyperthermia. Histol Histopathol. 1999;14:461–469. doi: 10.14670/HH-14.461. [DOI] [PubMed] [Google Scholar]
- [98].Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, Peeters M, Findlay M, Weaver A, Mills J, Wilson C, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159–1171. doi: 10.1016/S1470-2045(17)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lammers T, Subr V, Peschke P, Kühnlein R, Hennink WE, Ulbrich K, Kiessling F, Heilmann M, Debus J, Huber PE, Storm G. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br J Cancer. 2008;99:900–910. doi: 10.1038/sj.bjc.6604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mi Y, Shao Z, Vang J, Kaidar-Person O, Wang AZ. Application of nanotechnology to cancer radiotherapy. Cancer Nanotechnol. 2016;7 doi: 10.1186/s12645-016-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lammers T, Peschke P, Kühnlein R, Subr V, Ulbrich K, Debus J, Huber P, Hennink W, Storm G. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery systems. J Controlled Release. 2007;117:333–341. doi: 10.1016/j.jconrel.2006.10.032. [DOI] [PubMed] [Google Scholar]
- [102].Davies CdeL, Lundstrøm LM, Frengen J, Eikenes L, Bruland S ØS, Kaalhus O, Hjelstuen MHB, Brekken C. Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res. 2004;64:547–553. doi: 10.1158/0008-5472.can-03-0576. [DOI] [PubMed] [Google Scholar]
- [103].Miller MA, Chandra R, Cuccarese MF, Pfirschke C, Engblom C, Stapleton S, Adhikary U, Kohler RH, Mohan JF, Pittet MJ, Weissleder R. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].A S, Koukourakis Michael I, Koukouraki Sofia, Giatromanolaki Alexandra, Kakolyris Stelios, Georgoulias Vassilis, Velidaki Antigoni, Karkavitsas NN. High Intratumoral Accumulation of Stealth Liposomal Doxorubicin in Sarcomas: Rationale for Combination with Radiotherapy. Acta Oncol. 2000;39:207–211. doi: 10.1080/028418600430789. [DOI] [PubMed] [Google Scholar]
- [105].Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Oudinot P, Bertrand P. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- [107].Barker HE, Paget JTE, Khan AA, Harrington KJ. The Tumour Microenvironment after Radiotherapy: Mechanisms of Resistance and Recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Brown LC, Mutter RW, Halyard MY. Benefits, risks, and safety of external beam radiation therapy for breast cancer. Int J Womens Health. 2015;7:449–458. doi: 10.2147/IJWH.S55552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Koops HS, Vaglini M, Suciu S, Kroon BB, Thompson JF, Göhl J, Eggermont AM, Di Filippo F, Krementz ET, Ruiter D, Lejeune FJ. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16:2906–2912. doi: 10.1200/JCO.1998.16.9.2906. [DOI] [PubMed] [Google Scholar]
- [110].Emami B, Scott C, Perez CA, Asbell S, Swift P, Grigsby P, Montesano A, Rubin P, Curran W, Delrowe J, Arastu H, et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors: A prospectively controlled randomized study by the radiation therapy oncology group. Int J Radiat Oncol. 1996;34:1097–1104. doi: 10.1016/0360-3016(95)02137-X. [DOI] [PubMed] [Google Scholar]
- [111].Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymphnodes in stage IV head and neck patients. Int J Radiat Oncol. 1994;28:163–169. doi: 10.1016/0360-3016(94)90154-6. [DOI] [PubMed] [Google Scholar]
- [112].Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol. 1991;14:133–141. doi: 10.1097/00000421-199104000-00008. [DOI] [PubMed] [Google Scholar]
- [113].Wust P, Seebass M, Nadobny J, Deuflhard P, Mönich G, Felix R. Simulation studies promote technological development of radiofrequency phased array hyperthermia. Int J Hyperthermia. 1996;12:477–494. doi: 10.3109/02656739609023525. [DOI] [PubMed] [Google Scholar]
- [114].Dubois L, Sozanski JP, Tessier V, Camart JC, Fabre JJ, Pribetich J, Chive M. Temperature control and thermal dosimetry by microwave radiometry in hyperthermia. IEEE Trans Microw Theory Tech. 1996;44:1755–1761. doi: 10.1109/22.539932. [DOI] [Google Scholar]
- [115].Diederich CJ, Hynynen K. Ultrasound technology for hyperthermia. Ultrasound Med Biol. 1999;25:871–887. doi: 10.1016/S0301-5629(99)00048-4. [DOI] [PubMed] [Google Scholar]
- [116].Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27:365–374. doi: 10.1053/ctrv.2001.0232. [DOI] [PubMed] [Google Scholar]
- [117].Dewey WC, Thrall DE, Gillette EL. Hyperthermia and radiation--a selective thermal effect on chronically hypoxic tumor cells in vivo. Int J Radiat Oncol Biol Phys. 1977;2:99–103. doi: 10.1016/0360-3016(77)90013-x. [DOI] [PubMed] [Google Scholar]
- [118].Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol. 1998;177:137–147. doi: 10.1002/(SICI)1097-4652(199810)177:1<137::AID-JCP15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [119].Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60:4440–4445. [PubMed] [Google Scholar]
- [120].Kong G, Braun RD, Dewhirst MW. Characterization of the Effect of Hyperthermia on Nanoparticle Extravasation from Tumor Vasculature. Cancer Res. 2001;61:3027–3032. [PubMed] [Google Scholar]
- [121].Shi L, Palacio-Mancheno P, Badami J, Shin DW, Zeng M, Cardoso L, Tu R, Fu BM. Quantification of transient increase of the blood-brain barrier permeability to macromolecules by optimized focused ultrasound combined with microbubbles. Int J Nanomedicine. 2014;9:4437–4448. doi: 10.2147/IJN.S68882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Iwanaga K, Tominaga K, Yamamoto K, Habu M, Maeda H, Akifusa S, Tsujisawa T, Okinaga T, Fukuda J, Nishihara T. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Ther. 2007;14:354–363. doi: 10.1038/sj.cgt.7701026. [DOI] [PubMed] [Google Scholar]
- [123].Theek B, Gremse F, Kunjachan S, Fokong S, Pola R, Pechar M, Deckers R, Storm G, Ehling J, Kiessling F, Lammers T. Characterizing EPR-mediated passive drug targeting using contrast-enhanced functional ultrasound imaging. J Control Release Off J Control Release Soc. 2014;182:83–89. doi: 10.1016/j.jconrel.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Güvener N, Appold L, de Lorenzi F, Golombek SK, Rizzo LY, Lammers T, Kiessling F. Recent advances in ultrasound-based diagnosis and therapy with micro- and nanometer-sized formulations. Methods. 2017;130:4–13. doi: 10.1016/j.ymeth.2017.05.018. [DOI] [PubMed] [Google Scholar]
- [125].Koczera P, Appold L, Shi Y, Liu M, Dasgupta A, Pathak V, Ojha T, Fokong S, Wu Z, van Zandvoort M, Iranzo O, et al. PBCA-based polymeric microbubbles for molecular imaging and drug delivery. J Controlled Release. 2017;259:128–135. doi: 10.1016/j.jconrel.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Fokong S, Theek B, Wu Z, Koczera P, Appold L, Jorge S, Resch-Genger U, van Zandvoort M, Storm G, Kiessling F, Lammers T. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J Control Release Off J Control Release Soc. 2012;163:75–81. doi: 10.1016/j.jconrel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- [127].Kang J, Wu X, Wang Z, Ran H, Xu C, Wu J, Wang Z, Zhang Y. Antitumor effect of docetaxel-loaded lipid microbubbles combined with ultrasound-targeted microbubble activation on VX2 rabbit liver tumors. J Ultrasound Med Off J Am Inst Ultrasound Med. 2010;29:61–70. doi: 10.7863/jum.2010.29.1.61. [DOI] [PubMed] [Google Scholar]
- [128].Tinkov S, Coester C, Serba S, Geis NA, Katus HA, Winter G, Bekeredjian R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Release Off J Control Release Soc. 2010;148:368–372. doi: 10.1016/j.jconrel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- [129].Hernot S, Klibanov AL. Microbubbles in Ultrasound-Triggered Drug and Gene Delivery. Adv Drug Deliv Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bloch SH, Wan M, Dayton PA, Ferrara KW. Optical observation of lipid- and polymer-shelled ultrasound microbubble contrast agents. Appl Phys Lett. 2004;84:631–633. doi: 10.1063/1.1643544. [DOI] [Google Scholar]
- [131].Dimcevski G, Kotopoulis S, Bjånes T, Hoem D, Schjøtt J, Gjertsen BT, Biermann M, Molven A, Sorbye H, McCormack E, Postema M, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Controlled Release. 2016;243:172–181. doi: 10.1016/j.jconrel.2016.10.007. [DOI] [PubMed] [Google Scholar]
- [132].KVUS at Neoadjuvant CTx of Breast Cancer - Full Text View - ClinicalTrials.gov. [accessed May 28, 2018]; (n.d.). https://clinicaltrials.gov/ct2/show/NCT03385200.
- [133].Lammers T, Koczera P, Fokong S, Gremse F, Ehling J, Vogt M, Pich A, Storm G, van Zandvoort M, Kiessling F. Theranostic USPIO-Loaded Microbubbles for Mediating and Monitoring Blood-Brain Barrier Permeation. Adv Funct Mater. 2015;25:36–43. doi: 10.1002/adfm.201401199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Dasgupta A, Liu M, Ojha T, Storm G, Kiessling F, Lammers T. Ultrasound-mediated drug delivery to the brain: principles, progress and prospects. Drug Discov Today Technol. 2016;20:41–48. doi: 10.1016/j.ddtec.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev. 2014;72:94–109. doi: 10.1016/j.addr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved Anti-Tumor Effect of Liposomal Doxorubicin After Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound Med Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Blood-Brain Barrier Disruption Using Transcranial MRI-Guided Focused Ultrasound - Full Text View - ClinicalTrials.gov. [accessed May 11, 2017]; (n.d.). https://clinicaltrials.gov/ct2/show/study/NCT02343991.
- [138].Blood-Brain Barrier Opened Non-Invasively With Focused Ultrasound for the First Time. Focused Ultrasound Foundation; [accessed May 11, 2017]. (n.d.). https://www.fusfoundation.org/news/129-press-room/1677-blood-brain-barrier-opened-non-invasively-with-focused-ultrasound-for-the-first-time. [Google Scholar]
- [139].Blood-Brain-Barrier Opening Using Focused Ultrasound With IV Contrast Agents in Patients With Early Alzheimer’s Disease - Full Text View - ClinicalTrials.gov. [accessed February 27, 2018]; (n.d.). https://clinicaltrials.gov/ct2/show/NCT02986932.
- [140].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- [142].Kessel D, Luo Y. Mitochondrial photodamage and PDT-induced apoptosis. J Photochem Photobiol B. 1998;42:89–95. doi: 10.1016/s1011-1344(97)00127-9. [DOI] [PubMed] [Google Scholar]
- [143].Hamblin MR, Miller JL, Ortel B. Scavenger-Receptor Targeted Photodynamic Therapy. Photochem Photobiol. 2000;72:533–540. doi: 10.1562/0031-8655(2000)072<0533:SRTPT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [144].Kessel D, Woodburn K, Henderson BW, Chang CK. Sites of photodamage in vivo and in vitro by a cationic porphyrin. Photochem Photobiol. 1995;62:875–881. doi: 10.1111/j.1751-1097.1995.tb09150.x. [DOI] [PubMed] [Google Scholar]
- [145].Sano K, Nakajima T, Choyke PL, Kobayashi H. The Effect of Photoimmunotherapy Followed by Liposomal Daunorubicin in a Mixed Tumor Model: A Demonstration of the Super-Enhanced Permeability and Retention Effect after Photoimmunotherapy. Mol Cancer Ther. 2014;13:426–432. doi: 10.1158/1535-7163.MCT-13-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sano K, Nakajima T, Choyke PL, Kobayashi H. Markedly Enhanced Permeability and Retention Effects Induced by Photo-immunotherapy of Tumors. ACS Nano. 2013;7:717–724. doi: 10.1021/nn305011p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Tumor Vascular Permeabilization by Vascular-Targeting Photosensitization: Effects, Mechanism, and Therapeutic Implications. Clin Cancer Res. 2006;12:917–923. doi: 10.1158/1078-0432.CCR-05-1673. [DOI] [PubMed] [Google Scholar]
- [148].Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- [149].Lammers T, Subr V, Ulbrich K, Hennink WE, Storm G, Kiessling F. Polymeric nanomedicines for image-guided drug delivery and tumor-targeted combination therapy. Nano Today. 2010;5:197–212. doi: 10.1016/j.nantod.2010.05.001. [DOI] [Google Scholar]
- [150].Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7:216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- [151].Zhao M, Ding X, Shen J, Zhang X, Ding X, Xu B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J Zhejiang Univ Sci B. 2017;18:15–26. doi: 10.1631/jzus.B1600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Linton SS, Sherwood SG, Drews KC, Kester M. Targeting cancer cells in the tumor microenvironment: opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:208–222. doi: 10.1002/wnan.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Liu Y, Maccarini P, Palmer GM, Etienne W, Zhao Y, Lee C-T, Ma X, Inman BA, Vo-Dinh T. Synergistic Immuno Photothermal Nanotherapy (SYMPHONY) for the Treatment of Unresectable and Metastatic Cancers. Sci Rep. 2017;7 doi: 10.1038/s41598-017-09116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Frei E, III, Chou TC, Rideout DC. Clinical studies of combination chemotherapy for cancer. Synerg Antagon Chemother. 1991;1:103–108. [Google Scholar]
- [155].Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- [156].Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- [157].Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, Stuart RK, Strickland SA, Hogge D, Solomon SR, Stone RM, et al. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. [accessed May 12, 2017];J Clin Oncol. 2016 34 http://meetinglibrary.asco.org/content/165504-176. [Google Scholar]
- [159].C. for D.E. and Research. Approved Drugs - FDA approves liposome-encapsulated combination of daunorubicin-cytarabine for adults with some types of poor prognosis AML. [accessed April 25, 2018]; (n.d.). https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm569950.htm.
- [160].Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Batist G, Sawyer M, Gabrail N, Christiansen N, Marshall JL, Spigel DR, Louie A. A multicenter, phase II study of CPX-1 liposome injection in patients (pts) with advanced colorectal cancer (CRC) J Clin Oncol. 2008;26:4108–4108. doi: 10.1200/jco.2008.26.15_suppl.4108. [DOI] [Google Scholar]
- [162].ABOUT ABRAXANE. Abraxane Pro. [accessed May 24, 2017]; (n.d.). https://www.abraxanepro.com/about-abraxane/
- [163].Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Krishna K, Blazer MA, Wei L, Ahn DH, Wu CS-Y, Ciombor KK, Mikhail S, Noonan AM, Goldberg RM, Bekaii-Saab TS. Modified gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer (MPC): A single-institution experience. [accessed May 24, 2017];J Clin Oncol. 2015 33 doi: 10.1177/1758834016676011. http://meetinglibrary.asco.org/content/140419-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol Off J Am Soc Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- [166].Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- [167].Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet Lond Engl. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- [168].Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Down-Regulation of the erbB-2 Receptor by Trastuzumab (Herceptin) Enhances Tumor Necrosis Factor-related Apoptosis-inducing Ligand-mediated Apoptosis in Breast and Ovarian Cancer Cell Lines that Overexpress erbB-2. Cancer Res. 2001;61:4892–4900. [PubMed] [Google Scholar]
- [169].Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- [170].Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, Munn LL, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res BCR. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, Green M, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Talelli M, Oliveira S, Rijcken CJF, Pieters EHE, Etrych T, Ulbrich K, van Nostrum RCF, Storm G, Hennink WE, Lammers T. Intrinsically active nanobody-modified polymeric micelles for tumor-targeted combination therapy. Biomaterials. 2013;34:1255–1260. doi: 10.1016/j.biomaterials.2012.09.064. [DOI] [PubMed] [Google Scholar]
- [174].Nakamura T, Harashima H. Integration of nano drug-delivery system with cancer immunotherapy. Ther Deliv. 2017;8:987–1000. doi: 10.4155/tde-2017-0071. [DOI] [PubMed] [Google Scholar]
- [175].Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L, Engelman JA, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- [176].Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X, Zhang Y, Cheng K, Liu S, Hao J, Ren H, et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187–197. doi: 10.1016/j.biomaterials.2016.06.032. [DOI] [PubMed] [Google Scholar]
- [177].Yoon HY, Selvan ST, Yang Y, Kim MJ, Yi DK, Kwon IC, Kim K. Engineering nanoparticle strategies for effective cancer immunotherapy. Biomaterials. 2018 doi: 10.1016/j.biomaterials.2018.03.036. [DOI] [PubMed] [Google Scholar]
- [178].Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S, Florindo HF, Barata TS. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front Chem. 2014;2:105. doi: 10.3389/fchem.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol Baltim Md 1950. 1991;147:2860–2863. [PubMed] [Google Scholar]
- [180].Keller S, Wilson JT, Patilea GI, Kern HB, Convertine AJ, Stayton PS. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8(+) T cell responses. J Control Release Off J Control Release Soc. 2014;191:24–33. doi: 10.1016/j.jconrel.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J Am Chem Soc. 2016;138:16686–16695. doi: 10.1021/jacs.6b09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7 doi: 10.1038/ncomms12499. 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- [185].Shen H, Sun T, Hoang HH, Burchfield JS, Hamilton GF, Mittendorf EA, Ferrari M. Enhancing cancer immunotherapy through nanotechnology-mediated tumor infiltration and activation of immune cells. Semin Immunol. 2017;34:114–122. doi: 10.1016/j.smim.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Jiang W, Yuan H, Chan CK, von Roemeling CA, Yan Z, Weissman IL, Kim BYS. Lessons from immuno-oncology: a new era for cancer nanomedicine? Nat Rev Drug Discov. 2017;16:369–370. doi: 10.1038/nrd.2017.34. [DOI] [PubMed] [Google Scholar]
- [187].Jiang W, von Roemeling CA, Chen Y, Qie Y, Liu X, Chen J, Kim BYS. Designing nanomedicine for immuno-oncology. Nat Biomed Eng. 2017;1 doi: 10.1038/s41551-017-0029. 0029. [DOI] [Google Scholar]
- [188].Kunjachan S, Pola R, Gremse F, Theek B, Ehling J, Moeckel D, Hermanns-Sachweh B, Pechar M, Ulbrich K, Hennink WE, Storm G, et al. Passive vs. Active Tumor Targeting using RGD-and NGR-modified Polymeric Nanomedicines. Nano Lett. 2014;14:972–981. doi: 10.1021/nl404391r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Danhier F, Le Breton A, Préat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–2973. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- [190].Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR, Ino Y, Nomoto T, Matsumoto Y, Koyama H, Cabral H, et al. Cyclic RGD-Linked Polymeric Micelles for Targeted Delivery of Platinum Anticancer Drugs to Glioblastoma through the Blood–Brain Tumor Barrier. ACS Nano. 2013;7:8583–8592. doi: 10.1021/nn402662d. [DOI] [PubMed] [Google Scholar]
- [191].Jin Z, Lv Y, Cao H, Yao J, Zhou J, He W, Yin L. Core-shell nanocarriers with high paclitaxel loading for passive and active targeting. Sci Rep. 2016;6 doi: 10.1038/srep27559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular Damage and Anti-angiogenic Effects of Tumor Vessel-Targeted Liposomal Chemotherapy. Cancer Res. 2003;63:7400–7409. [PubMed] [Google Scholar]
- [193].Pastorino F, Brignole C, Paolo DD, Nico B, Pezzolo A, Marimpietri D, Pagnan G, Piccardi F, Cilli M, Longhi R, Ribatti D, et al. Targeting Liposomal Chemotherapy via Both Tumor Cell–Specific and Tumor Vasculature–Specific Ligands Potentiates Therapeutic Efficacy. Cancer Res. 2006;66:10073–10082. doi: 10.1158/0008-5472.CAN-06-2117. [DOI] [PubMed] [Google Scholar]
- [194].Dunne M, Zheng J, Rosenblat J, Jaffray DA, Allen C. APN/CD13-targeting as a strategy to alter the tumor accumulation of liposomes. J Controlled Release. 2011;154:298–305. doi: 10.1016/j.jconrel.2011.05.022. [DOI] [PubMed] [Google Scholar]
- [195].Manzoor AA, Lindner LH, Landon CD, Park J-Y, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17:738–750. doi: 10.1038/nrc.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Dewhirst MW, Kirsch D. Technological Advances, Biologic Rationales, and the Associated Success of Chemotherapy With Hyperthermia in Improved Outcomes in Patients With Sarcoma. JAMA Oncol. 2018;4:493–494. doi: 10.1001/jamaoncol.2017.4941. [DOI] [PubMed] [Google Scholar]
- [198].Gasselhuber A, Dreher MR, Rattay F, Wood BJ, Haemmerich D. Comparison of conventional chemotherapy, stealth liposomes and temperature-sensitive liposomes in a mathematical model. PloS One. 2012;7:e47453. doi: 10.1371/journal.pone.0047453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, Bally MB, Dewhirst MW. Magnetic Resonance Imaging of Temperature-Sensitive Liposome Release: Drug Dose Painting and Antitumor Effects. J Natl Cancer Inst. 2007;99:53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- [200].Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, Wood BJ, Dreher MR. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Controlled Release. 2012;158:487–494. doi: 10.1016/j.jconrel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Dunne M, Hynynen K, Allen C. Thermosensitive nanomedicines could revolutionize thermal therapy in oncology. Nano Today. 2017 doi: 10.1016/j.nantod.2017.08.001. [DOI] [Google Scholar]
- [202].Wood BJ, Poon RT, Locklin JK, Dreher MR, Ng KK, Eugeni M, Seidel G, Dromi S, Neeman Z, Kolf M, Black CDV, et al. Phase I Study of Heat-Deployed Liposomal Doxorubicin during Radiofrequency Ablation for Hepatic Malignancies. J Vasc Interv Radiol. 2012;23:248–255.e7. doi: 10.1016/j.jvir.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Zagar TM, Vujaskovic Z, Formenti S, Rugo H, Muggia F, O’Connor B, Myerson R, Stauffer P, Hsu I-C, Diederich C, Straube W, et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int J Hyperthermia. 2014;30:285–294. doi: 10.3109/02656736.2014.936049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Lencioni R, Cioni D. RFA plus lyso-thermosensitive liposomal doxorubicin: in search of the optimal approach to cure intermediate-size hepatocellular carcinoma. Hepatic Oncol. 2016;3:193–200. doi: 10.2217/hep-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Ehling J, Theek B, Gremse F, Baetke S, Möckel D, Maynard J, Ricketts S-A, Grüll H, Neeman M, Knuechel R, Lederle W, et al. Micro-CT imaging of tumor angiogenesis: quantitative measures describing micromorphology and vascularization. Am J Pathol. 2014;184:431–441. doi: 10.1016/j.ajpath.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Karageorgis A, Dufort S, Sancey L, Henry M, Hirsjärvi S, Passirani C, Benoit J-P, Gravier J, Texier I, Montigon O, Benmerad M, et al. Coll, An MRI-based classification scheme to predict passive access of 5 to 50-nm large nanoparticles to tumors. Sci Rep. 2016;6 doi: 10.1038/srep21417. 21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Sulheim E, Kim J, van Wamel A, Kim E, Snipstad S, Vidic I, Grimstad IH, Widerøe M, Torp SH, Lundgren S, Waxman DJ, et al. Multi-modal characterization of vasculature and nanoparticle accumulation in five tumor xenograft models. J Control Release Off J Control Release Soc. 2018 doi: 10.1016/j.jconrel.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- [209].Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2 doi: 10.1038/natrevmats.2017.24. 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Lammers T, Rizzo LY, Storm G, Kiessling F. Personalized nanomedicine. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:4889–4894. doi: 10.1158/1078-0432.CCR-12-1414. [DOI] [PubMed] [Google Scholar]
- [211].Pérez-Medina C, Abdel-Atti D, Tang J, Zhao Y, Fayad ZA, Lewis JS, Mulder WJM, Reiner T. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat Commun. 2016;7 doi: 10.1038/ncomms11838. 11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Lee H, Gaddy D, Ventura M, Bernards N, de Souza R, Kirpotin D, Wickham T, Fitzgerald J, Zheng J, Hendriks BS. Companion Diagnostic 64Cu-Liposome Positron Emission Tomography Enables Characterization of Drug Delivery to Tumors and Predicts Response to Cancer Nanomedicines. Theranostics. 2018;8:2300–2312. doi: 10.7150/thno.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH, Yang KS, Laughney AM, Wojtkiewicz G, Kamaly N, Bhonagiri S, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med. 2015;7:314ra183. doi: 10.1126/scitranslmed.aac6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Ramanathan RK, Korn R, Raghunand N, Sachdev JC, Newbold RG, Jameson G, Fetterly GJ, Prey J, Klinz SG, Kim J, Cain J, et al. Correlation Between Ferumoxytol Uptake in Tumor Lesions by MRI and Response to Nanoliposomal Irinotecan in Patients With Advanced Solid Tumors: A Pilot Study. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-1990. clincanres.1990.2016. [DOI] [PubMed] [Google Scholar]
- [215].Lee H, Shields AF, Siegel BA, Miller KD, Krop I, Ma CX, LoRusso PM, Munster PN, Campbell K, Gaddy DF, Leonard SC, et al. (64)Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- [217].Choi CHJ, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Lammers T, Kiessling F, Ashford M, Hennink W, Crommelin D, Storm G. Cancer nanomedicine: is targeting our target? Nat Rev Mater. 2016 doi: 10.1038/natrevmats.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Taurin S, Nehoff H, van Aswegen T, Greish K. Tumor Vasculature, EPR Effect, and Anticancer Nanomedicine: Connecting the Dots. In: Bae YH, Mrsny RJ, Park K, editors. Cancer Target Drug Deliv. Springer; New York: 2013. [accessed October 23, 2014]. pp. 207–239. [DOI] [Google Scholar]
- [220].Lucas AT, White TF, Deal AM, Herity LB, Song G, Santos CM, Zamboni WC. Profiling the relationship between tumor-associated macrophages and pharmacokinetics of liposomal agents in preclinical murine models. Nanomedicine Nanotechnol Biol Med. 2017;13:471–482. doi: 10.1016/j.nano.2016.09.015. [DOI] [PubMed] [Google Scholar]
- [221].May M. Cancer research with a human touch. Nature. 2018 doi: 10.1038/d41586-018-04161-w. [DOI] [PubMed] [Google Scholar]
- [222].Huszthy PC, Daphu I, Niclou SP, Stieber D, Nigro JM, Sakariassen PØ, Miletic H, Thorsen F, Bjerkvig R. In vivo models of primary brain tumors: pitfalls and perspectives. Neuro-Oncol. 2012;14:979–993. doi: 10.1093/neuonc/nos135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Stewart E, Federico SM, Chen X, Shelat AA, Bradley C, Gordon B, Karlstrom A, Twarog NR, Clay MR, Bahrami A, Freeman BB, et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature. 2017;549:96–100. doi: 10.1038/nature23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Caretti V, Sewing ACP, Lagerweij T, Schellen P, Bugiani M, Jansen MHA, van Vuurden DG, Navis AC, Horsman I, Vandertop WP, Noske DP, et al. Human pontine glioma cells can induce murine tumors. Acta Neuropathol (Berl.) 2014;127:897–909. doi: 10.1007/s00401-014-1272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Roerink SF, Sasaki N, Lee-Six H, Young MD, Alexandrov LB, Behjati S, Mitchell TJ, Grossmann S, Lightfoot H, Egan DA, Pronk A, et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature. 2018;1 doi: 10.1038/s41586-018-0024-3. [DOI] [PubMed] [Google Scholar]
- [226].Kuo CJ, Curtis C. Organoids reveal cancer dynamics. Nature. 2018 doi: 10.1038/d41586-018-03841-x. [DOI] [PubMed] [Google Scholar]
- [227].A A, et al. Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. [accessed January 26, 2018]; doi: 10.1016/j.biomaterials.2014.04.060. (n.d.) [DOI] [PubMed] [Google Scholar]
- [228].van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Weeber F, Ooft SN, Dijkstra KK, Voest EE. Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chem Biol. 2017;24:1092–1100. doi: 10.1016/j.chembiol.2017.06.012. [DOI] [PubMed] [Google Scholar]
- [230].In Vitro Models of Liver and Pancreatic Cancer - Full Text View - ClinicalTrials.gov. [accessed January 26, 2018]; (n.d). https://clinicaltrials.gov/ct2/show/NCT02436564.
- [231].Organoid Based Response Prediction in Esophageal Cancer - Full Text View - ClinicalTrials.gov. [accessed January 26, 2018]; (n.d). https://clinicaltrials.gov/ct2/show/NCT03283527.
- [232].Zhou H, Qian W, Uckun FM, Wang L, Wang YA, Chen H, Kooby D, Yu Q, Lipowska M, Staley CA, Mao H, et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano. 2015;9:7976–7991. doi: 10.1021/acsnano.5b01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Kim H, Samuel SL, Lopez-Casas PP, Grizzle WE, Hidalgo M, Kovar J, Oelschlager DK, Zinn KR, Warram JM, Buchsbaum DJ. SPARC independent delivery of nab-paclitaxel without depleting tumor stroma in patient-derived pancreatic cancer xenografts. Mol Cancer Ther. 2016;15:680–688. doi: 10.1158/1535-7163.MCT-15-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Patient-derived Xenograft (PDX) Modeling of Treatment Response for Triple Negative Breast Cancer - Full Text View - ClinicalTrials.gov. [accessed January 26, 2018]; (n.d.). https://clinicaltrials.gov/ct2/show/NCT02247037.
- [235].Clinical Studies by Using Accelerated PDX Model to Screen Drugs for Advanced Solid Tumor - Full Text View - ClinicalTrials.gov. [accessed January 26, 2018]; (n.d.). https://clinicaltrials.gov/ct2/show/NCT03299452.
- [236].Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo GM, Roman-Roman S, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Zitvogel L, Pitt JM, Daillère R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16:759–773. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- [238].Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- [239].Workman P, Draetta GF, Schellens JHM, Bernards R. How Much Longer Will We Put Up With $100,000 Cancer Drugs? Cell. 2017;168:579–583. doi: 10.1016/j.cell.2017.01.034. [DOI] [PubMed] [Google Scholar]