Abstract
Apathy, common in neurological disorders, is defined as disinterest and loss of motivation, with a reduction in self‐initiated activity. Research in diseased populations has shown that apathy is associated with variations in the volume of brain regions such as the anterior cingulate and the frontal lobes. The goal of this study was to determine the neural signatures of apathy in people with penetrating traumatic brain injuries (pTBIs), as to our knowledge, these have not been studied in this sample. We studied 176 male Vietnam War veterans with pTBIs using voxel‐based lesion‐symptom mapping (VLSM) and apathy scores from the UCLA Neuropsychiatric Inventory (NPI), a structured inventory of symptoms completed by a caregiver. Our results revealed that increased apathy symptoms were associated with brain damage in limbic and cortical areas of the left hemisphere including the anterior cingulate, inferior, middle, and superior frontal regions, insula, and supplementary motor area. Our results are consistent with the literature, and extend them to people with focal pTBI. Apathy is a significant symptom since it can reduce participation of the patient in family and other social interactions, and diminish affective decision‐making. Hum Brain Mapp 35:943–953, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: apathy, traumatic brain injury, voxel‐based lesion‐symptom mapping, cingulate cortex, insula, fatigue
INTRODUCTION
Apathy is a behavioral symptom defined as disinterest and loss of motivation; it is a reduction in self‐initiated activity [Marin, 1990]. It has been reported in 46% of people with traumatic brain injury (TBI) [Andersson et al., 1999]. TBI patients with a functional recovery status score of 3 (severe disability) on the Glasgow Outcome Scale [GOS; Jennett and Bond, 1975] had four times the risk of developing apathetic behaviors than TBI patients with less severe GOS scores [Ciurli et al., 2011]. Apathy can be distinguished from depression [Aalten et al., 2008; Kirsch‐Darrow et al., 2011; Levy et al., 1998; Mortby et al., 2011] and can lead to caregiver distress, since it increases dependence on the caregiver [Greene et al., 1982; Kaufer et al., 1998; Landes et al., 2001]. The signs of fatigue can overlap with those of apathy [Pardini et al., 2010].
We used the Neuropsychiatric Inventory [NPI, Cummings et al., 1994], a structured, caregiver‐based interview, to measure signs of apathy. The NPI is a validated instrument [Corrigan et al., 1990; Levin et al., 1987], and as those with TBI frequently lack insight into their difficulties, a caregiver‐based interview may present a more accurate picture of this area of dysfunction [Dujardin et al., 2008; Levin et al., 1987; Robert et al., 2002; Starkstein et al., 2001].
It has been postulated that apathy results from the disruption of connections between the anterior cingulate cortex (ACC) and other cortical and subcortical regions [Migneco et al., 2001]. The ACC and orbitofrontal cortex (OFC) receive input from the basolateral amygdala and nucleus accumbens, areas involved in setting reward values. These areas then feed into an ascending pathway to the dorsolateral prefrontal cortex, which selects and executes behavioral responses. When ACC or OFC are damaged, this circuit is disrupted, resulting in impaired decision‐making and response initiation, i.e., apathy [Guimarães et al., 2008; Tunnard et al., 2010].
Many methods have been used to identify brain regions where damage is associated with apathy. In Alzheimer's disease (AD), single photon emission computed tomography (SPECT), positron emission tomography (PET), postmortem, proton density imaging, fractional anisotropy (FA), and voxel‐based morphometry (VBM) studies have found apathy predominantly associated with the ACC and the frontal lobes [Apostolova et al., 2007; Benoit et al., 2002; Bruen et al., 2008; Craig et al., 1996; Kim et al., 2011; Lanctôt et al., 2007; Marshall et al., 2006; Marshall et al., 2007; Migneco et al., 2001; Starkstein et al., 2009]. VBM studies of patients with other types of dementia or Parkinson's disease (PD) found relationships between apathy and gray matter atrophy in these same areas [Massimo et al., 2009; Reijnders et al., 2010; Rosen et al., 2005; Zamboni et al., 2008], and a diffusion tensor imaging study of amyotrophic lateral sclerosis (ALS) patients found that FA and apathy change scores were negatively correlated in the ACC [Woolley et al., 2011]. In addition to the many studies of apathy in neurodegenerative disease, there have also been a few studies in stroke. A case study by Laplane et al. 1981 found docility and indifference in a patient after damage to ACC and prefrontal cortex (PFC). Another study of stroke also found that apathy was associated with damage to ACC [Kumral et al., 2002]. Manes et al. 1999 found that stroke damage in the insula resulted in a subjective lack of energy with anergia and tiredness.
In summary, reduction of function of the ACC and the frontal lobes has been associated with apathy in many studies using a variety of methods in populations with AD and other forms of dementia, ALS, or stroke. In this study, we used voxel‐based lesion‐symptom mapping (VLSM) to determine where pTBI lesions are associated with apathy. VLSM computes a t‐value comparing behavioral scores in those with damage to a voxel and those without damage to that voxel. It performs a whole‐brain, voxel‐by‐voxel analysis, rather than attempting to categorize patients based on lesion location.
MATERIAL AND METHODS
Subjects
Veterans were drawn from Phase III of the W.F. Caveness Vietnam Head Injury Study registry (VHIS), a longitudinal study of brain injured veterans, mainly with focal penetrating injuries, and uninjured combat veterans [Raymont et al., 2011]. Phase III (2003–2006) was conducted at the National Naval Medical Center in Bethesda, MD. The veteran population offers a number of methodological advantages including its large sample size, relative uniformity, and access to preinjury data for comparison with postinjury performance.
One hundred seventy‐six brain‐injured (TBI group) and 52 uninjured (control group) male combat veterans for whom CT and NPI data were available were included in this sample. There were no significant differences in age, years of education, and preinjury IQ between the TBI and control groups (see Table 1). In Phase II of the VHIS (1981–1984), 56% of the TBI group were working compared with 82% of the control group [Schwab et al., 1993], and equal percentages of the TBI and control groups were living with their wives (74%). All participants gave their written informed consent, which followed the Code of Ethics of the World Medical Association (Declaration of Helsinki). All study procedures were approved by Institutional Review Boards at the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke.
Table 1.
Comparison between veterans with TBI and control veterans on demographic information and neurobehavioral scores
| Variable | TBI Veterans | Control veterans | Statistics (2‐tailed) |
|---|---|---|---|
| Age | (N = 176) 58.44 ± 3.16 | (N = 52) 59.04 ± 3.47 | t(226) = 1.18; p = .24 |
| Years of education | (N = 174) 14.85 ± 2.55 | (N = 51) 15.15 ± 2.50 | t(223) = 0.74; p = .46 |
| Preinjury AFQT percentile score | (N = 165) 61.50 ± 25.54 | (N = 32) 67.25 ± 22.93 | t(195) = 1.18; p = .24 |
| Postinjury (Phase III) AFQT percentile score | (N = 171) 53.55 ± 24.50 | (N = 51) 69.63 ± 21.53 | t(220) = 4.22; p < .001 |
| WMS working memory primary index percentile score | (N = 169) 49.98 ± 28.22 | (N = 51) 65.06 ± 26.33 | t(218) = 3.40; p = .001 |
| MMSE total score | (N = 165) 28.48 ± 2.02 | (N = 49) 29.12 ± 1.32 | t(120.97) = 2.63; p = .01 |
| BDI total score | (N = 175) 9.16 ± 9.09 | (N = 51) 11.04 ± 9.68 | t(224) = 1.28; p = .20 |
| SCID‐I/NP major depressive disorder lifetime prevalence | (N = 174) 1.40 ± 0.80 | (N = 51) 1.57 ± 0.88 | t(223) = 1.32; p = .19 |
| Krupp's fatigue severity scale score | (N = 175) 36.33 ± 11.92 | (N = 52) 37.31 ± 12.17 | t(225) = 0.52; p = .60 |
| NRS fatigability score | (N = 161) 1.69 ± 1.24 | (N = 48) 1.40 ± 1.11 | t(207) = 1.47; p = .14 |
| NPI apathy frequency x severity score | (N = 176) 0.77 ± 2.11 | (N = 52) 1.10 ± 1.94 | z = 2.35; p = .02 |
Means and standard deviations. AFQT, Armed Forces Qualification Test; WMS, Wechsler Memory Scale; MMSE, Mini‐mental State Exam; BDI, Beck Depression Inventory; SCID‐I/NP, Structured Clinical Interview for DSM‐IV‐TR Axis I disorders, Non‐Patient edition; NRS, Neurobehavioral Rating Scale; NPI, Neuropsychiatric Inventory.
Computed Tomography (CT) Scans and Lesion Identification
As many of the TBI veterans had metal fragments in their heads, we performed CT scans rather than MRIs. Axial CT scans were acquired without contrast in helical mode on a GE LightSpeed Plus CT scanner. Images were reconstructed with an in‐plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm, and a 1 mm slice interval. Lesion location was determined from CT images by manual tracing on each slice using the Analysis of Brain Lesions (ABLe) software implemented in MEDx v3.44 (Medical Numerics) [Makale et al., 2002; Solomon et al., 2007]. Lesion volume was calculated by summing the traced areas and multiplying by slice thickness. The tracing was performed by an experienced physician (VR) on the de‐identified scans, and reviewed by an experienced observer (JG) who was blind to the clinical and behavioral data. Scans were normalized to a template in Montreal Neurological Institute (MNI) space, using the AIR algorithm with a 12‐parameter affine fit [Woods et al., 1998]. To optimize precision of the registration procedure, the brain images were first automatically skull‐stripped. Voxels inside the traced lesion were not included in the spatial normalization procedure. For each subject, the resulting normalized lesion mask image was used in the VLSM analysis. Specifically, the analysis examined whether there were any significant associations between apathy and lesion locations. Gray matter location was obtained from the AAL atlas [Tzourio‐Mazoyer et al., 2002] and white matter from the White Matter atlas [Mori et al., 2005].
Neuropsychological Testing
Neuropsychological functions were assessed in a battery of tests administered over 5–7 days. This study used a subset of these. Pre‐ and postinjury general intelligence scores were assessed with the Armed Forces Qualification Test (AFQT‐7A, United States Department of Defense, 1960). Scores on this test correlate highly with the Wechsler Adult Intelligence Scale [WAIS, Wechsler, 1997aa] intelligence quotient scores [Grafman et al., 1988]. Working memory was assessed with the Wechsler Memory Scale‐III (WMS) working memory primary index score [Wechsler, 1997bb]. Cognitive status was evaluated using the Mini‐Mental State Examination [MMSE; Folstein et al., 1975]. Depression and other psychiatric symptoms and diagnoses that could explain apathetic symptoms were evaluated using the Beck Depression Inventory [BDI; Beck, 1978] and the Structured Clinical Interview for DSM‐IV‐TR Axis I disorders, Non‐Patient edition lifetime prevalence for major depressive disorder scores [SCID‐I/NP; First et al., 2002]. Fatigue was measured using the Fatigue Severity Scale (FSS), a 9‐item self‐rating scale [Krupp et al., 1989]. Fatigability, defined as the degree to which someone “rapidly fatigues on challenging cognitive tasks or complex activities, (and is) lethargic,” was measured using a clinician rating on the Neurobehavioral Rating Scale [NRS; Levin et al., 1987]. For the NPI, the examiner read the questions aloud to a rater who knew the participant well. Raters were generally spouses, partners, or adult children, most of whom gave their answers via telephone. For each abnormal behavior present, a rating for that behavior was elicited on a severity scale from 1 (mild) to 3 (severe), and on a frequency scale from 1 (occasionally, less than once a week) to 4 (very frequently, once or more per day or continuously). We used apathy frequency × severity scores.
Statistical Analysis
Behavioral analyses
Analysis of the behavioral data was performed with IBM© SPSS© 19.0 (http://www.spss.com), and alpha was set to .05 (two‐tailed) for all analyses. Independent samples t‐tests were performed to compare the TBI and the control group in age, years of education, and neurobehavioral scores. Kolmogorov‐Smirnov testing on apathy scores showed that both groups had significantly non‐normal distributions, TBI group: D(176) = 0.48, P < 0.001; control group: D(52) = 0.39, P < 0.001, because most individuals showed no overt signs of apathy. For this reason, we performed Spearman's rho correlational analyses for the TBI group between apathy scores and each of the neurobehavioral scores that were significantly different between TBI and control groups, plus those that could influence apathy, including MMSE, WMS, fatigue, fatigability, lifetime history of major depressive disorder, BDI depression, level of consciousness at time of first exam after injury, and duration of loss or alteration of consciousness. In addition, we performed a Mann‐Whitney U‐test to compare apathy scores in the TBI group versus the control group, and between TBI veterans who had a lifetime history of major depression with TBI veterans who had not. The same analysis also was performed within the control group. As a follow‐up to our VLSM analysis, we performed independent samples t‐tests to compare the significantly correlated neurobehavioral scores between two resulting groups: those TBI veterans with lesions in areas significantly associated with apathy and those TBI veterans with lesions only in other areas. We also performed similar t‐tests for volume loss.
Neuroimaging analyses
We first created a lesion overlap map to show how many TBI veterans had damage at each voxel by overlaying their individual normalized lesion maps. We also created a lesion overlap map to show lesions for only those 29 veterans with apathy scores greater than zero. Next, we used VLSM to implement one‐tailed t‐tests at each voxel to find significant positive associations of lesioned voxels and apathy scores. Apathy scores for all TBI veterans were used in this analysis, including those with scores equal to zero. We also performed a VLSM analysis on white matter to find significant positive associations of lesioned white matter and apathy scores. FDR corrections of 0.05 were used for multiple comparisons [Genovese et al., 2002], and a minimum cluster size of 10 voxels.
Because of the likelihood that a subset of participants would have damage involving a given voxel, statistical power is often lacking in VLSM analyses. We tolerated low power in order to be able to test lesion locations over much of the brain. For our study, we chose the minimum number of cases with overlapping lesions to be 4 at any voxel [see Gläscher et al., 2009, who used a similar criterion]. If fewer than 4 TBI veterans had a lesion in a given voxel, that voxel was excluded from the analysis.
RESULTS
Behavioral Results
The control group had significantly higher postinjury AFQT, WMS working memory, and MMSE scores than the TBI group, although the mean scores of the TBI group were within the normal range. There were no significant group differences on preinjury AFQT, BDI, SCID‐I/NP major depressive disorder lifetime prevalence, fatigue, or fatigability. Apathy scores were significantly higher in the control group (mean rank 127.74) than the TBI group (110.59) (See Table 1). This was because relatively more TBI veterans were rated as having no apathy. There were no within group differences on apathy scores for either group between those who had major depression in their lifetimes and those who had not (TBI group: z = 0.27, P = 0.79; control group: z = 0.48, P = 0.64).
To further investigate the differences in postinjury AFQT, WMS working memory, and MMSE scores, we performed post‐hoc Spearman's correlations of apathy scores in the TBI group. There was a significant correlation between apathy and postinjury AFQT scores (r s= −0.17, P = 0.03). To rule out this being a lifelong tendency toward apathy, we performed a Spearman's correlation between apathy and pre‐injury AFQT, and found no significant correlation (r s = −0.06, P = 0.45). We found no significant correlations between apathy and WMS working memory scores (r s = −0.04, P = 0.63), or apathy and MMSE scores (r s = 0.05, P = 0.51). For the other neurobehavioral scores that could influence apathy, we found no significant correlations between apathy and fatigue (r s = 0.10, P = 0.17), apathy and lifetime history of major depressive disorder (r s = 0.10, P = 0.19), apathy and level of consciousness at first exam (r s = −0.04, P = 0.56), or apathy and duration of loss or alteration of consciousness (r s = 0.12, P = 0.11); however, the correlations between apathy and BDI scores (r s = 0.24, P = 0.001), and apathy and fatigability (r s = 0.16, P = 0.048) were significant.
Neuroimaging Results
Lesion overlap maps
Figure 1 shows the lesion overlap map associated with apathy for all 176 veterans with TBI. The maximum overlap of 28 subjects occurred in prefrontal areas. The frontal lobes and ACC, regions where damage is often cited as playing a role in apathy, were well covered.
Figure 1.
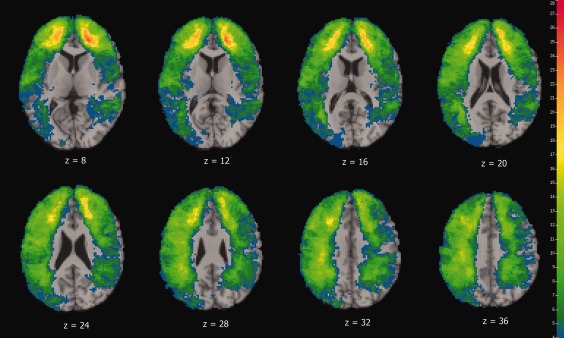
Color indicates the number of participants' lesions overlapping at each voxel for the apathy analysis. We required a minimum of four lesion cases at each voxel in order for that voxel to be included in VLSM analysis. The left is on the viewer's right.
Figure 2 shows the lesion overlap map associated with apathy for only those 28 veterans with any apathy symptoms reported on the NPI.
Figure 2.
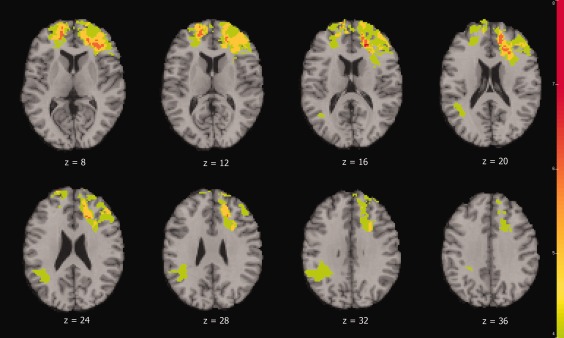
Color indicates the number of the 28 participants with apathy symptoms who had lesions overlapping at each voxel. We required a minimum of four lesion cases at each voxel for that voxel to be included in VLSM analysis. The left is on the viewer's right.
Grey matter VLSM analyses
Figures 3 and 4 as well as Table 2 show the results from the VLSM t‐test analysis for apathy. Lesioned regions in the left frontal lobe, ACC, insula, and supplementary motor cortex were associated with apathy. There were no lesioned regions in the right hemisphere associated with apathy.
Figure 3.
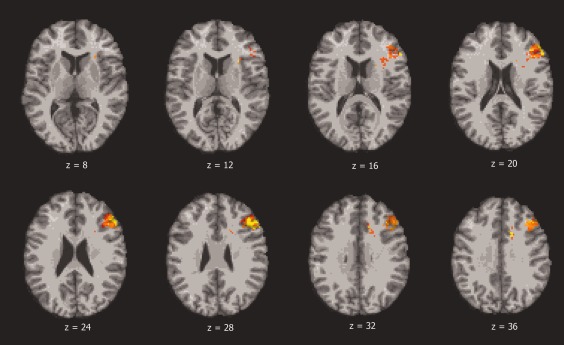
Color indicates brain regions where the association between voxel lesion location and apathy score is statistically significant, based on t‐tests and after correction for multiple comparisons (FDR). The left is on the viewer's right.
Figure 4.
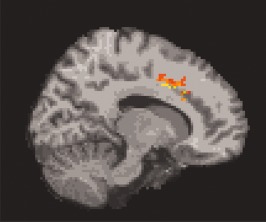
Sagittal image showing the cingulate lesions associated with apathy.
Table 2.
AAL labels of the damaged brain regions associated with apathy (t‐test, FDR = 0.05 correction for multiple comparisons)
| Location | Hemisphere | x | y | z | Volume (voxels) | Z |
|---|---|---|---|---|---|---|
| Middle frontal, inferior frontal operculum and triangular, insula | Left | −58 | 28 | 20 | 882 | 5.20 |
| Superior frontal, supplementary motor area, anterior cingulate | Left | −14 | 24 | 32 | 117 | 5.41 |
| Inferior frontal triangular, insula | Left | −36 | 28 | 2 | 14 | 4.24 |
Corresponds with Figure 3. x, y, z, Talairach coordinates.
White matter VLSM analysis
Injury to several areas of white matter, all in the left hemisphere, was significantly associated with apathy. These areas were the superior and anterior corona radiata (ACR), and the genus and body of the corpus callosum (Table 3).
Table 3.
White matter where damage was associated with apathy (t‐test, FDR = 0.05 correction for multiple comparisons)
| % | Structure | Hemisphere | Z‐value | Cohen's d |
|---|---|---|---|---|
| 3.06 | Anterior corona radiata | Left | 4.24 | 2.17 |
| 2.27 | Superior corona radiata | Left | 4.75 | 2.09 |
| 0.20 | Genu of corpus callosum | Left | 4.15 | 2.17 |
| 0.10 | Corpus callosum | Left | 4.75 | 2.05 |
Ad‐hoc analyses
To investigate whether damage to apathy‐related lesion areas was associated with cognitive deficits, we compared the control group with the two lesion groups resulting from our VLSM analysis (those with lesions in areas associated with apathy and those with lesions only in other areas), and found that the control group scored significantly better than both lesion groups [control group vs. nonapathy related lesion group: AFQT, 69.63 ± 21.53 vs. 55.28 ± 24.28, t(191) = 3.73, P < 0.001; WMS Working Memory, 65.06 ± 26.33 vs. 52.50 ± 27.96, t(190) = 2.79, P = 0.006; and MMSE, 29.12 ± 1.32 vs. 28.62 ± 1.70, t(107.69) = 2.13, P = 0.04]; [control group vs. apathy‐related lesion group: AFQT, 69.63 ± 21.53 vs. 45.07 ± 24.20, t(78) = 4.69, P < 0.001; WMS Working Memory, 65.06 ± 26.33 vs. 37.29 ± 26.47, t(77) = 4.48, P < 0.001; and MMSE, 29.12 ± 1.32 vs. 27.73 ± 3.18, t(29.63) = 2.14, P = 0.04]. The nonapathy‐related lesion group scored significantly better than the apathy‐related lesion group on the AFQT, t(169) = 2.07, P = 0.04, and the WMS Working Memory test, t(167) = 2.65, P = 0.009, but not the MMSE, t(27.71) = 1.39, P = 0.18.
To investigate whether damage to apathy‐related lesion areas was associated with depression in our subjects, we compared the two lesion groups (apathy‐related and not) in BDI scores and found no significant difference (9.97 ± 9.80 vs. 9.00 ± 8.97, t(173) = 0.52, P = 0.60). To investigate whether damage to apathy‐related lesion areas was associated with fatigability in our subjects, we compared the two lesion groups and found a significant difference (2.26 ± 1.56 vs. 1.57 ± 1.14, t(31.83) = 2.17, P = 0.038), meaning that those with lesions in the areas associated with apathy had higher fatigability scores than those with lesions only in other areas. To further investigate fatigability, we ran a post‐hoc VLSM analysis and found several areas that overlapped with our VLSM results for apathy. The overlapping areas include left middle frontal, inferior frontal operculum and triangular, insula, and superior frontal (medial and orbital) regions. The fatigability‐only results included left inferior and middle orbital frontal, rectus, pre‐ and post‐central, and middle temporal, plus right middle frontal orbital, middle frontal, superior medial frontal, and superior frontal regions.
Since lesion size could be a confounding variable, we investigated whether veterans with lesions in areas associated with apathy had greater volume loss than those with lesions only in other areas. We performed a t‐test comparing these two groups and found that veterans with lesions in areas associated with apathy had greater brain volume loss than those veterans with lesions only in other locations (5.46% ± 4.50% vs. 2.42% ± 2.90%, t(34.12) = 3.55, P = 0.001). Then, to investigate the potential impact of brain volume loss, we performed a correlational analysis between brain volume loss and apathy in the TBI group. The result was not significant (r s = 0.09, P = 0.25).
DISCUSSION
While pTBI affected intelligence, memory, and cognitive status, it did not increase apathy in general; however, damage to specific areas including the left middle, superior, and inferior frontal regions (including the operculum and pars triangularis), supplementary motor area, ACC, and insula, was associated with greater apathy. Damage to white matter near the ACC, that is, the corona radiata and corpus callosum, was also associated with greater apathy. Other studies using patients with AD, PD, ALS, and arteriopathy also found cingulate and frontal areas associated with apathy. Specifically, Apostolova et al. 2007 found gray matter atrophy in the bilateral cingulate (BA 24) and left medial frontal cortex/supplementary motor area (BA 8/9) correlated with apathy in AD patients. Reijnders et al. 2010 found lower gray matter density in inferior frontal gyrus (IFG) and insula associated with apathy in PD patients. Tunnard et al. 2010 found significantly greater cortical thinning in the left pars triangularis and ACC associated with apathy in AD patients. Woolley et al. 2011 found a negative correlation between FA and apathy scores in anterior cingulate white matter (although on the right) in ALS patients, and Jouvent et al. 2011 found the width of left anterior cingulate sulcus was associated with apathy in patients with arteriopathy.
Within the TBI group, we found a small correlation between apathy and postinjury (but not preinjury) intelligence as measured by the AFQT. When we divided the TBI group into those with lesions in areas associated with apathy and those with lesions only in other areas, those with lesions in areas associated with apathy had worse AFQT scores and working memory scores. We also found a correlation between apathy and current depression in the TBI group; this has been found in other patient studies as well [Jouvent et al., 2011; Reijnders et al., 2010]. We found, however, no significant differences in depression between those veterans with lesions in areas associated with apathy and those with lesions only in other areas. We did find that veterans with lesions in areas associated with apathy had greater brain volume loss than those with damage only in other areas; however, further testing showed no correlation between brain volume loss and apathy in the whole TBI group.
The signs of fatigue, defined as a feeling of constant exhaustion causing difficulty in initiation of or sustaining voluntary activities [Chaudhuri and Behan, 2004], can overlap those of apathy. While we found no significant correlation between apathy and Krupp's fatigue severity scale (FSS) scores, we did find a correlation between apathy and NRS fatigability scores. That the FSS is a self‐rating scale while a clinician completes the NRS may contribute to these differing results, as brain damage can decrease self‐insight [Dujardin et al., 2008; Levin et al., 1987; Robert et al., 2002; Starkstein et al., 2001]. Also, these differing results may be due to the third party having difficulty distinguishing apathy and fatigue (fatigability), since externally they may look similar. Supporting this, we found that veterans with lesions in areas associated with apathy had greater fatigability scores than those with lesions only in other areas, and the results from our apathy and fatigability VLSM analyses overlapped in several areas. Others have also found the areas we found associated with apathy to be associated with fatigue. Cook et al. 2007 found that greater activation in left middle cingulate and right IFG (we found left IFG) was associated with self‐ratings of mental fatigue made immediately after a difficult cognitive task in those suffering from chronic fatigue syndrome. Kohl et al. 2009, using a cognitive digit matching task in moderate‐to‐severe TBI patients and brain activation‐based measures of fatigue, found activation in the left middle frontal gyrus associated with within‐run fatigue, and in the left anterior cingulate associated with across‐run fatigue. Other studies have found brain areas associated with fatigue that differ from the brain areas we found associated with apathy, so there is not complete overlap for the two. For example, using the same task as Kohl et al. 2009, DeLuca at al. 2008 found a fatigue interaction (where patients had increasing activation and healthy controls had decreasing activation within runs) in medial/orbital frontal gyrus in multiple sclerosis patients; we did not find these regions associated with apathy. They also found across‐run fatigue interactions in superior, middle and inferior frontal areas, areas we did find associated with apathy. Using the same cohort as the current study, Pardini et al. 2010 compared participants with at least 15% damage to their ventromedial PFC (vmPFC) to those with dlPFC damage and to normal controls (NCs) and found FSS scores were higher in the vmPFC‐damaged group than the dlPFC‐damaged group and the NCs, while we did not find apathy associated with the vmPFC.
Apathy may be at least partially caused by reductions in attentional control or motivation. The ACC is involved in affective modulation of both, along with sensory and emotional information processing [Miller and Cummings, 1999; Sescousse et al., 2010; Woolley et al., 2011]. It is strongly implicated in reinforcement‐guided decision making, especially for decisions involving movement and effort [Rushworth et al., 2007]. ACC lesioned rats avoid choices involving greater effort even when the reward is four times greater [Rudebeck et al., 2006]. In humans, the ACC is involved in willingness to pay [Plassmann et al., 2007]. Guimarães et al.'s [2008] model proposes that dysfunction of the ACC is a key component in apathy due to its role in decision‐making, specifically in evaluating action and outcomes. Migneco et al. 2001 suggests that the cingulate is the cortical gateway through which limbic motivation influences goal‐directed behavior. The insula and the ACC work together [Nieuwenhuys, 2012]. The insula has a role in emotion, attention, cognitive control, awareness of body states, and time perception; in sum, awareness [Craig, 2009; Damasio, 1994; Nieuwenhuys, 2012]. Damage to the insula can result in anergia and tiredness [Manes et al., 1999]. Therefore, damage to the ACC and insula could lead to reduced motivated behavior, namely apathy, due to lack of awareness of emotional and motivational states. These areas feed into an ascending pathway to the dorsolateral PFC, which connects to the premotor and motor areas, to initiate a behavioral response [Guimarães et al., 2008; Tunnard et al., 2010]. The supplementary motor area is also involved in initiating motor responses. When any part of this circuit is damaged, decision making and/or response initiation are impaired, resulting in apathetic behavior [Guimarães et al., 2008; Tunnard et al., 2010]. Our results support this model, as we found damage to the ACC, frontal cortex, supplementary motor area, and insula associated with increased apathy.
The white matter tracts we found associated with apathy are all located near the ACC. The effect of white matter lesions in the anterior and superior corona radiata, and the body and genus of the corpus callosum is consistent with a diffusion tensor imaging (DTI) study of HIV patients, which found apathy associated with low FA values in the anterior and superior corona radiata and the genu of the corpus callosum [Hoare et al., 2010]. The corona radiata includes projection, association, and callosal fibers; the corpus callosum connects corresponding areas from opposite hemispheres [Wakana et al., 2004], and the ACR connects with the ACC [Niogi et al., 2008]. This white matter damage may be a factor in the lack of recovery in these veterans after more than 35 years, as extent of white matter damage has been shown to be one of the factors affecting plasticity and the likelihood for long term recovery [Ludlow et al., 1986].
The damaged areas that we found associated with apathy were all in the left hemisphere. Consistent with our results, Holthoff et al. 2005 found apathy associated with hypometabolism in left inferior and medial frontal cortex, and Benoit et al. 2004 found hypoperfusion in the left middle frontal gyrus; however, a study by Benoit et al. 2002 found hypoperfusion in the right IFG and bilaterally in the medial frontal cortex. Apathy has been associated with changes in the left ACC [Jouvent et al., 2011; Lanctôt et al., 2007; Tunnard et al., 2010], consistent with our results, although most studies report bilateral ACC changes [Apostolova et al., 2007; Benoit et al., 1999, 2002; Bruen et al., 2008; Marshall et al., 2007; Migneco et al., 2001; Robert et al., 2006]. Woolley reports reduced FA in right ACC white matter correlated with apathy changes scores [Woolley et al., 2011], contrary to the left hemisphere white matter lesions we found.
Strengths of our study include our data analysis method, VLSM, which allows whole brain analysis without being restricted to a priori regions of interest; our large sample size; and our homogeneous study population, which consisted of male Vietnam veterans of uniform age, education, and ethnicity; however, this homogeneity also makes the generalizability of the results to other populations uncertain. Another caveat is that our lesion groups differed in total brain volume loss, in that those with lesions in areas associated with apathy had significantly more volume loss on average than those with lesions only in other areas. This makes it difficult to disentangle the effects of lesion location and brain volume loss. However, across all lesioned participants, there was no significant correlation between brain volume loss and apathy. Another limitation is that the apathy measure was obtained from ratings of people who knew the participants well, and therefore reflects observed behavior, not first person feelings, and many of the participants scored zero for apathy. Finally, the necessary use of CT rather than MRI resulted in a less‐detailed picture of the neuroanatomy involved.
In summary, we detected an effect of focal brain damage on NPI apathy. In addition, apathy was negatively correlated with postinjury intelligence, and positively correlated with BDI scores and fatigability. We found that the neural correlates of fatigability and apathy overlapped to some degree in that both included ACC and the frontal cortex. Increased apathy was also associated with damage to the supplementary motor area and insula, and to white matter in the left corona radiata and corpus callosum. To our knowledge, this is the largest study of the neural correlates of apathy in those with focal brain lesions. Apathy is a significant symptom since it can reduce participation of the patient in family and other social interactions, and diminish affective decision‐making. The identification of a reliable relationship between symptoms of apathy and lesion location allows clinicians to inform caregivers of potential issues that can arise if a patient is apathetic and of techniques to help patients improve their day‐to‐day functioning.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, nor the United States Government.
ACKNOWLEDGMENTS
The authors especially thank the Vietnam War veterans who participated in this study. They thank the National Naval Medical Center for their support and provision of their facilities and S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for their invaluable help with the testing of participants and organization of this study. They also thank Michael Tierney for his help with the study database.
REFERENCES
- Aalten P, Verhey FRJ, Boziki M, Brugnolo A, Bullock R, Bryne EJ, Camus V, Caputo M, Collins D, De Deyn PP, et al. (2008): Consistency of neuropsychiatric syndromes dementias: Results from the European Alzheimer Disease Consortium. Dementia Geriatric Cogn Disorders 25:1–8. [DOI] [PubMed] [Google Scholar]
- Andersson S, Krogstad JM, Finset A (1999): Apathy and depressed mood in acquired brain damage: Relationship to lesion localization and psycholphysiological reactivity. Psychol Med 29:447–456. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM (2007): Structural correlates of apathy in Alzheimer's Disease. Dementia Geriatric Cogn Disorders 24:91–97. [DOI] [PubMed] [Google Scholar]
- Beck AT (1978): Beck Depression Inventory. San Antonio:Harcourt Brace and Company. [Google Scholar]
- Benoit M, Clairet S, Koulibaly PM, Darcourt J, Robert PH (2004): Brain perfusion correlates of the Apathy Inventory dimensions of Alzheimer's disease. Int J Geriatric Psychiatry 19:864–869. [DOI] [PubMed] [Google Scholar]
- Benoit M, Dygai I, Migneco O, Robert PH, Bertogliati C, Darcourt J, Benoliel J, Aubin‐Brunet V, Pringuey D (1999): Behavioral and psychological symptoms in Alzheimer's disease. Dementia Geriatric Cogn Disorders 10:511–517. [DOI] [PubMed] [Google Scholar]
- Benoit M, Koulibaly PM, Migneco O, Darcourt J, Pringuey DJ, Robert PH (2002): Brain perfusion in Alzheimer's disease with and without apathy: A SPECT study with statistical parametric mapping analysis. Psychiatry Res Neuroimaging 114:103–111. [DOI] [PubMed] [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A (2008): Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain 131:2455–2463. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO (2004): Fatigue in neurological disorders. Lancet 363:978–988. [DOI] [PubMed] [Google Scholar]
- Ciurli P, Formisano R, Bivona U, Cantagallo A, Angelelli P (2011): Neuropsychiatric disorders in persons with severe traumatic brain injury: Prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J Head Trauma Rehab 26:116–126. [DOI] [PubMed] [Google Scholar]
- Cook DB, O'Connor PJ, Lange G, Steffener J (2007): Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. NeuroImage 36:108–122. [DOI] [PubMed] [Google Scholar]
- Corrigan J, Dickerson J, Fisher E, Meyer P (1990): The Neurobehavioural Rating Scale: Replication in an acute, inpatient rehabilitation setting. Brain Injury 4:215–222. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel ‐ now? The anterior insula and human awareness. Neuroscience 10:59–70. [DOI] [PubMed] [Google Scholar]
- Craig AH, Cummings JL, Fairbanks L, Itti L, Miller BL, Li J, Mena I (1996): Cerebral blood flow correlates of apathy in Alzheimer disease. Arch Neurol 53:1116–1120. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega MS, Gray JA, Rosenberg‐Thompson S, Carusi DA, Gornbein J (1994): The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314. [DOI] [PubMed] [Google Scholar]
- Damasio A (1994): Descartes' Error: Emotion,Reason, and the Human Brain. New York:Grosset/Putnam. [Google Scholar]
- DeLuca J, Genova HM, Hillary FG, Wylie G (2008): Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurological Sci 270:28–39. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Sockeel P, Delliaux M, Destée A, Defebvre L (2008): The Lille Apathy Rating Scale: Validation of a caregiver‐based version. Mov Disorders 23:845–849. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, editors.2002. Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Non‐patient Edition. (SCID‐I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): Mini‐mental test. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R (2009): Lesion mapping of cognitive abilities linked to intelligence. Neuron 61:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Jonas BS, Martin A, Salazar AM, Weingartner H, Ludlow C, Smutok MA, Vance SC (1988): Intellectual function following penetrating head injury in Vietnam veterans. Brain 111:169–184. [DOI] [PubMed] [Google Scholar]
- Greene JG, Smith R, Gardiner M, Timbury GC (1982): Measuring behavioral disturbance of elderly demented patients in the community and its effects on relatives: A factor analytic study. Age Aging 11:121–126. [DOI] [PubMed] [Google Scholar]
- Guimarães HC, Levy R, Teixeira AL, Beato RG, Caramelli P (2008): Neurobiology of apathy in Alzheimer's disease. Arq Neuro‐psiquiatr 66:436–443. [DOI] [PubMed] [Google Scholar]
- Hoare J, Fouche J‐P, Spottiswoode B, Joska JA, Schoeman R, Stein DJ, Carey PD (2010): White matter correlates of apathy in HIV‐positive subjects: A diffusion tensor imaging study. J Neuropsychiatry Clin Neurosci 22:313–320. [DOI] [PubMed] [Google Scholar]
- Holthoff VA, Beuthien‐Baumann B, Kalbe E, Lüdecke S, Lenz O, Zündorf G, Spirling S, Schierz K, Winiecki P, Sorbi S,et al.(2005): Regional cerebral metabolism in early Alzheimer's disease with clinically significant apathy or depression. Biol Psychiatry 57:412–421. [DOI] [PubMed] [Google Scholar]
- Jennett B, Bond M (1975): Assessment of outcome after severe brain damage: A practical scale. Lancet 1:480–484. [DOI] [PubMed] [Google Scholar]
- Jouvent E, Reyes S, Mangin J‐F, Roca P, Perrot M, Thyreau B, Hervé D, Dichgans M, Chabriat H (2011): Apathy is related to cortex morphology in CADASIL. Neurology 76:1472–1477. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, MacMillan A, Ketchel P, DeKosky ST (1998): Assessing the impact of neuropsychiatric symptoms in Alzheimer's disease: The neuropsychiatric inventory caregiver distress scale. J Am Geriatrics Soc 46:210–215. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lee DY, Choo ILH, Seo EH, Kim SG, Park SY, Woo JM (2011): Microstructural alteration of the anterior cingulum is associated with apathy in Alzheimer Disease. Am J Geriatric Psychiatry 19:644–653. [DOI] [PubMed] [Google Scholar]
- Kirsch‐Darrow L, Marsiske M, Okun M, Bauer RM, Bowers D (2011): Apathy and depression: Separate factors in Parkinson's Disease. J Int Neuropsychol Soc 17:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl AD, Wylie GR, Genova HM, Hillary FG, Deluca J (2009): The neural correlates of cognitive fatigue in traumatic brain injury using functional MRI. Brain Injury 23:420–432. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD (1989): The Fatigue Severity scale. Arch Neurol 46:1121–1123. [DOI] [PubMed] [Google Scholar]
- Kumral E, Bayulkem G, Evyapan D, Yunten N (2002): Spectrum of anterior cerebral artery territory infarction: Clinical and MRI findings. Eur J Neurol 9:615–624. [DOI] [PubMed] [Google Scholar]
- Lanctôt KL, Moosa S, Herrmann N, Leibovitch FS, Rothenburg L, Cotter A, Black SE (2007): A SPECT study of apathy in Alzheimer's disease. Dementia Geriatric Cogn Disorders 24:65–72. [DOI] [PubMed] [Google Scholar]
- Landes AM, Sperry SD, Strauss ME, Geldmacher DS (2001): Apathy in Alzheimer's disease. J Am Geriatrics Soc 49:1700–1707. [DOI] [PubMed] [Google Scholar]
- Laplane D, Degos JD, Baulac M, Gray F (1981): Bilateral infarction of the anterior cingulate gyri and of the fornices. J Neurol Sci 51:289–300. [DOI] [PubMed] [Google Scholar]
- Levin HS, High WM, Goethe KE, Sisson R, Overall J, Rhoades H, Eisenberg HM, Kalisky Z, Gary HE (1987): Neurobehavioural rating scale: Assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry 50:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, Paulsen JS, Litvan I (1998): Apathy is not depression. J Neuropsychiatry Clin Neurosci 10:314–319. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Rosenberg J, Fair C, Buck D, Schesselman S, Salazar A. (1986): Brain lesions associated with nonfluent aphasia fifteen years following penetrating head injury. Brain 109:55–80. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J (2002): Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput 34:6–18. [DOI] [PubMed] [Google Scholar]
- Manes F, Paradiso S, Robinson RG (1999): Neuropsychiatric effects of insular stroke. J Nervous Mental Dis 187:707–712. [DOI] [PubMed] [Google Scholar]
- Marin RS (1990): Differential diagnosis and classification of apathy. Am J Psychiatry 147:22–30. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL (2006): Neuropathologic correlates of apathy in Alzheimer's disease. Dementia Geriatric Cogn Disorders 21:144–147. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL (2007): Positron emission tomography metabolic correlates of apathy in Alzheimer's disease. Arch Neurol 64:1015–1020. [DOI] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, Libon DJ, Grossman M (2009): Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dementia Geriatric Cogn Disorders 27:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migneco O, Benoit M, Koulibaly PM, Dygai I, Bertogliati C, Desvignes P, Robert PH, Malandain G, Bussiere F, Darcourt J (2001): Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: A study in Alzheimer's disase and nondemented patients. Neuroimage 13:896–902. [DOI] [PubMed] [Google Scholar]
- Miller BL, Cummings JL (1999): The Human Frontal Lobes: Functions and Disorders. New York:Guilford Press. [Google Scholar]
- Mori S, Wakana S, Nagae‐Poetscher LM, van Zijl PCM (2005): MRI Atlas of Human White Matter. Amsterdam:Elsevier. [Google Scholar]
- Mortby ME, Maercker A, Forstmeier S (2011): Apathy: A separate syndrome from depression in dementia? Aging Clin Exp Res. Epub ahead of print. DOI 10.3275/8105. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R (2012): The insular cortex: A review. Prog Brain Res 195:123–163. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Suh M, Zimmerman RD, Manley GT, McCandliss BD (2008): Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131:3209–3221. [DOI] [PubMed] [Google Scholar]
- Pardini M, Krueger F, Raymont V, Grafman J (2010): Ventromedial prefrontal cortex modulates fatigue after penetrating traumatic brain injury. Neurology 74:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A (2007): Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci 27:9984–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Krueger F, Grafman J (2011): “Studying injured minds” ‐ The Vietnam head injury study and 40 years of brain injury research. Frontiers Neurol 2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders JSAM, Scholtissen B, Weber WEJ, Aalten P, Verhey FRJ, Leentjens AFG (2010): Neuroanatomical correlates of apathy in Parkinson's Disease: A magnetic resonance imaging study using voxel‐based morphometry. Mov Disorders 25:2318–2325. [DOI] [PubMed] [Google Scholar]
- Robert PH, Clairet S, Benoit M, Koutaich J, Bertogliati C, Tible O, Caci H, Borg M, Brocker P, Bedoucha P (2002): The apathy inventory: Assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. Int J Geriatric Psychiatry 17:1099–1105. [DOI] [PubMed] [Google Scholar]
- Robert PH, Darcourt G, Koulibaly MP, Clairet S, Benoit M, Garcia R, Dechaux O, Darcourt J (2006): Lack of initiative and interest in Alzheimer's disease: A single photon emission computer tomography study. Eur J Neurol 13:729–735. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno‐Tempini ML, Weiner MW, Miller B (2005): Neuroanatomical correlates of behavioural disorders in dementia. Brain 128:2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth FS (2006): Separate neural pathways process different decision costs. Nat Neurosci 9:1161–1168. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME (2007): Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behavior. Trends Cogn Sci 11:167–176. [DOI] [PubMed] [Google Scholar]
- Schwab K, Grafman J, Salazar A, Kraft J (1993): Residual impairments and work status 15 years after penetrating head injury: Report from the Vietnam Head Injury Study. Neurology 43:95–103. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Redouté J, Dreher J‐C (2010): The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci 30:13095–13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J (2007): User‐friendly software for the analysis of brain lesions (ABLe). Comput Methods Programs Biomed 86:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD (2009): Neuroimaging correlates of apathy and depression in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 21:259–265. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Petracca G, Chemerinski E, Kremer J (2001): Syndromic validity of apathy in Alzheimer's Disease. Am J Psychiatry 158:872–877. [DOI] [PubMed] [Google Scholar]
- Tunnard C, Whitehead D, Hurt C, Wahlund lO, Mecocci P, Tsolaki M, Vellas B, Spenger C, Kloszewska I, Soininen H, et al. (2010): Apathy and cortical atrophy in Alzheimer's disease. Int J Geriatric Psychiatry 26:741–748. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PCM, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997a): Wechsler Adult Intelligence Scale, 3rd ed San Antonio, TX:Psychological Corporation. [Google Scholar]
- Wechsler D (1997b): Wechsler Memory Scale, 3rd ed San Antonio, TX:Psychological Corporation. [Google Scholar]
- Woods RP, Grafton ST, Watson J, Sicotte NL, Mazziotta JC (1998): Automated image registration. II. Intersubject validation of linear and nonlinear models. J Comput Assisted Tomography 22:153–165. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Zhang Y, Schuff N, Weiner MW, Katz JS (2011): Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotrophic Lateral Sclerosis 12:52–58. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J (2008): Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology 71:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]


