Abstract
The chemical composition of commercial Syzygium aromaticum, Cinnamomum verum, and Laurus nobilis essential oils as well as their antifungal activity against four pathogenic fungi isolated from Mediterranean rice grains has been investigated. Eighty nine compounds accounting for between 98.5 and 99.4% of the total essential oil were identified. The phenylpropanoids eugenol (89.37 ± 0.29%) and eugenol (56.34 ± 0.41%), followed by eugenol acetate (19.48 ± 0.13%) were, respectively, the main compounds in clove and cinnamon essential oils, whereas large amounts of the oxygenated monoterpenes 1,8-cineole (58.07 ± 0.83%) and α-terpinyl acetate (13.05 ± 0.44%) were found in bay leaf essential oil. Clove and cinnamon oils showed the best antifungal activity results against all tested fungi. Against Alternaria alternata, clove essential oil displayed the best antifungal effect, whereas against Curvularia hawaiiensis, cinnamon essential oil was more active. Both essential oils showed a similar antifungal effect towards Fusarium proliferatum and Fusarium oxysporum. In vitro studies in inoculated rice grains showed that clove and cinnamon totally inhibited pathogenic fungal development after 30 days of incubation. In vivo studies showed that eugenol used with a polysaccharide such as agar–agar formed a fine coat which wraps the inoculated rice grains, creating a natural biofilm and reducing the development of all pathogenic fungi (80–95%) for 30 days.
Introduction
Cereal fungal contamination causes both economic and human health problems. Economically important diseases, such as smuts, leaf spots, crown rots, and root rots are usually caused by Bipolaris, Curvularia, Fusarium, and Alternaria species.1 Infection of cereal seeds is a serious problem because these pathogens can remain viable for 10 years and are subsequently capable of propagating across other geographical areas, infecting further crops and achieving global dissemination.2,3Bipolaris and Curvularia are closely related genera of plant pathogens as well as emerging opportunistic human pathogens.4−6 Several species, according to the method of infection and immune status, have been reported, from mild skin and nail infections to severe invasive human diseases. Curvularia is an important dematiaceous fungus involved in phaeohyphomycosis; Curvularia australiensis, Ctenochaetus hawaiiensis, and Curvularia spicifera have frequently been isolated from human phaeohyphomycoses.7−9 These three species were formerly classified as members of the genus Bipolaris; however, phylogenetic studies have demonstrated that species previously placed in Bipolaris, especially those known as human pathogens, actually belong to the Curvularia genus.4
The genus Fusarium includes plant pathogens of agricultural crops, as well as mycotoxin-producing species.10 Several species of Fusarium, such as Fusarium verticillioides, Fusarium proliferatum, and Fusarium oxysporum are responsible for the higher fumonisin levels observed in cereals. It is well known that these mycotoxins constitute a principal health risk for domesticated animals, being also associated with a number of human health problems, probably due to the consumption of large amounts of cereal-based products.11 These emerging problems need increasing attention because of their toxic effects. Particularly worrying is the recently found high contamination levels of breakfast and infant cereals, such as muesli and cornflakes, which usually contain favorable ingredients for fungi colonization, with Fusarium mycotoxins such as ochratoxin A that has a studied impact on human health.12
Fusarium, together with Alternaria, Cladosporium, and Trichoderma genera, belongs to the allergenic fungi, important producers of outdoor airborne allergens which have been found in plant and soil samples from agricultural fields in which cereals are grown.13
On the other hand, Alternaria is the most common genus of endophytes in plants. Pathogenic Alternaria such as Alternaria alternata are used to control the host weed. A suspension of Alternaria J46 mycelial segments and culture filtrates of the fungi display marked seed germination inhibition against different species, including the most important cereal crops worldwide for human consumption, wheat and rice.14 This represents important economic losses as well as health problems due to Alternaria, which is also an important allergenic fungi and opportunistic human pathogen in immunocompromised patients.
Previous studies carried out by our research team showed significant antifungal effects of commercial oregano and thyme essential oils against phytopathogenic fungi.15,16 The aims of this work were to (1) analyze the chemical composition of commercial essential oils of bay leaf, cinnamon, and clove, (2) determine the most important isolates and to identify four pathogens from rice seeds by morphological and molecular techniques, (3) evaluate in vitro and in vivo antifungal activity of essential oils, and (4) evaluate a natural biofilm we have created as an antifungal product for rice conservation.
Results
Chemical Composition of Commercial Essential Oils
The identified components of commercial cinnamon, clove, and bay leaf essential oils are shown in Table 1. The individual compounds were identified by MS, and their identity was confirmed by comparison of their retention indices (RIs) and mass spectra with authentic samples or with data already available in the NIST 2005 Mass Spectral Library.17
Table 1. Chemical Composition of Commercial Clove, Cinnamon, and Bay Leaf Essential Oilsa.
| compound | RI | clove | cinnamon | bay |
|---|---|---|---|---|
| monoterpene hydrocarbons | 3.84 ± 0.11 | 12.63 ± 0.22 | ||
| tricyclene | 926 | 0.04 ± 0.00 | ||
| α-thujene | 930 | 0.06 ± 0.00 | 0.10 ± 0.00 | |
| α-pinene | 939 | 0.77 ± 0.03 | 2.93 ± 0.05 | |
| camphene | 952 | 0.37 ± 0.01 | 0.33 ± 0.00 | |
| sabinene | 976 | 5.56 ± 0.09 | ||
| β-pinene | 979 | 0.29 ± 0.01 | 2.44 ± 0.10 | |
| myrcene | 991 | 0.05 ± 0.00 | 0.38 ± 0.00 | |
| α-phellandrene | 1005 | 0.26 ± 0.01 | 0.10 ± 0.00 | |
| δ-3-carene | 1011 | 0.03 ± 0.00 | 0.04 ± 0.00 | |
| α-terpinene | 1019 | 0.10 ± 0.00 | 0.07 ± 0.00 | |
| p-cymene | 1027 | 0.89 ± 0.02 | 0.50 ± 0.07 | |
| limonene | 1029 | 0.01 ± 0.00 | ||
| β-phellandrene | 1030 | 1.00 ± 0.03 | ||
| cis-ocimene | 1039 | 0.04 ± 0.00 | ||
| trans-ocimene | 1052 | 0.03 ± 0.00 | ||
| γ-terpinene | 1061 | 0.06 ± 0.00 | ||
| terpinolene | 1089 | 0.03 ± 0.00 | 0.02 ± 0.00 | |
| oxygenated monoterpenes | 1.14 ± 0.04 | 81.76 ± 0.24 | ||
| 1,8-cineole | 1034 | 58.07 ± 0.83 | ||
| cis-sabinene hydrate | 1070 | 0.01 ± 0.00 | 0.13 ± 0.00 | |
| cis-linalool oxide | 1072 | 0.05 ± 0.00 | ||
| trans-linalool oxide | 1086 | 0.03 ± 0.00 | ||
| linalool | 1098 | 0.57 ± 0.01 | 3.82 ± 0.04 | |
| trans-sabinene hydrate | 1101 | 0.11 ± 0.00 | ||
| cis-p-menth-2-en-1-ol | 1121 | 0.02 ± 0.00 | 0.06 ± 0.00 | |
| trans-pinocarveol | 1140 | 0.04 ± 0.00 | ||
| trans-p-menth-2-en-1-ol | 1141 | 0.03 ± 0.00 | 0.04 ± 0.00 | |
| camphor | 1146 | 0.02 ± 0.00 | 0.01 ± 0.00 | |
| sabina ketone | 1157 | 0.03 ± 0.00 | ||
| pinocarvone | 1162 | 0.02 ± 0.00 | ||
| δ-terpineol | 1165 | 0.12 ± 0.01 | ||
| borneol | 1169 | 0.12 ± 0.00 | 0.05 ± 0.00 | |
| terpinen-4-ol | 1179 | 0.13 ± 0.00 | 2.00 ± 0.04 | |
| p-cymen-8-ol | 1185 | 0.03 ± 0.00 | 0.04 ± 0.00 | |
| α-terpineol | 1190 | 0.17 ± 0.00 | 2.26 ± 0.06 | |
| myrtenol | 1194 | 0.15 ± 0.01 | ||
| α-fenchyl acetate | 1219 | 0.18 ± 0.01 | ||
| nerol | 1229 | 0.31 ± 0.01 | ||
| linalyl acetate | 1258 | 0.28 ± 0.01 | ||
| p-menth-2-en-1,4-diol | 1268 | 0.02 ± 0.00 | ||
| bornyl acetate | 1288 | 0.52 ± 0.00 | ||
| carvacrol | 1299 | 0.05 ± 0.01 | ||
| δ-terpinyl acetate | 1315 | 0.25 ± 0.01 | ||
| α-terpinyl acetate | 1349 | 13.05 ± 0.44 | ||
| neryl acetate | 1361 | 0.15 ± 0.00 | ||
| sesquiterpene hydrocarbons | 8.06 ± 0.50 | 2.56 ± 0.02 | 0.29 ± 0.02 | |
| α-copaene | 1376 | 0.36 ± 0.01 | ||
| β-elemene | 1390 | 0.10 ± 0.01 | ||
| β-caryophyllene | 1419 | 6.02 ± 0.41 | 1.81 ± 0.01 | 0.10 ± 0.00 |
| α-humulene | 1454 | 1.70 ± 0.09 | 0.38 ± 0.00 | |
| allo-aromadendrene | 1460 | 0.02 ± 0.00 | ||
| γ-muurolene | 1479 | 0.02 ± 0.00 | ||
| β-selinene | 1490 | 0.02 ± 0.00 | ||
| α-muurolene | 1498 | 0.02 ± 0.00 | ||
| γ-cadinene | 1512 | 0.04 ± 0.01 | ||
| trans-calamenene | 1521 | 0.14 ± 0.01 | ||
| δ-cadinene | 1521 | 0.12 ± 0.01 | 0.06 ± 0.01 | |
| α-calacorene | 1544 | 0.02 ± 0.00 | ||
| oxygenated sesquiterpenes | 0.93 ± 0.03 | 1.40 ± 0.03 | 0.16 ± 0.01 | |
| spathulenol | 1578 | 0.18 ± 0.00 | 0.04 ± 0.00 | |
| caryophyllene oxide | 1583 | 0.77 ± 0.04 | 1.05 ± 0.03 | 0.12 ± 0.01 |
| humulene epoxide II | 1608 | 0.15 ± 0.01 | 0.15 ± 0.00 | |
| cubenol | 1646 | 0.01 ± 0.00 | ||
| aromatic compounds (C6–C3; C6–C1) | 89.54 ± 0.16 | 89.91 ± 0.54 | 4.24 ± 0.24 | |
| benzyl acetate | 1162 | 0.05 ± 0.00 | ||
| methyl chavicol | 1196 | 0.21 ± 0.01 | ||
| chavicol | 1250 | 0.04 ± 0.00 | ||
| trans-cinnamaldehyde | 1270 | 2.08 ± 0.00 | ||
| safrole | 1287 | 0.12 ± 0.03 | ||
| cinnamyl alcohol | 1304 | 0.08 ± 0.01 | ||
| eugenol | 1359 | 89.37 ± 0.29 | 56.34 ± 0.41 | 0.91 ± 0.07 |
| hydrocinnamyl acetate | 1368 | 0.34 ± 0.00 | ||
| dihydro eugenol | 1369 | 0.02 ± 0.01 | ||
| vanillin | 1396 | 0.08 ± 0.02 | 0.06 ± 0.00 | |
| methyl eugenol | 1405 | 0.02 ± 0.00 | 3.00 ± 0.15 | |
| trans-cinnamyl acetate | 1446 | 6.52 ± 0.03 | ||
| trans-methyl isoeugenol | 1494 | 0.12 ± 0.01 | ||
| eugenol acetate | 1522 | 19.48 ± 0.13 | ||
| 4-hydroxy-3-methoxy-cinnamaldehyde | 1729 | 0.06 ± 0.01 | ||
| benzyl benzoate | 1760 | 4.81 ± 0.03 | ||
| others | 0.52 ± 0.15 | 0.02 ± 0.00 | 0.31 ± 0.01 | |
| isopropyl-isobutyrate | 793 | 0.01 ± 0.00 | ||
| 2-methyl-ethyl-butanoate | 849 | 0.02 ± 0.00 | ||
| 3-hexen-1-ol | 853 | 0.06 ± 0.00 | ||
| 3-methyl-ethyl-butanoate | 858 | 0.02 ± 0.00 | ||
| 2-methyl-1-methylethyl-butanoate | 885 | 0.01 ± 0.00 | ||
| 2-heptanol | 896 | 0.03 ± 0.00 | ||
| 2-methyl-butyl-propanoate | 917 | 0.04 ± 0.00 | ||
| 2-nonanone | 1090 | 0.03 ± 0.00 | ||
| 2-methyl-2-methylbutyl-butanoate | 1100 | 0.02 ± 0.00 | ||
| monoacetin | 1263 | 0.04 ± 0.00 | ||
| diacetin | 1268 | 0.02 ± 0.00 | ||
| 2-undecanone | 1293 | 0.11 ± 0.00 | ||
| triacetin | 1376 | 0.47 ± 0.15 | ||
| total identified | 99.04 ± 0.08 | 98.55 ± 0.06 | 99.40 ± 0.06 | |
RI: retention index relative to C8–C32n-alkanes on an HP-5MS column; t: trace amount ≤0.01. Values are means ± standard deviation of three samples.
Eighty nine compounds accounting for between 98.5 and 99.4% of the total essential oil were identified. In clove and cinnamon essential oils, more than 89% of aromatic compounds biosynthesized by the shikimic acid pathway were found: the phenylpropanoid eugenol (89.37 ± 0.29%) and eugenol (56.34 ± 0.41%), followed by eugenol acetate (19.48 ± 0.13%) were, respectively, the main compounds, whereas large amounts of the oxygenated monoterpenes 1,8-cineole (58.07 ± 0.83%) and α-terpinyl acetate (13.05 ± 0.44%) were found in bay leaf essential oil.
Antifungal Activity in Solid Media
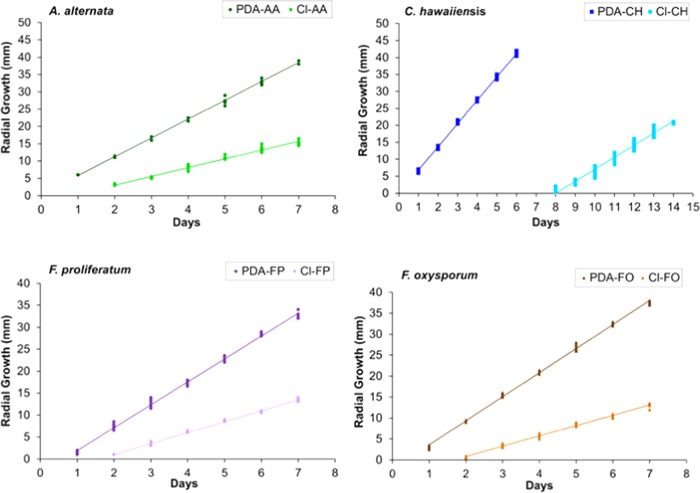
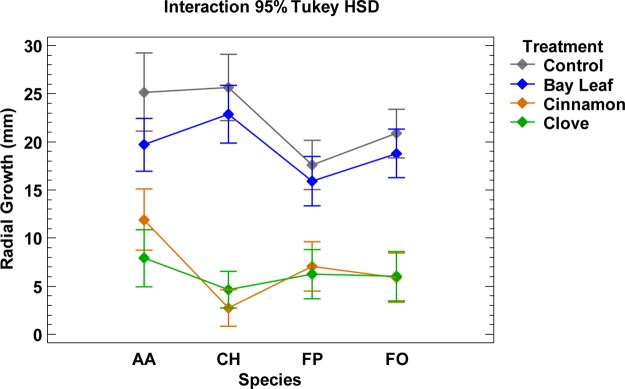
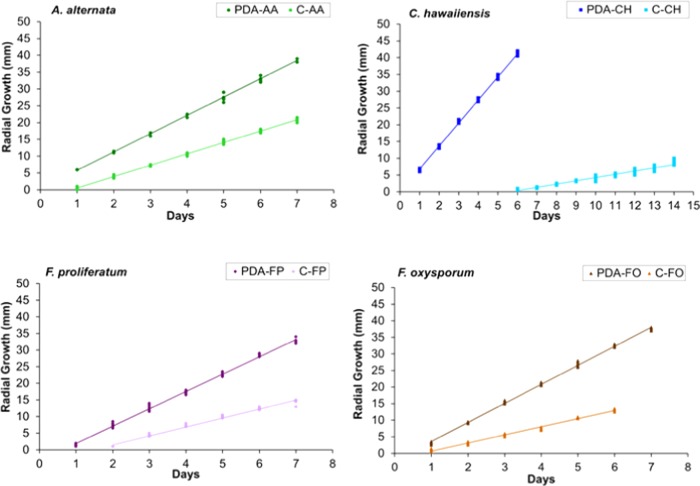
Clove essential oil was more effective against A. alternata, whereas cinnamon essential oil was more active against C. hawaiiensis (Table 2, Figures 1–3). Both essential oils displayed a similar antifungal effect against F. proliferatum and F. oxysporum. Cinnamon and clove completely inhibited the growth of C. hawaiiensis to days 7 and 8 (Figures 1 and 2). In model conditions assayed at 300 μg/mL, bay leaf essential oil showed no significant antifungal activity against the tested phytopathogenic fungi, whereas clove and cinnamon showed significant antifungal activity against all tested fungi, with a similar behavior pattern (Figure 3).
Table 2. Effects of Clove (Cl), Cinnamon (C), and Bay Leaf (BL) essential oils (300 μg/mL) on Radial Growth and Growth Rates of A. alternata, C. hawaiiensis, F. proliferatum, and F. oxysporum. Confidence Intervals with a Probability of 0.95a.
| species-treatment | mean | lower limit | upper limit | GR |
|---|---|---|---|---|
| A. alternata-PDA | 25.11 ± 2.24 | 20.71 | 29.51 | 5.44 (0.99) |
| A. alternata-Cl | 7.88 ± 1.26 | 5.40 | 10.36 | 2.53 (0.97) |
| A. alternata-C | 11.85 ± 1.29 | 9.32 | 14.41 | 3.39 (0.99) |
| A. alternata-BL | 19.67 ± 1.52 | 16.69 | 22.65 | 4.80 (0.99) |
| C. hawaiiensis-PDA | 25.61 ± 1.89 | 21.90 | 29.32 | 7.00 (0.99) |
| C. hawaiiensis-CL | 4.61 ± 0.82 | 2.99 | 6.22 | 1.10 (0.96) |
| C. hawaiiensis-C | 2.72 ± 0.78 | 1.19 | 4.24 | 0.97 (0.94) |
| C. hawaiiensis-BL | 22.83 ± 1.66 | 19.57 | 26.09 | 6.20 (0.99) |
| F. proliferatum-PDA | 17.56 ± 1.40 | 14.80 | 20.31 | 5.21 (0.99) |
| F. proliferatum-CL | 6.22 ± 1.09 | 4.08 | 8.37 | 2.49 (0.99) |
| F. proliferatum-C | 7.03 ± 1.03 | 5.00 | 9.07 | 2.71 (0.99) |
| F. proliferatum-BL | 15.88 ± 1.40 | 13.12 | 18.64 | 5.13 (0.99) |
| F. oxysporum-PDA | 20.82 ± 1.40 | 18.06 | 23.58 | 5.74 (0.99) |
| F. oxysporum-CL | 5.98 ± 1.09 | 3.84 | 8.13 | 2.46 (0.99) |
| F. oxysporum-C | 5.85 ± 1.03 | 3.81 | 7.88 | 2.44 (0.98) |
| F. oxysporum-BL | 18.77 ± 1.40 | 16.01 | 21.53 | 5.26 (0.99) |
Mean: mean radius ± standard error; GR: growth rate (R2).
Figure 1.
Growth rate (mm/day) of fungi on potato-dextrose agar (PDA) and clove (Cl) essential oil (300 μg/mL). A. alternata (PDA-AA: control; Cl-AA: clove), C. hawaiiensis (PDA-CH: control; Cl-CH: clove), F. proliferatum (PDA-FP: control; Cl-FP: clove), and F. oxysporum (PDA-FO: control; Cl-FO: clove)
Figure 3.
Interaction plot (mean radius, species, and treatment) at 300 μg/mL of bay leaf, cinnamon, and clove against A. alternata (AA), Curvularia hawaiiensis (CH), F. proliferatum (FP), and F. oxysporum (FO).
Figure 2.
Growth rate (mm/day) of fungi on PDA and cinnamon (C) essential oil (300 μg/mL). A. alternata (PDA-AA: control; C-AA: cinnamon), C. hawaiiensis (PDA-CH: control; C-CH: cinnamon), F. proliferatum (PDA-FP: control; C-FP: cinnamon), and F. oxysporum (PDA-FO: control; C-FO: cinnamon).
For the measured mycelial growth inhibition (MGI), the antifungal effect of cinnamon at doses of 100 and 200 μg/mL against C. hawaiiensis is noteworthy (Table 3). At the highest concentration (300 μg/mL), pure eugenol showed the best antifungal activity results against all tested phytopathogenic fungi (Table 3).
Table 3. Mycelial Growth Inhibition (MGI) of A. alternata (AA), C. hawaiiensis (CH), F. proliferatum (FP) and F. oxysporum (FO) with Cinnamon (C), Clove (Cl), and Eugenol (E)a.
| AA |
CH |
FP |
FO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| concentration (μg/mL) | C | Cl | E | C | Cl | E | C | Cl | E | C | Cl | E |
| 100 | 19.81 | 33.61 | 39.97 | 77.85 | 26.96 | 49.59 | 22.25 | 17.53 | 35.38 | 23.59 | 21.04 | 31.46 |
| 200 | 29.72 | 50.12 | 51.65 | 82.55 | 62.85 | 93.14 | 33.97 | 31.68 | 43.67 | 42.63 | 40.43 | 67.77 |
| 300 | 55.73 | 62.67 | 66.28 | 100 | 100 | 100 | 55.64 | 54.80 | 60.06 | 65.28 | 64.94 | 95.02 |
MGI: percentage inhibition.
Essential Oils on Rice Storage
In vitro studies showed that the disease produced in rice grains inoculated with all tested fungi (A. alternata, C. hawaiiensis, F. proliferatum, and F. oxysporum) was totally inhibited when the kernels were placed into PDA-clove and PDA-cinnamon (Figure 4). Both essential oils completely inhibited the growth of pathogenic fungi in the caryopsis rice at 300 μg/mL after 30 days of incubation.
Figure 4.
Experiment 1. Effect of essential oils on rice grain conservation after 30 days. From left to right: A. alternata on PDA, PDA-bay leaf, PDA-clove, and PDA-cinnamon (300 μg/mL). The PDA and PDA-bay leaf plates show the development of the inoculated fungus A. alternata plus endophytic mycobiota, whereas the PDA-cinnamon and PDA-clove plates show total inhibition of the development of A. alternata and endophytic mycobiota.
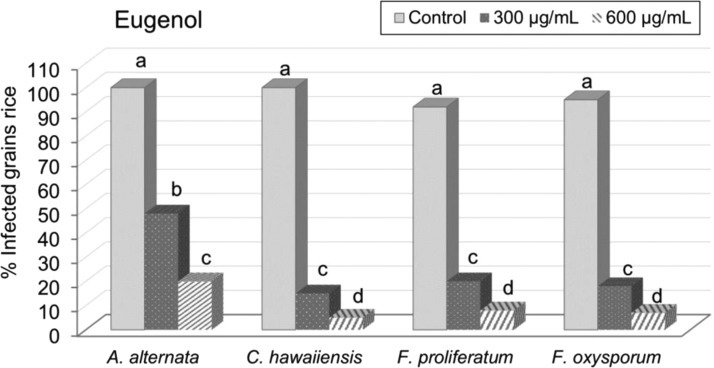
In vivo studies showed that eugenol used with a polysaccharide such as agar–agar (0.25%) formed a fine coat which wraps the inoculated rice grains, creating a natural biofilm and reducing the development of all pathogenic fungi at 300 and 600 μg/mL (Figure 4). Eugenol significantly (P < 0.05) reduced fungal growth in stored rice, depending on the dose used after 30 days of incubation at 28 °C. At 300 μg/mL, eugenol showed high antifungal activity, reducing C. hawaiiensis, F. proliferatum, and F. oxysporum by between 85 and 82%. At 600 μg/mL, A. alternata was reduced by 80% and the highest antifungal activity was 95 and 92% in C. hawaiiensis, F. proliferatum, and F. oxysporum, showing an antifungal effect after 30 days of incubation at 28 °C (Figure 5).
Figure 5.
Efficacy of different concentrations of eugenol (300 and 600 μg/mL) on fungal development of A. alternata, C. hawaiiensis, F. proliferatum, and F. oxysporum in inoculated rice grains after 30 days. Significant difference at 95% level probability using Fisher’s least significant difference.
Discussion
Eugenol, the main compound in both essential oils, is a natural phenolic compound characterized, among a wide range of biological properties, by its antifungal activity;16 in the year 2013 it was approved as a fungicide by the European Food Safety Authority (EFSA), (Reg. EU No. 546/2013). Also, the use of eugenol coated with polysaccharides such as agar–agar enhances the shelf life of rice during the storage period.18
The chemical versatility of its structure has led to the use of this compound as a starting biological material for the synthesis of new antifungal eugenol derivative agents to reduce nosocomial infections caused by Candida spp., especially in patients admitted to an intensive care unit.19 The antifungal activity of eugenol against clinically relevant fungi, including fluconazole-resistant strains, has also been observed for clove essential oil, which is able to inhibit Aspergillus and Candida species (such as C. albicans, C. tropicalis, and Candida parapsilosis) and fluconazole-resistant C. albicans isolates, as well as clinical dermatophyte strains.20
According to samples of Syzygium aromaticum leaf essential oil grown in Madagascar21 and in the commercial clove oil analyzed here, the main compound is the phenylpropanoid eugenol (89.37 ± 0.29%), followed by the sesquiterpene hydrocarbons β-caryophyllene (6.02 ± 0.41%) and α-humulene (1.70 ± 0.09%). Only two compounds, caryophyllene oxide (0.77 ± 0.04%) and humulene epoxide (0.15 ± 0.01%), were detected in the oxygenated sesquiterpene fraction. In addition, neither hydrocarbons nor oxygenated monoterpenes were present in the commercial S. aromaticum essential oil analyzed here. However, the main compounds found in Cinnamomum verum were the phenylpropanoid eugenol (56.34 ± 0.41%), eugenol acetate (19.48 ± 0.13%), E-cinnamyl acetate (6.52 ± 0.03%), and E-cinnamaldehyde (2.08 ± 0.00%), along with a large amount of the aromatic compound benzyl benzoate (4.81 ± 0.03%). On the other hand, in this essential oil, 30 compounds biosynthesized from the mevalonic acid pathway were identified but only the monoterpene hydrocarbon β-phellandrene (1.00 ± 0.03%), the sesquiterpene hydrocarbon β-caryophyllene (1.81 ± 0.01%), and the oxygenated sesquiterpene caryophyllene oxide (1.05 ± 0.03%) reached percentages close to or higher than 1%. Finally, oxygenated monoterpenes were quantitatively the minor fraction (1.14 ± 0.04%) in cinnamon essential oil, with linalool (0.57 ± 0.01%), α-terpineol (0.17 ± 0.01%), terpinen-4-ol (0.1 3± 0.00%), and borneol (0.12 ± 0.00%) as the main compounds. Although both essential oils have the same main compound, eugenol (89.37 ± 0.29 vs 56.34 ± 0.41%), commercial clove essential oil contains a higher quantity of sesquiterpene hydrocarbons (8.06 ± 0.50 vs 2.56 ± 0.02%), whereas commercial cinnamon essential oil has more oxygenated sesquiterpenes (1.40 ± 0.03 vs 0.90 ± 0.03%) and both hydrocarbons (3.84 ± 0.13%) and oxygenated monoterpenes (1.14 ± 0.04%) that were not detected in clove essential oil. S. aromaticum and Cinnamomum zeylanicum were the most active tested essential oils against stem and ear rot caused by Stenocarpella maydis responsible for severe losses in maize, reducing the pathogen incidence in the seeds by 39.0 and 28.0%, respectively.22 Although C. zeylanicum has a lower content of eugenol than S. aromaticum, the presence of eugenol acetate (19.48 ± 0.13%), trans-cinnamyl acetate (6.52 ± 0.03%), benzyl benzoate (4.81 ± 0.03%), and trans-cinnamaldehyde (2.08 ± 0.00%) in the commercial cinnamon essential oil here analyzed may contribute to the antifungal activity.23,24 In fact, cinnamaldehyde was found to have more antifungal activity than eugenol against Aspergillus fumigatus and Trichophyton rubrum(25) and a study about the antifungal effects against Fusarium spp. and C. zeylanicum with trans-cinnamaldehyde as the main compound showed more anti-Fusarium activity than with Citrus limon, Juniperus communis, Eucalyptus citriodora, Gaultheria procumbens, Melaleuca alternifolia, Origanum majorana, Salvia sclarea, and Thymus vulgaris essential oils.26
On the other hand, eugenol (57.0%) has been described as the main compound in commercial bay leaf essential oil27 as well as in samples of Laurus nobilis essential oil (eugenol 44.13%), followed by a large amount of cinnamaldehyde (30.28%)28 that has antifungal effects against fungi (Eurotium, Aspergillus, and Penicillium) commonly responsible for spoilage of bakery products or postharvest diseases produced by A. alternata in cherry tomatoes, respectively. However, the commercial bay leaf essential oil analyzed here is characterized by a high monoterpene fraction content (94.39%), mainly oxygenated monoterpenes (81.76 ± 0.24%), with 1,8-cineol (58.07 ± 0.83%) and α-terpinyl acetate (13.05 ± 0.44%) as the main compounds. A similar composition was found in commercial L. nobilis essential oil from Spain with medicinal items 1,8-cineole (51%) and α-terpinyl acetate (10%),29 with L. nobilis essential oil from Brazil 1,8-cineole (35.50%), linalool (14.10%), sabinene (9.45%), and terpinyl acetate (9.65%)30 or with dried bay leaves purchased from a local market in Tunisia, with 1,8-cineole (39.76%) and α-terpinyl acetate (13.35%)31 as the main compounds. Bay leaf essential oils with a high content of 1,8-cineole, linalool and terpinyl acetate were shown to have antibacterial activity toward foodborne pathogens, such as Escherichia coli and Yersinia enterocolitica in fresh Tuscan sausage,30 and also antifungal activity against Botrytis cinerea, Monilinia laxa, and Penicillium digitatum.32 However, the tested bay leaf essential oil with a high content of the oxygenated monoterpene 1,8-cineol did not show a significant antifungal effect against the four pathogenic fungi isolated from rice grains. These results are in accordance with the potency level of antifungal activity measured as in vitro mycelial growth of the main compounds (thymol > eugenol > carvone > terpinen-4-ol > 1,8-cineole) of essential oils.33
Conclusions
Clove and cinnamon essential oils with the highest amounts (89.37 and 56.34%, respectively) of eugenol, contrary to bay leaf essential oil containing 58.07% of the oxygenated monoterpene 1,8-cineole, have a significant antifungal effect against the four pathogenic fungi isolated from rice grains. In vivo studies showed that eugenol used with a polysaccharide such as agar–agar forms a fine coat that wraps the inoculated rice grains, creating a natural biofilm and reducing the development of all pathogenic fungi (80–95%) for 30 days. Eugenol could be used as an effective nontoxic preservative in stored rice grains against A. alternata, C. hawaiiensis, F. proliferatum, and F. oxysporum contamination, increasing their shelf life.
Materials and Methods
Plant Material
Commercial samples of clove leaf (Syzygium aromaticum L.) were supplied by Guinama and cinnamon leaf (C. verum J. Presl) and bay leaf (Laurus nobilis L.) essential oils were supplied by Essential Arôms. The essential oils were stored at 4 °C until chemical analysis and antifungal studies were done.
Gas Chromatography (GC)–Mass Spectrometry (MS)
A gas chromatography–mass spectrometry (GC/MS) analysis was carried out with Agilent 5973N apparatus, equipped with a capillary column (95 dimethylpolysiloxane-5% diphenyl) and an HP-5MS UI (30 m long and 0.25 mm i.d. with 0.25 μm film thickness). The column temperature program was 60 °C for 5 min, with 3 °C/min increases to 180 °C and then 20 °C/min increases to 280 °C. This program was maintained for 10 min. Helium was the carrier gas used at a flow rate of 1 mL/min. Split mode injection (ratio 1:30) was employed. Mass spectra were taken over the m/z 30–500 range, with an ionizing voltage of 70 eV. Kovat’s retention index (RI) was calculated using cochromatographed standard hydrocarbons. The individual compounds were identified by MS, and their identity was confirmed by comparison of their RIs, relative to C8–C32n-alkanes, and mass spectra with authentic samples or with data already available in the NIST 2005 Mass Spectral Library and in the literature.17
Fungal Species
Four phytopathogenic fungi, A. alternata (Fr.) Keissler CECT 20943 (LBEA 2103), Curvularia hawaiiensis Manamgoda, Cai & Hyde CECT 20934 (LBEA 2105) F. proliferatum (Matsush.) Nirenberg CECT 20944 (LBEA 2170), and F. oxysporum (Sacc.) Snyder & Hansen CECT 2715 (LBEA 2004) were isolated in the Laboratorio Botánica of the Departament of Ecosistemas Agroforestales (LBEA) Universitat Politècnica de València from Bomba rice samples collected in a Mediterranean region producing rice (Valencia, Spain). The fungal species were morphologically and molecularly identified and then deposited in the Spanish Type Culture Collection (CECT).
Fungal Strain Identification
Morphological analysis consisted of inoculation, incubation, and validation of culture characteristics, as well as microscopic observation, growing data, or colony morphology. Molecular analysis was based on amplification, sequencing, and BLAST alignment comparison of target regions in fungal DNA for forward and reverse directions: internal transcribed spacer regions ITS1 and ITS2 of ribosomic DNA, including the 5.8S rRNA gene, using the primers its1 and its4;34,35 D1/D2 domains on the 5′ end of the gene which codifies 28S rRNA, using nl1 and nl4 primers;36,37 a 0.3 kb fragment of the EF-1α gene (transcription elongation factor), with EF-1728F and EF-986R primers38 and, finally, partial sequencing of the β-tubulin gene using Bt2a and Bt2b primers.39
Sequence comparison between the amplified regions and those available in the NCBI Taxonomy Database (http://www.ncbi.nlm.nih.gov/taxonomy) and BLAST analysis of the sequences against assembling the fungal tree of life and MycoBank/CBS-KNAW Fungal Biodiversity Center (BioloMICSNet Software) databases showed that the isolate LBEA 2103 (CECT 20943) showed 99% identity for ITS regions and 100% identity for 28S rRNA 5′ domains with C. hawaiiensis (synonymous with Curvularia oryzae); the isolate LBEA 2105 (CECT 20923) showed 100% identity for ITS regions, 99% identity for EF-1α elongation factor, and 100% identity for the β-tubulin gene with the complex Alternaria sp. aff. A. alternata; the isolate LBEA 2170 (CECT 20944) showed 100% identity for ITS regions and 99.67% identity for β-tubulin gene with the species F. proliferatum (teleomorph Gibberella intermedia), and finally, the isolate LBEA 2004 (CECT 2715) showed 100% identity for ITS regions and 99.65% identity for EF-1α elongation factor with F. oxysporum.
Antifungal Activity in Solid Media
Growth Rate and Mycelial Growth Inhibition (MGI)
Essential oils were dissolved, mixed, and homogenized in previously sterilized and still liquid PDA/Tween 20 (0.1%) at 300 μg/mL. Then, it was distributed in 90 × 15 and 150 × 15 mm2 Petri dishes. Fungi were sowed in the center of each Petri dish with 8 mm discoid explants from a 7 day culture. Petri dish plates were incubated in the dark at 25 °C for 7 and 14 days. Control Petri dishes only had PDA/Tween 20 (0.1%). Fungi growth was evaluated by measuring the daily diameter of the colony in two perpendicular directions, calculating the speed of growth. Six repetitions were made per treatment. MGI also was calculated according to the following formula at 100, 200, and 300 μg/mL
CD: average diameter of colonies in nontreated dishes (without essential oil); OD: average diameter of colonies in treated dishes (with essential oil).
Essential Oils on Rice Storage
Valencian rice healthy grains were washed with sodium hypochlorite (20%) for 5 min, rinsed twice with distilled water, and air-dried at room temperature (25 ± 2 °C). Then, 150 seeds of rice for each tested fungus were dipped into a flask containing 50 mL of a spore suspension of 5 × 105 conidia/mL prepared in water–Tween 20 (0.1%) for 30 min; finally, they were air-dried to complete dryness. Two experiments were carried out.
In Vitro Study of Essential Oil Antifungal Effect on Rice Caryopsis
The eugenol (98%) used in this study was supplied by Sigma-Aldrich. Inoculated rice caryopsis were placed into Petri dishes containing PDA-bay leaf, PDA-clove, and PDA-cinnamon, 300 μg/mL (5 seeds per plate). For each fungus, six replicate dishes were used. Plates were incubated in the dark at 25 °C and high relative humidity (90–95%) for 30 days. Control Petri dishes contained equal amounts of sterilized water/Tween 20 (0.1%) on PDA but without essential oils. Fungal growth was evaluated through observation for 30 days.
In Vivo Study of Eugenol Antifungal Effect on Rice Caryopsis
Rice caryopsis inoculated with the molds were placed inside 150 × 150 mm2 plastic boxes, 100 seeds per box. Two concentrations (300 and 600 μg/mL) of eugenol were prepared in Tween 20 (0.1%)–agar 0.25%. Then, 5 mL of each solution was sprayed into the boxes. The seeds were wetted with the prepared solutions and dried to complete dryness, forming a fine coating. Controls were prepared similarly for volatile treatment with equal amounts of sterilized water/Tween 20 (0.1%)–agar 0.25% but without eugenol. All boxes were then transferred to storage at 28 °C and high relative humidity (90–95%) for 30 days. The percentage of infected rice grains was recorded after 30 days of incubation with an Olympus SZX10 magnifying glass.
Statistical Analysis
The fungal growth results were submitted to an analysis of variance (ANOVA). Furthermore, HSD Tukey intervals were represented with significant values at P < 0.05. Data analysis was performed using Statgraphics Centurion XVI.
Acknowledgments
This study has been financed by the Ministerio de Economía y Competitividad; Subdirección General de Proyectos de Investigación; Convocatoria de Ayudas a Proyectos de I+D+i; Research Challenges and Program-Oriented Societal Challenges 2014-2017, reference number AGL2013-42989-R-AR. The authors also thank the Central Service for Experimental Research of the University of Valencia (SCSIE) for providing the gas chromatography–mass spectrometry equipment and the Colección Española de Cultivos Tipo (CECT) for providing equipment for molecular identification of strains.
The authors declare no competing financial interest.
References
- Al-Sadi A. M.; Deadman M. L. Influence of seed-borne Cochliobolus sativus (Anamorph Bipolaris sorokiniana) on crown rot and root rot of barley and wheat. J. Phytopathol. 2010, 158, 683–690. 10.1111/j.1439-0434.2010.01684.x. [DOI] [Google Scholar]
- Farr D. F.; Rossman A. Y.. Fungal Databases, Systematic Mycology and Microbiology Laboratory; ARS, USDA, 2013. http://nt.ars-grin.gov/fungaldatabases. [Google Scholar]
- Zhang N.; Rossman A. Y.; Seifert K.; Bennett J. W.; Cai G.; Cai L.; Hillman B.; Hyde K. D.; Luo J.; Manamgoda D.; Meyer W.; Molnar T.; Schoch C.; Tadych M.; White J. F. Jr.. Impacts of the International Code of Nomenclature for Algae, Fungi and Plants (Melbourne Code) on the Scientific Names of Plant Pathogenic Fungi; American Phytopathological Society, 2013, APS Feature. http://www.apsnet.org/publications/apsnetfeatures/Pages/Melbourne.aspx. [Google Scholar]
- Madrid H.; da Cunha K. C.; Gené J.; Dijksterhuis J.; Cano J.; Sutton D. A.; Guarro J. P.; Crous W. Novel Curvularia species from clinical specimens. Persoonia 2014, 33, 48–60. 10.3767/003158514X683538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manamgoda D. S.; Rossman A. Y.; Castlebury L. A.; Crous P. W.; Madrid H.; Chukeatirote E.; Hyde K. D. The genus Bipolaris. Stud. Mycol. 2014, 79, 221–288. 10.1016/j.simyco.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar S. G.; Patterson J. E.; Sutton D. A.; Pullen R.; Rinaldi M. G. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin. Infect. Dis. 2002, 34, 467–476. 10.1086/338636. [DOI] [PubMed] [Google Scholar]
- Krizsán K.; Tóth E.; Nagy L. G.; Galgóczy L.; Manikandan P.; Chandrasekaran M.; Kadaikunnan S.; Alharbi N. S.; Vágvölgyi C.; Papp T. Molecular identification and antifungal susceptibility of Curvularia australiensis, C. hawaiiensis and C. spicifera isolated from human eye infections. Mycoses 2015, 58, 603–609. 10.1111/myc.12367. [DOI] [PubMed] [Google Scholar]
- Mikosz C. A.; Smith R. M.; Kim M.; Tyson C.; Lee E. H.; Adams E.; Straif-Bourgeois S.; Sowadsky R.; Arroyo S.; Grant-Greene Y.; Duran J.; Vasquez Y.; Robinson B. F.; Harris J. R.; Lockhart S. R.; Török T. J.; Mascola L.; Park B. J. Fungal endophthalmitis outbreak response team. Fungal endophthalmitis associated with compounded products. Emerging Infect. Dis. 2014, 20, 248–56. 10.3201/eid2002.131257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes K.; Capilla J.; Sutton D. A.; Mayayo E.; Fothergill A. W.; Guarro J. Experimental treatment of Curvularia infection. Diagn. Microbiol. Infect. Dis. 2014, 79, 428–431. 10.1016/j.diagmicrobio.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Aoki T.; O’Donnell K.; Geiser D. M. Systematics of key phytopathogenic Fusarium species: current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. 10.1007/s10327-014-0509-3. [DOI] [Google Scholar]
- Vismer H. F.; Shephard G. S.; Rheeder J. P.; van der Westhuizen L.; Bandyopadhyay R. Relative severity of fumonisin contamination of cereal crops in West Africa. Food Addit. Contam. 2015, 11, 1952–1958. 10.1080/19440049.2015.1084654. [DOI] [PubMed] [Google Scholar]
- Mahnine N.; Meca G.; Elabidi A.; Fekhaoui M.; Saoiabi A.; Font G.; Mañés J.; Zinedine A. Further data on the levels of emerging Fusarium mycotoxins enniatins (A, A1, B, B1), beauvericin and fusaproliferin in breakfast and infant cereals from Morocco. Food Chem. 2011, 124, 481–485. 10.1016/j.foodchem.2010.06.058. [DOI] [Google Scholar]
- Weikl F.; Radl V.; Munch J. C.; Pritsch K. Targeting allergenic fungi in agricultural environments aids the identification of major sources and potential risks for human health. Sci. Total Environ. 2015, 529, 223–230. 10.1016/j.scitotenv.2015.05.056. [DOI] [PubMed] [Google Scholar]
- Hao S. H.; Wei Y.; Wang J.; Zhou Y. M. Allelopathy and the active metabolites of the endophytic fungus, Alternaria J46, from Platycladus orientalis. Weed Biol. Manage. 2015, 15, 95–101. 10.1111/wbm.12072. [DOI] [Google Scholar]
- Roselló J.; Sempere F.; Sanz-Berzosa I.; Chiralt A.; Santamarina M. P. Antifungal activity and potential use of essential eils egainst Fusarium culmorum and Fusarium verticillioides. J. Essent. Oil-Bear. Plants 2015, 18, 359–367. 10.1080/0972060X.2015.1010601. [DOI] [Google Scholar]
- Santamarina M. P.; Ibañez M. D.; Marques M.; Roselló J.; Giménez S.; Blázquez M. A. Bioactivity of essential oils in phytopathogenic and post-harvest fungi control. Nat. Prod. Res. 2017, 31, 2675–2679. 10.1080/14786419.2017.1286479. [DOI] [PubMed] [Google Scholar]
- Adams R. P.Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, Illinois, 2007. [Google Scholar]
- Mohammadi A.; Hashemi M.; Hosseini S. M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Technol. 2015, 110, 203–213. 10.1016/j.postharvbio.2015.08.019. [DOI] [Google Scholar]
- Abrão P. H. O.; Pizi R. B.; de Souza T. B.; Silva N. C.; Fregnan A. M.; Silva F. N.; Coelho L. F. L.; Malaquias L. C. C.; Dias A. L. T.; Dias D. F.; Veloso M. P.; Carvalho D. T. Synthesis and biological evaluation of new eugenol Mannich bases as promising antifungal agents. Chem. Biol. Drug Des. 2015, 86, 459–465. 10.1111/cbdd.12504. [DOI] [PubMed] [Google Scholar]
- Pinto E.; Vale-Silva L.; Cavaleiro C.; Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- Srivastava A. K.; Srivastava S. K.; Syamsundar K. V. Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flavour Fragrance J. 2005, 20, 51–53. 10.1002/ffj.1364. [DOI] [Google Scholar]
- Teixeira G. A.; Alves E.; Amaral D. C.; Machado J. D.; Perina F. J. Essential oils on the control of stem and ear rot in maize. Cienc. Rural 2013, 43, 1945–1951. 10.1590/S0103-84782013001100004. [DOI] [Google Scholar]
- Lee H. C.; Cheng S. S.; Chang S. T. Antifungal property of the essential oils and their constituents from Cinnamomum osmophloeum leaf against tree pathogenic fungi. J. Sci. Food Agric. 2005, 85, 2047–2053. 10.1002/jsfa.2216. [DOI] [Google Scholar]
- Sumalan R. M.; Alexa E.; Poiana M. A. Assessment of inhibitory potential of essential oils on natural mycoflora and Fusarium mycotoxins production in wheat. Chem. Cent. J. 2013, 7, 32 10.1186/1752-153X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S. A.; Ahmad I. In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum, Syzygium and Cymbopogon species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine 2011, 19, 48–55. 10.1016/j.phymed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Homa M.; Fekete I. P.; Boszormenyi A.; Singh Y. R. B.; Selvam K. P.; Shobana C. S.; Manikandan P.; Kredics L.; Vagvolgyi C.; Galgoczy L. Antifungal effect of essential oils against Furarium keratitis isolates. Planta Med. 2015, 81, 1277–1284. 10.1055/s-0035-1546272. [DOI] [PubMed] [Google Scholar]
- Guynot M. E.; Ramos A. J.; Setó L.; Purroy P.; Sanchis V.; Marín S. Antifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products. J. Appl. Microbiol. 2003, 94, 893–899. 10.1046/j.1365-2672.2003.01927.x. [DOI] [PubMed] [Google Scholar]
- Blázquez M. Role of natural essential oils in sustainable agriculture and food preservation. J. Sci. Res. Rep. 2014, 3, 1843–1860. 10.9734/JSRR/2014/11376. [DOI] [Google Scholar]
- Peris I.; Blázquez M. A. Comparative GC-MS analysis of bay leaf (Laurus nobilis L.) essential oils in commercial samples. Int. J. Food Prop. 2015, 18, 757–762. 10.1080/10942912.2014.906451. [DOI] [Google Scholar]
- Da Silveira S. M.; Bittencourt F. L.; Fronza N.; Cunha A. Jr.; Scheuermann G. N.; Werneck Vieira C. R. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT--Food Sci. Technol. 2014, 59, 86–93. 10.1016/j.lwt.2014.05.032. [DOI] [Google Scholar]
- Boulila A.; Hassen I.; Haouari L.; Mejri F.; Amor I. B.; Casabianca H.; Hosni K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Ind. Crops Prod. 2015, 74, 485–493. 10.1016/j.indcrop.2015.05.050. [DOI] [Google Scholar]
- De Corato U.; Maccioni O.; Trupo M.; Di Sanzo G. Use of essential oil of Laurus nobilis obtained by means of a supercritical carbon dioxide technique against postharvest spoilage fungi. Crop Prot. 2010, 29, 142–147. 10.1016/j.cropro.2009.10.012. [DOI] [Google Scholar]
- Morcia C.; Malnati M.; Terzi V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam., Part A 2012, 29, 415–422. 10.1080/19440049.2011.643458. [DOI] [PubMed] [Google Scholar]
- Schoch C. L.; Seifert K. A.; Huhndorf S.; Robert V.; Spouge J. L.; Levesque C. A.; Chen W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 6241–6246. 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. J.; Bruns T.; Lee S.; Taylor J.. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protocols; Innis M. A., Gelfand D. H., Sninsky J. J., White T. J., Eds.; A Guide to Methods and Applications; Academic Press: San Diego, 1990; pp 315–322. [Google Scholar]
- Kurtzman C. P.; Robnett C. J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 1998, 73, 331–371. 10.1023/A:1001761008817. [DOI] [PubMed] [Google Scholar]
- O’Donnell K.; Cigelnik E.; Nirenberg H. I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. 10.2307/3761407. [DOI] [Google Scholar]
- Carbone I.; Kohn L. M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. 10.2307/3761358. [DOI] [Google Scholar]
- Glass N. L.; Donaldson G. C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]