Introduction
Neuropathic pain (NeuP) is defined as pain arising from a lesion or disease of the somatosensory nervous system[39; 88]. NeuP is common, affecting approximately 6-8% of the general population[14; 86] and currently treatment is inadequate due to both poor drug efficacy and tolerability[38]. Many different types of injury can cause neuropathic pain including genetic (e.g. SCN9A gain of function variants), metabolic (e.g. diabetic polyneuropathy), infective (e.g. HIV associated neuropathy, hepatitis), traumatic and toxic (e.g. chemotherapy induced neuropathy) causes. Such injurious events can impact on anatomically distinct regions of the somatosensory nervous system ranging from the terminals of nociceptive afferents (in small fiber neuropathy) to the thalamus (in post-stroke pain). Classification of neuropathic pain using etiology and location remains an important aspect of routine clinical practice; however, pain medicine is coming to the realization that we need more precision in this classification. The hope is that improved classification will lead to better understanding of risk, prognosis and optimal treatment of NeuP.
Patient stratification is the process of identifying subgroups of patients, suffering from a disorder (such as NeuP) in order to better target medical intervention[92]. Such sub-groups may map to a particular pathogenic mechanism but could also simply be a constellation of clinical symptoms and signs or biomarker, which are predictive of treatment response. Personalized medicine aims to target intervention to individual patients and is therefore even more ambitious in scope[68]. Personalized medicine may be possible in rare cases of NeuP (usually associated with specific gene mutations) but for the most part we will discuss stratified pain medicine in this review.
Both preclinical and clinical science, have identified an array of pathogenic mechanisms underlying NeuP ranging from ectopic activity in primary afferents to defective central pain modulation pathway (for a comprehensive review see [18]). It is not a new idea that we should be trying to understand pain mechanisms in patients [106; 107] although there are challenges in being able to assess specific mechanisms in individual patients. Stratification aims to achieve patient subgroupings that have utility in terms of diagnosis, prognosis or treatment and this may not relate to a single pathogenic mechanism. Fortunately our armamentarium for identifying patient subgroups (and in some cases directly assaying pathogenic mechanisms) in patients has greatly improved. In the first section of this manuscript we will review the means by which NeuP patients may be stratified and in the second section the potential benefits of stratification. Thomas Lewis said, ‘Diagnosis is a system of more or less accurate guessing, in which the endpoint achieved is a name. These names applied to disease come to assume the importance of specific entities, whereas they are for the most part no more than insecure and therefore temporary conceptions’. He was likely exaggerating for effect but we hope that patient stratification will not only reduce the uncertainty in diagnosis but also help improve prevention, prognostication and treatment.
How can we stratify NeuP patients?
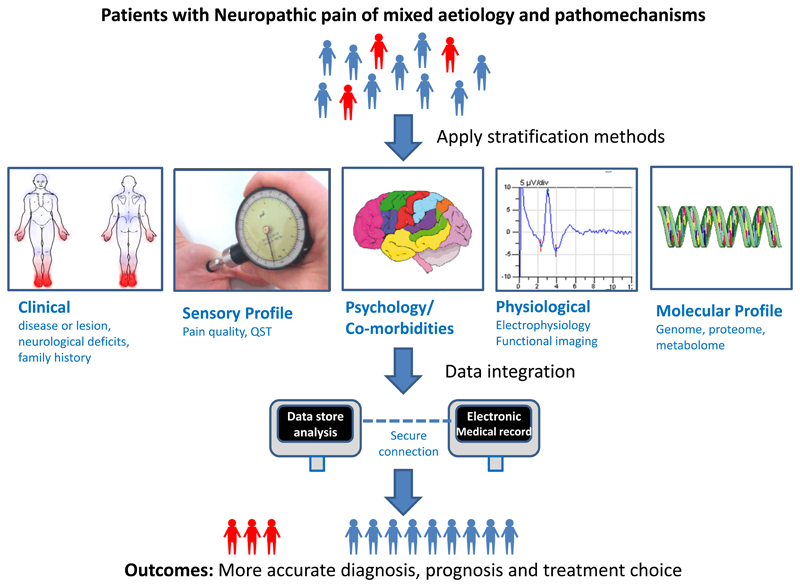
As in all medicine detailed clinical history and examination remain important in the assessment of neuropathic pain. An important aspect on history is the temporal course of pain onset and its relationship to the underlying disease process. The examination should be comprehensive and relevant to the disease process and history. For example, the presence of limb erythema with a diagnosis of erythromelalgia or absent lower limb reflexes as a consequence of peripheral neuropathy. Stratification of NeuP patients incorporates a multidisciplinary approach. Figure 1 provides a schematic representation of some of the techniques that can be used to stratify NeuP patients. A detailed description of the techniques will be discussed below.
Figure 1.
Schematic representation of some of the techniques that can be used to stratify neuropathic pain patients. Techniques to stratify patients in the context of neuropathic pain have been developed over the last decade. These include: detailed clinical assessment, psychophysical tools to assess sensory profiles; questionnaires to assess pain quality, pain severity, comorbidities and psychological impact; neurophysiological tools that can include nerve conduction studies, somatosensory evoked potentials and functional brain imaging; and, molecular profiling. Integration of data from diverse sources such as electronic health records, routine investigation and specialised investigations from biobank material, followed by downstream multivariate analysis provides a framework that will yield improvements in diagnosis, prognosis and treatment outcomes.
Sensory phenotype
In the last decade significant advances in techniques to define somatosensory phenotype in the context of NeuP have been developed. These include questionnaires to assess pain quality, psychophysical tools to assess sensory perception, and alteration of experimental pain through conditioned pain modulation.
Pain quality
A variety of tools have been developed to both screen and characterize the qualities of NeuP. Screening questionnaires, such as the DN4 [12], painDETECT [43] and LANSS[7; 8] are used to identify patients with neuropathic pain. The screening questionnaires incorporate descriptors of sensory symptoms to generate a score that helps predict whether the pain is likely to be neuropathic. Examples include “burning” quality to pain or the presence of paresthesias. The DN4 also includes an examination component to test for sensory loss and/or allodynia. The above questionnaires can be used to screen for neuropathic pain at a primary care level[1]. For example, the DN4 questionnaire has demonstrated excellent sensitivity and specificity in screening for NeuP in patients diagnosed with diabetic neuropathy (DPN) [81; 85]. The screening questionnaires have been validated to discriminate between neuropathic and non-neuropathic pain and translated in to over 90 languages[1]. The Neuropathic Pain Symptom Inventory (NPSI) [13], is a self-administered questionnaire developed to characterize the qualities of NeuP.
A major advantage of these questionnaires is that they are self-administered and can be used to capture data from large cohorts of patients. Analysis of large datasets has shown that NeuP caused by different etiologies share sensory symptom profiles [5; 42]. The profiles may reflect different pathophysiological pathways, independent of etiology, that cause NeuP. Hierarchical cluster analysis based on the painDETECT questionnaire of 2100 patients diagnosed with painful DPN or postherpetic neuralgia revealed five distinct symptom profile subgroups [5]. The different subgroups occurred in both groups of patients. Principal component analysis and hierarchical cluster analysis of individual pain dimensions based on NPSI descriptors completed by 1225 patients (diagnosed with central post stroke pain, painful DPN, painful HIV neuropathy and posttraumatic peripheral pain) identified three clusters with distinct symptom profiles [42]. The three clusters represented three different subgroups of patients that were seen across the different NeuP syndromes. A smaller study identified six distinct NeuP profiles, based on the NPSI, among patients with a variety of NeuP syndromes [80]. Although different clusters were identified in each study, grouping of patients based on sensory symptom profiles rather than solely etiology may yield new understanding of NeuP neurobiology and improve response to pain therapies. A step forward is the integration of questionnaires and sensory testing to better capture somatosensory profiles [99].
Quantitative sensory testing
Quantitative sensory testing (QST) is a psychophysical tool that assesses evoked sensory perception in response to a defined sensory stimulus [69]. The German research network of neuropathic pain (DFNS) developed and validated a standardized QST protocol that tests 13 parameters of sensory function [70]. The sensory modalities include small fiber sensory function, such as thermal detection/pain thresholds and pinprick sensitivity, and large fiber sensory function, such as mechanical and vibration detection thresholds. The standardization of QST data collection has significant advantages. Data collected across different centers can be compared against a large control population cohort, controlling for age and gender effects, and be combined to significantly increase statistical power [58; 59; 98]. A limitation of QST is that it requires a significant investment in equipment and examinations are lengthy. In a recent study, QST profiles of 1135 patients collected from multiple centers with peripheral neuropathic pain revealed three distinct phenotypes [4]. The three phenotypes were characterized by sensory loss, thermal hyperalgesia and mechanical hyperalgesia. These phenotypes can be found across different etiologies of NeuP but vary in frequency [100]. For example, the most common phenotype in diabetic polyneuropathy is sensory loss (64%), followed by mechanical hyperalgesia (20%) and thermal hyperalgesia (17%). In contrast, post herpetic neuralgia is characterized by the mechanical hyperalgesia phenotype (45%), followed by thermal hyperalgesia (35%) and sensory loss (20%). Such stratification of neuropathic pain may yield a greater understanding of the pathophysiological mechanisms that are shared across somatosensory phenotypes or specific to etiology. Somatosensory profiles can also be used to predict treatment response (discussed below).
Conditioned pain modulation
Conditioned pain modulation (CPM) refers to the dynamic psychophysical protocols that provide insight into an individual’s inhibitory pain modulation processes [46]. If a patient is asked to rate the pain intensity of a certain “test stimulus” (such as contact heat applied to the volar surface of the forearm), and then given the combination of a noxious “conditioning stimulus” (such as immersion of the opposite hand in a hot water bath) and a repeated similar “test stimulus”, the perceived pain intensity of the latter “test stimulus” will generally be lower than when given alone. CPM efficiency refers to the reduction of pain intensity between the two “test stimuli”. Less efficient CPM, was reported for chemotherapy-induced neuropathic pain [63] and peripheral neuropathy patients [93] when compared to healthy control participants. Thus impaired inhibitory pain modulation processes may be present in patients from suffering from NeuP. There is a growing body of evidence suggesting that CPM may be an important biomarker of chronic pain and a predictor of treatment response. Less efficient pre-operative CPM may predict chronic post-operative pain [105; 111]. Less efficient CPM was observed in a group of patients with painful DPN that reported a larger analgesic response to duloxetine (see below) [112]. While CPM holds great promise, limiting factors include the heterogeneity of protocols, significant variability reported in the size and stability of the CPM effect in healthy volunteers, and the inability to disentangle different mechanisms in individuals with different causes of chronic pain [55].
Physiological measures: Electrophysiology and functional brain imaging
Standard neurophysiological techniques, such as nerve conduction studies, investigation of trigeminal reflexes (including the blink reflex) and measurement of somatosensory evoked potentials, are commonly used to investigate neuropathic pain[25]. These techniques are broadly designed to assess the non-nociceptive pathways. They are most helpful in confirming a lesion within the peripheral or central somatosensory nervous system. Despite not assessing the pain pathways directly (as C-fibre activity is poorly represented in these outputs) emerging evidence does implicate focal demyelination of non-nociceptive Aβ fibers in neuropathic pain related to carpal tunnel syndrome [91] and ophthalmic post-herpetic neuralgia [89], as these abnormalities are correlated with paroxysmal pain and abnormal sensations. Laser Evoked Potentials (LEPs) is the preferred technique for assessment of nociceptive pathway function, due to ease of use and reliability [24]. Pulses of laser generated radiant heat are used to selectively excite free nerve endings in the superficial skin layers, which activates Aδ and C nociceptors and gives rise to brain evoked potentials specifically related to activation of ascending thermal-pain systems. Suppression of LEPs suggests a diagnosis of neuropathic pain [89–91]. LEP amplitudes are correlated to the severity of constant pain in patients with carpal tunnel syndrome [91] and ophthalmic post-herpetic neuralgia [89]. Microneurography is a unique neurophysiological technique that uses a microelectrode to record nerve activity directly from a peripheral nerve fascicle. It has been used to directly study nociceptor afferent activity in a wide range of neuropathic pain conditions [31]. Abnormal patterns of firing and distribution of nociceptive afferent subclasses have been identified in conditions such as painful DPN[65], painful neuropathy [57], small fiber neuropathy [73] and erythromelalgia [66]. Such aberrant activity is thought to be a key driver of peripheral neuropathic pain. The functional brain imaging field has adopted stratification of patients to identify pathological mechanisms of pain [87]. The descending pain modulatory system (DPMS) is a brainstem–subcortical–cortical network that can modulate nociceptive input to the brain. Pre-clinical studies have shown that DPMS is important in chronic pain states. Studies that have stratified patients according to NeuP contribution have shown that persistent pain may be linked to an imbalance in DPMS function, either due to a diminished inhibitory and/or an enhanced facilitatory capacity of the DPMS [47; 61; 72]. Patients with hip osteoarthritis pain [47] (before hip replacement surgery) that scored higher on painDETECT (i.e. neuropathic pain contribution more likely) demonstrated increased facilitatory DPMS activity when compared to patients that scored lower on painDTECT (i.e. neuropathic pain contribution less likely). Furthermore functional brain imaging has been used to disambiguate the efficacy of different pain treatments using an experimental model of central sensitization, which is a contributory pathomechanism of neuropathic pain [102]. After capsaicin induced central sensitization, gabapentin (a neuropathic pain medication), when compared to placebo and ibuprofen (non-neuropathic pain medication), suppressed resting state connectivity and secondary mechanical hyperalgesia evoked neural response in a region of the brainstem DPMS.
Molecular profiling
Genomics is having a growing influence on medical practice in providing a molecular pathogenic link to disease as well as clinically relevant outcomes such as treatment response. There are many genes that have a role in the pathogenesis of neuropathic pain however we will focus on variants in the gene SCN9a which provides one of the best examples of modern genomics applied to pain medicine [116]. SCN9a encodes Nav1.7 [6; 30] which is a voltage gated sodium channel (VGSC) expressed by sensory neurons. A number of rare pain disorders which are inherited in a Mendelian fashion are associated with mutations in this gene. Bi-allelic inactivating mutations in NaV1.7 result in congenital insensitivity to pain (CIP) and anosmia [20]. Heterozygous gain of function mutations in the same channel can lead to: inherited erythromelalgia[110] (IEM, characterized by pain and erythema of the extremities exacerbated by warmth) or paroxysmal extreme pain disorder (PEPD, associated with episodic pain and erythema of the sacrum and mandible triggered by mechanical stimulation)[37]. IEM provides an excellent example of how a molecular mechanism links to a pathophysiological pain driver. Nav1.7 mutations causing IEM result in gain of function of Nav1.7[26] resulting in hyperexcitability of sensory neurons been demonstrated both experimentally and by microneurographic recordings from IEM patients). There is a broad correlation between the biophysical dysfunction of the ion channel and the associated pain syndrome: IEM mutations causing a greater hyperpolarising shift in the voltage dependence of activation result in a more severe clinical phenotype [22; 49].
Small fiber neuropathy (SFN) is a more common condition than IEM presenting with burning pain of the extremities associated with small fiber degeneration[84]. A number of rare Nav1.7 variants (which are distinct to those causing IEM) have now also been linked to SFN and lead to gain of function in this ion channel[36]. Nav1.7 also provides a good example of how certain gene variants may not cause Mendelian pain disorders but contribute as risk factors for the development and severity of much more common acquired NeuP states. The concept being that such variants would not cause symptoms in the naïve state but can contribute to NeuP in the context of an environmental stressor such as the development of DPN. Studying a carefully phenotyped cohort of patients with DPN there was a higher prevalence of rare Nav1.7 variants in those patients with painful (10% of patients) versus painless DPN (0 patients) [10]. Two of these novel variants associated with painful DPN were shown to impair inactivation of Nav1.7 resulting in gain of function providing a physiological link to the development of pain.
Because not all Nav1.7 variants are likely to be pathogenic careful genetic counselling is required and functional analysis of Nav1.7 variants remains critical [103]. Genomics is now increasingly been integrated into clinical practice and the ‘100 000 genomes’ project will sequence the whole genomes of 75 000 people suffering from rare disorders (including familial pain disorders) as part of routine NHS care within the UK[78]. In the future it may become routine to sequence the genomes of large populations in order to appropriately target health care. The technology for such sequencing is available although there are still great challenges in information processing and ascribing pathogenicity to the variants found. Techniques are also being developed for high throughput assessment of epigenetic changes as well as the downstream effects of gene function including mRNA expression (transcriptome), protein expression (proteome) and metabolites (metabolome)[71]. In the future these may also be helpful in stratifying NeuP patients. One issue is that unlike oncology pathological material from the somatosensory nervous system is not easily accessible. However it is becoming possible to generate induced pleuripotent stem cells from patients which can provide a scalable source of sensory neurons[17] for molecular, physiological[104] and even pharmacological profiling[16]. This really would be an example of ‘personalized’ pain medicine however it is likely to have most utility in situations where there are strong genetic drivers of neuropathic pain and the workflow would need to be streamlined before this could be used in routine clinical practice.For now this is restricted to research practice.
Psychological profile and co-morbidities
NeuP, as with every form of pain, alarms, demands attention and interferes with ongoing activities[32]. Consequently, patients with neuropathic pain experience a lower ability to accomplish tasks of daily living, a lower quality of life, a lower mood and more sleep problems than those without pain[52; 79]. It may be expected that the presence of NeuP triggers a cascade of psychosocial processes that may finally maintain or exacerbate suffering, distress and disability.
To a large extent, these processes are similar to those involved in other forms of pain [23; 33]. Just as with musculoskeletal pain, anxiety or worrying about the pain and its possible consequences may lead to avoidance, and to more pain, distress and disability[97]. Nevertheless, the experience of NeuP has some particularities [27]. Avoidance seems to be less triggered by a fear that physical activities will increase pain or worsen their condition. Patients with neuropathic pain may rather avoid social situations because the feeling of clothes against the skin is uncomfortable. The unpredictable nature of paroxysmal pain may turn patients generally anxious and uncertain. These specific features in the phenomenology of NeuP need to be further explored.
We should go beyond a documentation of the comorbidities that patients experience. We need to understand how exactly these problems come about. It will be useful to put the assessment and treatment of neuropathic pain within the psychological context of the primary disorder. The patient struggling with diabetes and painful DPN has different needs from the patient with HIV neuropathy, who both have different needs from the patient with post-mastectomy pain syndrome. That way, we will identify what exactly patients are worried about, their specific beliefs about illness, pain and treatment, and how these factors impact their life. Pain management programs will need to be tailored and adapted to account for the specific contexts of NeuP [27]. Unfortunately, there are yet insufficient clinical trials allowing us to conclude that psychological treatments for NeuP work[35].
Overall, research on the role of psychological variables in NeuP is a relatively unexplored territory. It largely consists of cross-sectional studies. We do not know yet whether and how exactly psychological variables causally contribute to the development or maintenance of NeuP [52]. Neuropathic pain may well be a condition in which biobehavioural variables interact from onset. Anxiety, depression and stress may have a direct impact on disease processes and pain. No study has yet explored this hypothesis. Notwithstanding, there is strong evidence that anxiety, depression and stress contribute to disease onset and may delay wound healing via the immune and neuroendocrine system[56]. Psychological factors may also indirectly affect disease. Cognitions and emotions may be obstacles for the adoption of a healthy lifestyle, treatment adherence and optimal self-management. Each of these pathways may affect underlying disease mechanisms. In diabetes mellitus, patients who are anxious and depressed are less physically active and eat less healthy, exacerbating disease processes. Patients who have a low mood, are less adherent to their medication regime[45]. Inappropriate beliefs about the illness and treatment, may lead to suboptimal treatment and poor self-management [96].
A more context sensitive approach to the psychology of chronic NeuP is needed that builds on what we know from general behavioural science and behavioural pain medicine[34], but that translates it to the needs of the specific patient group.
Data integration
An important question is to what extent is stratification based on different modalities correlated? Taking genotype and sensory profile as an example there is a link between the two but this is not an exact match. Patients with IEM with known mutations in Nav1.7 actually showed surprising diversity in their sensory profile although the vast majority did show heat pain hypersensitivity measured by quantitative sensory testing at unaffected skin sites[60]. In painful DPN there was a correlation between genotypes and sensory profile but only to one measure: enhanced pressure pain sensitivity was noted in those patients with painful DPN with rare Nav1.7 variants compared to those patients without rare Nav1.7 variants [10]. Taking the approach of starting with the sensory profile of NeuP patients and then sequencing candidate genes Binder et al., showed that variants in TRPA1 (an ion channel activated by environmental irritants and cold) were associated with paradoxical heat sensation [9].
Ultimately the intersection between different modalities may be particularly helpful in stratification. We are in the era of ‘big data’ (data generated in large volume, at high velocity and in a variety of formats) in which bioinformatics approaches can be used to integrate prospective electronic health records, routine investigations and specialized tests using biobank material[19; 71]. This requires significant computing power as well as the ability to deal with the security and ethical challenges associated with such large amounts of personal data. It will be extremely powerful in generating hypotheses that can then be tested in focused cohorts providing potent opportunities for future research.
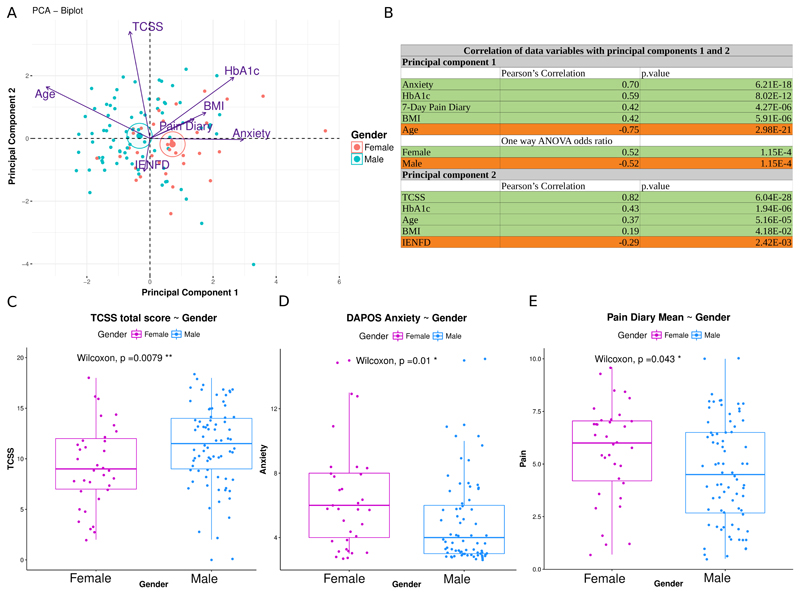
Multivariate analysis enables the study of multiple different, possibly correlated, factors as a cause of variation within a population and their relationship to pain. To provide an example we undertook principal components analysis in patients with painful DPN [76]. This revealed that the relationship between pain and different clinical and psychological factors were dependent on gender in patients with painful DPN. Multivariate principal components analysis, showed that anxiety (as measured with the DAPOS questionnaire), poor glucose control (high HbA1c), high BMI and high 7-Day pain diary scores were more prevalent in females, while more severe neuropathy (as assessed using the Toronto Clinical Scoring System TCSS and IENFD) was more prevalent in males (Figure 2). These findings emphasise the importance of one of the simplest forms of stratification: gender, but also the utility in studying multiple variables.
Figure 2.
A: PCA biplot of individuals and variables based on the first 2 principal components in a cohort of patients with neuropathic pain secondary to diabetic neuropathy. Dots represent individuals projected on the 2-dimensional plot. Individuals are colour coded according to gender. Active variables used for constructing the components are projected on the 2-dimensional plot, with arrows proportional to the variable’s contribution to the principal component. Angles between variables (co-sinus) represent their correlation. Arrows pointing in opposite directions indicate negatively correlated variables, pointing in the same direction indicating positively correlated variables and perpendicular are the uncorrelated variables. Centroids of the groups are shown with large dots and ellipses.
B: Correlation of original variables with the principal components. Pearson's R correlation coefficient is shown only when significant (p value < 0.05) for active continuous variables. Principal component’s association with the supplementary categorical variables “Male” and “Female” was calculated using one-way ANOVA. The first component (PC1) was strongly and significantly associated with anxiety, body mass index (BMI), high self-reported scores, younger age, and high HbA1c (glycosylated haemoglobin) and the second component (PC2) was associated with more severe neuropathy and low intra-epidermal nerve fibre density (IENFD) (Figure B). Principal component 1, was also significantly associated with females.
The rows highlighted in green are for positively correlated variables. The rows highlighted in orange are for negatively correlated variables.
C-E: Boxplots showing the median and the interquartile range for TCSS (Toronto Clinical Scoring System a measure of neuropathy severity) total score, DAPOS anxiety score and 7-Day pain diary mean score for males and females. The two-sided Mann-Whitney Wilcoxon rank sum test was used to compare groups. Males had significantly higher TCSS scores (Figure C) while women reported significantly higher anxiety (Figure D) and scores in the 7-Day pain diary (Figure E) (p value < 0.05 *, p value < 0.01 **).
The analysis completed in this figure adopted the same techniques as applied in Sieberg et al. [76]. The current data was restricted to only those patients with neuropathic pain.
If a stratification measure only has a small effect size or is overly complex and time consuming it will not be adopted in clinical practice. For final clinical use therefore stratification measures will require extensive optimization and field testing.
Utility of patient stratification
Diagnosis of neuropathic pain
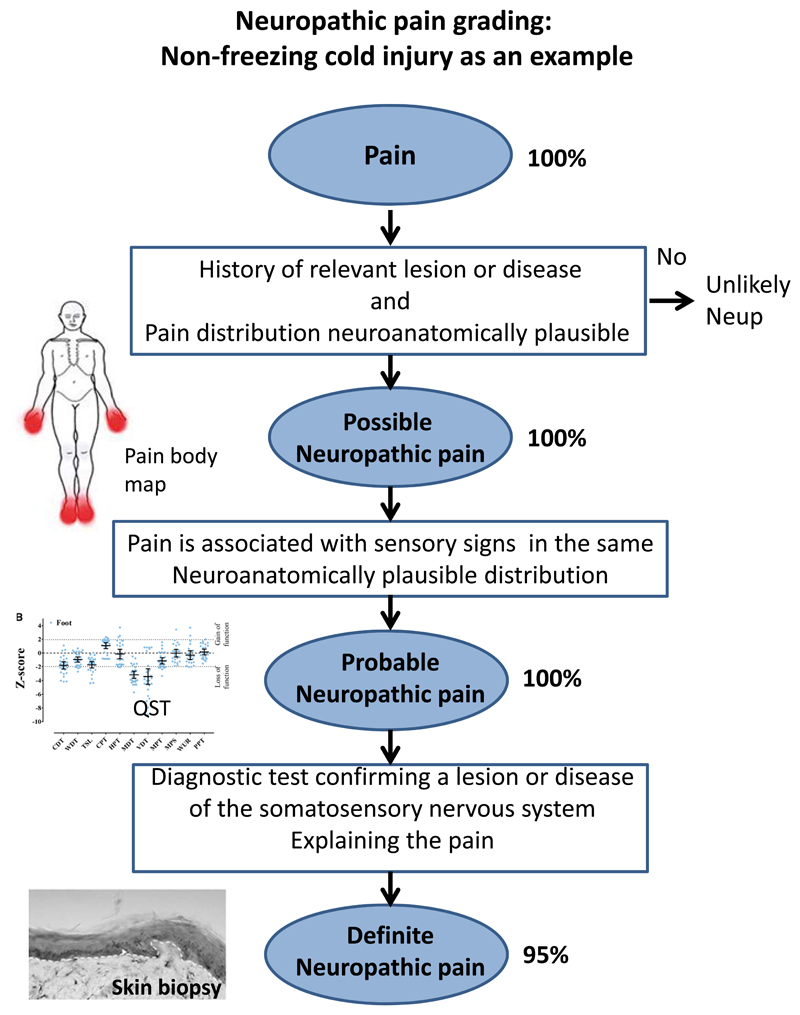
An important step in the stratification of patients is to determine the certainty of neuropathic pain diagnosis on an individual basis. A revised grading of neuropathic pain has been developed by Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain to facilitate the correct classification of pain as neuropathic [40]. The grading is based on the following criteria. Possible neuropathic pain must fulfil criteria 1 and 2. Probable neuropathic pain must fulfil criteria 1, 2 and 3. Definite neuropathic pain must fulfil all 4 criteria.
Pain with a distinct neuroanatomically plausible distribution.
A history suggestive of a relevant lesion or disease affecting the peripheral or central somatosensory system.
Demonstration of distinct neuroanatomically plausible distribution of neuropathic pain
Demonstration of the relevant lesion or disease by at least one confirmatory test
Neuropathic pain has been shown to be present in a number of previously poorly understood conditions, such as recessive dystrophic epidermolysis bullosa and non-freezing cold injury [95; 101], in which a neuropathic component may not have been suspected or described. Recessive dystrophic epidermolysis bullosa is an inherited dermal condition characterized by bullous eruption of the skin and is associated with severe, debilitating pain. Application of the new NeupSIG grading system demonstrated that 62% of patients with epidermolysis bullosa had a definite diagnosis of neuropathic pain, 24% had a probable diagnosis of neuropathic pain, and 13.7% had a possible diagnosis of neuropathic pain [101]. Based on this finding, inherited epidermolysis bullosa was shown to cause a small fibre neuropathy and patients were started on appropriate neuropathic pain therapies. Non-freezing cold injury is an umbrella term used to describe an environmental injury in which soldiers that are exposed to cold and wet conditions can develop pain and sensory disturbance of the feet and hands. We showed using detailed clinical examination, quantitative sensory testing and skin biopsy to determine intra-epidermal nerve fibre density (IENFD) that the sensory disturbance is caused by a sensory neuropathy and application of the new NeupSIG grading system demonstrated that 95.2% of patients with non-freezing cold injury had a definite diagnosis of neuropathic pain (Figure 3) [95]. The demonstration of impaired small fiber function in fibromyalgia in particular is interesting. Fibromyalgia is a syndrome characterized by widespread pain. Careful phenotyping using the NPSI questionnaire, clinical examination, electrophysiology including pain evoked potentials, skin biopsy for IENFD, and microneurography demonstrated that the pain experienced in fibromyalgia has a significant neuropathic component caused by dysfunction within small fibers [74; 94].
Figure 3.
This provides an example of how detailed phenotyping and application of the NeupSIG grading system was applied to non-freezing cold injury. Non-freezing cold injury arises following exposure to a cold wet environment, most commonly in army service personnel. This condition is associated with disabling chronic pain but the basis of this pain remained mysterious. All study participants gave a history of exposure to a cold wet environment with acute onset of sensory symptoms (pain, numbness and paresthesia) that then persisted for at least 3 months. Pain was present symmetrically in the hands and feet (a body map is shown). Possible neuropathic pain was fulfilled in 100% of cases. Bed-side clinical sensory examination and quantitative sensory examination revealed sensory loss in the hands and feet and all subjects met criteria for probable neuropathic pain. Skin biopsy revealed reduced intra-epidermal nerve fibers confirming a lesion at structural level and 95% of study participants met criteria for definite neuropathic pain. Data used in figure derived from Vale et al., [92].
The revised neuropathic pain grading is a significant improvement on previous approaches as it offers a methodical and hierarchical process of diagnosis that can be applied in clinical and research settings. It provides a rational basis to prioritize investigations and to commence appropriate neuropathic pain treatment.
Understanding pathogenic mechanisms underlying neuropathic pain in patients
Neuropathic pain is a complex multidimensional clinical entity and the underlying pathogenic mechanisms that cause neuropathic pain are not understood. A number of pathogenic mechanisms, based on pre-clinical studies, are postulated to play a role in acquired neuropathic pain disorders, such as painful DPN [82]. We believe that a stratified approach can help translate findings between the clinical and pre-clinical arenas. As an example of the strength of patient stratification we describe how a large multicenter observational study incorporated a complex multi-disciplinary approach to explore the pathophysiological mechanisms of chronic painful DPN. The first step was the recruitment of a large of cohort of patients that satisfied criteria for definite DPN [83]. A total of 191 patients with DPN underwent neurological examination, quantitative sensory testing, nerve conduction studies, and skin biopsy for IENFD assessment. A set of questionnaires assessed the presence of pain, pain intensity, pain distribution, and the psychological and functional impact of pain [85]. We then used the NeupSIG grading system of NeuP to separate the cohort. Participants were divided into those with painful DPN (NeuP present for at least three months) and painless DPN (those without NeuP). We showed that there was a positive correlation between greater neuropathy severity, poorer diabetic control, and the presence (and severity) of NeuP. This link to neuropathy severity has been independently confirmed by Raputova et al. [67]. DPN sensory phenotype was characterized by hyposensitivity to applied stimuli that was more marked in those with more severe painful DPN. Therefore, the sensory profile of patients with painful DPN was distinct from those patients with painless DPN. Once our patient cohort was carefully phenotyped and stratified we investigated underlying pathogenic mechanisms. We first explored the contribution of genetic variability in NeuP and examined the relationship between variants in Nav1.7 and NeuP [11]. No rare variants were found in participants with painless DPN, we identified twelve rare Nav1.7 variants in ten (out of 111) study participants with painful DPN. Five of these variants had previously been described in the context of other NeuP disorders and seven have not previously been linked to NeuP. Those patients with rare variants reported more severe pain and greater sensitivity to pressure stimuli on quantitative sensory testing. In vitro electrophysiological characterisation for two of the novel variants demonstrated gain of function changes as a consequence of markedly impaired channel fast inactivation. We were therefore able to link the patient phenotype/genotype to changes within the biophysical properties of Nav1.7. We then went on to use functional brain imaging to study the neural correlates of chronic NeuP in those with painful DPN using a carefully matched group of patients with painless diabetic neuropathy as control[72]. We found that the ventrolateral periaqueductal grey which is an important centre for descending pain modulation was dysfunctional in those patients with painful DPN. The dysfunction refers to altered connectivity between the ventrolateral periaqueductal grey and the descending pain modulatory system that may enhance incoming nociceptive input. The degree of dysfunction correlated with the intensity of spontaneous pain and the size of cortical response to an experimental tonic heat pain. This suggests that a brain based pain facilitating mechanism contributes to chronic NeuP in DPN. In aggregate these findings illustrate how patient stratification and multi-disciplinary investigation can yield important insights into potential pathogenic mechanisms underlying neuropathic pain.
How can patient stratification aid treatment selection?
The most obvious example where improved patient stratification is already aiding treatment selection is using screening tools and (in some cases) more specialized, investigations to recognize pain as neuropathic (as opposed to nociceptive) in order to initiate appropriate therapy. Once pain is recognized as neuropathic how can we better target therapies to optimize the likelihood of response and minimize side effects? Currently first line agents for the treatment of neuropathic pain include: tricyclic antidepressants (eg. amitriptyline), dual serotonin and noradrenaline reuptake inhibitors (eg. duloxetine) and the Gabapentinoids (eg. pregabalin and gabapentin) [38]. Unfortunately for NeuP conditions these agents have failure rates of ≥70% in painful DPN and post-herpetic neuralgia[62]. However, when patients do respond this is usually within the first month of treatment, the response is lasting and is often accompanied by improved sleep and mood. Currently initial treatment selection is usually empirical and is not guided by predicted efficacy but by pragmatic decisions on tolerability and often the personal experience of the prescriber (see figure 4). Furthermore there is a growing list of second line agents such as opiates, the high dose capsaicin patch, lidocaine plasters and Botulinum toxin[38]. In certain cases, antiepileptic drugs which block VGSCs may be beneficial. One good example is trigeminal neuralgia which responds to Carbamazepine[114]. The hope is that NeuP patient stratification will facilitate initial treatment selection to optimize early pain relief and also reduce exposure to drugs which are unlikely to be effective. Such an approach could also enhance clinical trial design by stratifying patients into those that are most likely to respond to the study medication.
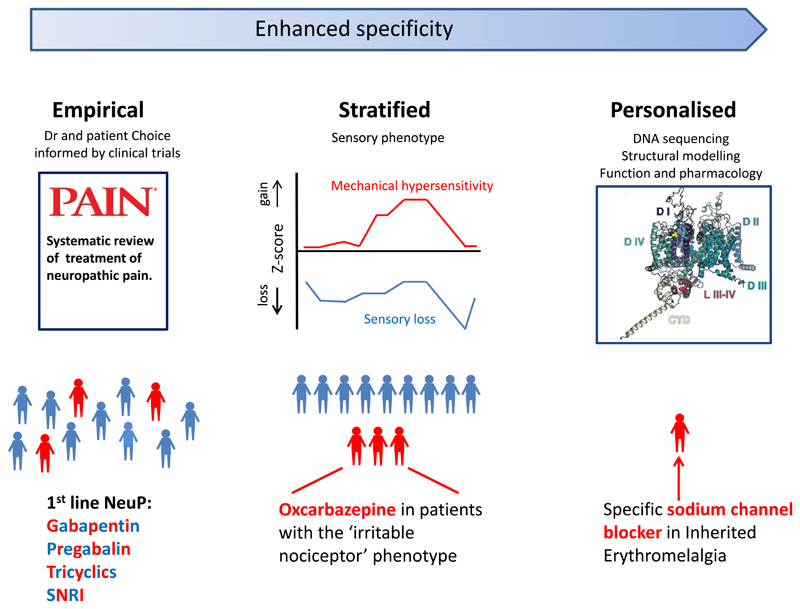
Figure 4.
A schematic showing the continuum of improved targeting of pain therapies.
Current first line agents for the treatment of neuropathic pain include: tricyclic antidepressants (eg. amitriptyline), dual serotonin and noradrenaline reuptake inhibitors (eg. duloxetine) and the Gabapentinoids (eg. pregabalin and gabapentin). Initial treatment selection is usually empirical and is not guided by predicted efficacy in individual patients.
Sensory profiling is a stratification measure which may help target treatment. Patients with an “irritable nociceptor” phenotype, a profile with preserved small-fiber function together with hyperalgesia, obtain a greater analgesic response to oxcarbazepine than to placebo. The most specific and personalized treatments are based on the identification of genetic variants that are functionally responsive to specific drugs. Structural modelling of the ion channel (illustrated) can aid in predicting such a treatment response. The use of carbamazepine or mexiletine, which can normalize the gain of function effects in certain Nav1.7 mutations associated with inherited erythromelagia, is the prototypical example of such a personalized approach.
SNRI- Serotonin and Noradrenaline Reuptake Inhibitors
(Figure of sodium channel adapted with permission from Blesneac I, Themistocleous AC, Fratter C, Conrad LJ, Ramirez JD, Cox JJ, Tesfaye S, Shillo PR, Rice ASC, Tucker SJ, Bennett DLH. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain. 2018 Mar; 159(3):469-480. doi: 10.1097/j.pain.0000000000001116, https://journals.lww.com/pain/fulltext/2018/03000/Rare_NaV1_7_variants_associated_with_painful.10.aspx)
The pain channelopathies provide an excellent example as to how identifying gene mutations, assessing their impact on channel biophysics, pharmacology and structure in vitro and in silico then enables us to predict treatment response. IEM is notoriously difficult to treat. Mexiletine is a drug related to the local anesthetic lidocaine and is active orally. General NeuP treatment guidelines actually advise against the use of mexiletine[38] because of poor efficacy and cardiac side effects. However, mexiletine’ss activity in blocking mutant Nav1.7, demonstrated in vitro for certain IEM related mutations [21], means that it can be helpful in certain cases of IEM. There are more than 30 mutations which can cause IEM and there are a variety of drugs that can block VGSCs (including both local anesthetics and anti-epileptic drugs). Is there any means of predicting which mutations (and hence which patients) will respond to which drug?
The fact that the structure of VGSCs has recently been solved at near atomic-resolution [75] means that we are now able to visualize where a single mutated residue resides within the 3-D structure of Nav1.7 and potentially model its impact. Most IEM related Nav1.7 mutations do not respond to the non-selective VGSC blocker Carbamazepine; however, patients with the V400M were found to clinically respond [41; 109] and the effects of this mutation on the channel (hyperpolarizing the voltage dependence of activation) could be reversed by Carbamazepine. Structural modelling of Nav1.7 was used to show that two other mutations (S241T and I234T) were in close proximity to V400M in 3D space (but note not in the linear amino acid sequence)[108; 109]. Both of these mutations led to gain of function in Nav1.7 and DRG cell hyper-excitability and in accordance with the structural prediction these effects were normalized by Carbamazepine. The acid-test of this hypothesis was the subsequent finding that two patients carrying the S241T mutation responded to carbamazepine in a double-blind randomized placebo-controlled study[44]. This is a small trial in a rare condition but provides proof of concept as to how molecular genetics and structural modelling can provide insights relevant to distinct ion channels and clinical disorders. Such molecular profiling is now becoming relevant to more common acquired neuropathic pain conditions [10]In genetic analysis of a patient suffering from NeuP secondary to painful DPN we discovered a mutation (S242T) in Nav1.8. This VGSC is also expressed in sensory neurons and is distinct from but shares homology with Nav1.7. Indeed this Nav1.8 mutation is homologous to the Carbamazepine responsive S241T mutation in Nav1.7. This variant was found to cause gain of function in Nav1.8 and DRG neuron hyperexcitability by our collaborators S Waxman and S Dib-Hajj and as predicted from in silico analysis these changes could be normalized by Carbamazepine [50]. A recent trial using Lacosamide in small fibre neuropathy provides another example of using molecular genetics to stratify NeuP patients. Lacosamide is an anti-epileptic drug which has activity against VGSCs including Nav1.7[54]. Lacosamide when used in un-stratified NeuP cohorts such as painful diabetic neuropathy has at best limited efficacy[51]. This randomized, double-blind, placebo controlled trial recruited patients with small fibre neuropathy and specifically those with mutation in Nav1.7. Lacosamide treatment in this group showed significant analgesic efficacy in comparison to placebo[28]. The fact that more specific blockers of VGSCs are under clinical development [115] will give added impetus to using genetics to identify mutations in these ion channels. Pharmacogenomics is not restricted to prediction of efficacy but can also be used as a means of predicting adverse outcomes (to take a topical example the risk of opiate addiction) and this may be a further application of genomics to pain medicine in the future[48].
Sensory profiling is a further stratification measure which may help target treatment[3]. This has been incorporated into a number of clinical trials in order to determine in retrospective analysis whether stratification according to sensory profile at baseline can predict response to the study medication. This has proved the case in a number of studies (for examples see [2; 77]) although the findings vary depending on the drug class analyzed [53]. One recent study was designed to test ‘a priori’ that patient stratification using sensory profiling could help predict treatment efficacy[29]. Patients with painful neuropathy underwent QST at baseline which was used to assign patients to an irritable nociceptor or the non-irritable nociceptor group. They were then treated with oxcarbazepine versus placebo. The initial hypothesis was: that those patients with irritable nociceptor profile would be more responsive to oxcarbazepine (a drug which blocks VGSCs and reduces ectopic activity). This proved to be the case: there was a significant interaction between phenotype and treatment response with a lower NNT for oxcarbazepine in the irritable nociceptor (NNT=3.9) versus non-irritable nociceptor (NNT=11) group. CPM is a means of assessing whether some patients may have insufficient endogenous pain modulation as a pathophysiological driver of NeuP. The mechanism of action of duloxetine is to restore such modulation and indeed those patients with defective CPM were found to be more responsive to duloxetine [113].
Data on sensory symptoms is easier to collect than QST however it is not necessarily a surrogate[29] and provides different information about the somatosensory nervous system. For instance sensory symptoms more are informative about spontaneous pain than evoked pains and are not as effective at assessing sensory loss[3]); however, assessment of neuropathic pain symptoms (for instance NPSI) can reveal different responses between distinct drug classes [15].
In summary both genomics and sensory profiling show some promise in predicting treatment efficacy. A stratified approach is not used routinely to inform clinical decision making; however, if found to be informative in large scale clinical trials of common acquired NeuP states this is likely to facilitate clinical adoption. A schematic showing the continuum of improved targeting of pain therapies is shown in Figure 4.
Summary and future directions
We have an impressive array of techniques to identify different patient sub-groups of NeuP patients ranging from the relatively simple such as pain symptoms to highly complex genomics. In both cases we have seen examples of stratified medicine being employed in clinical pain practice whether screening for patients with NeuP or identifying those patients with very rare monogenic pain disorders likely to respond to a particular drug. These opportunities are likely to grow especially with standardized sensory phenotyping, the use of electronic health records and the increased adoption of large scale genome sequencing. One challenge will be to understand the relationship between these different stratification methods. For instance, if a patient was found to have a gain of function mutation in a VGSC, would this take precedence over a sensory profile which showed deafferentation, which we would normally predict would reduce the likelihood of response? Data storage and integration within the health service remains a challenge certainly at national scale which would provide the greatest traction for stratification. Large scale genomics requires data security and also robust procedures for dealing with incidental findings that may not be relevant to pain but could be highly relevant to the health of the patient and their family. Stratified pain medicine has important implications for drug development and these techniques are increasingly being adopted in clinical trials. Although restricting a therapy to a sub-group of patients may initially seem an economic disincentive to pharma companies the advantage is that this may make the difference between trial success and failure; certainly the era of targeted biologics in cancer therapy has set a positive precedent for better patient stratification. We hope that Thomas Lewis would be impressed by progress over the last 70 years and in particular that we will be taking some of the ‘guessing’ out of diagnosis; our aspiration is that the end point will have a more tangible link both to the pathophysiological mechanisms driving pain but also be predictive of patient prognosis and treatment response.
Acknowledgements
Figure 1 was made using items from the Servier Medical Art Powerpoint Image Bank under a creative commons attribution license. DLB, GC and A.C.T. are members of the DOLORisk consortium funded by the European Commission Horizon 2020 (ID633491). DLB and ACT are members of the International Diabetic Neuropathy Consortium, the Novo Nordisk Foundation (Ref. NNF14SA0006). DLB is a senior Wellcome clinical scientist (Ref. 202747/Z/16/Z). ACT is an Honorary Research Fellow of the Brain Function Research Group, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Footnotes
Conflict of interest
DLB has undertaken consultancy and advisory board work for Oxford innovation—in the past 36 months, this has included work for Abide, Biogen, GSK, Lilly, Mitsubishi Tanabe, Mundipharma, Teva, and Pfizer.
References
- [1].Attal N, Bouhassira D, Baron R. Diagnosis and assessment of neuropathic pain through questionnaires. The Lancet Neurology. 2018;17(5):456–466. doi: 10.1016/S1474-4422(18)30071-1. [DOI] [PubMed] [Google Scholar]
- [2].Attal N, de Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, Raicher I, Uceyler N, Sommer C, Bouhassira D. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. The Lancet Neurology. 2016;15(6):555–565. doi: 10.1016/S1474-4422(16)00017-X. [DOI] [PubMed] [Google Scholar]
- [3].Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. The Lancet Neurology. 2012;11(11):999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- [4].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpää M, Hansson P, Hüllemann P, Jensen TS, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN. 2017;158(2):261–272. doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. PAIN. 2009;146(1–2):34–40. doi: 10.1016/j.pain.2009.06.001. [DOI] [PubMed] [Google Scholar]
- [6].Bennett DL, Woods CG. Painful and painless channelopathies. The Lancet Neurology. 2014;13(6):587–599. doi: 10.1016/S1474-4422(14)70024-9. [DOI] [PubMed] [Google Scholar]
- [7].Bennett M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92(1–2):147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- [8].Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: Validation for use in clinical and postal research. Journal of Pain. 2005;6(3):149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [9].Binder A, May D, Baron R, Maier C, Tolle TR, Treede RD, Berthele A, Faltraco F, Flor H, Gierthmuhlen J, Haenisch S, et al. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PloS one. 2011;6(3):e17387. doi: 10.1371/journal.pone.0017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blesneac I, Themistocleous AC, Fratter C, Conrad LJ, Ramirez JD, Cox JJ, Tesfaye S, Shillo PR, Rice ASC, Tucker SJ, Bennett DLH. Rare Nav1.7 variants associated with painful diabetic peripheral neuropathy. Pain. 2017 doi: 10.1097/j.pain.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blesneac I, Themistocleous AC, Fratter C, Conrad LJ, Ramirez JD, Cox JJ, Tesfaye S, Shillo PR, Rice ASC, Tucker SJ, Bennett DLH. Rare Nav1.7 variants associated with painful diabetic peripheral neuropathy. PAIN. 2018;159(3):469–480. doi: 10.1097/j.pain.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) PAIN. 2005;114(1–2):29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- [13].Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108(3):248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- [14].Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- [15].Bouhassira D, Wilhelm S, Schacht A, Perrot S, Kosek E, Cruccu G, Freynhagen R, Tesfaye S, Lledo A, Choy E, Marchettini P, et al. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: data from the randomized, double-blind, COMBO-DN study. Pain. 2014;155(10):2171–2179. doi: 10.1016/j.pain.2014.08.020. [DOI] [PubMed] [Google Scholar]
- [16].Cao L, McDonnell A, Nitzsche A, Alexandrou A, Saintot PP, Loucif AJ, Brown AR, Young G, Mis M, Randall A, Waxman SG, et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med. 2016;8(335):335ra356. doi: 10.1126/scitranslmed.aad7653. [DOI] [PubMed] [Google Scholar]
- [17].Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nature biotechnology. 2012;30(7):715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, et al. Neuropathic pain. Nature reviews Disease primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Costa FF. Big data in biomedicine. Drug discovery today. 2014;19(4):433–440. doi: 10.1016/j.drudis.2013.10.012. [DOI] [PubMed] [Google Scholar]
- [20].Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cregg R, Cox JJ, Bennett DL, Wood JN, Werdehausen R. Mexiletine as a treatment for primary erythromelalgia: normalization of biophysical properties of mutant L858F NaV 1.7 sodium channels. British journal of pharmacology. 2014;171(19):4455–4463. doi: 10.1111/bph.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cregg R, Laguda B, Werdehausen R, Cox JJ, Linley JE, Ramirez JD, Bodi I, Markiewicz M, Howell KJ, Chen YC, Agnew K, et al. Novel mutations mapping to the fourth sodium channel domain of Nav1.7 result in variable clinical manifestations of primary erythromelalgia. Neuromolecular medicine. 2013;15(2):265–278. doi: 10.1007/s12017-012-8216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. The Clinical journal of pain. 2012;28(6):475–483. doi: 10.1097/AJP.0b013e3182385392. [DOI] [PubMed] [Google Scholar]
- [24].Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD, Garcia-Larrea L. Recommendations for the clinical use of somatosensory-evoked potentials. Clinical Neurophysiology. 2008;119(8):1705–1719. doi: 10.1016/j.clinph.2008.03.016. [DOI] [PubMed] [Google Scholar]
- [25].Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpää M, Jørum E, Serra J, Jensen TS. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11(3):153–162. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- [26].Cummins TR, Dib-Hajj SD, Waxman SG. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci. 2004;24(38):8232–8236. doi: 10.1523/JNEUROSCI.2695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Daniel HC, Narewska J, Serpell M, Hoggart B, Johnson R, Rice AS. Comparison of psychological and physical function in neuropathic pain and nociceptive pain: implications for cognitive behavioral pain management programs. European journal of pain. 2008;12(6):731–741. doi: 10.1016/j.ejpain.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [28].DeGreef BT, Hoeijmakers JG, Geerts M, Oakes M, Church TJ, Waxman SG, Dib-Hajj SD, Faber CG, Merkies IS. Lacosamide in patients with gain-of-function Nav1.7 mutations-related small fiber neuropathy (LENSS): a randomized, double-blind, placebo-controlled, crossover trial. 2018 Submitted. [Google Scholar]
- [29].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155(11):2263–2273. doi: 10.1016/j.pain.2014.08.014. [DOI] [PubMed] [Google Scholar]
- [30].Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Na v 1.7 and human pain disorders. Trends in neurosciences. 2007;30(11):555–563. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- [31].Donadio V, Liguori R. Microneurographic recording from unmyelinated nerve fibers in neurological disorders: An update. Clinical Neurophysiology. 2015;126(3):437–445. doi: 10.1016/j.clinph.2014.10.009. [DOI] [PubMed] [Google Scholar]
- [32].Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychological bulletin. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- [33].Eccleston C, Crombez G. Worry and chronic pain: a misdirected problem solving model. Pain. 2007;132(3):233–236. doi: 10.1016/j.pain.2007.09.014. [DOI] [PubMed] [Google Scholar]
- [34].Eccleston C, Crombez G. Advancing psychological therapies for chronic pain. F1000Research. 2017;6:461. doi: 10.12688/f1000research.10612.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Eccleston C, Hearn L, Williams AC. Psychological therapies for the management of chronic neuropathic pain in adults. The Cochrane database of systematic reviews. 2015;(10):CD011259. doi: 10.1002/14651858.CD011259.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, Dib-Hajj S, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71(1):26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- [37].Fertleman CR, Ferrie CD, Aicardi J, Bednarek NA, Eeg-Olofsson O, Elmslie FV, Griesemer DA, Goutieres F, Kirkpatrick M, Malmros IN, Pollitzer M, et al. Paroxysmal extreme pain disorder (previously familial rectal pain syndrome) Neurology. 2007;69(6):586–595. doi: 10.1212/01.wnl.0000268065.16865.5f. [DOI] [PubMed] [Google Scholar]
- [38].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DLH, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, et al. Neuropathic pain: an updated grading system for research and clinical practice. PAIN. 2016;157(8):1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fischer TZ, Gilmore ES, Estacion M, Eastman E, Taylor S, Melanson M, Dib-Hajj SD, Waxman SG. A novel Nav1.7 mutation producing carbamazepine-responsive erythromelalgia. Ann Neurol. 2009;65(6):733–741. doi: 10.1002/ana.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. PAIN. 2014;155(2):367–376. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- [43].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- [44].Geha P, Yang Y, Estacion M, Schulman BR, Tokuno H, Apkarian AV, Dib-Hajj SD, Waxman SG. Pharmacotherapy for Pain in a Family With Inherited Erythromelalgia Guided by Genomic Analysis and Functional Profiling. JAMA neurology. 2016;73(6):659–667. doi: 10.1001/jamaneurol.2016.0389. [DOI] [PubMed] [Google Scholar]
- [45].Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Granovsky Y. Conditioned Pain Modulation: A Predictor for Development and Treatment of Neuropathic Pain. Current Pain and Headache Reports. 2013;17(9):361. doi: 10.1007/s11916-013-0361-8. [DOI] [PubMed] [Google Scholar]
- [47].Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Care & Research. 2009;61(9):1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- [48].Haerian BS, Haerian MS. OPRM1 rs1799971 polymorphism and opioid dependence: evidence from a meta-analysis. Pharmacogenomics. 2013;14(7):813–824. doi: 10.2217/pgs.13.57. [DOI] [PubMed] [Google Scholar]
- [49].Han C, Dib-Hajj SD, Lin Z, Li Y, Eastman EM, Tyrrell L, Cao X, Yang Y, Waxman SG. Early- and late-onset inherited erythromelalgia: genotype-phenotype correlation. Brain. 2009;132(Pt 7):1711–1722. doi: 10.1093/brain/awp078. [DOI] [PubMed] [Google Scholar]
- [50].Han C, Themistocleous AC, Macala LJ, Blesneac I, Fratter C, Bennett DL, Dib-Hajj SD, Waxman SG. From Nav1.7 to Nav1.8: A case for precision medicine. Proceedings of the Society for Neuroscience. 2017;288(08):D29. [Google Scholar]
- [51].Hearn L, Derry S, Moore RA. Lacosamide for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews. 2012;(2) doi: 10.1002/14651858.CD009318.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hebert HL, Veluchamy A, Torrance N, Smith BH. Risk factors for neuropathic pain in diabetes mellitus. Pain. 2017;158(4):560–568. doi: 10.1097/j.pain.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Holbech JV, Bach FW, Finnerup NB, Jensen TS, Sindrup SH. Pain phenotype as a predictor for drug response in painful polyneuropathy-a retrospective analysis of data from controlled clinical trials. Pain. 2016;157(6):1305–1313. doi: 10.1097/j.pain.0000000000000563. [DOI] [PubMed] [Google Scholar]
- [54].Jo S, Bean BP. Lacosamide Inhibition of Nav1.7 Voltage-Gated Sodium Channels: Slow Binding to Fast-Inactivated States. Molecular Pharmacology. 2017;91(4):277–286. doi: 10.1124/mol.116.106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC. Reliability of conditioned pain modulation: a systematic review. Pain. 2016;157(11):2410–2419. doi: 10.1097/j.pain.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annual review of psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- [57].Kleggetveit IP, Namer B, Schmidt R, Helås T, Rückel M, Ørstavik K, Schmelz M, Jørum E. High spontaneous activity of C-nociceptors in painful polyneuropathy. PAIN®. 2012;153(10):2040–2047. doi: 10.1016/j.pain.2012.05.017. [DOI] [PubMed] [Google Scholar]
- [58].Magerl W, Krumova EK, Baron R, Tölle T, Treede R-D, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN. 2010;151(3):598–605. doi: 10.1016/j.pain.2010.07.026. [DOI] [PubMed] [Google Scholar]
- [59].Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN. 2010;150(3):439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [60].McDonnell A, Schulman B, Ali Z, Dib-Hajj SD, Brock F, Cobain S, Mainka T, Vollert J, Tarabar S, Waxman SG. Inherited erythromelalgia due to mutations in SCN9A: natural history, clinical phenotype and somatosensory profile. Brain. 2016;139(Pt 4):1052–1065. doi: 10.1093/brain/aww007. [DOI] [PubMed] [Google Scholar]
- [61].Mills EP, Di Pietro F, Alshelh Z, Peck CC, Murray GM, Vickers ER, Henderson LA. Brainstem Pain-Control Circuitry Connectivity in Chronic Neuropathic Pain. The Journal of Neuroscience. 2018;38(2):465–473. doi: 10.1523/JNEUROSCI.1647-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. Bmj. 2013;346:f2690. doi: 10.1136/bmj.f2690. [DOI] [PubMed] [Google Scholar]
- [63].Nahman-Averbuch H, Yarnitsky D, Granovsky Y, Sprecher E, Steiner M, Tzuk-Shina T, Pud D. Pronociceptive Pain Modulation in Patients with Painful Chemotherapy-Induced Polyneuropathy. Journal of Pain and Symptom Management. 2011;42(2):229–238. doi: 10.1016/j.jpainsymman.2010.10.268. [DOI] [PubMed] [Google Scholar]
- [64].Namer B, Ørstavik K, Schmidt R, Kleggetveit I-P, Weidner C, Mørk C, Kvernebo MS, Kvernebo K, Salter H, Carr TH, Segerdahl M, et al. Specific changes in conduction velocity recovery cycles of single nociceptors in a patient with erythromelalgia with the I848T gain-of-function mutation of Nav1.7. PAIN. 2015;156(9):1637–1646. doi: 10.1097/j.pain.0000000000000229. [DOI] [PubMed] [Google Scholar]
- [65].Ørstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jørum E, Torebjörk HE. Abnormal Function of C-Fibers in Patients with Diabetic Neuropathy. The Journal of Neuroscience. 2006;26(44):11287–11294. doi: 10.1523/JNEUROSCI.2659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ørstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jørum E, Handwerker H, Torebjörk E. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126(3):567–578. doi: 10.1093/brain/awg060. [DOI] [PubMed] [Google Scholar]
- [67].Raputova J, Srotova I, Vlckova E, Sommer C, Üçeyler N, Birklein F, Rittner HL, Rebhorn C, Adamova B, Kovalova I, Kralickova Nekvapilova E, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain. 2017;158(12):2340–2353. doi: 10.1097/j.pain.0000000000001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Redekop WK, Mladsi D. The faces of personalized medicine: a framework for understanding its meaning and scope. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16(6 Suppl):S4–9. doi: 10.1016/j.jval.2013.06.005. [DOI] [PubMed] [Google Scholar]
- [69].Rolke R, Baron R, Maier C, Tölle TR, Treede RDD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- [70].Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. European journal of pain (London, England) 2006;10(1):77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [71].Sandhu C, Qureshi A, Emili A. Panomics for Precision Medicine. Trends in molecular medicine. 2018;24(1):85–101. doi: 10.1016/j.molmed.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Segerdahl AR, Themistocleous AC, Fido D, Bennett DL, Tracey I. A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain. 2018;141(2):357–364. doi: 10.1093/brain/awx337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Serra J, Bostock H, Solà R, Aleu J, García E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. PAIN. 2012;153(1):42–55. doi: 10.1016/j.pain.2011.08.015. [DOI] [PubMed] [Google Scholar]
- [74].Serra J, Collado A, Solà R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Annals of Neurology. 2014;75(2):196–208. doi: 10.1002/ana.24065. [DOI] [PubMed] [Google Scholar]
- [75].Shen H, Zhou Q, Pan X, Li Z, Wu J, Yan N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science. 2017;355(6328) doi: 10.1126/science.aal4326. [DOI] [PubMed] [Google Scholar]
- [76].Sieberg CB, Taras C, Gomaa A, Nickerson C, Wong C, Ward C, Baskozos G, Tesfaye S, Rice ASC, Bennett DLH, Ramirez JD, et al. Neuropathic pain drives anxiety behavior in mice and diabetic neuropathy patients. PAIN Reports. 2018 doi: 10.1097/PR9.0000000000000651. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R, Group HIVNS Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology. 2010;74(5):413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Siva N. UK gears up to decode 100,000 genomes from NHS patients. Lancet. 2015;385(9963):103–104. doi: 10.1016/S0140-6736(14)62453-3. [DOI] [PubMed] [Google Scholar]
- [79].Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Current pain and headache reports. 2012;16(3):191–198. doi: 10.1007/s11916-012-0256-0. [DOI] [PubMed] [Google Scholar]
- [80].Sommer C, Richter H, Rogausch JP, Frettlöh J, Lungenhausen M, Maier C. A modified score to identify and discriminate neuropathic pain: a study on the German version of the neuropathic pain symptom inventory (NPSI) BMC Neurology. 2011;11:104–104. doi: 10.1186/1471-2377-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Spallone V, Morganti R, D'Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med. 2012;29(5):578–585. doi: 10.1111/j.1464-5491.2011.03500.x. [DOI] [PubMed] [Google Scholar]
- [82].Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456–2465. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Themistocleous AC, Ramirez JD, Serra J, Bennett DL. The clinical approach to small fibre neuropathy and painful channelopathy. Pract Neurol. 2014;14(6):368–379. doi: 10.1136/practneurol-2013-000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice ASC, Bennett DLH. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. PAIN. 2016;157(5):1132–1145. doi: 10.1097/j.pain.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7(4):281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- [87].Tracey I. Neuroimaging mechanisms in pain: from discovery to translation. PAIN. 2017;158:S115–S122. doi: 10.1097/j.pain.0000000000000863. [DOI] [PubMed] [Google Scholar]
- [88].Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- [89].Truini A, Galeotti F, Haanpaa M, Zucchi R, Albanesi A, Biasiotta A, Gatti A, Cruccu G. Pathophysiology of pain in postherpetic neuralgia: A clinical and neurophysiological study. PAIN. 2008;140(3):405–410. doi: 10.1016/j.pain.2008.08.018. [DOI] [PubMed] [Google Scholar]
- [90].Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans—how symptoms help disclose mechanisms. Nature Reviews Neurology. 2013;9:572. doi: 10.1038/nrneurol.2013.180. [DOI] [PubMed] [Google Scholar]
- [91].Truini A, Padua L, Biasiotta A, Caliandro P, Pazzaglia C, Galeotti F, Inghilleri M, Cruccu G. Differential involvement of A-delta and A-beta fibres in neuropathic pain related to carpal tunnel syndrome. Pain. 2009;145(1–2):105–109. doi: 10.1016/j.pain.2009.05.023. [DOI] [PubMed] [Google Scholar]
- [92].Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nature reviews Drug discovery. 2007;6(4):287–293. doi: 10.1038/nrd2251. [DOI] [PubMed] [Google Scholar]
- [93].Tuveson B, Leffler AS, Hansson P. Heterotopic noxious conditioning stimulation (HNCS) reduced the intensity of spontaneous pain, but not of allodynia in painful peripheral neuropathy. European Journal of Pain. 2007;11(4):452–462. doi: 10.1016/j.ejpain.2006.06.007. [DOI] [PubMed] [Google Scholar]
- [94].Üçeyler N, Zeller D, Kahn A-K, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136(6):1857–1867. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- [95].Vale TA, Symmonds M, Polydefkis M, Byrnes K, Rice ASC, Themistocleous AC, Bennett DLH. Chronic non-freezing cold injury results in neuropathic pain due to a sensory neuropathy. Brain. 2017;140(10):2557–2569. doi: 10.1093/brain/awx215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Vedhara K, Dawe K, Wetherell MA, Miles JN, Cullum N, Dayan C, Drake N, Price P, Tarlton J, Weinman J, Day A, et al. Illness beliefs predict self-care behaviours in patients with diabetic foot ulcers: a prospective study. Diabetes research and clinical practice. 2014;106(1):67–72. doi: 10.1016/j.diabres.2014.07.018. [DOI] [PubMed] [Google Scholar]
- [97].Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- [98].Vollert J, Attal N, Baron R, Freynhagen R, Haanpää M, Hansson P, Jensen TS, Rice ASC, Segerdahl M, Serra J, Sindrup SH, et al. Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. PAIN. 2016;157(3):750–758. doi: 10.1097/j.pain.0000000000000433. [DOI] [PubMed] [Google Scholar]
- [99].Vollert J, Kramer M, Barroso A, Freynhagen R, Haanpää M, Hansson P, Jensen TS, Kuehler BM, Maier C, Mainka T, Reimer M, et al. Symptom profiles in the painDETECT Questionnaire in patients with peripheral neuropathic pain stratified according to sensory loss in quantitative sensory testing. PAIN. 2016;157(8):1810–1818. doi: 10.1097/j.pain.0000000000000588. [DOI] [PubMed] [Google Scholar]
- [100].Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmühlen J, Haanpää M, Hansson P, et al. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. PAIN. 2017;158(8):1446–1455. doi: 10.1097/j.pain.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].von Bischhoffshausen S, Ivulic D, Alvarez P, Schuffeneger VC, Idiaquez J, Fuentes C, Morande P, Fuentes I, Palisson F, Bennett DLH, Calvo M. Recessive dystrophic epidermolysis bullosa results in painful small fibre neuropathy. Brain. 2017;140(5):1238–1251. doi: 10.1093/brain/awx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wanigasekera V, Mezue M, Andersson J, Kong Y, Tracey I. Disambiguating pharmacodynamic efficacy from behaviour with neuroimaging: implications for analgesic drug development. Anesthesiology. 2016;124(1):159–168. doi: 10.1097/ALN.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Waxman SG, Merkies IS, Gerrits MM, Dib-Hajj SD, Lauria G, Cox JJ, Wood JN, Woods CG, Drenth JP, Faber CG. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. The Lancet Neurology. 2014;13(11):1152–1160. doi: 10.1016/S1474-4422(14)70150-4. [DOI] [PubMed] [Google Scholar]
- [104].Weir GA, Middleton SJ, Clark AJ, Daniel T, Khovanov N, McMahon SB, Bennett DL. Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source. Brain. 2017;140(10):2570–2585. doi: 10.1093/brain/awx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with Chronic Pain After Abdominal Surgery Show Less Preoperative Endogenous Pain Inhibition and More Postoperative Hyperalgesia: A Pilot Study. Journal of Pain & Palliative Care Pharmacotherapy. 2010;24(2):119–128. doi: 10.3109/15360281003706069. [DOI] [PubMed] [Google Scholar]
- [106].Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, Lipton R, Loeser JD, Payne R, Torebjork E. Towards a mechanism-based classification of pain? Pain. 1998;77(3):227–229. doi: 10.1016/S0304-3959(98)00099-2. [DOI] [PubMed] [Google Scholar]
- [107].Woolf CJ, Decosterd I. Implications of recent advances in the understanding of pain pathophysiology for the assessment of pain in patients. Pain. 1999;(Suppl 6):S141–147. doi: 10.1016/S0304-3959(99)00148-7. [DOI] [PubMed] [Google Scholar]
- [108].Yang Y, Adi T, Effraim PR, Chen L, Dib-Hajj SD, Waxman SG. Reverse pharmacogenomics: carbamazepine normalizes activation and attenuates thermal hyperexcitability of sensory neurons due to Nav 1.7 mutation I234T. British journal of pharmacology. 2017 doi: 10.1111/bph.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yang Y, Dib-Hajj SD, Zhang J, Zhang Y, Tyrrell L, Estacion M, Waxman SG. Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a Na(V)1.7 mutant channel. Nature communications. 2012;3:1186. doi: 10.1038/ncomms2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41(3):171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L-A, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. PAIN. 2008;138(1):22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- [112].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. PAIN. 2012;153(6):1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- [113].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- [114].Zakrzewska JM, Linskey ME. Trigeminal neuralgia. BMJ clinical evidence. 2014;2014 [PMC free article] [PubMed] [Google Scholar]
- [115].Zakrzewska JM, Palmer J, Morisset V, Giblin GM, Obermann M, Ettlin DA, Cruccu G, Bendtsen L, Estacion M, Derjean D, Waxman SG, et al. Safety and efficacy of a Nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: a double-blind, placebo-controlled, randomised withdrawal phase 2a trial. The Lancet Neurology. 2017;16(4):291–300. doi: 10.1016/S1474-4422(17)30005-4. [DOI] [PubMed] [Google Scholar]
- [116].Zorina-Lichtenwalter K, Parisien M, Diatchenko L. Genetic studies of human neuropathic pain conditions: a review. PAIN. 2018;159(3):583–594. doi: 10.1097/j.pain.0000000000001099. [DOI] [PMC free article] [PubMed] [Google Scholar]