Abstract
Tumors are characterized by leaky blood vessels, and by an abnormal and heterogeneous vascular network. These pathophysiological characteristics contribute to the enhanced permeability and retention (EPR) effect, which is one of the key rationales for developing tumor-targeted drug delivery systems. Vessel abnormality and heterogeneity, however, which typically result from excessive pro-angiogenic signaling, can also hinder efficient drug delivery to and into tumors. Using histidine-rich glycoprotein (HRG) knockout and wild type mice, and HRG-overexpressing and normal t241 fibrosarcoma cells, we evaluated the effect of genetically induced and macrophage-mediated vascular normalization on the tumor accumulation and penetration of ~10 nm-sized polymeric drug carriers based on poly(N-(2-hydroxypropyl) methacrylamide). Multimodal and multiscale optical imaging was employed to show that normalizing the tumor vasculature improves the accumulation of fluorophore-labeled polymers in tumors, and promotes their penetration out of the blood vessels deep into the tumor interstitium.
Keywords: Nanomedicine, Tumor-targeting, Vascular normalization, EPR, HRG, pHPMA
Introduction
One of the biggest challenges in systemic anticancer drug therapy relates to the inefficient accumulation of intravenously (i.v.) administered chemotherapeutic agents at pathological sites. Classical chemotherapeutic drugs typically suffer from a short circulation half-life (as a result of rapid renal excretion), and from a large volume of distribution (resulting in systemic side effects) [1, 2]. For several decades, scientists have been developing materials and methods to overcome these problems, primarily via the incorporation of low-molecular-weight chemotherapeutic agents in 1-100(0) nm-sized drug delivery systems. These carrier materials, which typically include polymers, micelles and liposomes, are larger than the renal clearance threshold, they present with a completely different biodistribution profile, and accumulate more efficiently at pathological sites than free drug molecules [3–5].
In case of drug targeting to tumors, pathophysiological characteristics such as hypervascularity, hyperpermeability and impaired lymphatic drainage allow nanomedicines to accumulate more efficiently and more selectively at sites of malignancy, via the so-called enhanced permeability and retention (EPR) effect [6, 7]. The EPR effect, however, is met with increasing scrutiny, and an increasing number of papers has questioned its presence, prominence and relevance [8–11]. One of the potential problems associated with leaky blood vessels, for instance, is the relatively high interstitial fluid pressure (IFP) which is typical for tumors [12, 13]. Consequently, convective transport processes, in particular the penetration of drugs and drug delivery systems from the tumor blood vessels deep into the interstitium, are impaired. Furthermore, the EPR effect is not omni-present in all tumors, resulting in a very high degree of inter- and intratumoral heterogeneity in the “passive” accumulation of nanomedicine formulations in tumors [14, 15].
To improve the accumulation and distribution of nanomedicines in tumors, and to eventually enhance their therapeutic efficacy, several pharmacological and physical vessel modulation strategies have been explored over the years [8, 16]. In this context, a popular recent pharmacological strategy is vascular normalization, which aims to improve tumor-directed drug delivery via partially counteracting pro-angiogenic signaling in tumors [17–19]. Angiogenesis, i.e. the formation of new blood vessels from pre-existing ones, is a tightly controlled process under normal physiological conditions. However, during tumor development, proliferating cancer cells and hypoxia result in the overproduction of vascular growth factors, such as VEGF. Already in the 1970s, the importance of angiogenesis for the development of tumors was realized, and strategies to starve tumors to death by pharmacologically inhibiting angiogenesis were suggested [20]. While anti-angiogenic (anti-VEGF) monotherapies failed in the clinic, they presented with clear added value when combined with chemotherapy and/or radiotherapy [21–23]. This enhanced efficacy can be explained - at least in part - by a phenomenon termed vascular normalization, which helps to improve the delivery of drug molecules and oxygen to tumors [17, 24–26].
The first vessels which respond to anti-angiogenic therapy are immature and leaky blood vessels. In a recent clinical study, Jain and colleagues showed that neoadjuvant bevacizumab (anti-VEGF antibody) treatment in HER2-negative breast cancer patients decreased the IFP and increased the fraction of pericyte-covered vessels, leading to a more normalized and better perfused vascular network, and to improved drug delivery [27]. Both standard chemotherapeutic drugs and metronomically administered anticancer agents have been shown to profit from vascular normalization [28], and also in case of nanomedicines, vascular normalization has already been shown to be able to improve drug delivery and anti-tumor efficacy [29–31]. However, it seems to occur in a size-dependent manner, with the normalization-induced reduction in pore size hindering the extravasation of relatively large nanomedicines, such as 100 nm-sized Doxil® liposomes, while promoting the accumulation and efficacy of smaller sized nanomedicines, such as the ones obtained upon the i.v. administration of Abraxane®, which in the blood stream disintegrates into 10 nm-sized self-assemblies of paclitaxel and endogenous albumin [32].
Tumor-associated macrophages (TAM) play a key role in vascular normalization. TAM are typically polarized towards a more M2-like phenotype, which promotes angiogenesis and immunosuppression [33]. One of the first studies investigating the effect of vascular normalization mediated via the repolarisation of TAM, from an alternatively activated and pro-angiogenic M2-like phenotype towards a more classically activated and anti-angiogenic M1-like phenotype, focused on the histidine-rich glycoprotein (HRG) [34]. HRG is a highly abundant plasma protein which suppresses the expression of the pro-angiogenic placental growth factor (PlGF). PIGF is overexpressed in several tumor types and fuels angiogenesis by binding to VEGFR1 [35–37]. By inhibiting PlGF signaling, HRG expression protects against tumor growth and metastasis, via modulation of macrophage polarization and induction of vascular normalization [19, 33, 38, 39].
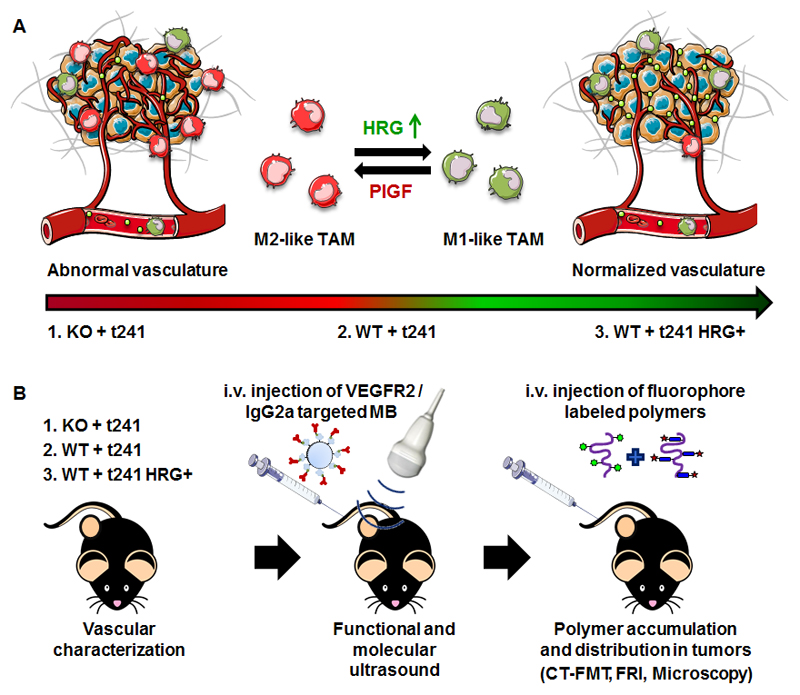
We here used HRG-knockout and wild type mice, and HRG-overexpressing and normal t241 fibrosarcoma cells, to systematically study the effect of macrophage-mediated vascular normalization on the tumor accumulation and penetration of ~10 nm-sized polymeric drug carriers based on poly(N-(2-hydroxypropyl) methacrylamide) (pHPMA). As exemplified by Figure 1, we hypothesized that increased expression of HRG drives the polarization of TAM towards an anti-angiogenic M1-like phenotype, resulting in normalized tumor blood vessels, and in improved tumor-targeted drug delivery.
Fig. 1. Study design.
(A) Expression of histidine-rich glycoprotein (HRG) affects the polarization of tumor-associated macrophages (TAM) and induces an M1-like anti-angiogenic (and vascular normalization) phenotype in tumors, via the downregulation of the placental growth factor (PIGF). (B) Three different genetic model systems were used to assess the impact of HRG knockout and (over-) expression on the accumulation and penetration of fluorophore-labeled 10 nm-sized polymeric drug carriers in t241 fibrosarcoma tumors. Knockout mice (KO) lacking HRG and wild type (WT) mice expressing HRG at physiological levels were implanted with t241 fibrosarcoma tumor cells. The latter mice were also implanted with t241 cells overexpressing HRG, to further promote local HRG-mediated vascular normalization. In these three mouse models, functional and molecular ultrasound, as well as multimodal and multiscale optical imaging, were employed to assess the effect of HRG-mediated vascular normalization on the accumulation and penetration of 10 nm-sized pHPMA-based polymeric drug delivery systems.
Materials & Methods
Polymer synthesis
The synthesis of the pHPMA-based polymeric drug carrier was performed as described previously [40–42]. In short, a copolymer precursor was synthesized by radical copolymerization of (N-2-hydroxypropyl)methacrylamide (HPMA, 85 mol %) and 3-(N-methacryloyl glycylglycyl) thiazolidine-2-thione (Ma-GG-TT, 15 mol %) in DMSO at 50 °C for 6 h. Poly(HPMA-co-Ma-GG-TT) was dissolved in N,N'-dimethylacetamide and N,N’-diisopropylethylamine, together with the fluorophores ATTO 488-NH2 and Dy750-NH2. After a reaction time of 30 min, the polymer was aminolyzed with 1-aminopropan-2-ol, followed by precipitation using diethylether and centrifugation. Purification was performed using HPLC on Chromolith SemiPrep 100-10mm and then by gel filtration on PD-10 desalting columns containing Sephadex G-25 resin in water (GE Healthcare, Solingen, Germany). The molecular weight of the polymer was 67 kDa, the polydispersity index was 1.7, and the content of dye ATTO 488 was 2.1% (w/w) and of DY-750 was 1.6% (w/w).
Animal experiments
All animal experiments were performed according to the regulations of the local and national ethical committee for animal welfare. C57BL/6J mice (WT) were obtained from Charles River (The Netherlands). B6-(HRG33)tmwja1 mice (KO), a viable and fertile HRG-/- null mutation have been described previously [43]. For both phenotypes 5-8 week-old male and female mice were used. Group sizes ranged from 3-6. The mice were kept in individually ventilated cages with food and water ad-libitum as well as controlled light–dark cycles. One million t241 cells (i.e. murine fibrosarcoma) or HRG-overexpressing t241 cells (t241-HRG+) [34] were inoculated subcutaneously into the right flank of the mice, and tumors were allowed to grow to 6-7 mm in diameter. Three days before the start of the drug delivery experiment, the diet was changed to chlorophyll-free food (ssniff Spezialdiäten GmbH, Germany), to reduce background fluorescence in the FMT analyses.
Contrast-enhanced ultrasound imaging
All in vivo ultrasound (US) experiments were conducted under continuous inhalation anesthesia, using 2% (vol/vol) isoflurane. The US device employed in this study was the VisualSonics Vevo2100 imaging system (Fujifilm Sonosite, The Netherlands). During the examination, a heatable pad (Terra ComfortHeat Mat, Geilenkirchen, Germany) was used to avoid hypothermia of the animals. For the recording both linear and non-linear contrast mode were chosen with a transmission frequency of 21 MHz, power of 4% and 10 frames per second. To determine the VEGFR2 expression the monoclonal biotin anti-mouse CD309 antibody (BioLegend, San Diego, USA) was coupled to Vevo Micromarker® target-ready contrast agent (Fujifilm Sonosite, The Netherlands) according to the manufacturer's instructions. Via a tail vein catheter, 1×108 MB were injected and the inflow was simultaneous recorded to allow maximum intensity over time (MIOT) calculation. This procedure was repeated with an IgG2a-biotin control antibody (eBioscience, Frankfurt a.M., Germany). The signal increase after the injection of control microbubbles was used to calculate the relative blood volume. The signal difference before and after the high intensity burst was used to determine the relative amount of MB which bound to the target receptor, thereby providing information in the level of angiogenic marker expression.
Hybrid CT-FMT and FRI measurements
Hybrid computed tomography–fluorescence molecular tomography (CT-FMT) and fluorescence reflectance imaging (FRI) analyses were performed as described in [42, 44, 45]. Anesthetized mice were placed into a custom-made mouse bed enabling both CT (CT Imaging, Germany) and FMT (PerkinElmer, USA) imaging. CT scans with 720 projections in 1.1 full rotations on a flat panel detector with the size of 1032 × 1012 pixels were obtained. Mice were then transferred to the FMT and FRI device, to assess the tumor accumulation and the biodistribution of the fluorescent polymers. For a partial body scan covering the tumor region, 45–60 FMT scan points lying on a grid with an inter-individual distance of 3 mm, were acquired from top and bottom orientation of each mouse. This procedure was performed directly, 4, 24 and 48 h after the i.v. injection of the polymers, in order to monitor the polymer accumulation longitudinally. After the last measurement, the mice were i.v. injected with rhodamine-lectin (to stain functional blood vessels and facilitate microscopy analyses) and sacrificed 10 min later. Tumors were excised and imaged ex vivo, in fluorescence reflectance mode at 750 nm, to obtain a high-resolution image of the polymer distribution in the tumor as a whole. Afterwards, they were embedded in a TissueTek O.C.T. (Sakura Finetek Europe, The Netherlands) mixture containing 1% (vol/vol) Imeron (Bracco, Konstanz, Germany) and 5% (vol/vol) Intralipid (Fresenius Kabi, India) for ex vivo CT-FMT imaging and cryopreservation at -80 °C. One hundred μm-thick sections were freshly prepared from the frozen tumor samples and fluorescence reflectance images acquired, to monitor polymer distribution, using FRI and two-photon laser scanning microscopy (TPLSM).
Image analysis
The hybrid CT-FMT data sets were reconstructed, fused and analyzed using Imalytics Preclinical (Gremse-IT GmbH, Germany). Tumor segmentation was based on the anatomical information obtained by CT. The FMT-based biodistribution data was loaded as an overlay, and the fluorescence signal allocated to the tumor was quantified and expressed as % of the injected dose (%ID) per 250 mm3 tumor (corresponding to the average volume of the tumors used in this study). In case of the FRI of 100 µm-thick sections the periphery was defined as a 5-pixel wide outer zone of the slice segmentation.
Two-photon laser scanning microscopy
Image stacks of 50 images with a step size of 1 μm were acquired using a 25× water-immersed objective mounted on the Olympus FV1000MPE multiphoton microscopy system and processed as described previously [46]. In each image stack, fluorescent polymers as well as perfused blood vessels (via rhodamine-lectin staining). TPLSM images were analyzed using the Imaris Software, Version 7.4 (Bitplane AG, Zurich, Switzerland). The micro-distribution of the ATTO488-containing fluorescent polymers, i.e. their extravasation from blood vessels into the tumor interstitium, was analyzed using a modified version of the “Dilate Surface” XTension in Imaris. For this purpose, initially, thresholding was applied on the basis of the rhodamine-lectin signal (to create a 3D vessel segmentation), and on the ATTO488 signal (to determine the volume of polymer distribution). The vessel segmentation was then automatically dilated, at step-sizes of 10 μm, 30 μm and 50 μm. Finally, the total amount of polymers detected within a 50 μm radius around the vessels was quantified by multiplying the relative polymer volume and the signal intensity. The relative micro-distribution of the polymers was calculated, by determining the relative amount of carrier material detected in each individual 3D segmentation, to compare the distribution of the polymers in tumors varying in HRG levels and vascular normalization status.
Immunofluorescence
Eight µm-thick tumor cryosections were fixed with 80% methanol (v/v) aqueous solution for 5 min and afterwards with -20 °C cold acetone for 2 min. Immunofluorescent stainings were performed with the following primary monoclonal antibodies: rat anti-mouse CD31 (PECAM-1) antibody (BD Biosciences, Germany); biotin anti-mouse smooth muscle actin (SMA) antibody (Progen Biotechnik, Heidelberg, Germany); rat anti-mouse F4-80 antibody (AbD Serotec, Puchheim, Germany); and rat anti-mouse CD206/Mrc-1 antibody (Acris Antibodies, Herford, Germany). Secondary antibodies were obtained from Dianova (Hamburg, Germany). DAPI (Sigma Aldrich, Taufkirchen, Germany) was used for nuclear counterstaining and sections were embedded with Mowiol® 4-88 (Roth, Karlsruhe). Images were acquired using the Axio Imager M2 microscopy system (Carl Zeiss AG, Jena, Germany).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5. All results are shown as average ± standard deviation. US and immunofluorescence analysis were performed using one-way ANOVA corrected for multiple comparisons (Bonferroni). In vivo CT-FMT analysis on polymer accumulation and ex vivo measurements were evaluated as well with one-way ANOVA including the Bonferroni correction. For the polymer penetration analysis in TPLSM the two-way ANOVA with Bonferroni post-test was applied to investigate the interaction between the groups and the distance from the vessel surface. P-values of less than 0.05 were considered to be statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Results & Discussion
Effect of HRG expression on tumor vasculature
To study the role of HRG-mediated vascular normalization on EPR-mediated tumor targeting with 10 nm-sized polymeric drug carriers, we first characterized the tumor vasculature in the three different mouse models, i.e. KO+t241, WT+t241 and WT+t241-HRG+. This was done applying contrast-enhanced functional (Fig. 2A-C) and molecular (Fig. 2D-F) ultrasound (US) imaging, as well as using microscopy. For functional US, the maximum intensity over time (MIOT) image sequence was used, to determine the relative blood volume of tumors (Fig 2A). Representative US images obtained at the beginning and at the end of MIOT analysis are shown in Figure 2B. The images after contrast agent (i.e. microbubble; MB) injection exemplify the perfused regions within the tumors. Multiple perfused blood vessels could be identified in t241 tumors implanted in HRG-knockout and wild-type mice. In WT mice implanted with t241 cells overexpressing HRG, the relative blood vessel density was found to be lower than for the two other models, which hints towards pruning of highly angiogenic vessels. In line with this, when performing molecular US imaging using MB surface-modified with antibodies recognizing VEGFR2, we also observed lower angiogenic marker expression in t241 tumors overexpressing HRG. As exemplified by Fig. 2D, the signal difference before and after the destructive pulse is a relative measure for the number of MB bound to VEGFR2, and thus for the angiogenic status of the tumor vasculature. As hypothesized, t241 tumors grown in HRG-knockout mice (KO+t241) showed the highest signal difference and thus the highest expression of pro-angiogenic VEGFR2 on tumor blood vessels. They were followed by t241 tumors grown in WT mice (WT+t241), and the lowest number of VEGFR2-targeted MB were detected in HRG-overexpressing t241 tumors grown in WT mice (WT+t241-HRG+) (Fig 2E-F). This shows that the (over-) expression of HRG results in less angiogenic and more normalized blood vessels in tumors.
Fig. 2. Functional and molecular ultrasound (US) imaging to assess the effect of HRG expression on tumor vascularization and angiogenic marker expression.
(A) An exemplary time-intensity curve of a maximum-intensity over time (MIOT) image sequence upon the i.v. injection of microbubbles (MB) as US contrast agents. MIOT analysis was used to determine the relative blood volume of tumors. (B-C) Representative MIOT images before and after MB injection (B) and the respective quantification of the signal differences (C) are shown for the three different mouse models. (D) To assess the expression of the angiogenic marker VEGFR2, MB surface-modified with anti-VEGFR2 antibodies were i.v. injected and allowed to bind to their target before they were destroyed using an US burst. (E-F) Imaging (E) and quantification (F) of the amount of VEGFR2-targeted MB which bound to tumor blood vessels in the three different models, indicating reduced levels of angiogenic marker expression in tumors with increased levels of HRG. Green circles indicate the tumors. Values represent average ± standard deviation of n=3-6 tumors.
To validate the in vivo US findings, we subsequently characterized the tumor vasculature via ex vivo immunohistochemistry and microscopy (Fig. 3). Staining of the endothelial cell marker CD31 (platelet-endothelial cell adhesion molecule 1; PECAM-1) showed that the average vessel density was significantly higher in t241 tumors grown in KO mice than in both t241 tumors grown in WT mice and in HRG-overexpressing t241 tumors grown in WT mice (P<0.05; Fig. 3A,C). To investigate the functionality of tumors vessels, the relative fraction of lectin-positive vessels was determined. The lectin/CD31 positive fraction in the KO+t241 group was 46 ± 17 % (Fig. 3D). In the WT+t241-HRG+ group, this was found to be significantly higher (61 ± 21 %; Fig. 3D). Tumors in the WT+t241 group had a lectin/CD31 positive fraction of 58 ± 18 %. Since pericyte coverage is a key measure of vessel maturity, we also stained for α-smooth muscle actin (αSMA). In tumors grown in WT mice, we observed higher levels of αSMA than in mice lacking HRG, with the highest mean value observed in WT mice implanted with t241 overexpressing HRG (0.2 ± 0.1 %; Fig. 3E). This tendency was also reflected in the vessel maturity score, in which the αSMA/CD31 positive fraction was substantially higher for the WT+t241-HRG+ group (24 ± 18 %) than for the KO+t241 group (15 ± 11 %) (Fig. 3F).
Fig. 3. Characterization of tumor blood vessels and tumor-associated macrophages in the three different mouse models.
(A) Representative fluorescence microscopy images of the vasculature of t241 tumors in HRG-KO and WT mice, as well as in HRG-overexpressing t241 tumors in WT mice. Endothelial cells are stained with CD31 (blue), perfused blood vessels with rhodamine-lectin (red), and pericytes with αSMA (green). Scale bar: 50 µm. (B) Representative fluorescence microscopy images of lectin-stained perfused blood vessels (red), of all tumor-associated macrophages (TAM; via F4-80+ staining (green)) and of pro-angiogenic M2-like TAM (via Mrc-1+ staining (blue)). Scale bar: 50 µm. (C) The microvessel density, corresponding to the CD31 positive area fraction, is significantly lower in the presence of HRG. (D) Vessel functionality, depicted as % of rhodamine-lectin positive area / CD31 positive vessel area, increases with HRG presence. (E-F) Quantification of the pericyte marker αSMA shows a tendency towards more mature blood vessels in the presence of HRG. (G-H) F4-80 and Mrc-1 staining revealed similar amounts of TAM in the three groups (G), but a significantly higher ratio of Mrc-1+ pro-angiogenic M2-likeTAM in tumors characterized by low levels of HRG (H). Values represent average ± standard deviation based on quantifying n=4 sections in n=3-6 tumors. * indicates p<0.05.
Since it is known that tumor-associated macrophages (TAM) play a key role in angiogenesis and HRG-mediated vascular normalization [47, 48], we subsequently assessed the levels and the polarization status of TAM. This was done via staining the pan-macrophage marker F4-80 and the macrophage mannose receptor-1 (Mrc-1), which is specifically expressed by pro-angiogenic M2-like macrophages. As shown in Fig. 3B and Fig. 3G, no significant differences in the overall amount of TAM were observed in the three different groups. In HPG-overexpressing t241 tumors grown in WT mice, however, a significantly lower fraction of Mrc-1-positive M2-like macrophages (52 ± 17 %) was detected than in normal t241 tumors grown in HRG-knockout mice (65 ± 17 %) (Fig. 3H), confirming that HRG expression contributes to the skewing of macrophages towards a more M1-like anti-angiogenic phenotype [34, 38, 39].
Effect of HRG-mediated vascular normalization on the tumor accumulation of polymers
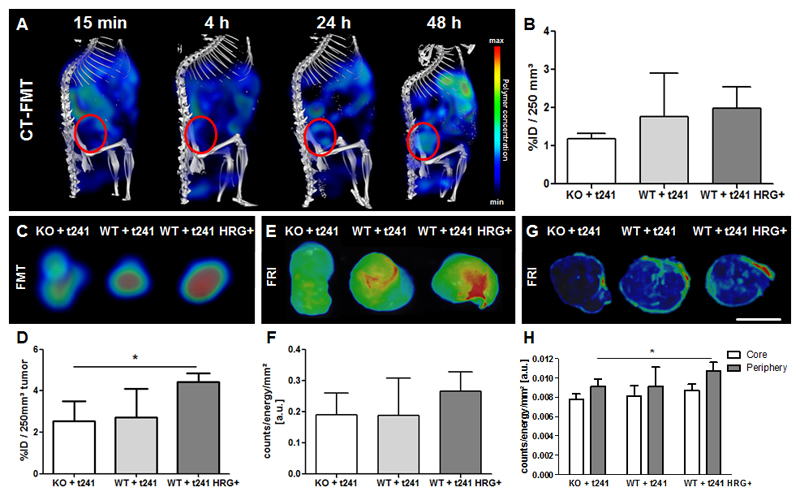
To investigate how HRG-mediated vascular normalization influences the tumor accumulation of 67 kDa-sized HPMA copolymer-based drug delivery systems, hybrid CT-FMT imaging was performed (Fig. 4A). In line with the findings above on vascular normalization status, the highest polymer accumulation was found in the WT+t241-HRG+ group, with 2.0 ± 0.6 % of the injected dose (ID) per 250 mm3 tumor at 48 h after the i.v. administration of the polymers (Fig. 4B). The second highest accumulation was found for the WT+t241 group, with 1.8 ± 1.1 % ID per 250 mm3. Polymer accumulation was least efficient in the most angiogenic tumor type, i.e. KO+t241, with 1.2 ± 0.2 % ID per 250 mm3 (Fig. 4B).
Fig. 4. In vivo and ex vivo optical imaging of the tumor accumulation of fluorophore-labeled polymers in the three tumor models.
(A) Representative CT-FMT images of polymer accumulation in a WT+t241 mouse at several different time points after i.v. injection. The tumor region is encircled in red. (B) Quantification of the tumor accumulation of the polymeric drug carrier at 48 h post i.v. injection in KO and WT mice implanted with t241 tumors, and in WT mice implanted with HRG-overexpressing t241 tumors, demonstrating a tendency towards higher accumulation in tumors with normalized blood vessels. (C-F) Ex vivo FMT (C-D) and FRI (E-F) images and quantifications reveal a higher polymer accumulation in tumors expressing higher levels of HRG. (G-H) FRI analysis of 100 µm sections of tumors, demonstrating improved polymer accumulation particularly in the periphery of tumors expressing higher amounts of HRG. Values represent average ± standard deviation of n=3-6 tumors. Scale bar: 5 mm. * indicates p<0.05.
Next, the in vivo CT-FMT findings were validated ex vivo. As shown in Fig. 4C-F, we were able to confirm that HRG-mediated vascular normalization positively affected polymer accumulation. To this end, tumors were excised, embedded in appropriate matrix material, and scanned ex vivo using both 3D-FMT and 2D-FRI, showing a significantly improved nanocarrier accumulation in tumors in the WT+t241-HRG+ group. As compared to the KO+t241 group, values were up to 70% higher (P<0.05). FRI was performed using 100 µm-thick tissue sections, differentiating between the peripheral zone (the outermost 5 pixels) and the remaining tumor core. Qualitative and quantitative assessment of these images shows that the polymers indeed accumulated better in the presence of HRG, and that this effect was strongest in the peripheral zone in HRG-overexpressing tumors (Fig. 4G-H). The observation that higher nanocarrier amounts were always detected in the tumor periphery is in line with the literature, and likely results from the fact that the periphery typically contains larger and better perfused blood vessels (and a lower IFP) than the tumor core [49–52].
Effect of HRG-mediated vascular normalization on the tumor penetration of polymers
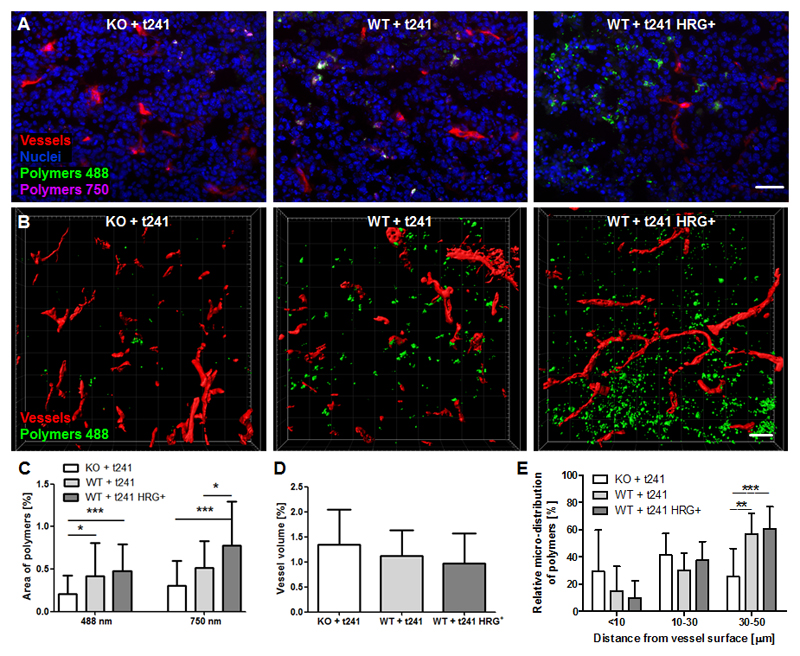
Besides macroscopically assessing tumor accumulation, we also analyzed the penetration of the polymeric drug carriers in tumors at the microscopic level. This was done using fluorescence and two-photon laser scanning microscopy (TPLSM) [53]. Homogenous and deep penetration of polymers out of the blood vessels into the tumor interstitium is highly important for efficient drug delivery and successful therapy [54]. Conversely, insufficient tumor penetration, not exceeding multiple cell layers beyond the blood vessel wall, is one of the most common pitfalls in tumor-targeted drug delivery [55, 56]. To analyze polymer extravasation and penetration, the polymer-positive area percentages were determined at both 488 and 750 nm. Figure 5A shows representative 2D images acquired with a standard fluorescence microscope. It was found that the more normalized the vasculature in the tumor was, the more polymers accumulated (Fig. 5C). Based on quantification at both 488 and 750 nm, the amount of polymers accumulating in tumors in the WT+t241-HRG+ group (0.5 % for 488 nm; 0.8 % for 750 nm) more than doubled in comparison to the KO+t241 group (0.2 % for 488 nm; 0.3 % for 750 nm).
Fig. 5. Penetration of polymeric drug carriers in tumors with normalized blood vessels.
(A) Exemplary immunofluorescence images showing the amount of blood vessels (red), cell nuclei (blue), ATTO 488-labeled (green) and Alexa-750-labeled (violet) polymers. Scale bar: 50 µm. (B) Surface-rendered two-photon laser scanning microscopy (TPLSM) image stacks with perfused blood vessels (red) and polymers (green). Scale bar: 50 µm. (C) A good correlation between the accumulation of polymers labeled with ATTO 488 and Alexa-750 is observed, in both cases indicating that polymer accumulation increases as a result of HRG-mediated vascular normalization. (D) TPLSM-based quantification indicates a somewhat lower blood volume upon HRG-mediated vascular normalization. (E) The penetration of polymers out of the blood vessels deep into the tumor interstitium significantly increases as a result of HRG-mediated vascular normalization. Values represent average ± standard deviation based on quantifying n=3-4 sections in n=3-6 different tumors. *p<0.05, **p < 0.01, ***p < 0.001.
We also visualized and quantified polymer penetration using TPLSM (Fig. 5B). This allowed us to assess, in a three-dimensional manner, the micro-distribution and penetration of polymers at distances of up to 50 µm around functional rhodamine-lectin-perfused tumor blood vessels. In line with the US and microscopy analyses in the three models, the perfused vessel volume decreased upon HRG-mediated vascular normalization (Fig. 5D). In spite of this, the amount of polymers accumulating in tumors with normalized blood vessels substantially increased (Fig. 5B). On top of this, and arguably even more importantly, the 3D-TPLSM analysis showed that HRG-mediated vascular normalization improved the ability of polymers to penetrate deeper into the tumor tissue (Fig. 5E). In the WT+t241 and WT+t241-HRG+ groups, the relative polymer accumulation in tumor tissue located 30-50 µm away from the nearest blood vessel was more than twice as high than for the KO+t241 group. For the former two, the polymer-positive area fraction was 57 ± 15 % (P<0.001) and 61 ± 16 % (P<0.01), respectively, as compared to only 25 ± 21 % for the latter. This finding demonstrates that vascular normalization strongly potentiates the penetration and the micro-distribution of 10 nm-sized polymeric drug carriers in tumors.
Overall, we show that HRG-induced vascular normalization improves the delivery of prototypic polymeric drug carriers to and into tumors. Our vascular normalization strategy (i.e. genetically induced vs. pharmacologically mediated) is based on the skewing of TAM towards an anti-tumoral M1-like macrophage phenotype. The effect of HRG relies on the downregulation of PIGF, and its enhanced expression has been shown to play a key role in the inhibition of tumor growth and metastatic spread [34, 38]. We used three different mouse models, with gradually increasing HRG levels in tumors, to analyze how normalized blood vessels affect polymer-based drug delivery to tumors at the macroscopic and microscopic level. Strategies to normalize the tumor vasculature are frequently discussed as an emerging concept to modulate the EPR effect [16, 30, 32, 57, 58]. However, finding the normalization window using antiangiogenic drug therapies is difficult, as these effects are highly dose- and time-dependent. Our genetic vascular normalization setup helps to overcome such limitations, and appears to be well suited for systematically studying the effect of vascular normalization on tumor accumulation of drugs and drug delivery systems.
Our findings are in line with the outcome of several clinical studies in which standard chemotherapeutic agents, metronomically administered anticancer drugs and radiotherapy have been shown to profit from vascular normalization [22, 23, 27, 28]. In the future, neoadjuvant therapies which induce vascular normalization may also turn out to be useful for priming the tumor microenvironment for improved delivery and efficacy of targeted anticancer agents, including e.g. nanomedicinal drug formulations and immunomodulating antibodies [47, 59, 60].
Conclusion
High heterogeneity in EPR-mediated tumor accumulation and poor tissue penetration are among the key reasons explaining the relatively disappointing clinical performance of tumor-targeted nanomedicines. To reduce the high inter- and intratumoral heterogeneity in nanomedicine accumulation and penetration, several different EPR modulation strategies have been explored. Vascular normalization, which can be induced by anti-angiogenic agents, is a promising neoadjuvant therapy that may help to make the tumor more amenable to nanomedicine therapies. Using HRG-knockout mice and HRG-overexpressing cancer cells, we generated mouse tumors which contain a progressively normalized tumor vasculature, and we show that HRG-mediated vascular normalization improves the accumulation and the penetration of 10 nm-sized polymeric drug carriers. These findings indicate that priming tumor blood vessels and the microenvironment may help to improve the outcome of nanomedicine-based anticancer therapy.
Acknowledgements
The authors gratefully acknowledge financial support by the European Research Council (ERC-StG-309495-NeoNaNo), by the German Research Foundation (DFG: LA 2937/1-2, SFB 1066, SFB/TRR 57, and GR 5027/2-1) and by the IZKF Aachen (Projects O3-1 and O3-2, and Multiphoton Imaging Core Facility (Prof. Marc van Zandvoort and Dr. Michael Vogt)). CT-FMT reconstructions were performed with computing resources granted by RWTH Aachen University under project rwth0178. HRG-overexpressing t241 and empty vector t241 fibrosarcoma cells were provided by Lena Claesson-Welsh (Department of Immunology, Genetics and Pathology, Rudbeck Laboratory, Uppsala University). Image templates, provided by Servier Medical Art (http://smart.servier.com/), were employed for preparing the graphical abstract and figure 1.
References
- [1].Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- [2].Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- [3].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- [4].Nie S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine (Lond) 2010;5:523–528. doi: 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Theek B, Rizzo LY, Ehling J, Kiessling F, Lammers T. The Theranostic Path to Personalized Nanomedicine. Clin Transl Imaging. 2014;2:66–76. doi: 10.1007/s40336-014-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- [7].Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- [9].Wilhelm S, Tavares AJ, Dai Q, Ohta S. Analysis of nanoparticle delivery to tumours. Nat Rev Mat. 2016;1 [Google Scholar]
- [10].Lammers T, Kiessling F, Ashford M, Hennink W, Crommelin D, Storm G. Cancer nanomedicine: Is targeting our target? Nat Rev Mater. 2016;1 doi: 10.1038/natrevmats.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mohammadi M, Chen P. Effect of microvascular distribution and its density on interstitial fluid pressure in solid tumors: A computational model. Microvasc Res. 2015;101:26–32. doi: 10.1016/j.mvr.2015.06.001. [DOI] [PubMed] [Google Scholar]
- [13].Zhao J, Salmon H, Sarntinoranont M. Effect of heterogeneous vasculature on interstitial transport within a solid tumor. Microvasc Res. 2007;73:224–236. doi: 10.1016/j.mvr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [14].Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- [15].Perry JL, Reuter KG, Luft JC, Pecot CV, Zamboni W, DeSimone JM. Mediating Passive Tumor Accumulation through Particle Size, Tumor Type, and Location. Nano Lett. 2017;17:2879–2886. doi: 10.1021/acs.nanolett.7b00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CTW, Storm G, Kiessling F, Lammers T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017;119:44–60. doi: 10.1016/j.addr.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- [18].Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- [19].Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- [21].Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Willett CG, Kozin SV, Duda DG, di Tomaso E, Kozak KR, Boucher Y, Jain RK. Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin Oncol. 2006;33:S35–40. doi: 10.1053/j.seminoncol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- [24].Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006486. a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 2011;21:73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- [27].Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, Ancukiewicz M, Barry WT, Goel S, Lahdenrata J, Isakoff SJ, Yeh ED, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci U S A. 2015;112:14325–14330. doi: 10.1073/pnas.1518808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mpekris F, Baish JW, Stylianopoulos T, Jain RK. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci U S A. 2017;114:1994–1999. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- [30].Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang Y, Snuderl M, Jain RK. Polarization of tumor-associated macrophages: a novel strategy for vascular normalization and antitumor immunity. Cancer Cell. 2011;19:1–2. doi: 10.1016/j.ccr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- [35].Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- [36].Poon IK, Patel KK, Davis DS, Parish CR, Hulett MD. Histidine-rich glycoprotein: the Swiss Army knife of mammalian plasma. Blood. 2011;117:2093–2101. doi: 10.1182/blood-2010-09-303842. [DOI] [PubMed] [Google Scholar]
- [37].Olsson AK, Larsson H, Dixelius J, Johansson I, Lee C, Oellig C, Bjork I, Claesson-Welsh L. A fragment of histidine-rich glycoprotein is a potent inhibitor of tumor vascularization. Cancer Res. 2004;64:599–605. doi: 10.1158/0008-5472.can-03-1941. [DOI] [PubMed] [Google Scholar]
- [38].Tugues S, Honjo S, Konig C, Noguer O, Hedlund M, Botling J, Deschoemaeker S, Wenes M, Rolny C, Jahnen-Dechent W, Mazzone M, et al. Genetic deficiency in plasma protein HRG enhances tumor growth and metastasis by exacerbating immune escape and vessel abnormalization. Cancer Res. 2012;72:1953–1963. doi: 10.1158/0008-5472.CAN-11-2194. [DOI] [PubMed] [Google Scholar]
- [39].Jones AL, Hulett MD, Parish CR. Histidine-rich glycoprotein: A novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol Cell Biol. 2005;83:106–118. doi: 10.1111/j.1440-1711.2005.01320.x. [DOI] [PubMed] [Google Scholar]
- [40].Rihova B, Jelinkova M, Strohalm J, Subr V, Plocova D, Hovorka O, Novak M, Plundrova D, Germano Y, Ulbrich K. Polymeric drugs based on conjugates of synthetic and natural macromolecules. II. Anti-cancer activity of antibody or (Fab')(2)-targeted conjugates and combined therapy with immunomodulators. J Control Release. 2000;64:241–261. doi: 10.1016/s0168-3659(99)00140-6. [DOI] [PubMed] [Google Scholar]
- [41].Etrych T, Mrkvan T, Rihova B, Ulbrich K. Star-shaped immunoglobulin-containing HPMA-based conjugates with doxorubicin for cancer therapy. J Control Release. 2007;122:31–38. doi: 10.1016/j.jconrel.2007.06.007. [DOI] [PubMed] [Google Scholar]
- [42].Kunjachan S, Gremse F, Theek B, Koczera P, Pola R, Pechar M, Etrych T, Ulbrich K, Storm G, Kiessling F, Lammers T. Noninvasive optical imaging of nanomedicine biodistribution. ACS Nano. 2013;7:252–262. doi: 10.1021/nn303955n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tsuchida-Straeten N, Ensslen S, Schafer C, Woltje M, Denecke B, Moser M, Graber S, Wakabayashi S, Koide T, Jahnen-Dechent W. Enhanced blood coagulation and fibrinolysis in mice lacking histidine-rich glycoprotein (HRG) J Thromb Haemost. 2005;3:865–872. doi: 10.1111/j.1538-7836.2005.01238.x. [DOI] [PubMed] [Google Scholar]
- [44].Gremse F, Doleschel D, Zafarnia S, Babler A, Jahnen-Dechent W, Lammers T, Lederle W, Kiessling F. Hybrid microCT-FMT imaging and image analysis. J Vis Exp. 2015:e52770. doi: 10.3791/52770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gremse F, Stark M, Ehling J, Menzel JR, Lammers T, Kiessling F. Imalytics Preclinical: Interactive Analysis of Biomedical Volume Data. Theranostics. 2016;6:328–341. doi: 10.7150/thno.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Theek B, Baues M, Ojha T, Mockel D, Veettil SK, Steitz J, van Bloois L, Storm G, Kiessling F, Lammers T. Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. J Control Release. 2016;231:77–85. doi: 10.1016/j.jconrel.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jarosz-Biej M, Kaminska N, Matuszczak S, Cichon T, Pamula-Pilat J, Czapla J, Smolarczyk R, Skwarzynska D, Kulik K, Szala S. M1-like macrophages change tumor blood vessels and microenvironment in murine melanoma. PLoS One. 2018;13:e0191012. doi: 10.1371/journal.pone.0191012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stapleton S, Milosevic M, Tannock IF, Allen C, Jaffray DA. The intra-tumoral relationship between microcirculation, interstitial fluid pressure and liposome accumulation. J Control Release. 2015;211:163–170. doi: 10.1016/j.jconrel.2015.06.008. [DOI] [PubMed] [Google Scholar]
- [50].Li L, Sun J, He Z. Deep penetration of nanoparticulate drug delivery systems into tumors: challenges and solutions. Curr Med Chem. 2013;20:2881–2891. doi: 10.2174/09298673113209990004. [DOI] [PubMed] [Google Scholar]
- [51].Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2 doi: 10.1038/natrevmats.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci U S A. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sun Q, Ojha T, Kiessling F, Lammers T, Shi Y. Enhancing Tumor Penetration of Nanomedicines. Biomacromolecules. 2017;18:1449–1459. doi: 10.1021/acs.biomac.7b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- [55].Popovic Z, Liu W, Chauhan VP, Lee J, Wong C, Greytak AB, Insin N, Nocera DG, Fukumura D, Jain RK, Bawendi MG. A nanoparticle size series for in vivo fluorescence imaging. Angew Chem Int Ed Engl. 2010;49:8649–8652. doi: 10.1002/anie.201003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- [57].Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4:81–89. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chen Q, Xu L, Chen J, Yang Z, Liang C, Yang Y, Liu Z. Tumor vasculature normalization by orally fed erlotinib to modulate the tumor microenvironment for enhanced cancer nanomedicine and immunotherapy. Biomaterials. 2017;148:69–80. doi: 10.1016/j.biomaterials.2017.09.021. [DOI] [PubMed] [Google Scholar]
- [60].Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis. 2017;20:185–204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]